Abstract
Small numbers of the papers have studied the association between organophosphate (OP) poisoning and the subsequent acute kidney injury (AKI). Therefore, we used the National Health Insurance Research Database (NHIRD) to study whether patients with OP poisoning are associated with a higher risk to have subsequent AKI.
The retrospective cohort study comprised patients aged ≥20 years with OP poisoning and hospitalized diagnosis during 2000–2011 (N = 8924). Each OP poisoning patient was frequency-matched to 4 control patients based on age, sex, index year, and comorbidities of diabetes, hypertension, hyperlipidemia, chronic obstructive pulmonary disease, coronary artery disease, and stroke (N = 35,696). We conducted Cox proportional hazard regression analysis to estimate the effects of OP poisoning on AKI risk.
The overall incidence of AKI was higher in the patients with OP poisoning than in the controls (4.85 vs 3.47/1000 person-years). After adjustment for age, sex, comorbidity, and interaction terms, patients with OP poisoning were associated with a 6.17-fold higher risk of AKI compared with the comparison cohort. Patients with highly severe OP poisoning were associated with a substantially increased risk of AKI.
The study found OP poisoning is associated with increased risk of subsequent AKI. Future studies are encouraged to evaluate whether long-term effects exist and the best guideline to prevent the continuously impaired renal function.
INTRODUCTION
For the last 60 years, organophosphate (OP) poisoning has been one of the most crucial public health concerns worldwide. According to the World Health Organization's estimates, there are >3 million cases of OP pesticide poisoning annually; among these, >250,000 deaths are caused by intentional self-poisoning, accounting for 30% of suicides worldwide.1–3 OP pesticides are used to eliminate or control various pests, and most contact poisonings occur in the agricultural societies of developing countries where such toxic compounds are readily available.1 The mechanism of OP toxicity is the irreversible inhibition of the enzyme cholinesterase, leading to massive accumulation of the neurotransmitter acetylcholine within the synaptic cleft, and, thus, overstimulation of nicotinic and muscarinic receptors in the central and peripheral nervous system, which form various toxidromes.1,4,5 Even with adequate medical care in the setting of well-equipped intensive care units, the mortality rate could reach 40%, and respiratory failure represents the leading cause of death.1,6 Other toxicity-related complications include motor neuropathy, arrhythmia, pulmonary edema, pneumonia, pancreatitis, and renal failure.3,7,8
Acute kidney injury (AKI) is a worldwide concern with diverse etiologies and clinical presentations. Misidentifying or underestimating the problem may lead to severe adverse outcomes. According to the literature, critically ill patients who develop AKI are associated with high morbidity, resulting in long hospital stays, high medical expenditure, and a risk of long-term dialysis.9 However, few studies have investigated the correlation between OP poisoning and the subsequent risk of AKI. To obtain sufficient statistical power, we used a large-scale, nationwide database for analysis and sought to delineate the possible role of OP poisoning in the development of AKI in patients with no history of prior kidney disease.
METHODS
Data Source
The National Health Insurance (NHI) program was launched in Taiwan on March 1, 1995, and it consists of detailed healthcare data from >23 million enrollees, representing >99% of the population of Taiwan (http://www.nhi.gov.tw/english/index.aspx). The National Health Insurance Research Database (NHIRD) contains comprehensive information regarding clinical visits for each insured person, including the scrambled patient identification number, demographic characteristics, inpatient and outpatient dates, prescription details, and diagnostic codes based on the International Classification of Diseases, Ninth revision, Clinical Modification (ICD-9-CM). To protect the privacy of all the persons registered in the NHI program, the National Health Research Institutes encrypts and converts the identification numbers of all NHIRD records before releasing them for researchers. For this study, we used a subset of the NHIRD containing health care data including files of inpatient claims and the registry of beneficiaries. This study was exempted from informed consent by the institutional review board of China Medical University (CMUH-104-REC2-115).
Sampled Patients
Patients in the inpatient database with newly identified OP poisoning (ICD-9 code 989.3) from 2000–2011 were selected as the OP poisoning cohort. The date of the first admission for OP poisoning was used as the index date. For each patient with OP poisoning, we randomly selected 4 persons without OP poisoning from all NHI beneficiaries and frequency-matched them according to age (every 5-year span), sex, the year of index date, and comorbidities of diabetes, hypertension, hyperlipidemia, chronic obstructive pulmonary disease (COPD), coronary artery disease (CAD), and stroke. The index date in non–OP poisoning controls was randomly assigned a date in OP poisoning cases. In both cohorts, patients who were diagnosed with AKI (ICD-9 code 584), chronic kidney disease (ICD-9 codes 580–589), or end-stage renal disease (ICD-9 code 585) before the index date, or who had missing information for age and sex, were excluded from the data analysis. Those patients with a history of AKI were also excluded from the study (Fig. 1).
FIGURE 1.
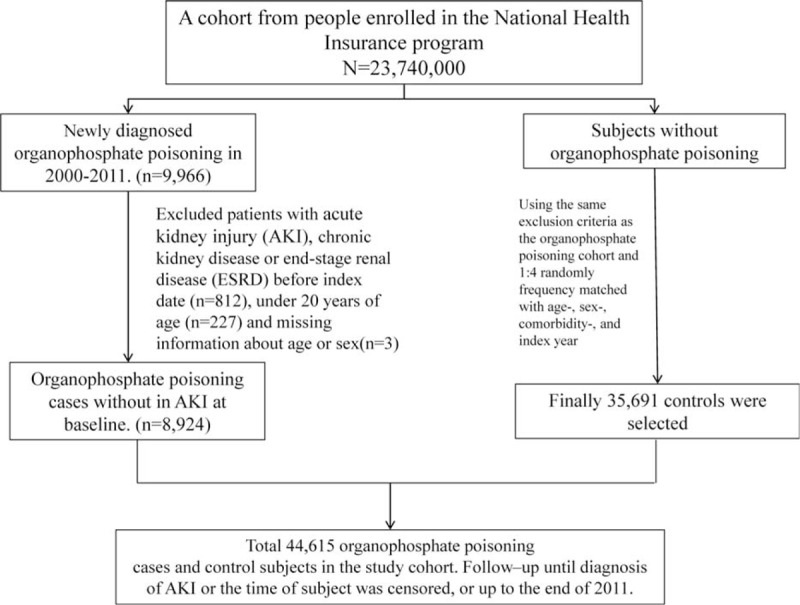
Derivation of our study cohort.
Outcome and Comorbidities
The main outcome was hospitalization with a new diagnosis of AKI (ICD-9 code 584) during the follow-up. All the studied patients were followed from the index date to the occurrence of AKI, withdrawal from the database, or December 31, 2011.
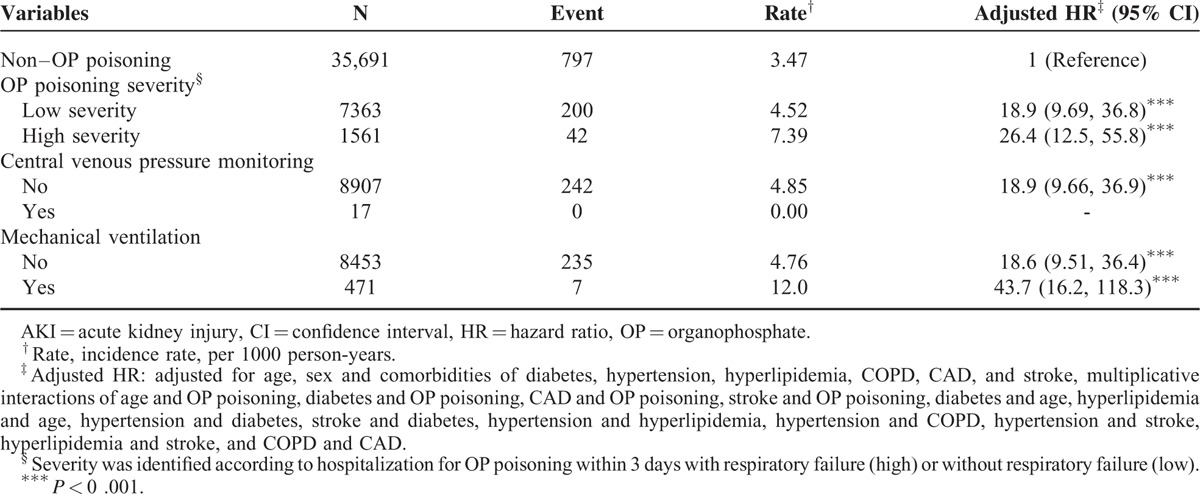
We also incorporated an inpatient diagnosis file to ascertain the comorbidities including diabetes (ICD-9 code 250), hypertension (ICD-9 codes 401–405), hyperlipidemia (ICD-9 code 272), COPD (ICD-9 code 491, 492, and 496), congestive heart failure (CHF) (ICD-9 code 428), CAD (ICD-9 codes 410–414), and stroke (ICD-9 codes 430–438). In addition, acute respiratory failure (ICD-9-CM code 518.81), central venous pressure monitoring (ICD-9-OP code 8962), and mechanical ventilation (ICD-9-OP codes 9390 and 967) were considered as severity indicators based on diagnoses in the admission records within the first 3 days of hospitalization.
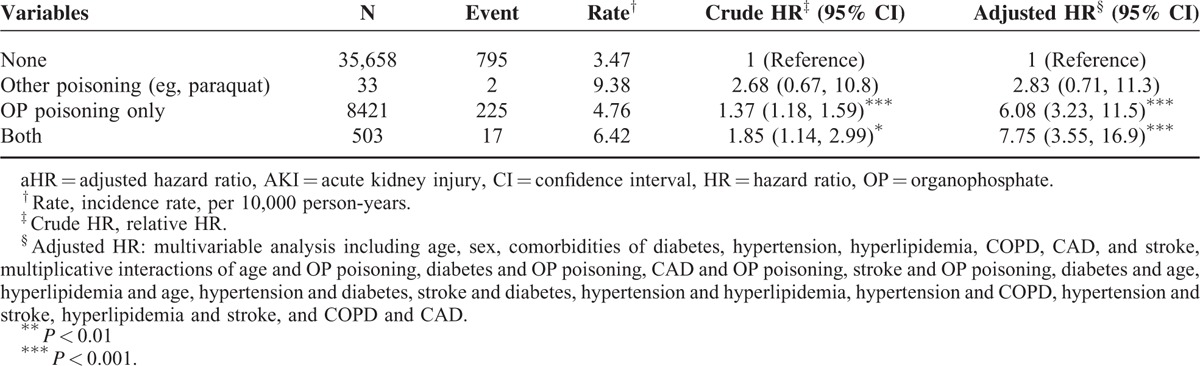
In the present study, we used ICD-9-CM code 989.3 (toxic effect of OP and carbamate) to identify patients with OP poisoning, which comprised our study cohort and separated from other types of poisoning (such as paraquat), which were classified and coded into another group (ICD-9-CM code 989.4). We also conducted an additional analysis to clarify the effect of other types of poisoning (ICD-9-CM code 989.4) on AKI.
Statistical Analysis
Distributions of age (≤34, 35–49, 50–64, and ≥65 years), sex, and comorbidities were compared between the OP poisoning and non–OP poisoning cohorts, and examined using the χ2 test. The median ages and median follow-up years of both cohorts were measured and compared using the Mann–Whitney U test. The follow-up times were used to estimate the incidence density rates of AKI in both cohorts. Univariate and multivariable Cox proportional hazards regression models were used to examine the effect of OP poisoning on the risk of AKI development, as shown by hazard ratios (HRs) with 95% confidence intervals (CIs). The multivariable models simultaneously adjusted for age, sex, comorbidities of diabetes, hypertension, hyperlipidemia, COPD, CHF, CAD, and stroke, and multiplicative interactions of age and OP poisoning, diabetes and OP poisoning, CAD and OP poisoning, stroke and OP poisoning, diabetes and age, hyperlipidemia and age, hypertension and diabetes, stroke and diabetes, hypertension and hyperlipidemia, hypertension and COPD, hypertension and stroke, hyperlipidemia and stroke, and COPD and CAD. The HRs of the OP poisoning and non–OP poisoning cohort and the 95% CIs of AKI were estimated using the Cox model. The Kaplan–Meier method was used to estimate the cumulative incidence of AKI for the OP poisoning and non–OP poisoning cohorts, and the log-rank test was used to test the difference between the curves. All the statistical analyses were performed using SAS software (version 9.3 for Windows; SAS Institute, Inc, Cary, NC). A 2-tailed P < 0.05 was considered significant.
RESULTS
Table 1 displays the distributions of age, sex, and comorbidities of the 2 cohorts. Most patients were aged ≥50 years (57.1% in both cohorts) and men (69.8% in both cohorts). The median ages of the OP poisoning and non–OP poisoning cohorts were 53.7 and 53.3 years, respectively. The OP poisoning cohort and the non–OP poisoning cohort were well matched for comorbidities. The median duration of follow-up was 5.73 (95% CI 5.51–5.67) years in the OP poisoning cohort and 6.71 (95% CI 6.39–6.46) years in the non–OP poisoning cohort. The cumulative incidence of AKI was higher in the OP poisoning cohort than in the non–OP poisoning cohort (log-rank test P < 0.001) by the end of the follow-up (Fig. 2).
TABLE 1.
Demographic characteristics and comorbidities in cohorts with and without organophosphate poisoning

FIGURE 2.
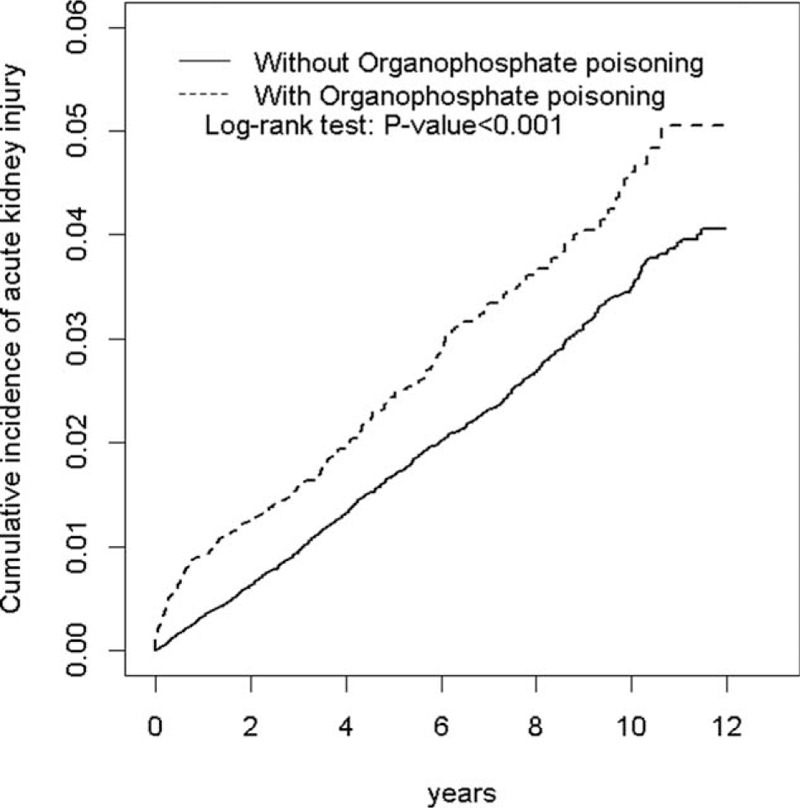
Cumulative incidence comparison of AKI for patients with (dashed line) or without (solid line) OP poisoning. AKI = acute kidney injury, OP = organophosphate.
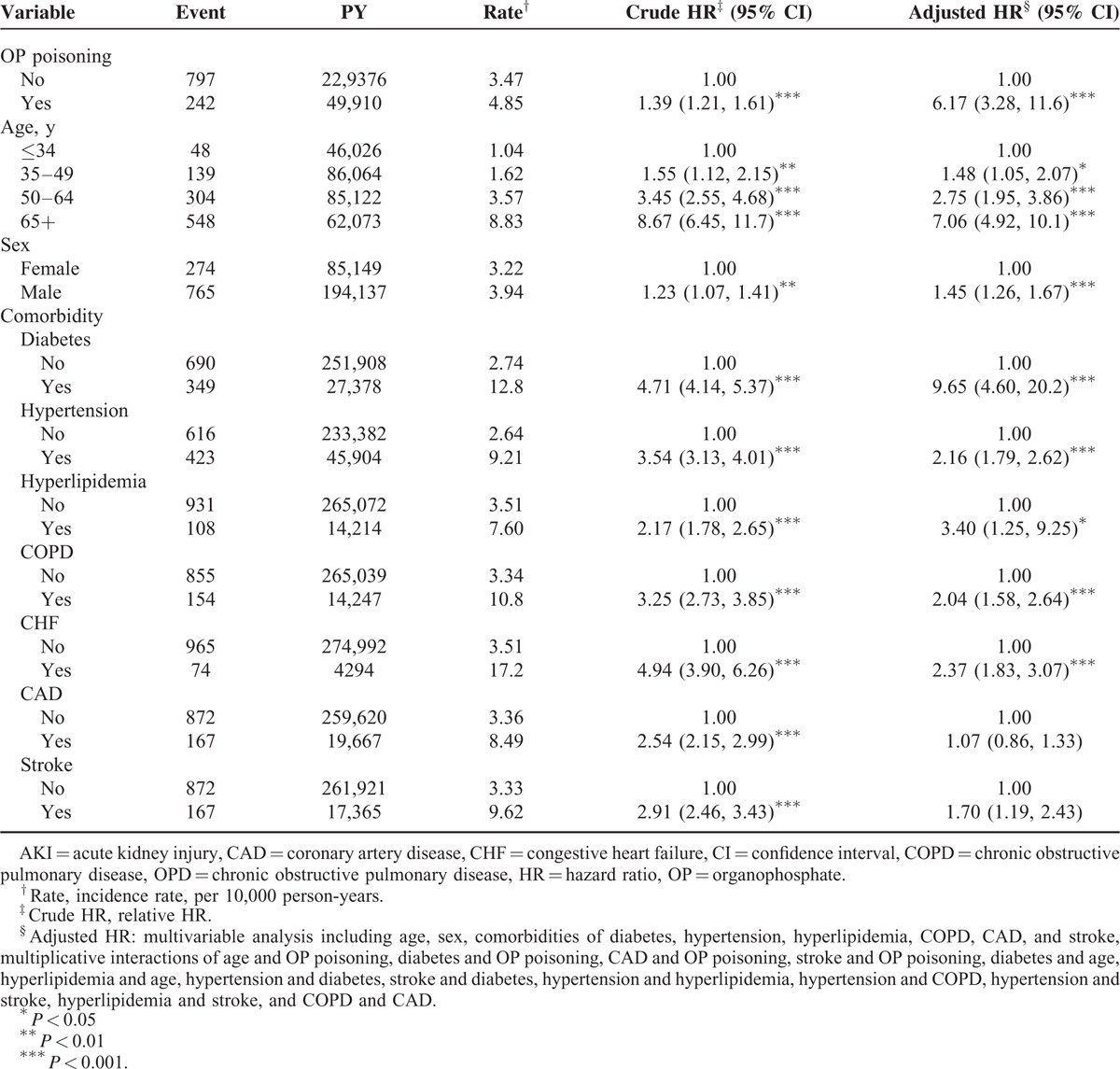
The incidence of AKI in the OP poisoning cohort was 4.85 per 1000 person-years, which was 1.39-fold higher than that in the non–OP poisoning cohort (3.47/1000 person-years), with an adjusted HR of 6.17 (95% CI 3.28–11.6) (Table 2). The incidence of AKI increased as age increased, in men more than in women and among patients with comorbidity. In the multivariable model, the adjusted HR of AKI was 7.06-fold (95% CI 4.92–10.1) higher for patients aged ≥65 years, compared with patients aged <34 years, and 1.45-fold higher for men than for women (95% CI 1.26–1.67). Patients with diabetes, hypertension, hyperlipidemia, COPD, and CHF were also significantly associated with an increased risk of AKI.
TABLE 2.
Incidence (per 1000 Person-Years) and HR for AKI and AKI-associated Risk Factor
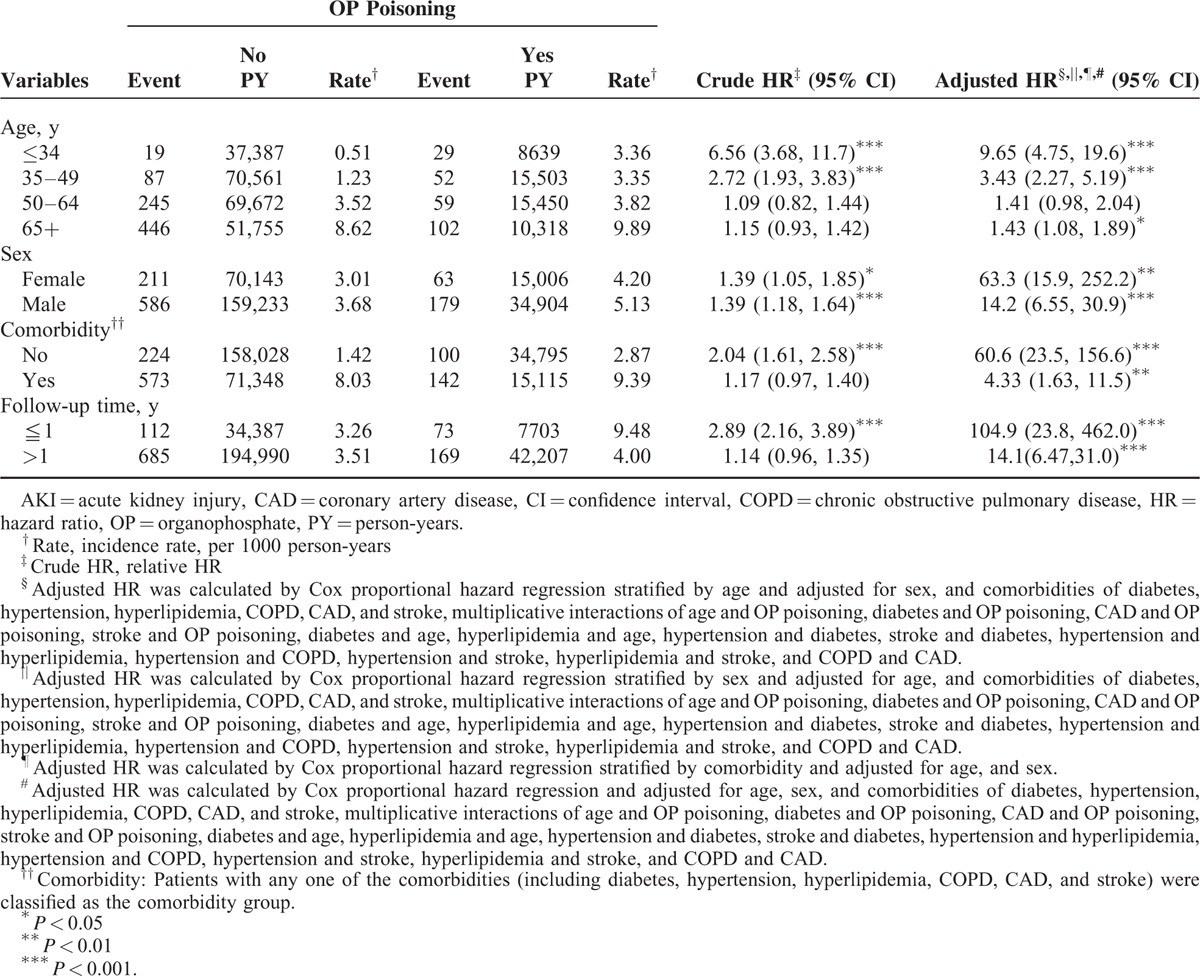
The relative risk of AKI in the age-specific OP poisoning cohort compared with the non–OP poisoning cohort was higher for all age groups, except for the group aged 50 to 64 years (adjusted HR 9.65, 95% CI 4.75–19.6 for ages ≤34 years; adjusted HR 3.43, 95% CI 2.27–5.19 for ages 35–49 years; adjusted HR 1.43, 95% CI 1.08–1.89 for ages 65+ years, respectively) (Table 3). The adjusted HR of AKI in the sex-specific OP poisoning cohort and non–OP poisoning cohort was significant for both women and men. The risk of AKI was higher in the OP poisoning cohort than in the non–OP poisoning cohort for patients without comorbidity and with comorbidity. In the first year of follow-up, the OP poisoning cohort had a higher risk of AKI than that of the non–OP poisoning cohort (adjusted HR 104.9, 95% CI 23.8–462.0). Moreover, the risk of developing AKI in the OP poisoning cohort was significantly higher than that in the non–OP poisoning cohort with >1 year follow-up (adjusted HR 14.1, 95% CI 6.47–31.0).
TABLE 3.
Incidence and Cox Model–Measured HR of AKI for Patients With OP Poisoning Compared With Those Without OP Poisoning by Age, Sex, Comorbidity and Follow-Up Time
Compared with patients without OP poisoning, patients with severe OP poisoning were 26.4-fold more likely to develop AKI (95% CI 12.5–55.8), followed by patients with less severe OP poisoning (adjusted HR 18.9, 95% CI 9.69–36.8). Compared with patients without OP poisoning, patients with OP poisoning and mechanical ventilation were 43.7-fold more likely to develop AKI (95% CI, 16.2–118.3) (Table 4).
TABLE 4.
Cox Proportional Hazard Regression Analysis for the Risk of AKI Stratified by the Severity of OP Poisoning
Compared with patients without OP and without other poisoning (eg, paraquat), patients with both OP and other poisoning (eg, paraquat) poisoning were 7.75-fold more likely to develop AKI (95% CI 3.55–16.9), followed by patients with OP poisoning only (adjusted HR 6.08, 95% CI 3.23–11.5) (Table 5). In addition, there was absence of renal tumor events (ICD-9-CM codes 189.0 and 189.1) in our study cohort during the following period.
TABLE 5.
Incidence and aHR of AKI Between Different Types of Poisoning
DISCUSSION
Based on a literature review, this is the first population-based, retrospective, and longitudinal cohort study to show an association between OP poisoning and the subsequent risk of AKI development. The results indicate that patients with OP poisoning have a higher overall incidence and crude risk of developing AKI. After adjustment for possible confounders including age, sex, comorbidity, and interaction terms, the data remained statistically significant, indicating that patients with OP poisoning were at a 6.17-fold higher risk of developing AKI compared with the general population.
OP compounds are frequently used as pesticides, herbicides, and even chemical warfare agents, accounting for the greatest poison-related morbidity and mortality in agricultural societies.6 Dharmani and Jaga10 concluded from an epidemiologic review that suicidal and occupational poisonings were more prevalent in developing countries, whereas accidental poisonings were more prevalent in developed countries. In 1995, the Aum Shinrikyo Tokyo subway attack was one of the most lethal acts of chemical terrorism ever committed. The nerve agent sarin, a potent highly toxic OP compound was released by a religious cult and ultimately resulted in 12 deaths and ∼5500 injuries.11,12 Nowadays, the spread of terrorism around the world has highlighted the crucial role in the management of OP poisoning.
The clinical process of acute OP poisoning can be characterized by the following 3 phases: acute cholinergic crisis, intermediate syndrome (IMS), and OP-induced delayed neuropathy (OPIDN). Acute cholinergic crisis develops within a few minutes to hours after exposure to OP pesticides, with typical manifestations including nausea, vomiting, abdominal cramps, urinary incontinence, miosis, salivation, lacrimation, bronchorrhea, bradycardia, bronchospasm, hypotension, muscle fasciculation, paralysis, confusion, seizure, coma, and death.3,13 OPIDN occurs 2 to 5 weeks after acute OP exposure, with presentations of motor neuropathy, numbness, and weakness of the lower extremities, followed by progression to the proximal limbs and perhaps paraplegia, quadriplegia, or impotence,3 which may last for years. The specific toxidromes that occur between the end of a cholinergic crisis and the onset of OPIDN, termed IMS, are characterized by prominent weakness of proximal limb muscles, neck flexors, respiratory muscles, and motor cranial nerve involvement.3,6 Most fatalities from acute OP poisoning could be attributed substantially to respiratory failure through the following effects: depression of the respiratory center in the brainstem, neuromuscular paralysis, excessive respiratory secretions, and bronchoconstriction.1,14,15 Reportedly, renal impairment complicated by OP poisoning is rare or may be overlooked clinically but is more frequent and strongly related to late mortality in critically ill patients or patients with severe OP poisoning.16–18 In September 2004, the term AKI was proposed to reflect the entire spectrum of acute decline in renal function. AKI is currently defined by either change in serum creatinine or urine output,19 which affects 1% of the general population and 15% of hospitalized patients.20 Persistent decline of renal function without recovery can lead to end-stage renal disease and increase the short-term and long-term risk of death.21–23 In our study, we used the Cox regressions model to adjust for potential confounders and revealed that old age, male sex, comorbidities of hypertension, diabetes, COPD, hyperlipidemia, and CHF remained significant risk factors for AKI, which is consistent with previous studies.21,24,25
The major finding of our study is that patients with OP poisoning compared with patients without OP poisoning had a higher overall crude and adjusted risk of subsequent AKI. In the subgroup analysis, although those who were old, male, and had comorbidities had a significantly higher incidence rate of AKI in both the study and comparison groups, the young and productive age group (≤49 years), who were male or female without coexisting comorbidities, exhibited a considerable risk of developing subsequent AKI after exposure to OP pesticide compared with those without OP poisoning, implying that physicians should carefully examine the urine output and variations of renal function when managing acute OP poisoning in such patients. Few studies on the association between OP poisoning and AKI have been published. Faiz et al16 surveyed 300 patients with OP poisoning in an intensive care unit and reported that only 1.66% of them had acute renal failure. Arefi et al26 investigated 1500 poisoned patients referred to the emergency department in 2010 and indicated that rhabdomyolysis was a strong risk factor for renal failure. Gokel27 reported 2 cases of OP poisoning complicated with rhabdomyolysis and acute renal failure. Moreover, Agostini and Bianchin17 reported a case of intentional ingestion of OP pesticide, who developed acute renal failure and multiple organ dysfunction syndrome, suggesting that high intratubular OP concentrations and hypovolemia might be responsible for AKI. Similarly, Cavari et al18 proposed that OP effects on the renal system could be due to direct parenchymal intoxication, secondary to hemodynamic instability, or seizure-induced rhabdomyolysis. In summary, we hypothesize that bradycardia, dehydration with hypovolemia, and hypotension that induced renal hypoperfusion, direct toxic effects on renal parenchyma and convolutions, endothelial cell damage, activation of immune and inflammatory responses, formation of free radicals, convulsive seizure, and muscular fasciculation–related rhabdomyolysis may all contribute to the decline of renal function after acute poisoning from OP pesticide. In addition, hemodilution induced by massive fluid resuscitation, which impairs the oxygen supply to the kidney may also play a role.9,28 Notably, OP effects are not confined to the acute stage. Our data revealed a significant increased risk of AKI after 1 year of follow up, indicating the possible presence of long-term effects. Moreover, the median follow-up time for AKI events in the OP poisoning cohort (5.73 years) was shorter than that in the non–OP poisoning cohort (6.71 years), suggesting that OP played a role in facilitating the development of AKI.
Bardin and Van Eeden29 developed the clinical severity grading system of OP poisoning and defined life-threatening poisoning as suicidal attempts, stupor, hypoxic respiratory distress, and abnormal chest roentgenogram.30 Because of the unavailability of certain history and clinical information from our claims dataset, including radiological and laboratory data, acute respiratory failure (ICD-9-CM code 518.81) diagnosed within the first 3 days of hospitalization was considered the indicator for distinguishing between the high- and low-severity groups and for calculating the relative risk of AKI for each group. Expectedly, the result exhibited a substantially higher risk of AKI in the high-severity group, with adequate statistical significance. Moreover, patients with OP poisoning, who required mechanical ventilation support during the first 3 days of hospitalization, exhibited a considerably higher risk of developing AKI than those without OP poisoning. Previous studies have proposed the harmful bidirectional interaction between acute lung injury and AKI in critically ill patients.28,31,32 We believe that the renal effects of acute lung injury and mechanical ventilation, termed “ventilator-induced kidney injury,” could play a role in the pathogenesis of AKI in patients with severe OP-poisoning.
Previous studies have shown that poisoning other than with OP compound (eg, paraquat) are strongly correlated with acute kidney damage.33–35 In our additional analysis, dual intoxication with OP and other types of poisoning exhibited a 7.75-fold higher risk of subsequent AKI development, whereas other poisoning alone failed to show a statistically significant renal effect. We suppose that it may be due to insufficient numbers of cases for data analysis (Table 5).
In our study, patients with newly developed AKI events since the index date and during the follow-up period till the end of 2011 were documented. Actually, it is hard to separate the events from acute effect (may have developed few minutes to hours after poisoning) from late effect (may have developed days to weeks after poisoning) in various clinical settings and disease severity. However, the follow-up time analysis (Table 3) showed the significant increased risk of AKI after a 1-year follow-up, might indicate the presence of long-term effect. In addition, the median follow-up time for AKI events were 5.73 years (OP cohort) and 6.71 years (non-OP cohort), which also suggested the possible existence of long-term effect that promoted the subsequent development of AKI.
LIMITATIONS
A major advantage of this study is the use of a large-scale claims database that covers >99% of the population in Taiwan. Although not originally designed for academic research, this database facilitated accurate and clear observations for each event occurrence and served as an appropriate representative of the population. In addition, disease definitions were based on ICD-9-CM codes, which were meticulously and repeatedly reviewed by physicians and medical reimbursement specialists throughout the hospital course; thus, most selection bias could be avoided. However, miscoding and misclassification may still exist. Next, the NHIRD lacks individual information regarding family history, socioeconomic background, cigarette smoking habits, body mass index, severity of comorbidities and laboratory data, which were considered highly associated with AKI development; hence, we could not adjust for them. Furthermore, in our study, the outcome measure depended upon hospitalization with a newly diagnosed AKI, which raises the problem that patients diagnosed in outpatients might be missed and led to an underestimation of the occurrence of AKI. However, we believe such bias could be omitted due to the highly developed health care facilities in Taiwan that most of the patients with suspicious or newly recognized AKI would be referred for hospitalization for further investigation. Moreover, factors influencing the severity of poisoning regarding the agent, route, amount, and duration of exposure could not be validated. Therefore, because the only 3 indicators we selected for severity grading were acute respiratory failure, central venous pressure monitoring, and mechanical ventilation, drawing a conclusion may be inadequate. Furthermore, an observational cohort study is unable to fully explain causal relationships. Finally, our study sampling was based on the population of Taiwan; thus, our results may not be applicable to other countries and regions. All of the aforementioned factors constitute the major limitations of our study.
CONCLUSION
In conclusion, our research revealed the possible association between OP poisoning and the subsequent development of AKI. Although the exact mechanism underlying this correlation has not yet been confirmed, we believe our results could be valuable for physicians and intensivists in managing acute OP poisoning. Further investigation is warranted to determine the exact mechanism of the OP effect on the urinary system, the existence of long-term effects, and whether antidote therapy (atropine and oximes) would be beneficial in preventing the development of AKI.
Footnotes
Abbreviations: AKI = acute kidney injury, BNHI = Bureau of National Health Insurance, CI = confidence interval, ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification, NHI = National Health Insurance, NHIRD = National Health Insurance Research Database, OP = organophosphate.
C-HK provided administrative support. All the authors were involved in data analysis and interpretation, writing of this article, and final approval of this article.
I-CL and C-HK contributed equally to this article.
This study is supported in part by the Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW104-TDU-B-212-113002); China Medical University Hospital, Academia Sinica Taiwan Biobank, and Stroke Biosignature Project (BM104010092); NRPB Stroke Clinical Trial Consortium (MOST 103-2325-B-039-006); Tseng-Lien Lin Foundation, Taichung, Taiwan; Taiwan Brain Disease Foundation, Taipei, Taiwan; Katsuzo and Kiyo Aoshima Memorial Funds, Japan; CMU under the Aim for Top University Plan of the Ministry of Education, Taiwan. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the article. No additional external funding was received for this study.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Carey JL, Dunn C, Gaspari RJ. Central respiratory failure during acute organophosphate poisoning. Respir Physiol Neurobiol 2013; 189:403–410. [DOI] [PubMed] [Google Scholar]
- 2.Iyer R, Iken B, Leon A. Developments in alternative treatments for organophosphate poisoning. Toxicol Lett 2015; 233:200–206. [DOI] [PubMed] [Google Scholar]
- 3.Yang CC, Deng JF. Intermediate syndrome following organophosphate insecticide poisoning. J Chin Med Assoc 2007; 70:467–472. [DOI] [PubMed] [Google Scholar]
- 4.Leibson T, Lifshitz M. Organophosphate and carbamate poisoning: review of the current literature and summary of clinical and laboratory experience in southern Israel. Isr Med Assoc J 2008; 10:767–770. [PubMed] [Google Scholar]
- 5.Weissmann-Brenner A, Friedman LM, David A, et al. Organophosphate poisoning: a multihospital survey. Isr Med Assoc J 2002; 4:573–576. [PubMed] [Google Scholar]
- 6.Paudyal BP. Organophosphorus poisoning. JNMA J Nepal Med Assoc 2008; 47:251–258. [PubMed] [Google Scholar]
- 7.Karakus A, Celik MM, Karcioglu M, et al. Cases of organophosphate poisoning treated with high-dose of atropine in an intensive care unit and the novel treatment approaches. Toxicol Ind Health 2014; 30:421–425. [DOI] [PubMed] [Google Scholar]
- 8.King AM, Aaron CK. Organophosphate and carbamate poisoning. Emerg Med Clin North Am 2015; 33:133–151. [DOI] [PubMed] [Google Scholar]
- 9.Pakula AM, Skinner RA. Acute kidney injury in the critically ill patient: a current review of the literature. J Intensive Care Medicine 2015; [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 10.Dharmani C, Jaga K. Epidemiology of acute organophosphate poisoning in hospital emergency room patients. Rev Environ Health 2005; 20:215–232. [DOI] [PubMed] [Google Scholar]
- 11.Tokuda Y, Kikuchi M, Takahashi O, et al. Prehospital management of sarin nerve gas terrorism in urban settings: 10 years of progress after the Tokyo subway sarin attack. Resuscitation 2006; 68:193–202. [DOI] [PubMed] [Google Scholar]
- 12.Yanagisawa N, Morita H, Nakajima T. Sarin experiences in Japan: acute toxicity and long-term effects. J Neurol Sci 2006; 249:76–85. [DOI] [PubMed] [Google Scholar]
- 13.Peter JV, Sudarsan TI, Moran JL. Clinical features of organophosphate poisoning: a review of different classification systems and approaches. Indian J Crit Care Med 2014; 18:735–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karki P, Ansari JA, Bhandary S, et al. Cardiac and electrocardiographical manifestations of acute organophosphate poisoning. Singapore Med J 2004; 45:385–389. [PubMed] [Google Scholar]
- 15.Mishra A, Pandya HV, Dave N, et al. Multi-organ dysfunction syndrome with dual organophosphate pesticides poisoning. Toxicol Int 2013; 20:275–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Faiz MS, Mughal S, Memon AQ. Acute and late complications of organophosphate poisoning. J Coll Physicians Surg Pak 2011; 21:288–290. [PubMed] [Google Scholar]
- 17.Agostini M, Bianchin A. Acute renal failure from organophospate poisoning: a case of success with haemofiltration. Hum Exp Toxicol 2003; 22:165–167. [DOI] [PubMed] [Google Scholar]
- 18.Cavari Y, Landau D, Sofer S, et al. Organophosphate poisoning-induced acute renal failure. Pediatr Emerg Care 2013; 29:646–647. [DOI] [PubMed] [Google Scholar]
- 19.Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007; 11:R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Talabani B, Zouwail S, Pyart RD, et al. Epidemiology and outcome of community-acquired acute kidney injury. Nephrology (Carlton) 2014; 19:282–287. [DOI] [PubMed] [Google Scholar]
- 21.Poncio L, Balbi AL, Rocha EP, et al. The long-term outcome after acute kidney injury: a narrative review. J Bras Nefrol 2015; 37:115–120. [DOI] [PubMed] [Google Scholar]
- 22.Rewa O, Bagshaw SM. Acute kidney injury-epidemiology, outcomes and economics. Nat Rev Nephrol 2014; 10:193–207. [DOI] [PubMed] [Google Scholar]
- 23.Srisawat N, Kellum JA. Acute kidney injury: definition, epidemiology, and outcome. Curr Opin Crit Care 2011; 17:548–555. [DOI] [PubMed] [Google Scholar]
- 24.Liangos O, Wald R, O’Bell JW, et al. Epidemiology and outcomes of acute renal failure in hospitalized patients: a national survey. Clin J Am Soc Nephrol 2006; 1:43–51. [DOI] [PubMed] [Google Scholar]
- 25.Peres LA, Wandeur V, Matsuo T. Predictors of acute kidney injury and mortality in an Intensive Care Unit. J Bras Nefrol 2015; 37:38–46. [DOI] [PubMed] [Google Scholar]
- 26.Arefi M, Taghaddosinejad F, Salamaty P, et al. Renal failure prevalence in poisoned patients. Nephrourol Mon 2014; 6:e11910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gokel Y. Subarachnoid hemorrhage and rhabdomyolysis induced acute renal failure complicating organophosphate intoxication. Ren Fail 2002; 24:867–871. [DOI] [PubMed] [Google Scholar]
- 28.van den Akker JP, Egal M, Groeneveld AB. Invasive mechanical ventilation as a risk factor for acute kidney injury in the critically ill: a systematic review and meta-analysis. Crit Care 2013; 17:R98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bardin PG, Van Eeden SF. Organophosphate poisoning: grading the severity and comparing treatment between atropine and glycopyrrolate. Criti Care Med 1990; 18:956–960. [PubMed] [Google Scholar]
- 30.Yardan T, Baydin A, Acar E, et al. The role of serum cholinesterase activity and S100B protein in the evaluation of organophosphate poisoning. Hum Exp Toxicol 2013; 32:1081–1088. [DOI] [PubMed] [Google Scholar]
- 31.Koyner JL, Murray PT. Mechanical ventilation and the kidney. Blood Purif 2010; 29:52–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murray PT. The kidney in respiratory failure and mechanical ventilation. Contrib Nephrol 2010; 165:159–165. [DOI] [PubMed] [Google Scholar]
- 33.Mohamed F, Buckley NA, Jayamanne S, et al. Kidney damage biomarkers detect acute kidney injury but only functional markers predict mortality after paraquat ingestion. Toxicol Lett 2015; 237:140–150. [DOI] [PubMed] [Google Scholar]
- 34.Mohamed F, Endre Z, Jayamanne S, et al. Mechanisms underlying early rapid increases in creatinine in paraquat poisoning. PLoS One 2015; 10:e0122357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pavan M. Acute kidney injury following paraquat poisoning in India. Iran J Kidney Dis 2013; 7:64–66. [PubMed] [Google Scholar]


