Abstract
The aim of this study was to investigate the relationships between nocturnal variations in blood pressure (BP) and metabolic syndrome (MetS) in different gender.
This cross-sectional study involved 509 hypertensive patients (254 males and 255 females, 45 to 75 years old) from September 2013 to March 2014. BP values were acquired from ambulatory BP monitoring (ABPM). The dipper pattern of BP was defined as 10% to 20% reduction of the mean systolic BP (SBP) values at night compared with the daytime values. The diagnosis of MetS was made according to NCEP ATP-III definition. Multivariate logistic regression analyses were used to explore the relationships between ABPM results and MetS.
In our study, MetS were observed in 29.1% of male and 18.4% of female participants. The prevalence of MetS was higher in the patients with reverse-dipper pattern than in others. After multivariate logistic regression analysis, the reverse-dipper pattern of BP (odds ratio 2.298; P = 0.006) and 24-SBP (odds ratio 1.063; P = 0.021) were independently correlated with MetS in males. However, there was no association between MetS and BP reverse dipping in females.
Our cross-sectional study showed that the reverse-dipper pattern of BP is associated with MetS in male, while the underlying mechanism deserves further investigation.
INTRODUCTION
The ambulatory blood pressure monitoring (ABPM) is known as a noninvasive examination, which demonstrates circadian blood pressure (BP) level and improves the accuracy for diagnosing hypertension. Nowadays, it has been gradually accepted that BP variability might serve as potential risk factors for predicting cardiac and cerebrovascular events.1,2 As one of the short-term variabilities, BP dipping in most individuals happens when average night-time BP reduces 10% to 20% compared with their average daytime BP, while the abnormal nocturnal BP fall is commonly seen in a large number of hypertensive patients, which was regarded as nondipper.3 A few studies have reported that nondipper pattern of BP not only increases cardiovascular risk but also injures heart, brain, and kidney.4 To be noticed, reverse-dipper BP pattern (<0% SBP fall), which was used to be categorized as a variant of nondipper, plays a significant role in the severe renal and cardiovascular damages in patients with chronic kidney disease.5 Our previous studies also discovered that reverse-dipper BP pattern was closely related to the early formation of carotid plaque and lacunar infarction.6,7
According to NCEP ATP III definition,8 metabolic syndrome (MetS) includes at least 3 of the following medical conditions: abdominal obesity, hypertriglyceridemia, low high-density lipoprotein (HDL)-cholesterol, hypertension, and elevated fasting glucose. Each factors of MetS was shown to be associated with cardiovascular and cerebral diseases.9,10 Recently, a cross-sectional study demonstrated a significantly increased prevalence of blunted nocturnal BP in MetS patients,8 but little is known between elevated nocturnal SBP and MetS. The present study was carried out to investigate the relationship between reverse BP dipping and MetS in a general population of Chinese males and females.
METHODS
Subjects
The original data of this cross-sectional study were extracted from our entire in-patient ABPM service database (1024 patients in total) from September 2013 to March 2014. A total of 509 subjects (254 men, 255 women) were eventually recruited in our study. Hypertensive patients was defined as systolic BP (SBP) ≥140 mm Hg and/or diastolic BP (DBP) ≥90 mm Hg in casual office recording, or daytime (or awake) SBP ≥135 mm Hg and/or DBP ≥85 mm Hg or night-time (or asleep) SBP ≥120 mm Hg and/or DBP ≥70 mm Hg in ABPM.11 MetS was defined according to the revised ATP-III criteria, namely, the presence of at least 3 of the 5 following factors: waist circumference ≥102 cm in men or 88 cm in women, triglycerides ≥150 mg/dl or drug treatment for elevated triglycerides, HDL-cholesterol ≤40 mg/dl in men or 50 mg/dl in women or drug treatment for reduced HDL-cholesterol, SBP ≥130 mm Hg and/or DBP ≥85 mm Hg, and fasting glucose ≥100 mg/dl. Patients were excluded if the patients were <45 or >75 years old; were pregnant female; were under antihypertensive treatment; had BP measurements over 160/100 mm Hg; had night-work employment; had evidences of acute stroke or myocardial infarction within the past 6 months; had sleep apnea syndrome; had evidence of disease or conditions responsible for secondary hypertension; could not tolerate ABPM; had a history of any arrhythmia, congestive heart failure, hepatic failure, kidney failure, and significant systemic disease. BP patterns were evaluated using 24-hour ABPM. In addition, patients examined with poor-quality ABPM were eliminated. The study protocol was approved by the Ethics Committee of the Second Affiliated Hospital, Xi’an Jiaotong University School of Medicine.
Measurements
Blood samples were obtained from the antecubital vein of patients after overnight fasting. Blood was analyzed for the variables indexes (triglycerides, total cholesterol, HDL-cholesterol, LDL [low-density lipoprotein]-cholesterol, VLD-cholesterol, fasting glucose) using routine automatic techniques at the hospital laboratory. Waist and hip circumferences were measured by the same investigator by the methodology according to the ATP-III.12 Due to the cross-sectional nature of our study, we did not obtain part of the values of BMI and waistline data.
ABPM was recorded with a properly calibrated and validated SpaceLabs 90207 device (SpaceLabs, Redmond, Washington), which was set to measure every 30 min from 7:00 am to 11:00 pm and every 60 min from 11:00 pm to 7:00 am lasting 24 hours automatically. The patients were made to take activities as usual, avoid daytime napping, and sleep for 6 to 12 hours. The occurrence of unusual events or poor sleep should be noted. Fewer than 3% of the BP readings were rejected as artifacts on the basis of these criteria.13
Statistical Analysis
The present data were recorded using SPSS version 18.0 (SPSS, Chicago, Illinois). All analyses were conducted separately for men and women. To compare ordinal and continuous normally distributed variables between MetS (-) and MetS (+) in different gender, Chi-square test and analysis of variance (ANOVA) were employed, respectively. Our univariate models included age, smoking, circadian BP pattern, 24h-SBP, 24h-DBP, SBP-awakening, SBP-bedtime, DBP-awakening, and DBP-bedtime. Logistic regression analysis was use to predict the links between MetS and clinical variables. The variables found significantly in univariate models were included in the multivariate analyses. P values less than 0.05 were accepted to indicate statistically significant differences.
RESULTS
The baseline characteristics of subjects with and without MetS were summarized in Table 1 according to gender. This study totally recruited 509 patients (254 males and 355 females, 45–75 years old). MetS was observed in 74 (29.1%) men and 47 (18.4%) women, respectively. Subjects in both men and women with MetS had higher fasting glucose, triglycerides, VLD-C, and lower HDL-C. Women with MetS tended to have higher total cholesterol, ABPM (24 h-SBP), SBP-awakening, and SBP-bedtime (Table 1).
TABLE 1.
Characteristics of Males and Females With and Without Metabolic Syndrome
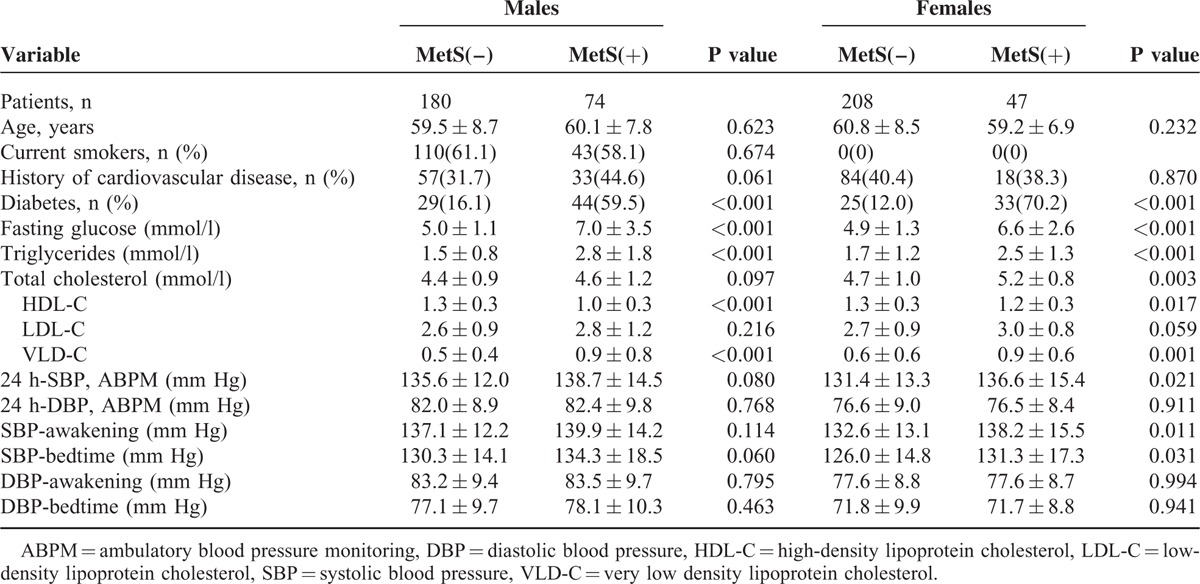
In our study, reverse-dipper pattern of BP was observed in 63 (24.8%) men and in 61 (23.9%) women. MetS was found in 42.9% of the patients with reverse-dipper pattern. Besides, only 24.6% was observed in nonreverse dippers. The distribution of patients with or without MetS in each circadian BP pattern was analyzed using Chi-square test. The difference between reversedippers and nonreverse dippers was statistically significants in males (P = 0.010) (Fig. 1A). However, there was no significant difference between Mets and control group comparing reverse dippers with nonreverse dippers in female (P = 0.850) (Fig. 1B).
FIGURE 1.
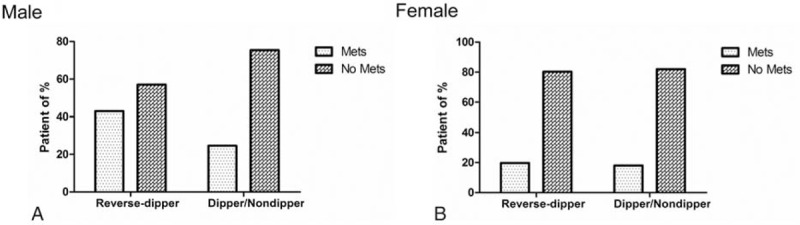
(A) The distribution of MetS and non-MetS patients in each circadian BP pattern group in males. (B) The distribution of MetS and non-MetS patients in each circadian BP pattern group in females.
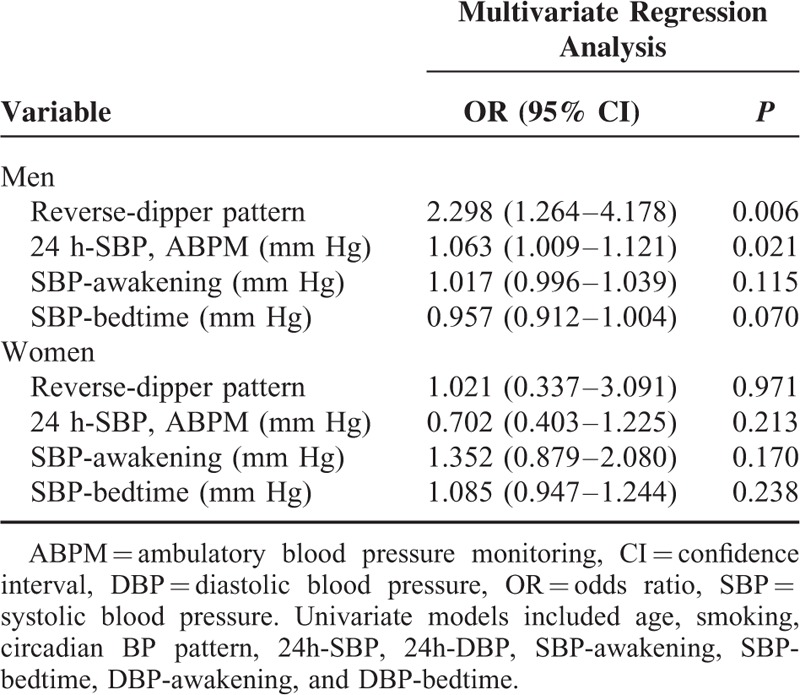
After using univariate and multivariate logistic regression analyses for MetS in different gender, reverse-dipper BP pattern (OR [odds ratio] = 2.298, P = 0.006) and 24 h-SBP (OR = 1.063, P = 0.021) were independently correlated with MetS in male. But there was no clear association between MetS and reverse dippers in women (Table 2).
TABLE 2.
Multivariate Logistic Regression Analysis for MetS in Different Gender
DISCUSSION
Recent studies have provided evidence that patients with abnormal circadian BP patterns had higher risks for left ventricular hypertrophy, myocardial infarction, cerebrovascular disease, and microalbuminuria, which could be observed in patients with MetS as well.14,15 Abnormal BP patterns were found quite common in severely normotensive obese patients and associated with chronically elevated blood glucose.16,17 We have also previously discovered that reverse-dipper pattern of BP was significantly associated with the type 2 diabetes (unpublished data). Then, we proposed that there might be some relationships between BP variations and MetS. This cross-sectional investigation was launched in 121 patients (74 males and 47 females) of MetS and 388 patients (180 males and 208 females) of non-MetS. It revealed that reverse dippers had a higher prevalence of MetS than nonreverse dippers. Furthermore, multivariate logistic regression analysis indicated that reverse-dipper BP pattern was associated with MetS in males (OR 2.298, P < 0.05).
However, the mechanism between MetS and BP reverse dipping is not clear and it is reasonable if there are other factors involved. For example, it was reported that the inflammation responses is implicated in the pathophysiology of hypertension.18 Another recent study showed that glia and neurons produced pro-inflammatory cytokines and may lead to the development of hypertension.19 Santulli et al20 also provided evidence that calcium/calmodulin-dependent kinase IV (CaMKIV) played a pivotal role in BP regulation via controlling endothelial nitric oxide synthase activity. Similarly, the sustaining hypothalamic stress and pro-inflammatory pathways, critically mediated by IKKβ/NF-κB, may cause central inflammatory state and contribute to the problem of over nutrition-induced obesity. Moreover, caloric excess induces persistent inflammation in the circulation and peripheral metabolic tissues, and thus results in MetS.21–25 It is also speculated that the role of neuroinflammation in the pathogenesis of obesity, insulin resistance, T2D/pre-T2D, and CVD may be related to reverse-dipper pattern.26
In addition, a nonignorable pathophysiological change of MetS is that the peripheral vascular networks cannot be able to adequately perfuse skeletal muscle. Subsequently, a series of consequences include endothelial dysfunction, oxidative stress, and inflammation would occur, which could affect the nocturnal variation of BP. It has also been reported that hyperinsulinemia is associated with MetS and may result in structural changes of arterioles, capillary network, and hypertension because of increased vascular resistance. Besides, the regulation of insulin secretion and glucose homeostasis mediated by RyR2 (type 2 ryanodine receptor) channels may play a crucial role in the process.27 Moreover, the sympathetic nervous system (SNS) also plays important roles in the regulation of BP and chronic SNS overactivity, which could contribute to the development of hypertension and may change the BP pattern.18,28 This is consistent with our results that MetS was associated with reverse-dipper pattern of BP (OR 1.910, P = 0.068; data not shown).
In our study, there is a correlation between reverse-dipper BP pattern and MS in male patients, but not in female patients. The circulatory differences between male and female involve either hormonal (estrogen) or nonhormonal (body height and size) factors. In premenopausal women, high estrogen level leads to vasodilatation.29 However, we did not divide females into groups according to menopause in our research. Perhaps this is the main reason why female gender have no correlations between MetS and BP reverse dipping. For limitations, our study only recruited the northern Chinese population with essential hypertension, so the results should not be extended to different ethnic groups. In addition, we only investigated the circadian BP pattern, whereas multiple ABPM over a longer period of time may provide more prognostic information. Due to the limitations of cross-sectional design, prospective clinical observation is necessary in the future investigations.
CONCLUSION
There is a significantly higher prevalence of MetS in hypertensive patients with reverse-dipper BP pattern than the nonreverse pattern. In male patients, the reverse-dipper pattern of BP was closely related to MetS, while in the female subjects, there was no association between MetS and BP reverse dipping.
Acknowledgments
We are grateful to Dr. Yuan Shen from the Department of Statistics, Xi’an Jiaotong University School of Medicine, for her advices and supports on the search strategy and statistics. We also thank Prof. Lingfang Zeng for his critical proof reading. We also acknowledge the supports from the patients who participated in our research.
Footnotes
Abbreviations: ABPM = ambulatory blood pressure monitoring, BP = blood pressure, CI = confidence interval, DBP = diastolic blood pressure, HDL-C = high-density lipoprotein cholesterol, IKKB = inhibitor of nuclear factor kappa-B kinase, LDL-C = low-density lipoprotein cholesterol, MetS = metabolic syndrome, NF-kB = nuclear factor-k-gene binding, OR = odds ratio, SBP = systolic blood pressure, TC = Total cholesterol, TG = Triglycerides, VLD-C = very low-density lipoprotein cholesterol.
Yan B and Yan H contributed to the work equally.
Funding for this study was provided by the National Natural Science Foundation of China (81300116), the Research Fund for the Young Scholars of the Higher Education Doctoral Program of China (20120201120083), the Fundamental Research Funds for the Central Universities of China (XJJ2013062), and the Scientific Fund for the Young talent of Shaanxi Province (2015KJXX-06).
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Kario K, Pickering TG, Matsuo T, et al. Stroke prognosis and abnormal nocturnal blood pressure falls in older hypertensives. Hypertension 2001; 38:852–857. [DOI] [PubMed] [Google Scholar]
- 2.Sander D, Kukla C, Klingelhofer J, et al. Relationship between circadian blood pressure patterns and progression of early carotid atherosclerosis: a 3-year follow-up study. Circulation 2000; 102:1536–1541. [DOI] [PubMed] [Google Scholar]
- 3.O’Brien E, Sheridan J, O’Malley K. Dippers and non-dippers. Lancet 1988; 2:397. [DOI] [PubMed] [Google Scholar]
- 4.Routledge F, McFetridge-Durdle J. Nondipping blood pressure patterns among individuals with essential hypertension: a review of the literature. Eur J Cardiovasc Nurs 2007; 6:9–26. [DOI] [PubMed] [Google Scholar]
- 5.Wang C, Zhang J, Liu X, et al. Reversed dipper blood-pressure pattern is closely related to severe renal and cardiovascular damage in patients with chronic kidney disease. PLoS One 2013; 8:e55419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yan B, Peng L, Han D, et al. Blood pressure reverse-dipping is associated with early formation of carotid plaque in senior hypertensive patients. Medicine 2015; 94:e604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yan B, Peng L, Dong Q, et al. Reverse-dipper pattern of blood pressure may predict lacunar infarction in patients with essential hypertension. Eur J Neurol 2015; 22:1022–1025. [DOI] [PubMed] [Google Scholar]
- 8.Ayala DE, Hermida RC, Chayan L, et al. Circadian pattern of ambulatory blood pressure in untreated hypertensive patients with and without metabolic syndrome. Chronobiol Int 2009; 26:1189–1205. [DOI] [PubMed] [Google Scholar]
- 9.Safar ME, Balkau B, Lange C, et al. Hypertension and vascular dynamics in men and women with metabolic syndrome. J Am Coll Cardiol 2013; 61:12–19. [DOI] [PubMed] [Google Scholar]
- 10.Chillaron JJ, Flores Le-Roux JA, Benaiges D, Pedro-Botet J. Type 1 diabetes, metabolic syndrome and cardiovascular risk. Metabolism 2014; 63:181–187. [DOI] [PubMed] [Google Scholar]
- 11.Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 2013; 31:1281–1357. [DOI] [PubMed] [Google Scholar]
- 12.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome. An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement Executive summary Cardiology in review 2005; 13:322–327. [PubMed] [Google Scholar]
- 13.Pickering TG, Shimbo D, Haas D. Ambulatory blood-pressure monitoring. N Engl J Med 2006; 354:2368–2374. [DOI] [PubMed] [Google Scholar]
- 14.Obayashi K, Saeki K, Iwamoto J, et al. Nocturnal urinary melatonin excretion is associated with non-dipper pattern in elderly hypertensives. Hypertens Res 2013; 36:736–740. [DOI] [PubMed] [Google Scholar]
- 15.Hermida RC. Ambulatory blood pressure monitoring in the prediction of cardiovascular events and effects of chronotherapy: rationale and design of the MAPEC study. Chronobiol Int 2007; 24:749–775. [DOI] [PubMed] [Google Scholar]
- 16.Lammertyn L, Schutte AE, Schutte R. Blood glucose and nocturnal blood pressure in African and Caucasian men: the SABPA study. Diabetes Res Clin Pract 2011; 93:235–242. [DOI] [PubMed] [Google Scholar]
- 17.Flores L, Janka M, Canivell S, et al. Glucose abnormalities associated with impaired nocturnal fall in blood pressure in normotensive severely obese patients. Diabetes Res Clin Pract 2013; 101:153–158. [DOI] [PubMed] [Google Scholar]
- 18.Pietri P, Vlachopoulos C, Tousoulis D. Inflammation and arterial hypertension: from pathophysiological links to risk prediction. Curr Med Chem 2015; 22:2754–2761. [DOI] [PubMed] [Google Scholar]
- 19.Winklewski PJ, Radkowski M, Wszedybyl-Winklewska M, Demkow U. Brain inflammation and hypertension: the chicken or the egg? J Neuroinflamm 2015; 12:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santulli G, Cipolletta E, Sorriento D, et al. CaMK4 gene deletion induces hypertension. J Am Heart Assoc 2012; 1:e001081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cai D, Liu T. Inflammatory cause of metabolic syndrome via brain stress and NF-kappaB. Aging 2012; 4:98–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cai D. One step from prediabetes to diabetes: hypothalamic inflammation? Endocrinology 2012; 153:1010–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hotamisligil GS. Inflammation and metabolic disorders. Nature 2006; 444:860–867. [DOI] [PubMed] [Google Scholar]
- 24.Hotamisligil GS. Inflammation and endoplasmic reticulum stress in obesity and diabetes. Int J Obesity 2008; 32 Suppl 7:S52–S54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Esser N, Legrand-Poels S, Piette J, et al. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract 2014; 105:141–150. [DOI] [PubMed] [Google Scholar]
- 26.Purkayastha S, Cai D. Neuroinflammatory basis of metabolic syndrome. Mol Metab 2013; 2:356–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santulli G, Pagano G, Sardu C, et al. Calcium release channel RyR2 regulates insulin release and glucose homeostasis. J Clin Invest 2015; 125:1968–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Canale MP, Manca di Villahermosa S, Martino G, et al. Obesity-related metabolic syndrome: mechanisms of sympathetic overactivity. Int J Endocrinol 2013; 2013:865965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Orshal JM, Khalil RA. Gender, sex hormones, and vascular tone. Am J Physiol Regul Int Comp Physiol 2004; 286:R233–R249. [DOI] [PubMed] [Google Scholar]


