Abstract
Severe sepsis remains the leading cause of mortality in the critically ill. Local epidemiological studies on sepsis are of paramount importance to increase our knowledge about sepsis features and to improve patient care and prognosis.
Adult patients (≥20 years) admitted to the surgical intensive care units with severe sepsis or septic shock from 2009 to 2010 were retrospectively retrieved and analyzed. The primary outcome of interest was 28-day mortality.
Of 7795 admissions, 536 (6.9%) patients had severe sepsis. The most common sites of infection were the respiratory tract (38%) and abdomen (33%). Gram-negative bacteria, particularly Klebsiella pneumoniae (8.6%) and Escherichia coli (6.0%), were the major infecting micro-organisms, responsible for approximately two-thirds of the severe sepsis episodes. The overall 28-day mortality rate was 61%, and a higher sequential organ failure assessment score and the use of mechanical ventilation were independently associated with a worse outcome.
Admissions with severe sepsis are not uncommon and are associated with substantial 28-day mortality in surgical intensive care units in northern Taiwan. Establishment and optimization of each institutional sepsis care standard to improve the outcome of sepsis are warranted.
INTRODUCTION
Despite the advances in medical therapeutics, severe sepsis and septic shock remain the leading cause of morbidity and mortality in intensive care units (ICUs).1,2 There have been a number of studies reporting the incidence, characteristics, and outcome of severe sepsis and septic shock from different regions and countries.3–6 Not surprisingly, the incidence and mortality rates widely vary throughout the world, and significant differences in the pattern of causative micro-organisms and infection sites have been observed.7 These facts reflect the importance of local epidemiological studies on sepsis to increase our knowledge about sepsis features in different areas and healthcare systems in order to improve patient care and prognosis.8
In Taiwan, a recent population-based study demonstrates that the incidence rate of severe sepsis increased from 135 per 100,000 in 1997 to 217 per 100,000 in 2006.9 During the same period, although the proportion of patients with multiorgan dysfunction increased from 11.7% in 1997 to 27.6% in 2006, the hospital mortality remained largely unchanged, averaging 30.8%.9 However, there is little, if any, information on the demographics and outcome of severe sepsis and septic shock in a specific geographical region of Taiwan. In the present work, we conducted a retrospective descriptive study to evaluate the characteristics, outcome, and prognostic factors in patients with severe sepsis and septic shock in the northern Taiwan. As epidemiology and surveillance data significantly differ between medical and surgical ICU patients,10 we further specifically focus on events in the surgical ICUs.
METHODS
Study Subjects
This retrospective descriptive study was conducted in the National Taiwan University Hospital, a tertiary referral center in the northern Taiwan. From 2009 to 2010, all patients admitted to the surgical ICUs were eligible for this study. The inclusion criteria were as follows: age ≥20 years, and an admission diagnosis of severe sepsis or septic shock. Severe sepsis and septic shock were defined according to the Surviving Sepsis Campaign guidelines.11 In brief, severe sepsis was defined as sepsis plus sepsis-induced organ dysfunction or tissue hypoperfusion and septic shock was defined as sepsis-induced hypotension persisting despite adequate fluid resuscitation. If a patient was admitted to the ICUs more than once, only the first episode of severe sepsis or septic shock was counted. This study was approved by the Research Ethics Committee of the National Taiwan University Hospital and the need for informed consent was waived.
Data Collection
Data were collected by a single experienced data collector using standardized forms. The following information was retrieved: demographics, body mass index, comorbidities, Charlson comorbidity index,12 no. of systemic inflammatory response syndrome (SIRS) met,13 sequential organ failure assessment (SOFA) score,14 smoking status (smoker or ex-smoker vs never-smoker), admission category (surgical scheduled vs surgical unscheduled vs medical), surgical procedure (neurosurgery vs thoracic surgery vs cardiovascular surgery vs general surgery), sites of infection, causative micro-organisms, and sepsis-associated complications (septic shock, dialysis-requiring acute kidney injury and need for invasive mechanical ventilation). The SOFA score was determined at the time of ICU admission. Surgical admissions were defined as surgery ≤2 weeks preceding ICU admission. Unscheduled surgery was defined as an operation performed ≤24 h of onset of symptoms or injury. Considered comorbidities included the presence of diabetes mellitus, chronic obstructive pulmonary disease, malignancy, liver cirrhosis, congestive heart failure, coronary artery disease, chronic kidney disease, and cerebrovascular disease. Sepsis-associated complications were counted if they occurred ≤7 days of ICU admission. The main outcome measure of interest was 28-day mortality.
Statistical Analysis
Data were analyzed using the SPSS 15.0 software program (SPSS Inc., Chicago, IL). Data were reported as the mean ± standard deviation or no. (%), as appropriate. Categorical variables were compared by the chi square or Fisher's exact test when necessary. The Student t test was applied to analyze continuous variables. Multivariate logistic regression analysis was used to determine independent predictors for 28-day mortality. Those variables with a P value of < 0.05 in the univariate analysis were entered into the multivariate model. A 2-tailed P value of < 0.05 was considered statistically significant.
RESULTS
Characteristics of The Study Population
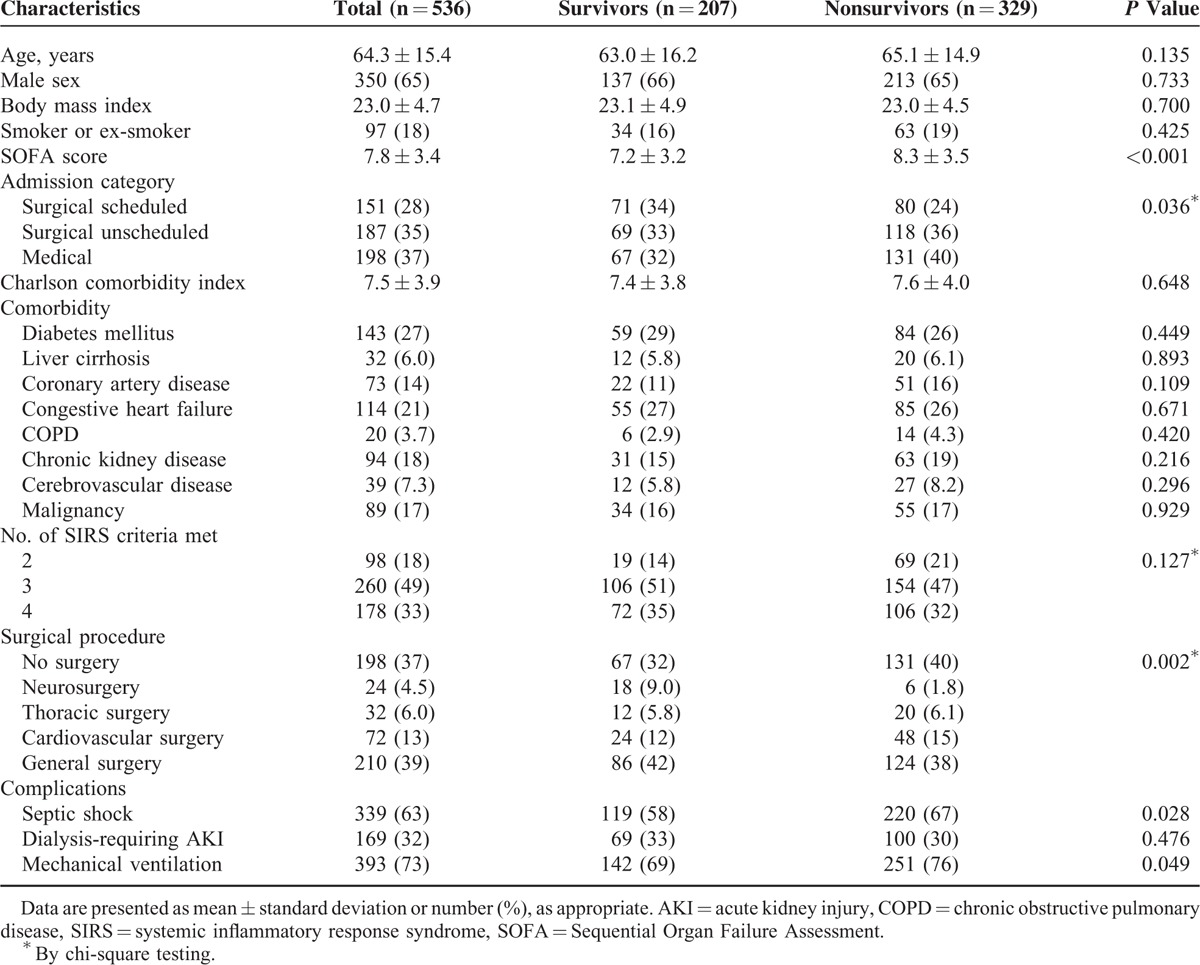
Among 7795 patients admitted to the surgical ICUs during the study period, 536 (6.9%) had severe sepsis and constituted the study population (Figure 1). Clinical characteristics of our patient population stratified by 28-day mortality are shown in Table 1. The average age of patients with severe sepsis was 64.3 years and about two-thirds of them were men. The mean SOFA score and Charlson comorbidity index were 7.8 and 7.5, respectively. The majority of patients had surgery, either scheduled or unscheduled, prior to ICU admission, and general surgery was the most commonly performed surgical procedure. Respiratory failure needing mechanical ventilation, septic shock, and acute kidney injury requiring dialysis were observed in 73%, 63%, and 32% of the severe sepsis patients. Compared with 28-day survivors, nonsurvivors had a higher SOFA score and were more likely to develop septic shock and be placed on mechanical ventilation.
FIGURE 1.
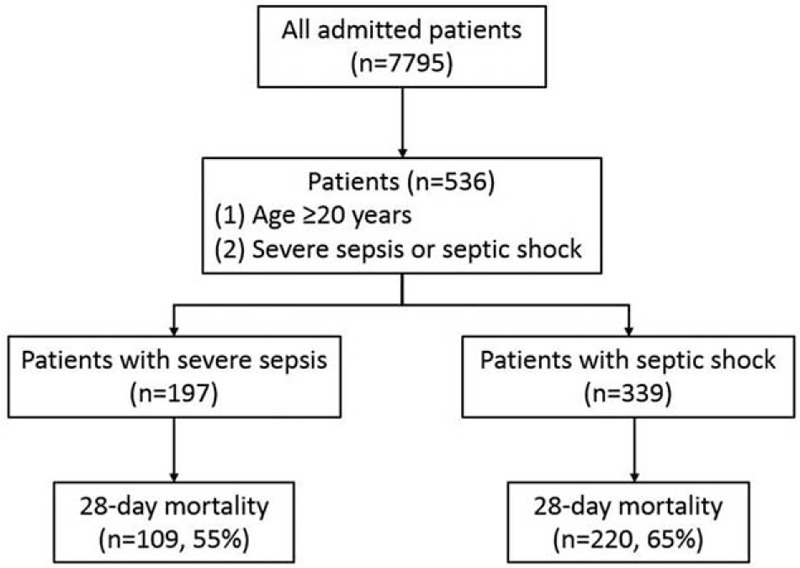
Study flow diagram.
TABLE 1.
Characteristics of Intensive Care Unit Patients With Severe Sepsis
Etiology and Site of Infection
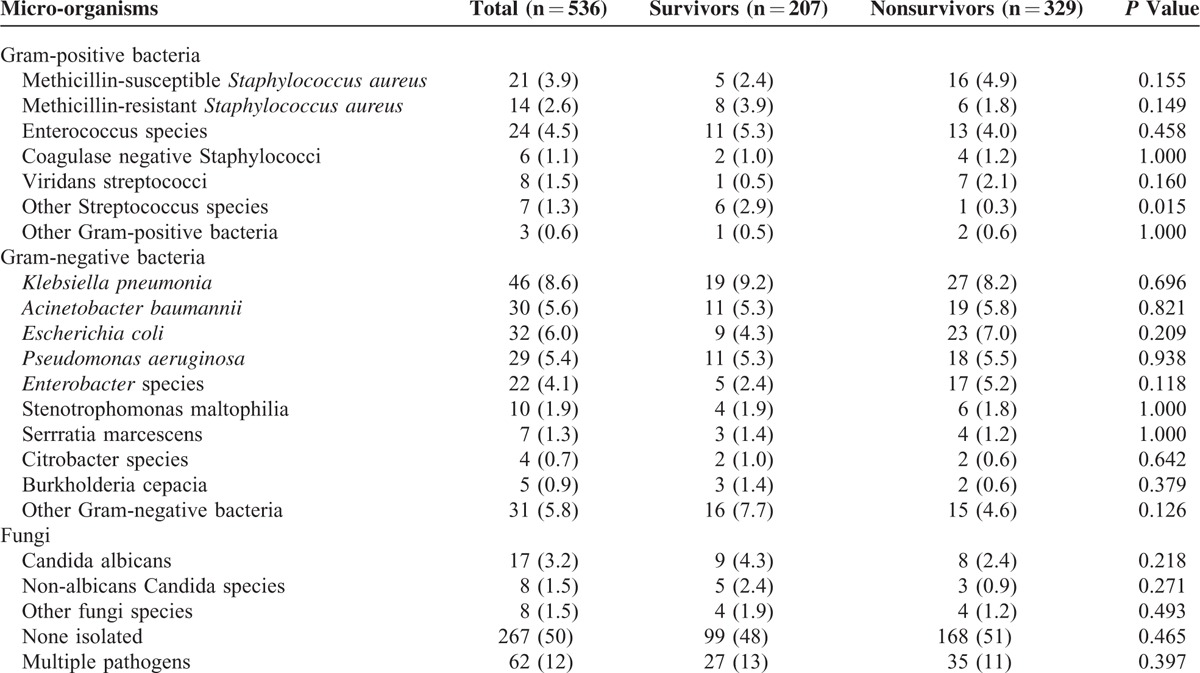
The respiratory tract (38%) and abdomen (33%) were the most frequent sites of infection (Table 2). In the 28-day nonsurvivors, intra-abdominal infection seemed more common than in survivors. Micro-organisms were identified in only half of the study population and 62 (12%) patients had polymicrobial infection (Table 3). Out of 269 patients with documented microbiological results, Gram-negative bacteria, Gram-positive bacteria, and fungi were isolated in 65%, 25%, and 10% of the severe sepsis patients. The most prevalent species were Klebsiella pneumoniae (8.6%), Escherichia coli (6.0%), Acinetobacter baumannii (5.6%), Pseudomonas aeruginosa (5.4%), and Enterococcus species (4.5%). The distribution of infecting microorganisms was not significantly different between survivors and nonsurvivors.
TABLE 2.
The Source of Infection in Severe Sepsis Patients

TABLE 3.
Distribution of Causative Micro-organisms Isolated From Severe Sepsis Patients
Outcome and Its Predictors
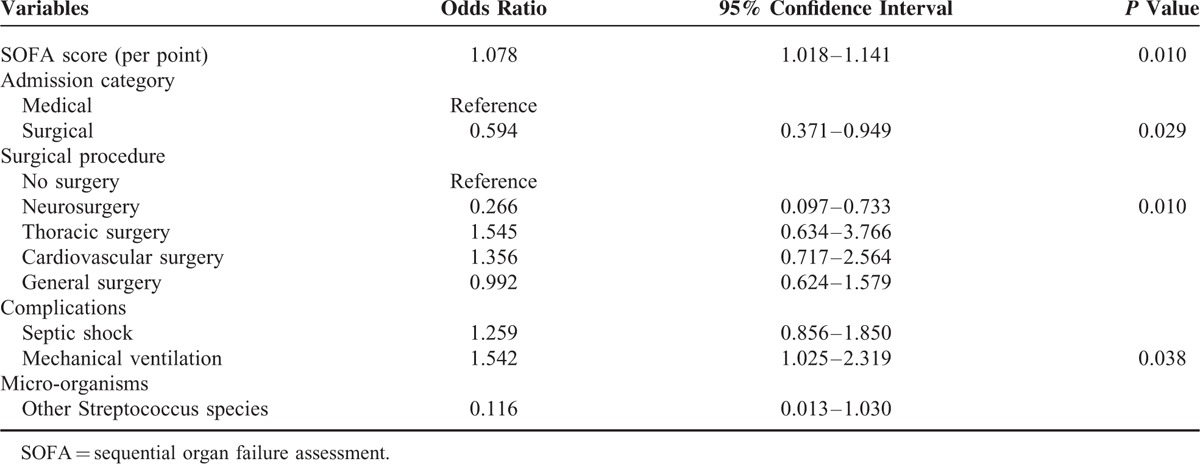
The overall 28-day mortality rate was 61%. The multivariate analysis of patient variables and 28-day mortality are shown in Table 4. Patient characteristics associated with increased mortality were a higher SOFA score and the need for mechanical ventilation. On the contrary, ICU admission after surgery, particularly neurosurgery, was associated with a reduced risk of mortality.
TABLE 4.
Multivariate Analysis of Risk Factors for 28-Day Mortality in Patients With Severe Sepsis
DISCUSSION
The objective of this descriptive study was to provide representative data on the incidence and mortality of severe sepsis in the surgical ICUs in northern Taiwan, and the main findings were summarized as follows. First, the incidence of severe sepsis was 69 per 1000 surgical ICU admissions in this study period. Second, respiratory tract infection and intra-abdominal infection were the most common sites of infection. Third, Gram-negative bacteria accounted for the majority episodes of severe sepsis, with predominant micro-organisms being K. pneumoniae and E. coli. Lastly, the patients with severe sepsis had a 28-day mortality rate of 61%, and a higher SOFA score and the need for mechanical ventilation were independent risk factors of death.
Previous studies have shown that the incidence rates of severe sepsis in ICUs ranged from 4.3% to 27%.15–17 Although the incidence of severe sepsis in our surgical ICUs was comparable to the reported data, it was undoubted that severe sepsis incidence varied significantly from place to place and from time to time.7 Moreover, patient characteristics are different among medical, surgical, or mixed ICUs. Thus, it is important for each ICU to access its own sepsis epidemiology and establish a management policy for patients with severe sepsis. Of note, the relatively lower incidence rate in this study may be explained by the strict inclusion criteria and characteristics of the total patient population. We only enrolled patients diagnosed with severe sepsis or septic shock on ICU admission and did not exclude those who stayed in the ICU for <24 h for routine postoperative observation as the whole study population.
Our data showed that the respiratory tract, abdomen, and wound and soft tissue accounted for >80% of patients with severe sepsis, and the respiratory tract was the principal source of infection, in accordance with the reports from several epidemiological studies of severe sepsis.5,15,18 Although respiratory tract infection increased over time and has been associated with the highest mortality,19,20 our results did not show a significant difference in 28-day mortality between sites of infection. In the present work, microbial isolates were obtained in only 50% of patients with severe sepsis and 12% of the episodes were polymicrobial. Both the figures were lower than those reported in the literature. The isolation rates were 60% to 70% and 16% to 55% of severe sepsis were associated with multiple micro-organisms in other studies.5,10,21–24 The case definition in this study may explain the discrepancy. Our patients had established severe sepsis or septic shock at the time of ICU admission, and this indicates that the culture specimens were mainly collected outside the ICU setting. In this way, a number of specimens may not be retrieved in a timely manner, more specifically, prior to antimicrobial administration. As we know, antibiotic therapy is anticipated to decrease the yield of cultures.25
The analysis of microbial characteristics of severe sepsis in this study showed that Gram-negative bacteria were the most common pathogens in severe sepsis, and the information was quite different from prior reports that Gram-positive bacteria were predominant.1,5,26 However, our microbial patterns were consistent with some studies in Asia; Tanriover et al27 and Zhou et al3 also reported that Gram-negative bacteria were isolated in >60% of sepsis patients. The reason for the diverse microbiological patterns of severe sepsis is probably a result of complex interactions involving the characteristics of the patient population, institutional infection control measures, hospital policies for antimicrobial therapy, invasive interventions, and healthcare quality provided. In addition, K. pneumoniae was the most prevalent pathogen in severe sepsis in this study, a finding reflecting the local-regional microbiological epidemiology. In Taiwan, K. pneumoniae is the major cause of liver abscess, brain abscess, lung abscess, thoracic empyema, prostatic abscess, deep neck infection, and complicated skin and soft tissue infection.28–30 In short, the results of this study may direct empirical antimicrobial therapy and draw attention to the importance of individual ICU sepsis guidelines.
Respiratory failure and septic shock were observed in >60% of the study subjects and were significantly more common in nonsurvivors than in survivors. In the multivariate model, need for mechanical ventilation was an independent risk factor for sepsis mortality. Previous studies have demonstrated that the use of mechanical ventilation was associated with increased disease severity and long-term mortality.31,32 It is speculative that mechanical ventilation per se may not only precipitate pre-existing lung injury, but contribute to the development of multiorgan dysfunction syndrome.33,34 Therefore, our finding emphasizes the prognostic role of the application of mechanical ventilation in severe sepsis and reminds clinicians of the downsides of invasive ventilatory support.
The outcome of severe sepsis patients varies considerably across different studies. In the past 2 decades, the observed 28-day mortality rates ranged from 13% to 61%,35,36 with a pooled mortality of 33%.37 In comparison, the mortality rate of 61% in patients with severe sepsis and septic shock in the present study was higher than that in most studies. A number of factors can explain the difference, such as the patient age, variation in the definition of severe sepsis, comorbidities, sites and types of infection, involved microorganisms, principles of antimicrobial therapy, severity of organ dysfunction, and standard of sepsis care. Several lines of evidence indicate a declining trend in severe sepsis mortality despite the absence of novel sepsis therapeutics.6,37 The reason behind this observation may be due to the improved care process, including early antimicrobial administration,38 adherence to early goal-directed therapy,39 and application of lung-protective ventilatory strategy.40 Accordingly, so-called standard of sepsis care may contribute to the wide range of mortality rates in various studies. However, this important confounding factor is hard to access from the literature.
Our study has some limitations. First, the generalizability of the single-center experience is limited, but we intend to establish local-regional epidemiology of severe sepsis to improve our understanding and patient care. Second, we studied only patients with severe sepsis and septic shock on ICU admission, but did not include those developing severe sepsis during the ICU stay. In this way, the composition of our study population is more homogeneous and we can provide more specific information to clinicians about severe sepsis, who will accommodate these critically ill patients.
In conclusion, the present study shows that severe sepsis was not an uncommon event in surgical ICUs in northern Taiwan and was frequently associated with a fatal outcome. Respiratory tract infection and intra-abdominal infection were predominant sources of severe sepsis, and Gram-negative bacteria, particularly K. pneumoniae, were the major causative micro-organisms. The use of mechanical ventilation in severe sepsis patients was associated with worse prognosis. Undoubtedly, severe sepsis remains an important public health issue, and establishment and optimization of each ICU's sepsis care standard to improve the outcome of sepsis are warranted.
ACKNOWLEDGMENTS
We thank the staff of the Eighth Core Lab, Department of Medical Research, National Taiwan University Hospital for technical support during the study.
Footnotes
Abbreviations: ICUs = intensive care units, SIRS = systemic inflammatory response syndrome, SOFA = sequential organ failure assessment.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Finfer S, Bellomo R, Lipman J, et al. Adult-population incidence of severe sepsis in Australian and New Zealand intensive care units. Intensive Care Med 2004; 30:589–596. [DOI] [PubMed] [Google Scholar]
- 2.Harrison DA, Welch CA, Eddleston JM. The epidemiology of severe sepsis in England, Wales and Northern Ireland, 1996 to 2004: secondary analysis of a high quality clinical database, the ICNARC Case Mix Programme Database. Crit Care 2006; 10:R42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou J, Qian C, Zhao M, et al. Epidemiology and outcome of severe sepsis and septic shock in intensive care units in mainland China. PLoS One 2014; 9:e107181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Engel C, Brunkhorst FM, Bone HG, et al. Epidemiology of sepsis in Germany: results from a national prospective multicenter study. Intensive Care Med 2007; 33:606–618. [DOI] [PubMed] [Google Scholar]
- 5.Alberti C, Brun-Buisson C, Burchardi H, et al. Epidemiology of sepsis and infection in ICU patients from an international multicentre cohort study. Intensive Care Med 2002; 28:108–121. [DOI] [PubMed] [Google Scholar]
- 6.Martin GS, Mannino DM, Eaton S, et al. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med 2003; 348:1546–1554. [DOI] [PubMed] [Google Scholar]
- 7.Mayr FB, Yende S, Angus DC. Epidemiology of severe sepsis. Virulence 2014; 5:4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lagu T, Rothberg MB, Shieh MS, et al. Hospitalizations, costs, and outcomes of severe sepsis in the United States 2003 to 2007. Crit Care Med 2012; 40:754–761. [DOI] [PubMed] [Google Scholar]
- 9.Shen HN, Lu CL, Yang HH. Epidemiologic trend of severe sepsis in Taiwan from 1997 through 2006. Chest 2010; 138:298–304. [DOI] [PubMed] [Google Scholar]
- 10.Sands KE, Bates DW, Lanken PN, et al. Epidemiology of sepsis syndrome in 8 academic medical centers. JAMA 1997; 278:234–240. [PubMed] [Google Scholar]
- 11.Dellinger RP, Levy MM, Rhodes A, et al. Surviving Sepsis Campaign: International Guidelines for Management of Ssevere Sepsis and Septic Shock, 2012. Intensive Care Med 2013; 39:165–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Charlson M, Szatrowski TP, Peterson J, et al. Validation of a combined comorbidity index. J Clin Epidemiol 1994; 47:1245–1251. [DOI] [PubMed] [Google Scholar]
- 13.Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. Am Coll Chest Phys/Soc Crit Care Med Chest 1992; 101:1644–1655. [DOI] [PubMed] [Google Scholar]
- 14.Vincent JL, de Mendonca A, Cantraine F, et al. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Med. Crit Care Med 1998; 26:1793–1800. [DOI] [PubMed] [Google Scholar]
- 15.Ogura H, Gando S, Saitoh D, et al. Epidemiology of severe sepsis in Japanese intensive care units: a prospective multicenter study. J Infect Chemother 2014; 20:157–162. [DOI] [PubMed] [Google Scholar]
- 16.Brun-Buisson C, Meshaka P, Pinton P, et al. EPISEPSIS: a reappraisal of the epidemiology and outcome of severe sepsis in French intensive care units. Intensive Care Med 2004; 30:580–588. [DOI] [PubMed] [Google Scholar]
- 17.Padkin A, Goldfrad C, Brady AR, et al. Epidemiology of severe sepsis occurring in the first 24 hrs in intensive care units in England, Wales, and Northern Ireland. Crit Care Med 2003; 31:2332–2338. [DOI] [PubMed] [Google Scholar]
- 18.Silva E, Pedro Mde A, Sogayar AC, et al. Brazilian Sepsis Epidemiological Study (BASES study). Crit Care 2004; 8:R251–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Esper AM, Moss M, Lewis CA, et al. The role of infection and comorbidity: factors that influence disparities in sepsis. Crit Care Med 2006; 34:2576–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Friedman G, Silva E, Vincent JL. Has the mortality of septic shock changed with time. Crit Care Med 1998; 26:2078–2086. [DOI] [PubMed] [Google Scholar]
- 21.Vincent JL, Bihari DJ, Suter PM, et al. The prevalence of nosocomial infection in intensive care units in Europe. Results of the European Prevalence of Infection in Intensive Care (EPIC) Study. EPIC International Advisory Committee. JAMA 1995; 274:639–644. [PubMed] [Google Scholar]
- 22.Bernard GR, Vincent JL, Laterre PF, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med 2001; 344:699–709. [DOI] [PubMed] [Google Scholar]
- 23.Alberti C, Brun-Buisson C, Goodman SV, et al. Influence of systemic inflammatory response syndrome and sepsis on outcome of critically ill infected patients. Am J Respir Crit Care Med 2003; 168:77–84. [DOI] [PubMed] [Google Scholar]
- 24.Maynard ND, Bihari DJ, Dalton RN, et al. Increasing splanchnic blood flow in the critically III. Chest 1995; 108:1648–1654. [DOI] [PubMed] [Google Scholar]
- 25.McKenzie R, Reimer LG. Effect of antimicrobials on blood cultures in endocarditis. Diagn Microbiol Infect Dis 1987; 8:165–172. [DOI] [PubMed] [Google Scholar]
- 26.Sundararajan V, Korman T, Macisaac C, et al. The microbiology and outcome of sepsis in Victoria, Australia. Epidemiol Infect 2006; 134:307–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanriover MD, Guven GS, Sen D, et al. Epidemiology and outcome of sepsis in a tertiary-care hospital in a developing country. Epidemiol Infect 2006; 134:315–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin YT, Chen TL, Siu LK, et al. Clinical and microbiological characteristics of community-acquired thoracic empyema or complicated parapneumonic effusion caused by Klebsiella pneumoniae in Taiwan. Eur J Clin Microbiol Infect Dis 2010; 29:1003–1010. [DOI] [PubMed] [Google Scholar]
- 29.Chang CM, Lee HC, Lee NY, et al. Community-acquired Klebsiella pneumoniae complicated skin and soft-tissue infections of extremities: emphasis on cirrhotic patients and gas formation. Infection 2008; 36:328–334. [DOI] [PubMed] [Google Scholar]
- 30.Liu KH, Lee HC, Chuang YC, et al. Prostatic abscess in southern Taiwan: another invasive infection caused predominantly by Klebsiella pneumoniae. J Microbiol Immunol Infect 2003; 36:31–36. [PubMed] [Google Scholar]
- 31.Lemay AC, Anzueto A, Restrepo MI, et al. Predictors of long-term mortality after severe sepsis in the elderly. Am J Med Sci 2014; 347:282–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knaus WA. Prognosis with mechanical ventilation: the influence of disease, severity of disease, age, and chronic health status on survival from an acute illness. Am Rev Respir Dis 1989; 140:S8–13. [DOI] [PubMed] [Google Scholar]
- 33.Slutsky AS, Tremblay LN. Multiple system organ failure. Is mechanical ventilation a contributing factor? Am J Respir Crit Care Med 1998; 157:1721–1725. [DOI] [PubMed] [Google Scholar]
- 34.Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J Med 2013; 369:2126–2136. [DOI] [PubMed] [Google Scholar]
- 35.Annane D, Sebille V, Charpentier C, et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA 2002; 288:862–871. [DOI] [PubMed] [Google Scholar]
- 36.Reinhart K, Gluck T, Ligtenberg J, et al. CD14 receptor occupancy in severe sepsis: results of a phase I clinical trial with a recombinant chimeric CD14 monoclonal antibody (IC14). Crit Care Med 2004; 32:1100–1108. [DOI] [PubMed] [Google Scholar]
- 37.Stevenson EK, Rubenstein AR, Radin GT, et al. Two decades of mortality trends among patients with severe sepsis: a comparative meta-analysis. Crit Care Med 2014; 42:625–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kumar A, Haery C, Paladugu B, et al. The duration of hypotension before the initiation of antibiotic treatment is a critical determinant of survival in a murine model of Escherichia coli septic shock: association with serum lactate and inflammatory cytokine levels. J Infect Dis 2006; 193:251–258. [DOI] [PubMed] [Google Scholar]
- 39.Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 2001; 345:1368–1377. [DOI] [PubMed] [Google Scholar]
- 40.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med 2000; 342:1301–1308. [DOI] [PubMed] [Google Scholar]


