Abstract
Primary gastrointestinal lymphoma (PGIL) is a rare malignant tumor without standard diagnosis and treatment methods. This study is aimed to systematically analyze its clinical characteristics and draw out an appropriate flow chart of diagnosis and treatment process for PGIL in China.
This study retrospectively analyzed the clinicopathological characteristics, diagnostic approaches, prognostic factors, and therapeutic modalities in 415 cases of PGIL in Chinese province of Guangdong. A systematic review was conducted in 118 studies containing 5075 patients to further identify clinical manifestations and mortalities of therapeutic modalities.
The most common clinical presentations were abdominal pain and bloody stools. Endoscopic biopsy was an important diagnostic means, and usually more than once to make a definite diagnosis. Retrospective multicenter clinical study showed that younger onset age (<60 years), female, one region involved, one lesion, early stage, International Prognostic Index (IPI ≤1), normal lactate dehydrogenase (LDH), normal albumin, and nonemergency operation were significant prognostic factors for B-cell lymphoma; non-B symptom, tumor restricted to gastric or ileocecal region, one lesion, performance status (PS ≤1), normal LDH, and nonsurgery alone were significant prognostic factors for T-cell lymphoma. Site of origin and IPI were independent prognostic factors for B-cell lymphoma; PS was the independent prognostic factor for T-cell lymphoma. And T-cell lymphoma had worse overall survival (OS) and progression-free survival (PFS) than B-cell lymphoma. Among different therapeutic modalities, chemotherapy alone or combined with surgery showed better OS and PFS than surgery alone for diffuse large B-cell lymphoma (DLBCL) of stage I/II E and T-cell lymphoma. For DLBCL of stage III E/IV and mucosa-associated lymphoid tissue lymphoma, OS and PFS did not differ among different therapeutic groups. In meta-analysis, surgery plus chemotherapy showed lowest mortality.
Chemotherapy alone or combined with surgery may be the first-line treatment for DLBCL of stage I/II E and T-cell lymphoma. A flow chart of diagnosis and treatment process for PGIL was approximately drew out.
INTRODUCTION
Primary gastrointestinal lymphoma (PGIL) is a tumor of gastrointestinal (GI) tract, as its primary lesion might invade the lymph nodes of the related drainage area, excluding tumors involving the liver, spleen, or lymphomas in patients who exhibit GI symptoms, and palpable lymph nodes.1,2 PGIL is a rare malignant tumor with an incidence of about 1505 per 100,000.3 However, PGIL is the most common type of extranodal lymphomas, accounting for 30% to 40% of extranodal lymphomas and 1% to 4% of the malignant tumors in GI tract.1,4 World Health Organization (WHO) classifications of 2008 have become widely accepted, and therapeutic modalities concerning immunophenotypes, involving site, are becoming increasingly popular.5–7Helicobacter pylori eradication therapy is the first-line treatment of gastric mucosa-associated lymphoid tissue (MALT) lymphoma; involved-field radiotherapy or surgery is recommended for patients of nongastric MALT with an early stage (I/II E); and R-CHOP regimen (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone) is recommended for gastric diffuse large B-cell lymphoma (DLBCL).6,7 However, treatments on primary intestinal lymphoma are still controversial.8,9 Furthermore, whether surgery should be a first-line therapy of PGIL has been debated for several years.3,10–14 Therefore, the optimal treatment strategy for PGIL is still not established.7,14,15
A great many clinical studies about PGIL have been published, but the sample size is usually small.7,14,16 There has been no diagnosis or treatment guideline for PGIL in China based on a large number of epidemiological investigations. To learn about the characteristics of PGIL, find out the prognostic factors for B-cell lymphoma and T-cell lymphoma, and evaluate the efficacy of different therapeutic modalities on the prognosis, we perform a retrospective multicenter clinical analysis of PGIL containing 415 cases of PGIL patients in Guangdong province of China and a systematic review containing 5075 Chinese PGIL patients.
METHODS
Patients
This study was subject to approval by the Research Ethics Committee of Sun Yat-Sen Memorial Hospital (IRB number: [2014] 56). PGIL cases were collected from four medical centers in Guangdong province of China from October 1998 to October 2013. The diagnosis of PGIL was based on the 2008 WHO classification.5,6 Patients <18 years of age were excluded from this study.17 Data concerning demographic, clinical, endoscopic features, biological and histological features, as well as treatments and clinical outcomes were recorded. A total of 415 patients were enrolled, and observed until death. All the patients but one (Manchu) was of the Han nationality. The follow-up data, including endpoint of collection, reasons for ending, and living statue, were collected. The median follow-up after diagnosis was 14 months (range, 0.25–185 months).
Histopathological Examination
Based on the immunohistochemical results, 355 cases were identified as B-cell phenotype and 60 cases as T-cell phenotype. Subsequently, B-cell lymphomas were classified as follows: MALT lymphoma, DLBCL, follicular lymphoma, mantle cell lymphoma, and Burkitt lymphoma. T-cell lymphomas were classified into 3 groups: enteropathy-associated T-cell lymphoma (EATL), NK/T-cell lymphoma (nasal type), and other T-cell types including anaplastic large-cell lymphoma.1
Staging and Clinical Manifestations
The staging workup comprised physical examination (inspection of Waldeyer's ring), hematological and biochemical routine investigations (full blood count, lactate dehydrogenase [LDH], albumin, beta2 microglobulin levels), endoscopy, x-rays (chest, small intestine), CT scan (thorax, abdomen, pelvic cavity), and bone marrow biopsy. The stage was defined according to the Ann Arbor staging system, 16,18 The macroscopic types of lymphoma were classified as follows: superficial, mass-forming, diffuse infiltrating, and unclassified.4 B symptoms were defined as fever (>38°C) lasting >3 days without explanation, weight loss (>10% within 6 months), and night sweat.8
Treatment Modalities and Survival Analysis
Therapeutic modalities were divided into 4 types: surgery alone (group A), chemotherapy and/without radiotherapy (nonsurgery) (group B), surgery plus chemotherapy and/without radiotherapy (group C), and supportive care (group D). Overall survival (OS) was measured from the date of diagnosis to death from any cause. Progression-free survival (PFS) was measured from the date of diagnosis to disease progression, disease relapse, or death from any cause.
Systematic Review of Chinese PGIL Patients
We searched for all published studies concerning Chinese PGIL patients using the following electronic databases: WANFANG (database in Chinese), CNKI (database in Chinese), and PubMed. The following medical search headings and free text words were used: “primary,” “gastrointestinal,” and “lymphoma.” We checked the reference lists of all relevant studies obtained from our search.
Studies were considered for inclusion based upon the following criteria: the patient was diagnosed with PGIL; all patients involved were Chinese; and studies concerning age of patient, sex, initial symptoms, diagnosis, phenotype, site of lesion, performance status (PS) score, International Prognostic Index (IPI) score, method of diagnosis-making, treatment, and outcome. The main exclusion criteria were as follows: studies reported not in the English or Chinese language; studies with irretrievable or unclear data; studies that were reviews, comments or replies, and meta-analyses; and studies used a considerable overlap data between authors and centers. Data on age of patient, sex, initial symptoms, diagnosis, phenotype, site of lesion, PS score, IPI score, method of diagnosis-making, treatment, and outcome were extracted.
The methodological quality of included studies was critically appraised using the Newcastle-Ottawa scale. The Newcastle–Ottawa scale is a quality assessment tool based on selection of cases and controls (0–4 points for cohort studies), comparability (0–2 points), and outcome (0–3 points in cohort studies). We defined the studies with <4 points as low quality, and these were excluded from the meta-analysis.
Statistical Analysis
OS and PFS rates were calculated by the Kaplan–Meier method, and the value was compared using the log-rank test. Prognostic factors (P < 0.1) were put into multivariate analysis using the Cox proportional hazards model.7 Other statistical differences were evaluated using the χ2 test and the Mann–Whitney U test. P value of <0.05 for each test was statistically significant. The statistical analyses above were performed using SPSS 19.0. The comparisons of mortalities in patients of PGIL from systematic review treated with different therapeutic modalities were calculated by meta-analysis using Review Manager 5.2. Treatment effects are represented as the odds ratio (OR) with 95% confidence interval. Heterogeneity across trials was evaluated using a standard χ2 test set at P < 0.05 and also assessed via I2 statistic set at I2 > 30%. Publication bias was estimated by visual inspection of a funnel plot. P value of <0.05 was statistically significant.
RESULTS
Patients
This study comprised 415 patients, and 355 (85.8%) were of B-cell and 60 (14.5%) were of T-cell. The B-cell lymphoma patients had a median age of 57 years (range, 19–92 years), and the T-cell patients had a median age of 44 years (range, 21–88 years). B-cell lymphoma patients comprised 221 male and 134 female (male:female, 1.65:1.00), and T-cell lymphoma patients comprised 39 male and 21 female (male:female, 1.86:1.00). There was no significant difference between B-cell and T-cell in sex distributions (P = 0.684). For 62 patients, cause of death or status at the last follow-up was unknown and these patients were excluded from survival analysis. The median follow-up after diagnosis was 14 months (range, 0.25–185 months).
Clinical Features
The most common clinical presentation was abdominal pain with a frequency of 65.5%, followed by bloody stools with a frequency of 20.2% (Fig. 1). As shown in Table 1, pathological subtypes were closely related with the age, incidence of emergency operation, B symptoms, PS score, LDH, IPI score, and lesion. Patients with T-cell lymphoma had younger onset age than patients with B-cell lymphoma (P = 0.000). There were totally 37 patients (8.9%) underwent emergency operations because of complications. The incidence of emergency operation in the B-cell group and T-cell group was 8.6% (19/221) and 43.9% (18/41), respectively (P = 0.000). B-cell group (10.4% [37/355]) had a lower incidence of B symptoms than T-cell group (33.3% [20/60]) (P = 0.000); 73.0% (259/355) B-cell group patients had PS ≤1, whereas 31.7% (19/60) T-cell group patients had a PS ≤1 (P = 0.000). Elevated LDH was observed in 91 (30.8%) patients of B-cell group and 27 (50.9%) patients of T-cell group (P = 0.004). One hundred seventy-seven (52.7%) patients in the B-cell group had IPI ≤1, whereas 16 (28.1%) patients in the T-cell group had IPI (P = 0.001). Multiple lesions appeared in 19.7% B-cell group patients, whereas it exhibited in 38.8% T-cell group (P = 0.001). No significant difference of sex, albumin levels, beta2 microglobulin levels, macroscopic types, or stage was found between B-cell group and T-cell group.
FIGURE 1.
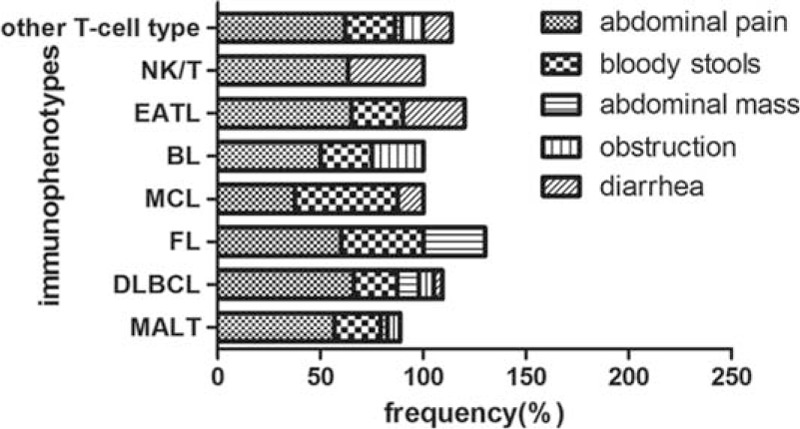
Initial clinical symptoms and immunophenotypes.
TABLE 1.
Comparison of Clinicopathologic Features and Histologic Type
Histologic Classification and Primary Site
The histologic classification and primary site are shown in Table 2. In the B-cell group, 299 patients had been classified histologically, consisting of 32.4% MALT lymphoma, 60.5% DLBCL, 3.3% follicular lymphoma, 2.7% mantle cell lymphoma, and 1.3% Burkitt lymphoma. The T-cell group comprised 33.3% patients who were classified as EATL, 18.3% as NK/T-cell lymphoma, and 48.3% as other T-cell types. The most frequent primary site of B-cell lymphoma was the stomach (169 patients [56.5%]), whereas T-cell lymphoma was the large intestine (21 patients [35.0%]). In the gastric group, DLBCL was the most frequent histologic type (52.0%), followed by MALT (37.9%). In the small intestinal group, DLBCL and MALT were the most frequent types (45.2% and 28.8%). DLBCL was the most frequent type in the ileocecal (53.8%) and large intestinal (48.0%) groups. As for the multiple regions involved group, DLBCL, MALT, and EATL were the most frequent types (47.4%, 15.8%, and 15.8%).
TABLE 2.
Histologic Classification and Sites of Origin
Diagnosis of Gastrointestinal Lymphoma
A total of 369 patients underwent endoscopy. Ulcer (47.0%) and mass (19.3%) were the most frequent gross endoscopic appearances. Endoscopic biopsy diagnosis was made in 214 patients (51.6%), including 187 B-cell lymphoma patients and 27 T-cell lymphoma patients. For B-cell group, 314 cases performed endoscopy, 124 (39.5%), 50 (55.4%), and 11 (58.9%) of these cases were respectively diagnosed at the first, second, and third time. On the contrary, 52 patients of T-cell lymphoma underwent endoscopy. Among these patients, 12 (23.1%), 12 (46.2%), and 3 (51.9%) cases were diagnosed at the first, second, and third time, respectively. In addition, 201 patients (48.4%) were diagnosed by surgery, and 18.4% of them received emergency operations. Out of the 201 patients diagnosed by surgery, 161 (80.1%) received radical surgery, 27 (13.4%) took palliative surgery, and 13 (6.5%) cases were lack of details on surgery. Among different immunophenotypes, patients of follicular lymphoma had the highest rate of being diagnosed by surgery. Patients of NK/T-cell lymphoma were the most probable to take emergency operations with a frequency of 54.5%, followed by those of other T-cell types with a frequency of 31.0%. Table 3 shows the characteristics of diagnosis-making.
TABLE 3.
Characteristics of Diagnosis-Making: Endoscopic Features, Stage at Diagnosis and Ways of Diagnosis-Making
Survival Analysis of B-Cell Lymphoma
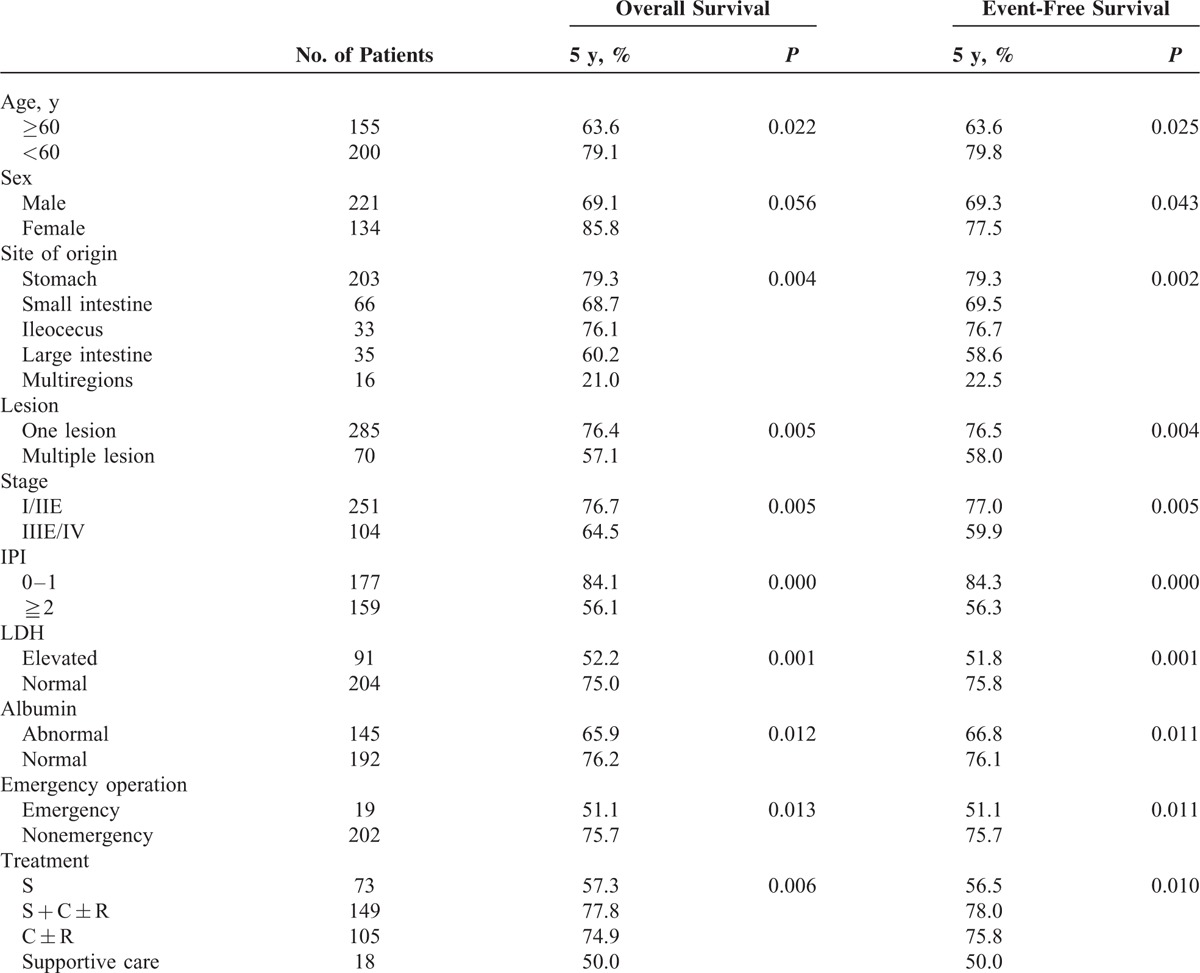
The 5-year OS and PFS rate of B-cell lymphoma was 72.3% and 72.5%, respectively. Patients of B-cell lymphoma with the following characteristics had significantly better OS and PFS rates: younger onset age (<60 years), female sex, tumors origin restricting to one region, one lesion, early stage, IPI ≤1, normal LDH, normal albumin, nonemergency operation, and chemotherapy with or without surgery (Table 4). Female had better PFS than male (P = 0.043), but such a result was not found in OS (P = 0.056). OS and PFS were poorer in patients with tumors origin from multiple regions than from one region (OS, P = 0.004; PFS, P = 0.002). Meanwhile, OS and PFS did not differ significantly among stomach, small intestine, ileocecal region, and large intestine (OS, P = 0.184). Therapeutic group D had poorer OS and PFS rates than other 3 groups (OS, P = 0.006). No significant difference of OS or PFS was found in the univariate analysis on B symptoms, immunophenotype, PS score, and beta2 micorglobulin. The Cox proportional hazard model was used for multivariate analysis of risk factors (P < 0.1) in single-factor analysis. The results demonstrated that site of origin (OS, P = 0.017, RR = 1.195, 95% CI [1.032, 1.384]) and IPI score (≥2 vs ≤1; OS, P = 0.000, RR = 0.311, 95% CI [0.177, 0.545]) were independent prognostic factors for B-cell lymphoma.
TABLE 4.
Significant Prognostic Factors of B-Cell Lymphoma on Univariate Analysis
For B-cell lymphoma, 73 patients received surgery alone (group A), and 149 patients received surgery plus chemotherapy with or without radiotherapy (group C). Sixty-four (87.7%) patients of group A received radical surgery, and 9 (12.3%) patients underwent palliative surgery. One hundred twenty-nine (86.6%) patients of group C received radical surgery, and 20 (13.4%) patients received palliative surgery. Among therapeutic groups A, B, and C, OS and PFS did not differ significantly in patients of B-cell lymphoma (Figure 2A; OS, P = 0.138), and similar result was observed when stratified with IPI score according to treatments (Figure 2D; OS, IPI ≤1, P = 0.086; Figure 2E; IPI ≥2, P = 0.082). When stratified with clinical stage, OS and PFS differed among these 3 therapeutic groups in stage IIIE/IV (Figure 2C; OS, P = 0.036). Patients in therapeutic group C showed better OS and PFS than group A (OS, P = 0.009), and OS and PFS rates did not differ between group B and C (OS, P = 0.273) and group A and B (OS, P = 0.121). And OS and PFS did not differ among groups A, B, and C in stage I/II E (Figure 2B; OS, P = 0.055). For DLBCL of stage I/II E, patients in therapeutic group A showed worse OS and PFS than group B and C (Figure 2F; OS, P = 0.000). OS and PFS rates did not differ between the latter 2 (OS, P = 0.060). Similar results were observed in patients with IPI ≤1 (Figure 2H; OS, P = 0.028), or in patients with IPI ≥2 (Figure 2I; OS, P = 0.006). For stage IIIE/IV, OS and PFS did not differ among therapeutic groups A, B, and C (Figure 2G; OS, P = 0.247). For MALT lymphoma, OS and PFS did not differ among therapeutic groups A, B, and C (Figure 2J; OS, P = 0.997). Similar results were observed when stratified with stage and IPI score.
FIGURE 2.
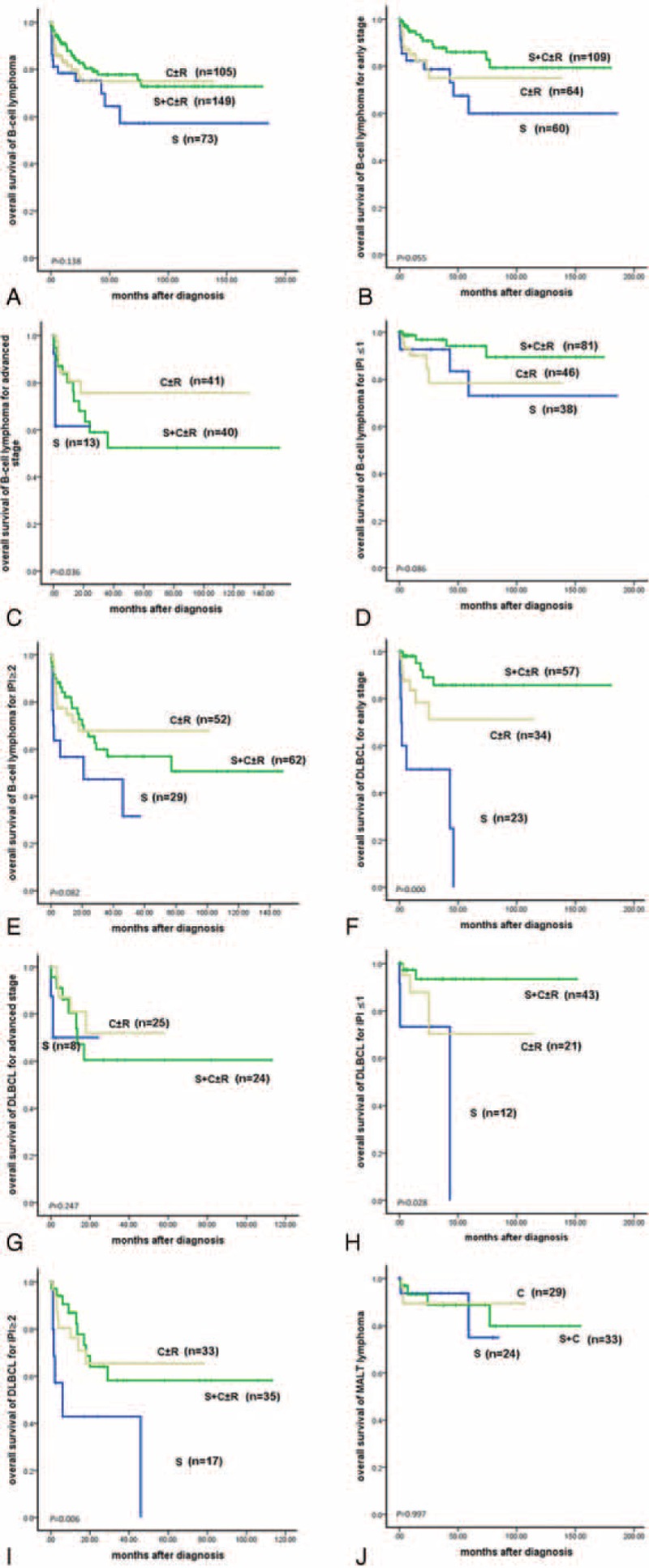
(A) Overall survival of B-cell lymphoma according to treatments (P = 0.138). (B) Overall survival of B-cell lymphoma for early stage (I/II E) according to treatments (P = 0.055). (C) Overall survival of B-cell lymphoma for advanced stage (III E/IV) according to treatments (P = 0.036). (D) Overall survival of B-cell lymphoma for IPI ≤1 according to treatments (P = 0.086). (E) Overall survival of B-cell lymphoma for IPI ≥2 according to treatments (P = 0.082). (F) Overall survival of DLBCL in an early stage (I/IIE) according to treatments (P = 0.000). (G) Overall survival of DLBCL in advanced stage (III E/IV) according to treatments (P = 0.247). (H) Overall survival of DLBCL for IPI ≤1 according to treatments (P = 0.028). (I) Overall survival of DLBCL for IPI ≥2 according to treatments (P = 0.006). (J) Overall survival of MALT according to treatments (P = 0.997). C = chemotherapy, R = radiotherapy, S = surgery.
Survival Analysis of T-Cell Lymphoma
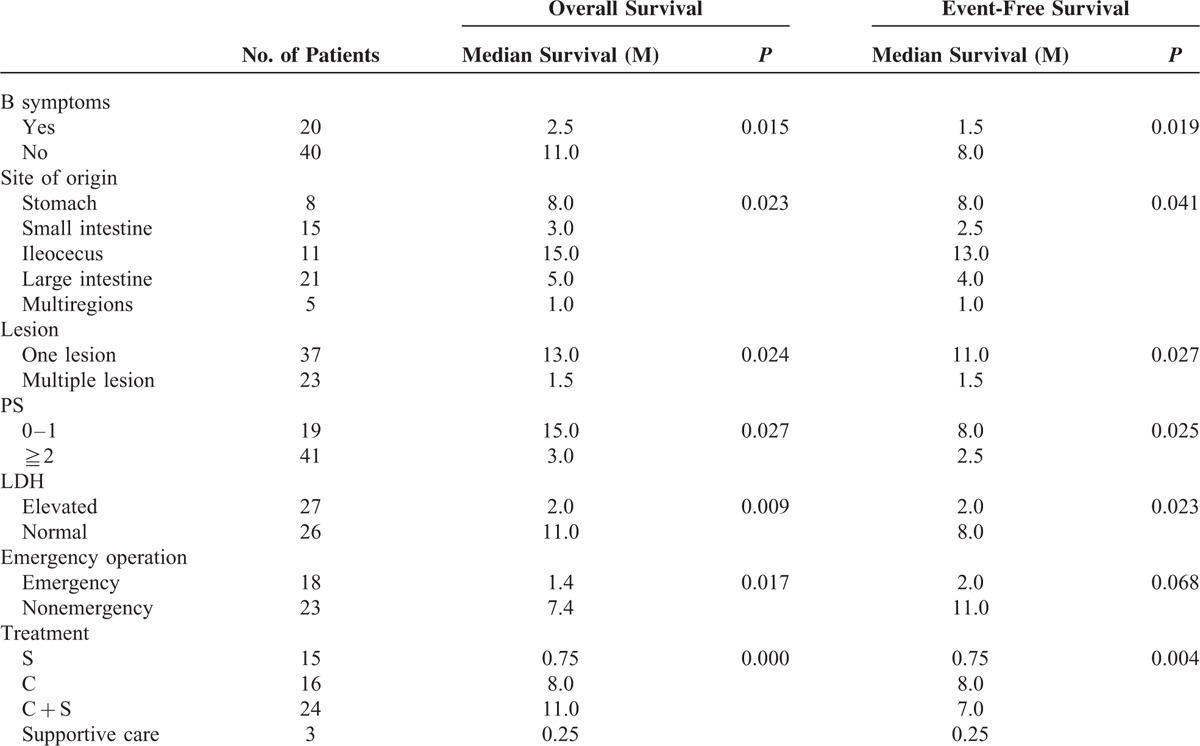
The median OS and PFS time of T-cell lymphoma was 5.0 months. Patients of T-cell lymphoma had worse OS and PFS than those of B-cell lymphoma (OS, P = 0.000; PFS, P = 0.000). For T-cell lymphoma, patients with the following characteristics had significantly longer median OS and PFS time: no B symptom, tumors origin restricting to gastric or ileocecal region, one lesion, PS ≤1, normal LDH, surgery plus chemotherapy, or chemotherapy alone (Table 5). Patients with B symptoms had shorter OS and PFS time than those without B symptoms (OS, P = 0.015). OS and PFS differed significantly among the 5 groups classified by site of origin (OS, P = 0.023). Furthermore, the gastric group and the ileocecal group did not differ significantly in the OS and PFS time (OS, P = 0.945), and the small intestine group and the large intestine group did not differ significantly (OS, P = 0.669). Patients undergoing emergency operations had shorter median OS time than those undergoing selective operation (P = 0.017). However, such a difference was not found in the PFS (P = 0.068). OS and PFS differed significantly among the 4 therapeutic groups (OS, P = 0.000), such a difference was not found between group B and C (OS, P = 0.873), or between group A and D (OS, P = 0.934). No significant difference of OS or PFS was found in the univariate analysis on age, sex, immunophenotype, stage, IPI score, or albumin. The Cox proportional hazard model showed that PS score (≥2 vs ≤1; OS, P = 0.045, RR = 0.325, 95% CI [0.108, 0.977]) was independent prognostic factors for T-cell lymphoma.
TABLE 5.
Significant Prognostic Factors of T-Cell Lymphoma on Univariate Analysis
For T-cell lymphoma, 15 patients received surgery alone (group A), and 24 patients received surgery plus chemotherapy with or without radiotherapy (group C). Eleven (73.7%) patients of group A received radical surgery, and 4 (26.3%) patients underwent palliative surgery. Seventeen (70.8%) patients of group C received radical surgery, and 7 (29.2%) patients took palliative surgery. Among therapeutic groups A, B, and C, OS and PFS differed significantly in patients of T-cell lymphoma (P = 0.000). Stratified with PS ≥2, there were significant differences among therapeutic groups A, B, and C for OS and PFS (OS, P = 0.008). Furthermore, OS and PFS were better for patients in therapeutic group C than A (OS, P = 0.005). There was no significant difference for OS or PFS between group A and B (OS, P = 0.051), or between group B and C (OS, P = 0.550). For patients with PS ≤1, no significant difference of OS or PFS was found between group B and C (OS, P = 0.255) (Fig. 3).
FIGURE 3.
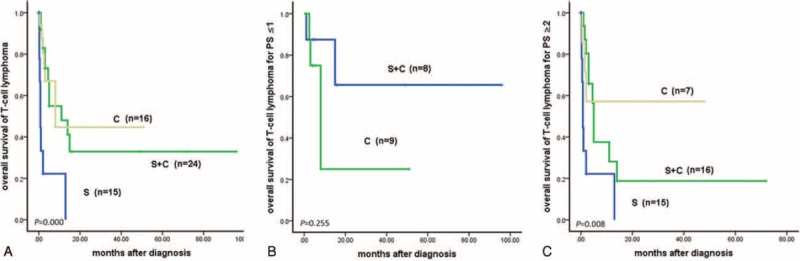
(A) Overall survival of T-cell lymphoma according to treatments (P = 0.000). (B) Overall survival of T-cell lymphoma with PS (0–1) according to treatments (P = 0.255). (C) Overall survival of T-cell lymphoma with PS ≥2 according to treatments (P = 0.008). C = chemotherapy, R = radiotherapy, S = surgery.
Characteristics and Mortality of Gastrointestinal Lymphoma in China
Using the search strategy listed above, 118 publications were identified, including 22 retrospective controlled studies (Fig. 4) comparing treatments (surgery alone vs chemotherapy alone vs surgery plus chemotherapy).13,19–25,32–57
FIGURE 4.
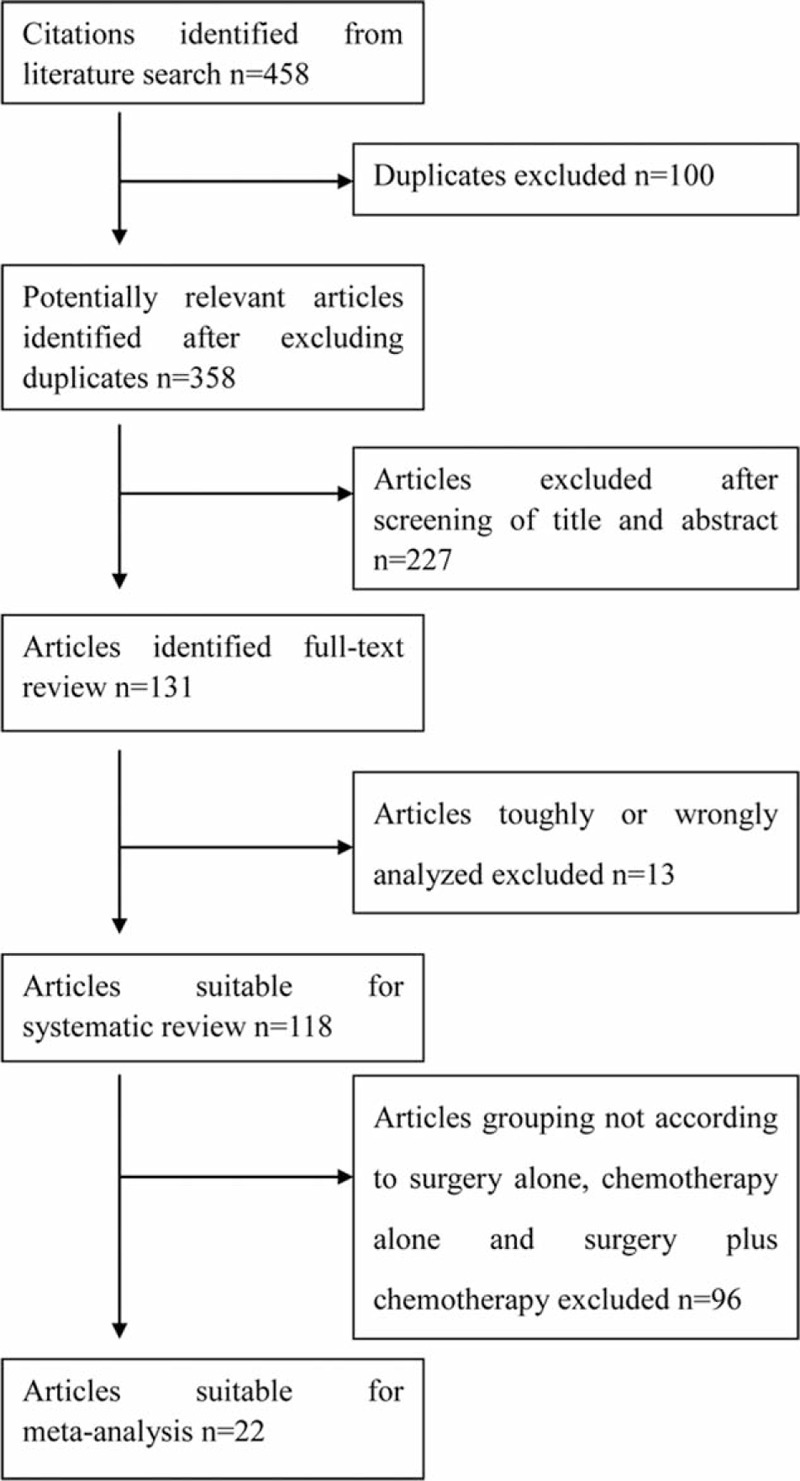
Flow chart showing literature search strategies.
The 118 studies contained 5075 patients. Three thousand nine hundred eighty-six (98.6%) patients were confirmed as non-Hodgkin lymphoma (NHL), and 58 (1.4%) as Hodgkin lymphoma (HL); 3323 (87.0%) patients were identified as B-cell lymphoma, and 496 (13.0%) as T-cell lymphoma; and 2340 patients of B-cell lymphoma had immunophenotyping tests. Of the 2340 patients, 826 (35.3%) were MALT lymphoma, 1290 (55.1%) were DLBCL, 38 (1.6%) were follicular lymphoma, 41 (1.8%) were mantle cell lymphoma, and 14 (0.6%) were Burkitt lymphoma. One hundred forty-one patients of T-cell lymphoma had immunophenotying tests, 43 (30.5%) were EATL, 18 (12.8%) were NK/T-cell lymphoma, and 69 (48.9%) were peripheral T-cell lymphoma, not Otherwise Specified (NOS). The sex ratio was 1.57:1 (male:female, 3087: 1963). Abdominal pain (72.7%, 2385/3282) was the most common present symptom, followed by melena or hematochezia (27.1%), abdominal mass (25.6%), and weight loss (23.9%). Stomach (56.9%) was the most common site of origin, and the small intestine (16.1%) was the most common site of origin for intestinal lymphoma. One thousand seven hundred ninety-one out of 2904 patients (61.7%) were diagnosed by surgery. Three thousand forty-six patients had detail information on treatment, 644 patients (21.1%) received surgery alone (group A), 486 (16.0%) received chemotherapy and/without radiotherapy (group B), and 1916 (62.9%) received surgery plus chemotherapy and/without radiotherapy (group C). Table 6 shows the comparisons of characteristics between data from systematic review group and that from retrospective study group. No obvious publication bias was found. The 2 groups did not differ in distribution of immunophenotype, sex, PS score, IPI score, or stage.
TABLE 6.
Comparison of Characteristics Between Data From Systematic Review Study Group and Retrospective Study Group
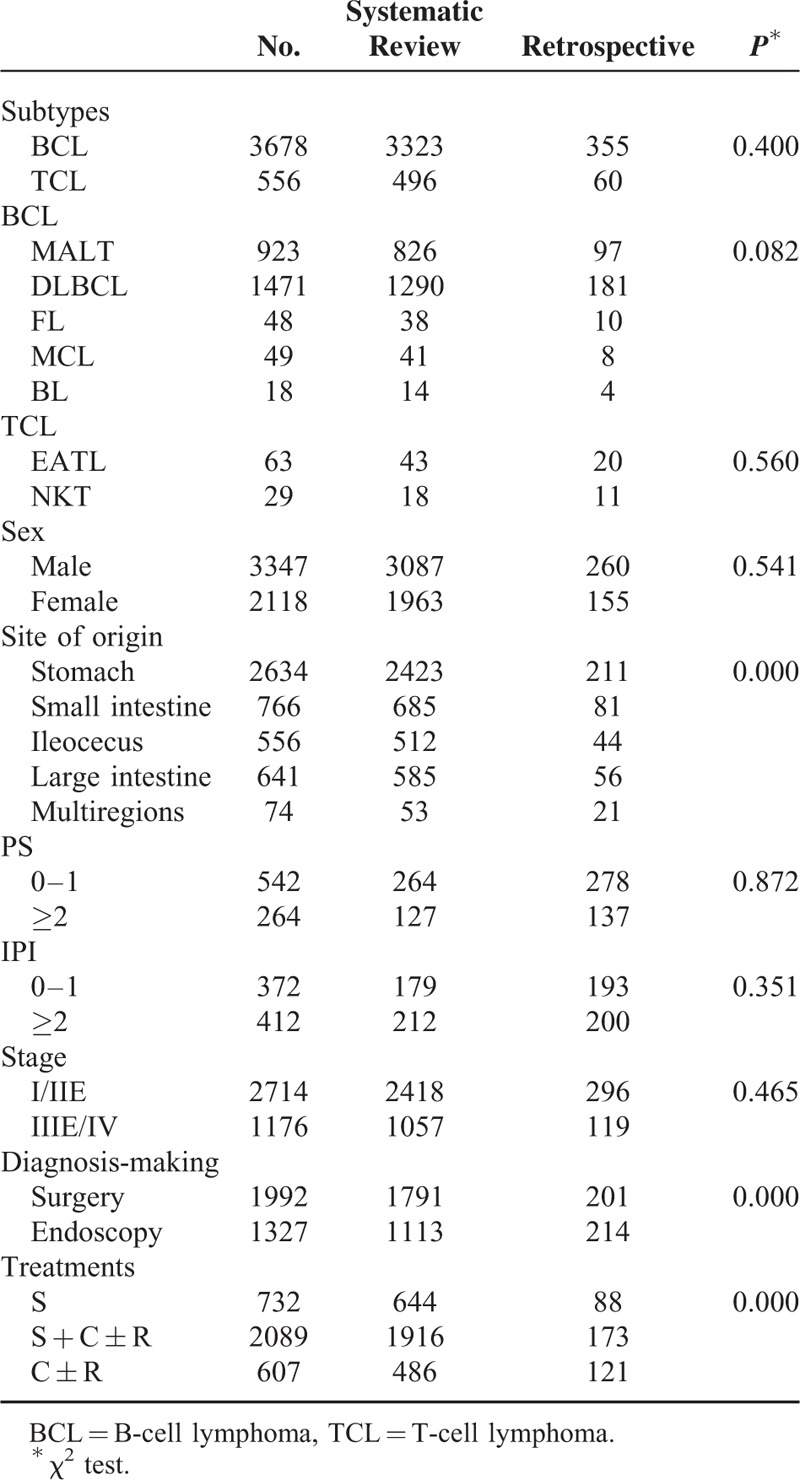
A higher mortality (5 years) was revealed in the therapeutic group B compared with group C (42.7% vs 35.0%, P = 0.02) (Fig. 5). Similar result was found in comparison of mortalities between group A and group C (67.5% vs 45.1%, P < 0.00001) (Fig. 6). A higher mortality (5 years) was observed in the group A compared with group C (65.5% vs 46.8%, P = 0.02) (Fig. 7).
FIGURE 5.
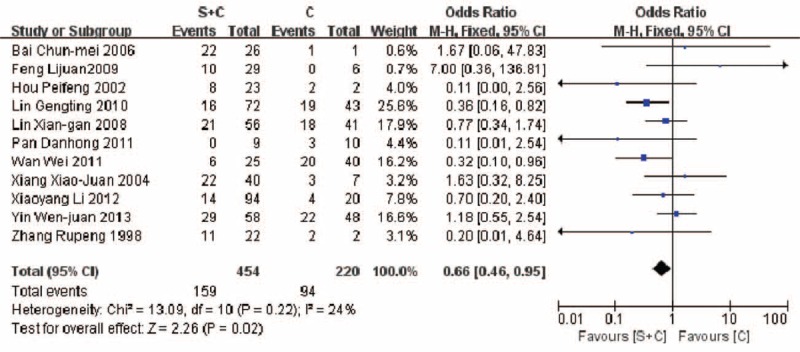
Comparison of mortality in patients of PGIL treated with surgery plus chemotherapy vs chemotherapy alone (35.0% vs 42.7%, P = 0.02).
FIGURE 6.
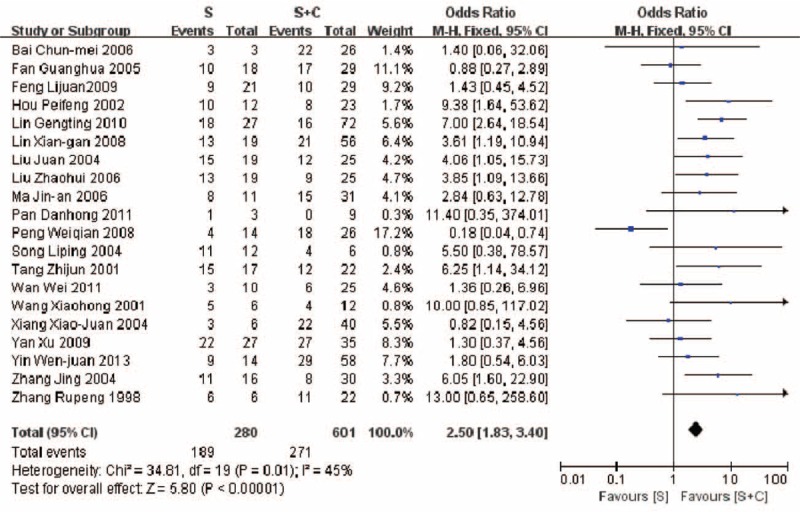
Comparison of mortality in patients of PGIL treated with surgery alone vs. surgery plus chemotherapy (67.5% vs 45.1%, P < 0.00001).
FIGURE 7.
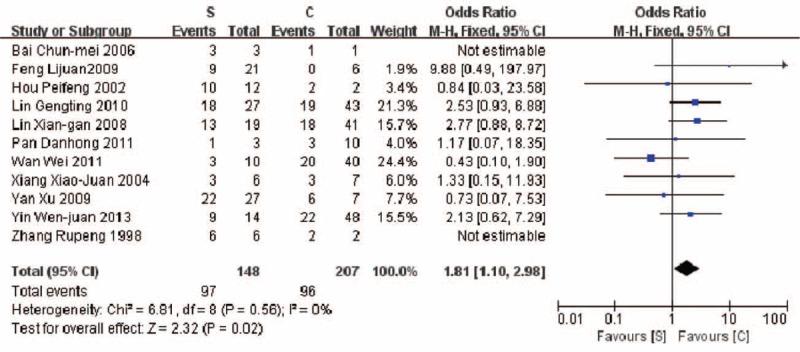
Comparison of mortality in patients of PGIL treated with surgery alone versus chemotherapy alone (65.5% vs 46.8%, P = 0.02).
The methodological quality of the included studies was moderate. Scores on the Newcastle–Ottawa scales were 5 to 6 points in cohort studies. Base on the results of the quality assessment, none of studies was excluded from the meta-analysis. As for the publication bias about the included studies, the funnel plot did not present obviously bias for mortality comparison between therapeutic group B and group C (Fig. 8A), or between therapeutic group A and group C (Fig. 8B). However, there might be publication bias for mortality comparison between therapeutic group A and group B (Fig. 8C). As there was no obviously heterogeneity (P = 0.56, I2 = 0%) or methodological quality, the reason for the asymmetrical funnel plot might be that the number of studies included was small, or that the sample of included studies was small.
FIGURE 8.
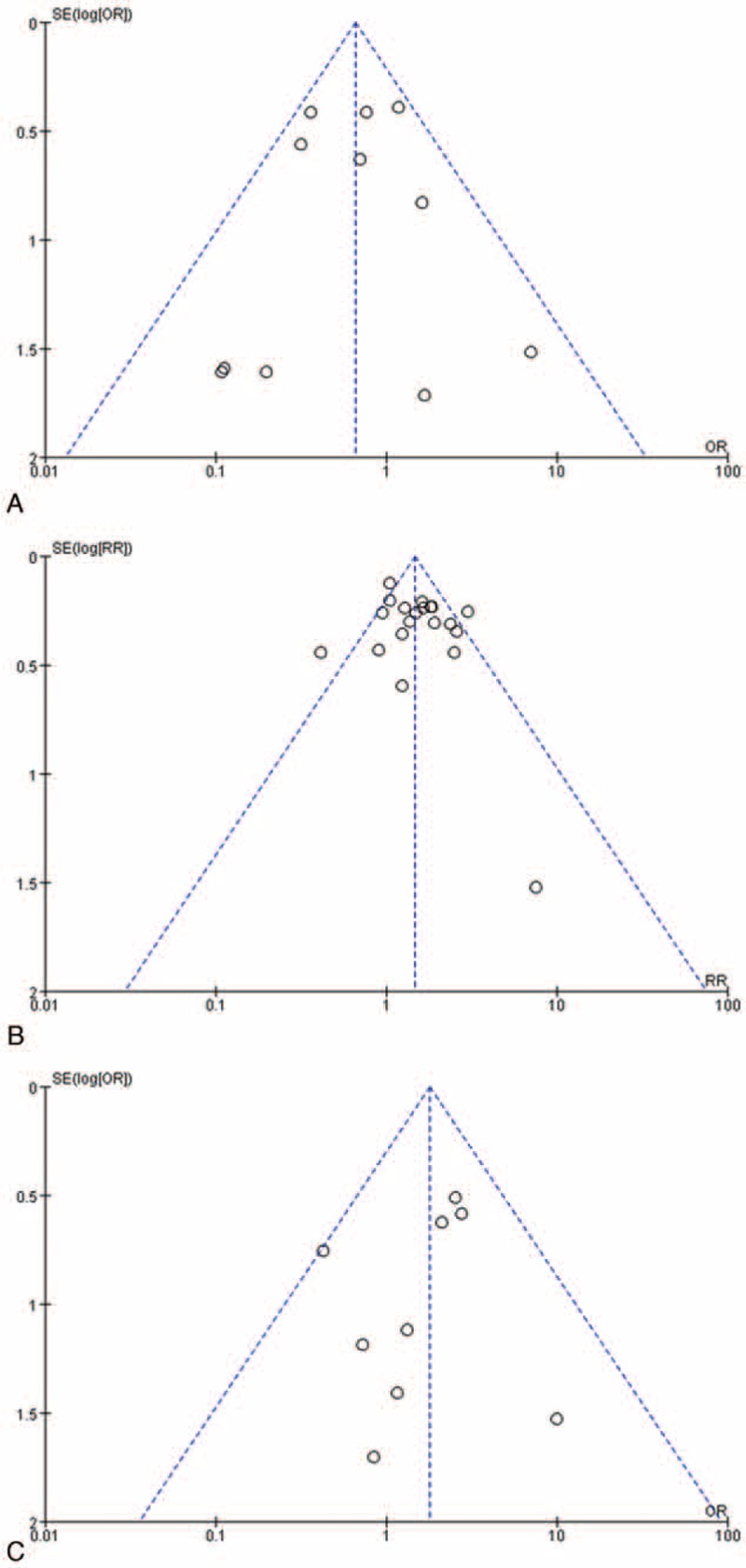
Funnel plot for comparisons of mortality in patients of PGIL treated by different therapeutic modalities. (A) Surgery plus chemotherapy versus chemotherapy alone. (B) Surgery alone versus surgery plus chemotherapy. (C) Surgery alone versus chemotherapy alone.
Base on the findings on the diagnosis, prognostic factors including independent prognostic factors, outcomes, and mortalities of different therapeutic modalities as mentioned above, a flow chart of diagnosis and treatment process for PGIL was approximately drew out (Fig. 9).
FIGURE 9.
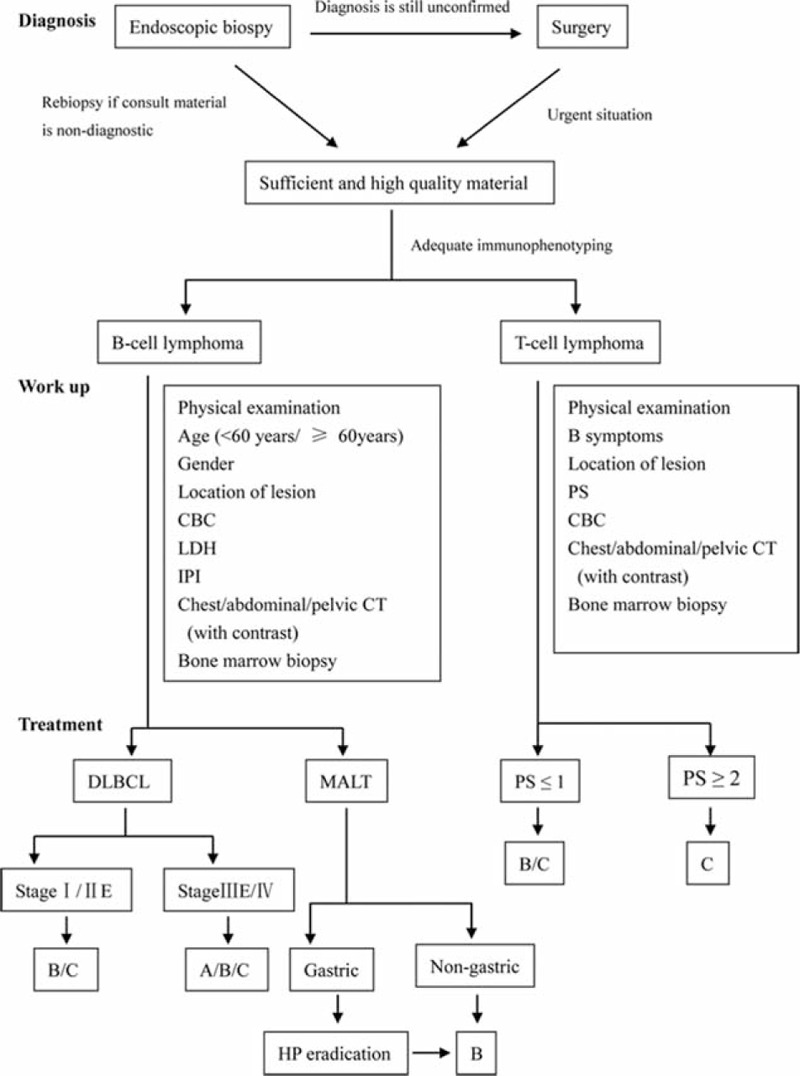
Flow chart of diagnosis and treatment process for PGIL. A = surgery alone, B = surgery plus chemotherapy and/without radiotherapy, C = chemotherapy and/without radiotherapy, CBC = count of blood cell, LDH = lactate dehydrogenase, IPI = International Prognostic Index, PS = performance status.
DISCUSSION
There are many publications concerning PGIL, as is true for China. However, most of them are in small scale.22,23,26,27 The current study is probably the largest series in China.
For PGIL, B-cell lymphoma is far more frequent (85.5%) than T-cell lymphoma. This is also demonstrated by the systematic review of previous studies in China in which the proportion of B-cell lymphoma was 87.0%. Among the different immunophenotypes of PGIL, DLBCL is the most frequent in our study as indicated by several previous studies.14,28,29 Stomach is the most frequently involved site varied from 66.7% to 76.1%, followed by the small intestine,4,7 which is consisted of the results in our study. As was reported by Nakamura et al,7 stomach was the most common primary site involved with a frequency of 60% to 70%, followed by the small intestine (20%–30%). In our study, the frequency of stomach involving was 56.4%, followed by the small intestine (16.4%), slightly lower than that reported in Japan. More patients received chemotherapy and/without radiotherapy in the retrospective study group than that in the systematic review group (31.7% vs 16.0%), which could be explained by the improved diagnosis ability, efficacy of chemotherapy, and that chemotherapy was increasingly an optimal treatment.7
The clinical presentations of PGIL in our study were dominated by abdominal pain, followed by bloody stools and abdominal mass, but differed depending on the histological type. The most common endoscopic appearances of PGIL are ulcerative and massive. However, the appearances are nonspecific and indistinguishable from carcinoma.1,4,30 51.6% of patients in our retrospective study group were diagnosed by endoscopic biopsy, which was consistent with a previous study.28 Furthermore, 36.9% of the patients diagnosed by endoscopic biopsy underwent repeated endoscopy. Therefore, our study reflects that repeat endoscopic biopsy is necessary when a malignant lesion is visualized; in addition, multiple biopsies of the tumor and multilevel biopsies are suggested. Apart from that, only 38.3% of patients of the systematic review group were diagnosed by endoscopic biopsy, which may reflect a selection bias existing in that our institutions are reference centers in general.
In our study, 201 patients (48.4%) were diagnosed by surgery, which makes surgery an important method for diagnosis of PGIL. Reasons for that surgery became a diagnosis method are as follows. On one hand, the lesions of PGIL mainly locate submucose, which improves the difficulty of diagnosis through endoscopic biopsy. Sometimes, when we still cannot confirm the diagnosis of a visualized malignant lesion after repeating endoscopic biopsy, surgery can be the choice. On the other hand, part of PGIL patients came to hospital because of acute abdomen, especially patients of T-cell lymphoma. Diagnosis could be confirmed after emergency operation. In our study, out of the 201 patients diagnosed by surgery, 37 (18.4%) patients received emergency operations, making surgery an essential method for diagnosis of PGIL, especially for the diagnosis of T-cell lymphoma because of its high frequency of acute abdomen.
The best OS and PFS were achieved in MALT lymphoma and follicular lymphoma, followed by DLBCL, and the poorest in EATL and other T-cell types. This observation is in concordance with previously published reports.4,14 Li et al13 identified treatment response, and elevated serum LDH level and PS score as independent prognostic factors of survival for DLBCL. Nakamura et al demonstrated that an earlier stage, younger age, gastric localization, B-cell phenotype, and absence of B symptoms were independent prognostic factors for better OS and EFS for PGIL.7 Gou et al12 reported that female, radical surgery, and B-cell phenotype were independent prognostic factors for better OS for primary intestinal non-Hodgkin lymphoma. Zhang et al29 identified PS score, and modified Ann Arbor stage and albumin as independent prognostic factors for primary gastric non-Hodgkin lymphoma. Delabie et al17 found that clinical sprue was a predictor of EFS for peripheral T-cell lymphoma and EATL (type1). Our multivariate analysis revealed site of multiple regions involved, IPI ≥2 to be independent prognostic factors for worse OS and PFS for B-cell lymphoma, and PS ≥2 to be independent prognostic factor for worse OS and PFS for T-cell lymphoma.
Nowadays, there is still no consensus on whether surgery should be a first-line treatment for PGIL. To evaluate the efficacy of surgery alone (group A), chemotherapy and/without radiotherapy (group B), and surgery plus chemotherapy and/without radiotherapy (group C), we performed a series of survival analysis according to subtypes of PGIL, lesion location, stage, and independent prognostic factors. In our study, the type of therapeutic modality did not influence OS or PFS of B-cell lymphoma as demonstrated in previous study, and the results were similar when regarding stage, IPI score.7 Such result was also found in patients of intestinal B-cell lymphoma, which was in concordance with that reported by Li et al.8 However, Kim et al found that therapeutic group C showed better survival in patients of intestinal B-cell lymphoma than group B; furthermore, Gou et al revealed that radical surgery was an independent prognostic index for primary intestinal non-Hodgkin lymphoma.9,12
Pathologically, therapeutic group A showed worse OS and PFS of DLBCL in an early stage (I/II E) than group B and C; type of therapeutic modality did not influence OS or PFS of DLBCL in advanced stage. Because of the chemo-sensitivity, chemotherapy is optimal for gastric DLBCL.4,6 OS and PFS in patients of intestinal DLBCL did not differ significantly between group B and C in our study, as was also indicated by Li et al,13 whereas Kim et al11 reported that therapeutic group C showed better OS than group B. According to Matysiak-Budnik et al,14 surgery may be the first-line treatment for intestinal DLBCL because of the probability of complications. Based on the survival results in our study and opinion of previous studies, surgery alone is not optimal for patients of DLBCL in an early stage.
Because stomach is the most common organ of MALT lymphoma, and gastric MALT lymphoma is closely related to H. pylori infection, which makes that H. pylori eradication therapy is the first-line treatment of gastric MALT lymphoma.6 Few patients of MALT lymphoma in our study first received H. pylori eradication therapy, making our study shot of evidence to evaluate it. In our study, therapeutic groups A, B, and C did not differ in OS or PFS for MALT lymphoma; surgery did not significantly improve the prognosis of MALT lymphoma. As an indolent lymphoma, MALT lymphoma can be excellently controlled by chemoimmunotherapy.58
Patients of T-cell lymphoma receiving therapeutic A presented worse OS and PFS than those of therapeutic group B and C, which was the same for those with lesion located in intestine, with PS ≥2. For patients of T-cell lymphoma with PS ≤1, therapeutic B and C showed equivalent efficacy. These findings demonstrated that therapeutic group C had an equivalent efficacy compared with group B as indicated by the previous studies.9,31 Despite that over half of patients of T-cell lymphoma were diagnosed by surgery, and 30% of patients of T-cell lymphoma received emergency operations; surgery is often mandatory as first-line treatment. Chemotherapy may improve the prognosis for patients with PS ≤1. A retrospective study of EATL (37 cases) reported by Malamut et al32 revealed that chemotherapy and surgical resection were predictors of good prognosis.
When performing the meta-analysis on the comparison of mortalities in patients treated with group A vs group C, sensitivity test identified that the article reported by Weiqian et al was heterogeneous from the other articles included. Because the article meet our including criteria, and it did not influence significantly the overall effect, this article was still included for meta-analysis. Our study revealed that patients in therapeutic group C had a lower mortality than those in group A and B, whereas group B showed lower mortality than group A. Taking the survival results into consideration, treatment of surgery plus chemotherapy promises the best prognosis for PGIL. Because the mortality analysis disregarded the subtypes, value of the results is limited. Patients’ quality of life is a point that is increasingly considered. Nonsurgical therapeutic modality is becoming increasingly optimal for PGIL6,7; 48.4% of patients in the retrospective study group of our study and 61.7% in the systematic review group were diagnosed by surgery; however, 8.9% of patients in the retrospective study group underwent emergency operations. From those above, surgery plays an important role in the diagnosis and treatment of PGIL, especially in patients of T-cell lymphoma.
Based on the results of our study, we propose a flow chart of diagnosis and treatment procedure for PGIL in China (Fig. 9). Endoscopic biopsy diagnosis and surgery diagnosis are the 2 main methods of confirming diagnosis of PGIL. Because of the limited successful rate of endoscopic biopsy diagnosis, we suggest repeat endoscopy and biopsy if necessary. Surgery plays an important role in diagnosing, especially for T-cell lymphoma. When it comes to the workup, we further make sure whether the GI lymphoma is “primary,” and emphasize prognostic factors for different subtypes of PGIL. As for treatment, chemotherapy alone or combined with surgery is the first choice for DLBCL of stage I/II E. For MALT lymphoma, our study showed that surgery did not significantly improve the prognosis; Hp eradication and chemotherapy are of great importance. For patients of T-cell lymphoma with PS ≥2, surgery plus chemotherapy shows the best OS and PFS, and for those with PS ≤1, chemotherapy and surgery plus chemotherapy show equivalent efficacy in OS and PFS.
Because our study is retrospective, this makes the observations of a limited value. Second, PGIL is a group of heterogeneity diseases, and each kind of phenotype presents certain characteristics, showing different clinical manifestations, effect to treatment, and survival. The case number of our study was not large enough for us to perform analysis completely according to the phenotypes. Third, the previous studies in China were usually of small scale, disregard the subtypes, leading to that our meta-analysis on mortality analysis of PGIL could not be further analyzed according to different subtypes. Further randomized prospective studies of large scale are needed.
Acknowledgments
The authors thank the staff of the First, Third, and Fifth Affiliated Hospitals of Sun Yat-Sen University, and Guangdong General Hospital for their partnership in this study.
Footnotes
Abbreviations: DLBCL = diffuse large B-cell lymphoma, EATL = enteropathy-associated T-cell lymphoma, IPI = International Prognostic Index, MALT = mucosa-associated lymphoid tissue, OS = overall survival, PFS = progression-free survival, PGIL = primary gastrointestinal lymphoma, PS = performance status.
YiC, YaC, and SC contributed equally to this work.
Authors’ contributors: YiC participated in the experimental design, data analysis, preparation, and review of the report; YaC participated in the experimental design, data collection, data analysis, preparation, and review of the report; SC participated in the data collection, data analysis, and review of the report; LW participated in the data collection of the report; LX participated in the data collection of the report; GL participated in the data analysis of the report; KY participated in the data collection of the report; YL participated in the data analysis of the report; LZ participated in the experimental design and review of the report; and KH participated in the experimental design, data analysis, and review of the report.
This work was supported by National Natural Science Foundation of China (Grant No. 81302140, 81572396, 81502503), National Natural Science Foundation of Guangdong, China (Grant No. 2014A030313050), and Specialized Research Fund for the Doctoral Program of Higher Education (Grant No. 20130171120093). Grant [2013]163 from Key Laboratory of Malignant Tumor Molecular Mechanism and Translational Medicine of Guangzhou Bureau of Science and Information Technology; Grant KLB09001 from the Key Laboratory of Malignant Tumor Gene Regulation and Target Therapy of Guangdong Higher Education Institutes were acknowledged.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Gurbuxani S, Anastasi J. What to do when you suspect gastrointestinal lymphoma: a pathologist's perspective. Clin Gastroenterol Hepatol 2007; 5:417–421. [DOI] [PubMed] [Google Scholar]
- 2.Kako S, Oshima K, Sato M, et al. Clinical outcome in patients with small-intestinal non-Hodgkin lymphoma. Leuk Lymphoma 2009; 50:1618–1624. [DOI] [PubMed] [Google Scholar]
- 3.Cheung MC, Housri N, Ogilvie MP, et al. Surgery does not adversely affect survival in primary gastrointestinal lymphoma. J Surg Oncol 2009; 100:59–64. [DOI] [PubMed] [Google Scholar]
- 4.Nakamura S, Matsumoto T. Gastrointestinal lymphoma: recent advances in diagnosis and treatment. Digestion 2013; 87:182–188. [DOI] [PubMed] [Google Scholar]
- 5.Sabattini E, Bacci F, Sagramoso C, et al. WHO classification of tumours of haematopoietic and lymphoid tissues in 2008: an overview. Pathologica 2010; 102:83–87. [PubMed] [Google Scholar]
- 6.Zelenetz AD, Abramson JS, Advani RH, et al. NCCN clinical practice guidelines in oncology: non-Hodgkin's lymphomas. J Natl Compr Canc Netw 2010; 8:288–334. [DOI] [PubMed] [Google Scholar]
- 7.Nakamura S, Matsumoto T, Iida M, et al. Primary gastrointestinal lymphoma in Japan: a clinicopathologic analysis of 455 patients with special reference to its time trends. Cancer 2003; 97:2462–2473. [DOI] [PubMed] [Google Scholar]
- 8.Li B, Shi YK, He XH, et al. Primary non-Hodgkin lymphomas in the small and large intestine: clinicopathological characteristics and management of 40 patients. Int J Hematol 2008; 87:375–381. [DOI] [PubMed] [Google Scholar]
- 9.Kim SJ, Choi CW, Mun YC, et al. Multicenter retrospective analysis of 581 patients with primary intestinal non-hodgkin lymphoma from the Consortium for Improving Survival of Lymphoma (CISL). BMC Cancer 2011; 11:321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lai YL, Lin JK, Liang WY, et al. Surgical resection combined with chemotherapy can help achieve better outcomes in patients with primary colonic lymphoma. J Surg Oncol 2011; 104:265–268. [DOI] [PubMed] [Google Scholar]
- 11.Kim SJ, Kang HJ, Kim JS, et al. Comparison of treatment strategies for patients with intestinal diffuse large B-cell lymphoma: surgical resection followed by chemotherapy versus chemotherapy alone. Blood 2011; 117:1958–1965. [DOI] [PubMed] [Google Scholar]
- 12.Gou HF, Zang J, Jiang M, et al. Clinical prognostic analysis of 116 patients with primary intestinal non-Hodgkin lymphoma. Med Oncol 2012; 29:227–234. [DOI] [PubMed] [Google Scholar]
- 13.Li X, Shen W, Cao J, et al. Treatment of gastrointestinal diffuse large B cell lymphoma in China: a 10-year retrospective study of 114 cases. Ann Hematol 2012; 91:1721–1729. [DOI] [PubMed] [Google Scholar]
- 14.Matysiak-Budnik T, Jamet P, Fabiani B, et al. Primary intestinal B-cell lymphoma: a prospective multicentre clinical study of 91 cases. Dig Liver Dis 2013; 45:947–952. [DOI] [PubMed] [Google Scholar]
- 15.Ahn MJ, Park YW, Han D, et al. A case of primary intestinal T-cell lymphoma involving entire gastrointestinal tract: esophagus to rectum. Korean J Intern Med 2000; 15:245–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daum S, Ullrich R, Heise W, et al. Intestinal non-Hodgkin's lymphoma: a multicenter prospective clinical study from the German Study Group on Intestinal non-Hodgkin's Lymphoma. J Clin Oncol 2003; 21:2740–2746. [DOI] [PubMed] [Google Scholar]
- 17.Delabie J, Holte H, Vose JM, et al. Enteropathy-associated T-cell lymphoma: clinical and histological findings from the international peripheral T-cell lymphoma project. Blood 2011; 118:148–155. [DOI] [PubMed] [Google Scholar]
- 18.Boot H. Diagnosis and staging in gastrointestinal lymphoma. Best Pract Res Clin Gastroenterol 2010; 24:3–12. [DOI] [PubMed] [Google Scholar]
- 19.Bai CM, Yang T, Xü Y, et al. Clinical analysis of 32 primary intestinal non-Hodgkin's lymphoma (Chinese). Zhonghua Zhong Liu Za Zhi 2006; 28:142–144. [PubMed] [Google Scholar]
- 20.Song LP, Hou HL, Zhao H, et al. Clinical study of 22 cases of primary gastrointestinal lymphoma (Chinese). Ai Zheng 2004; 23:685–688. [PubMed] [Google Scholar]
- 21.Xiang XJ, He YJ, Li YH, et al. Prognosis analysis of 53 cases with primary intestinal non-Hodgkin's lymphoma (Chinese). Ai Zheng 2004; 23:443–447. [PubMed] [Google Scholar]
- 22.Wan W, Wang J, Jing HM, et al. Analysis of clinical characteristics and prognostic factors of 110 cases with primary gastrointestinal tract non-Hodgkin's lymphoma (Chinese). Zhonghua Xue Ye Xue Za Zhi 2011; 32:652–655. [PubMed] [Google Scholar]
- 23.Yin WJ, Wu MJ, Yang HY, et al. Clinicopathological features and prognostic factors of 216 cases with primary gastrointestinal tract non-Hodgkin's lymphoma (Chinese). Zhonghua Xue Ye Xue Za Zhi 2013; 34:377–382. [DOI] [PubMed] [Google Scholar]
- 24.Zhang R, Wang D, Huo X. Primary ileocecal lymphoma: report of 30 cases (Chinese). Zhonghua Wai Ke Za Zhi 1998; 36:459–460. [PubMed] [Google Scholar]
- 25.Wu YX, Liu B, Chen L, et al. Prognostic factors of primary gastric diffuse large B cell lymphoma: a retrospective study of 75 cases in China (Chinese). Ann Hematol 2013; 92:861–862. [DOI] [PubMed] [Google Scholar]
- 26.Cui Q, Dong X, Wang D, et al. Diagnosis and treatment of primary gastric non-Hodgkin's lymphoma: analysis of 157 patients (Chinese). Zhonghua Yu Fang Yi Xue Za Zhi 2002; 36:502–504. [PubMed] [Google Scholar]
- 27.Wang T, Gui W, Shen Q. Primary gastrointestinal non-Hodgkin's lymphoma: clinicopathological and prognostic analysis. Med Oncol 2010; 27:661–666. [DOI] [PubMed] [Google Scholar]
- 28.Ran W, Ouyang Q. Primary intestinal non-Hodgkin's lymphoma: a retrospective study of 85 cases (Chinese). Nan Fang Yi Ke Da Xue Xue Bao 2012; 32:534–538. [PubMed] [Google Scholar]
- 29.Zhang J, Hu X, Liu X, et al. Prognostic factors in primary gastric non-Hodgkin's lymphoma—a single-center retrospective analysis of 103 cases from China. Hepatogastroenterology 2010; 57:989–996. [PubMed] [Google Scholar]
- 30.Xu W, Zhou C, Zhang G, et al. Repeating gastric biopsy for accuracy of gastric lymphoma diagnosis. Gastroenterol Nurs 2010; 33:313–317. [DOI] [PubMed] [Google Scholar]
- 31.Yang YL, Wang J, Zhao LZ, et al. Clinical characteristics, cell origin and prognosis of primary gastrointestinal diffuse large B-cell lymphoma: a report of 40 cases (Chinese). Ai Zheng 2008; 27:636–641. [PubMed] [Google Scholar]
- 32.Malamut G, Chandesris O, Verkarre V, et al. Enteropathy associated T cell lymphoma in celiac disease: a large retrospective study. Dig Liver Dis 2013; 45:377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu X, Liu LIY, et M. al. Clinical analysis of 33 cases of primary gastrointestinal malignant lymphoma (Chinese). Chin J Dig Endosc 2006; 23:433–437. [Google Scholar]
- 34.Lin G, Lin S. Analysis of 142 cases of primary gastrointestinal non-Hodgkin's lymphoma (Chinese). Mod Med J China 2010; 12:60–63. [Google Scholar]
- 35.Fan G, Liu Z. Clinical and prognosis analysis of 47 cases with primary gastrointestinal non-Hodgkin's lymphoma (Chinese). JIANGXI Med J 2005; 40:703–705. [Google Scholar]
- 36.Ma J, Ouyang M, Qiu Z, et al. Operation combined with adjunctive chemotherapy for primary gastrointestinal lymphoma (Chinese). J Clin Res 2006; 23:502–504. [Google Scholar]
- 37.Liu J, Mo Y. The clinical diagnosis and therapy of primary gastrointestinal malignant lymphoma (Chinese). Int Med Health Guidance News 2004; 10:12–14. [Google Scholar]
- 38.Zhang J, Lu Z, Zhang C, et al. Prognosis analysis of 66 cases of primary gastrointestinal non-Hodgkin's lymphoma (Chinese). J Jilin Univ 2004; 30:984–987. [Google Scholar]
- 39.Lin X, Huang K, Xie D, et al. Prognostic factor analysis of 116 cases of primary gastrointestinal non-Hodgkin's lymphoma (Chinese). J Southern Med Univ 2008; 28:243–247. [PubMed] [Google Scholar]
- 40.Zhuang Y, Chen P, Yang M, et al. Primary lymphoma of gastrointestinal: clinicopathological study of 46 cases (Chinese). FUJIAN Med J 2002; 24:125–129. [Google Scholar]
- 41.Song F, Xu Q. Clinical analysis and diagnosis of primary gastrointestinal malignant lymphoma in 43 patients (Chinese). J Clin Med Pract 2012; 16:138–140. [Google Scholar]
- 42.Yang J, Zhang X, Zhang X. Diagnosis and therapy of primary gastrointestinal lymphoma (Chinese). J Med Res 2006; 35:42–46. [Google Scholar]
- 43.Wand G, Li H. Diagnosis and treatment of primary gastrointestinal lymphoma (Chinese). Chin J Mod Operat Surgery 2006; 10:117–119. [Google Scholar]
- 44.Cheng Y, Luo H. Clinicopathologic features of primary gastrointestinal non-Hodgkin's lymphoma (Chinese). Chin J Gen Pract 2012; 11:145–148. [Google Scholar]
- 45.Gou H, Chen X, Hou M. Clinical study of primary gastrointestinal non-Hodgkin's lymphoma: a report of 45 cases (Chinese). Chin J Clin Oncol 2005; 32:451–457. [Google Scholar]
- 46.Xu X, Gong Z, Tan Q. Analysis of 38 cases with primary gastrointestinal non-Hodgkin's lymphoma (Chinese). HENAN J Oncol 2003; 16:268–271. [Google Scholar]
- 47.Xu G, Li Yu, Li Yan, et al. A clinical study of primary gastrointestinal non-Hodgkin's lymphoma: a report of 25 cases (Chinese). J Basic Clin Oncol 2008; 21:526–529. [Google Scholar]
- 48.Zhang H, Guo X, Sun A, et al. Diagnosis and treatment for primary gastrointestinal non-Hodgkin's lymphoma: a review analysis of 41 cases (Chinese). J Fourth Mil Med Univ 2001; 22:1875–1879. [Google Scholar]
- 49.Qin L, Zhang X, Wen J. Primary gastrointestinal malignant lymphoma (Chinese). Clin Med 2006; 26:4–6. [Google Scholar]
- 50.Zhang Y, Liang H. Diagnosis and treatment of the primary malignant lymphoma in gastrointestinal tract: a report of 64 cases (Chinese). Infect Inflamm Repair 2002; 3:103–106. [Google Scholar]
- 51.Zhou L, Huang L, Du J. Clinical analysis of 45 patients with primary gastrointestinal lymphomas (Chinese). Chin J Surg Integr Tradit Western Med 2003; 9:264–268. [Google Scholar]
- 52.Zhao S, Qi F. Clinicopathological analysis of 42 patients with primary gastrointestinal lymphomas (Chinese). Chin J Clin Oncol Rehab 2007; 14:506–509. [Google Scholar]
- 53.Li J, Zeng R, Yang Q. The analysis of 42 cases of primary malignant lymphoma in gastrointestinal tract (Chinese). J Basic Clin Oncol 2007; 20:435–438. [Google Scholar]
- 54.Jiang W, Zhang W, Zhu Y. Diagnosis and treatment of primary malignant lymphoma of gastrointestinal tract (Chinese). ZHEJIANG J Prev Med 2002; 14:9–11. [Google Scholar]
- 55.Tu L, Lin J, Yang G, et al. The clinical characteristics of primary gastrointestinal lymphoma on the different site of origin (Chinese). Chin J Dig 2008; 28:472–477. [Google Scholar]
- 56.Jiang W. Clinical analysis for 25 cases of primary malignant lymphoma in gastrointestinal tract (Chinese). China Mod Med 2013; 20:165–167. [Google Scholar]
- 57.Hou P, Zhang X, Zheng M, et al. Clinical analysis of 37 cases of malignant lymphoma of gastrointestinal tract neoplasm (Chinese). FUJIAN Med J 2002; 24:4–6. [Google Scholar]
- 58.Kiesewetter B, Ferreri AJ, Raderer M. Chemoimmunotherapy for mucosa-associated lymphoid tissue-type lymphoma: a review of the literature. Oncologist 2015; 20:915–925. [DOI] [PMC free article] [PubMed] [Google Scholar]


