Abstract
Several individual studies have reported the diagnostic accuracy of presepsin (sCD14-ST) for sepsis, but the results are inconsistent.
The present systematic review and meta-analysis pooled data to better ascertain the value of circulatory presepsin as a biomarker for sepsis.
Studies published in English before November 7, 2014 and assessing the diagnostic accuracy of presepsin for sepsis were retrieved from medical databases.
The quality of eligible studies was assessed using a revised Quality Assessment for Studies of Diagnostic Accuracy (QUADAS-2). The overall diagnostic accuracy of presepsin for sepsis was pooled according to a bivariate model. Publication bias was assessed using Deek funnel plot asymmetry test.
Eleven studies satisfied the inclusion criteria. The overall diagnostic sensitivity of presepsin for sepsis was 0.83 (95% CI: 0.77–0.88), and specificity was 0.78 (95% CI: 0.72–0.83). The area under the summary receiver operating characteristic curve was 0.88 (95% CI: 0.84–0.90). The pretest probability of sepsis was 0.56 among all subjects. When presepsin was introduced as the diagnostic test for sepsis, the posttest probabilities were 0.81 for a positive result and 0.19 for a negative. The major design deficits of the included studies were lack of prespecified thresholds and patient selection bias. The publication bias was negative.
Presepsin is an effective adjunct biomarker for the diagnosis of sepsis, but is insufficient to detect or rule out sepsis when used alone.
INTRODUCTION
Sepsis is a major challenge in emergency departments and intensive care units (ICUs), causing high mortality and morbidity.1,2 Early diagnosis and timely intervention are essential to improve the prognosis of septic patients.3 Bacterial culture is generally regarded as the gold standard for the diagnosis of sepsis, but it is time-consuming, frequently yields false-negative results, and microbial contamination can greatly affect its diagnostic value.4 Thus, the treatment of sepsis is often based on the clinician's experience, which risks an increase in antibiotic resistance and the cost of medical care. It is therefore necessary and urgent to develop a rapid and accurate method for the diagnosis of sepsis.
Various biomarkers have been reported useful in sepsis diagnosis, such as procalcitonin and C-reactive protein.5,6 However, these biomarkers may also be elevated in nonseptic conditions such as trauma, burn, and postoperative settings, and some are slow to rise after the onset of sepsis.5,6 It thus remains necessary to find reliable biomarkers to replace or improve those that are currently available.
Cluster of differentiation 14 (CD14) is a coreceptor working with toll-like receptor 4 (TLR4) on the surface of phagocytes such as monocytes, macrophages, and granulocytes.7 Once activated by lipopolysaccharide, CD14 with lipopolysaccharide binding protein (LBP) can induce the production of various proinflammatory cytokines.8,9 By shedding CD14 from the cell membrane (known as soluble CD14, or sCD14), the lipopolysaccharide–LBP–CD14 complex is released into the blood.10 In addition to phagocytes, hepatocytes have been reported able to secrete CD14.11,12 In blood, sCD14 can be cleaved by proteases, generating a truncated form of 64 amino acid residues known as sCD14 subtype (sCD14-ST), or presepsin.13,14 Therefore, the levels of presepsin in free circulation may reflect systemic inflammation, and therefore have diagnostic value in sepsis. Numerous studies have assessed the diagnostic value of presepsin for sepsis, with conflicting results.
To summarize the results of available studies and better guide future studies, we performed a systematic review and meta-analysis to ascertain the diagnostic value of presepsin for sepsis.
MATERIALS AND METHODS
Search Strategy and Study Selection
This meta-analysis was performed and reported in accordance with the guidelines of PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses).15 Two investigators independently retrieved eligible studies published before November 7, 2014. The electronic databases used for the literature search were Medline (using the PubMed search engine) and EMBASE. Search terms for PubMed were (“presepsin” or “sCD14” or “soluble CD14”) and “sepsis.” Similar terms were used for EMBASE. A manual search was also conducted using the references of the primary research and review articles retrieved.
The systematic review included studies concerned with the diagnostic value of presepsin for sepsis; and studies that provided diagnostic characteristics, that is, the area under the receiver operating characteristic (ROC) curve (AUC), or sensitivity and specificity. Excluded were animal studies, duplicate studies, non-English publications, studies with asepsis or control sample size <10 (since studies with small sample sizes yield great bias), and conference abstracts (for lack of sufficient details for analysis). The titles and abstracts of the retrieved papers were reviewed to identify the eligible studies. Any disagreement on study selection was resolved by full text review or discussion between the investigators.
Because all analyses were based on previous published studies, therefore, we did not obtain ethical approval or patient consent.
Data Extraction and Quality Assessment
The following information was extracted from the eligible studies: publication year, country of origin, inclusion criteria, control groups, test methods, reference standard, and diagnostic performances (AUC, threshold, sensitivity, and specificity).
Two investigators independently assessed the quality of eligible studies using the revised Quality Assessment for Studies of Diagnostic Accuracy tool (QUADAS-2).16 Studies that enrolled healthy individuals as a component of the controls were regarded as cross-sectional, if the data regarding the healthy individuals were not included in the final analysis; otherwise, they were considered to be case-control studies, and the risk of bias of the QUADAS-2 patient selection domain was labeled high. Any disagreement in data extraction and quality assessment was resolved by discussion.
Statistical Analyses
The overall diagnostic power of presepsin was pooled using the bivariate model.17 This model uses paired specificity and sensitivity as the starting point of the analysis; therefore, the trade-off between sensitivity and specificity had been considered. The positive and negative likelihood ratio was estimated according to the overall sensitivity and specificity.
Publication bias was assessed using a funnel plot.18 The possible sources of heterogeneity across all eligible studies were explored through a meta-regression analysis. All analyses were performed in Stata 12.0 (Stata Corp LP, College Station, TX), and the midas command was used for all statistical analyses.19
RESULTS
Summary of the Eligible Studies
At the end of the systematic literature search and study selection process (Fig. 1), 11 studies14,20–29 were included (Table 1). The sample size of the eligible studies ranged from 60 to 959. The inclusion criteria for research subjects were heterogeneous: emergency department patients who met at least two20,23–25,27 or one21 of the criteria for systemic inflammatory response syndrome (SIRS); ICU patients28,29; acute abdomen patients who met at least 2 criteria for SIRS22; and burn patients.26 One study did not report any inclusion or exclusion criteria.14
FIGURE 1.
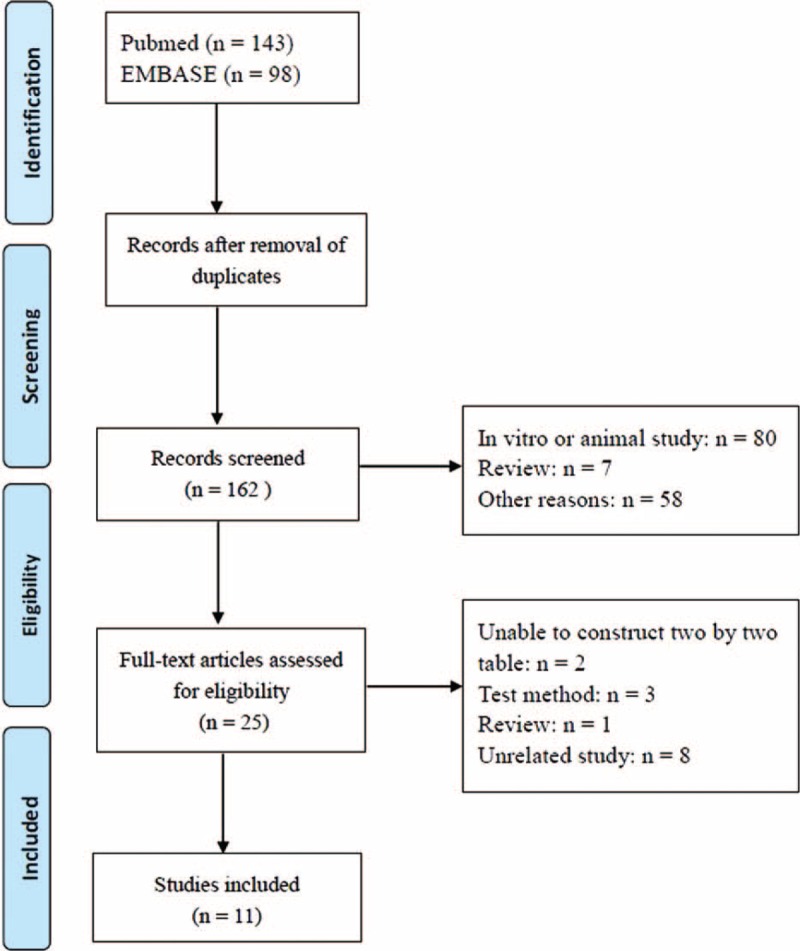
The systematic literature search and study selection process.
TABLE 1.
Summary of the Included Studies
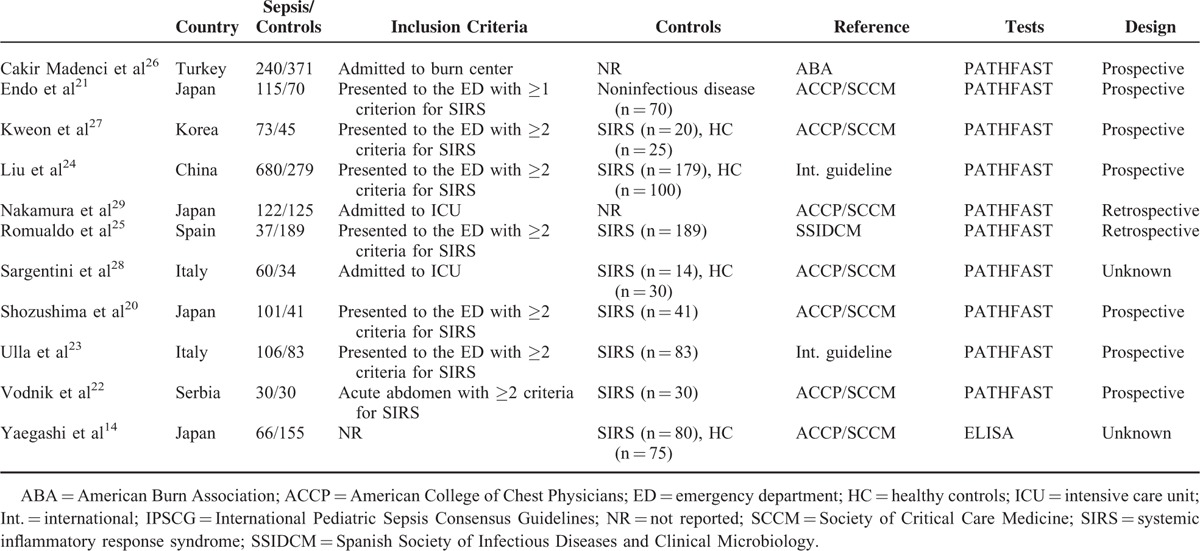
The characteristics of nonseptic controls were also heterogeneous, including those with SIRS,14,20,22–25,27,28 healthy individuals,14,20,24,27 and noninfected individuals.20,21
The references used in these studies for determining a diagnosis of sepsis were: criteria established by the American College of Chest Physicians/Society of Critical Care Medicine (ACCP/SCCM; the gold standard),30 applied in 5 studies14,20–22,27,28; the International Pediatric Sepsis Consensus Guidelines (IPSCG),31 used in 1 study32; international guidelines33–35 used in 2 studies23,24; criteria of the Spanish Society of Infectious Diseases and Clinical Microbiology (SSIDCM) applied in 1 study25; and those of the American Burn Association (ABA)36 applied in 1 study.26
Ten studies20–29 employed a chemiluminescence enzyme immunoassay with a PATHFAST analyzer37 (Mitsubishi Chemical Medience, Tokyo, Japan) to detect blood presepsin. One study was based on enzyme-linked immunosorbent assay (ELISA).14 Five studies used frozen samples for analysis.23–25,28,29
Quality Assessment
The patient selection risk of bias domain in 4 studies was labeled as high risk because healthy controls were included in the final analysis.14,24,27,28 Three studies were labeled as unknown because the authors did not report whether the subjects were consecutively enrolled21,22 (Table 2). In addition, 3 studies20,26,28 collected more than one sample from the same patient, which may affect the disease spectrum significantly; the patient selection domain of these studies was labeled high risk.
TABLE 2.
Quality Assessment of the 11 Studies
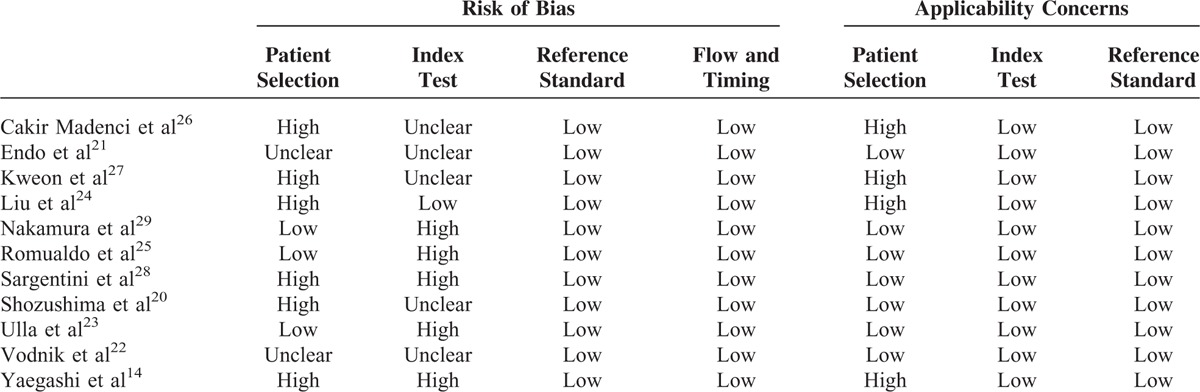
The index test domain of some studies was labeled as high risk because they did not use a prespecified threshold,14,23,25,28,29 or it was labeled as unclear risk because the authors did not report whether the thresholds used were prespecified,20–22,27,29 or whether the performers were blinded to the clinical status of the subjects.20–22,27,29
For applicability concerns, the patient selection domain of 4 studies was labeled as high risk because healthy individuals were enrolled.14,24,27,28
Diagnostic Accuracy of Presepsin for Sepsis
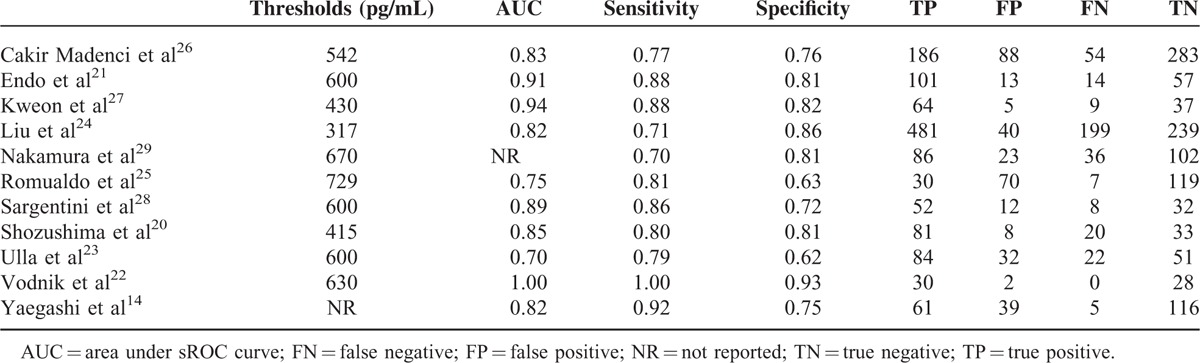
All the studies in this meta-analysis found that blood presepsin was an effective biomarker for the diagnosis of sepsis (Table 3). The threshold ranged from 317 to 729 pg/mL, the AUC ranged from 0.70 to 1.00, the sensitivity ranged from 0.70 to 1.00, and the specificity ranged from 0.62 to 0.93.
TABLE 3.
Diagnostic Accuracy of the Eligible Studies
We then performed a meta-analysis to estimate the diagnostic accuracy of presepsin for sepsis. The overall diagnostic sensitivity was 0.83 (95% CI: 0.77–0.88) and the specificity was 0.78 (95% CI: 0.72–0.83; Figure 2). In addition, the positive likelihood ratio was 3.9 (95% CI: 2.9–5.0), the negative likelihood ratio was 0.21 (95% CI: 0.15–0.30), and the diagnostic odds ratio was 18 (95% CI: 11–30).
FIGURE 2.
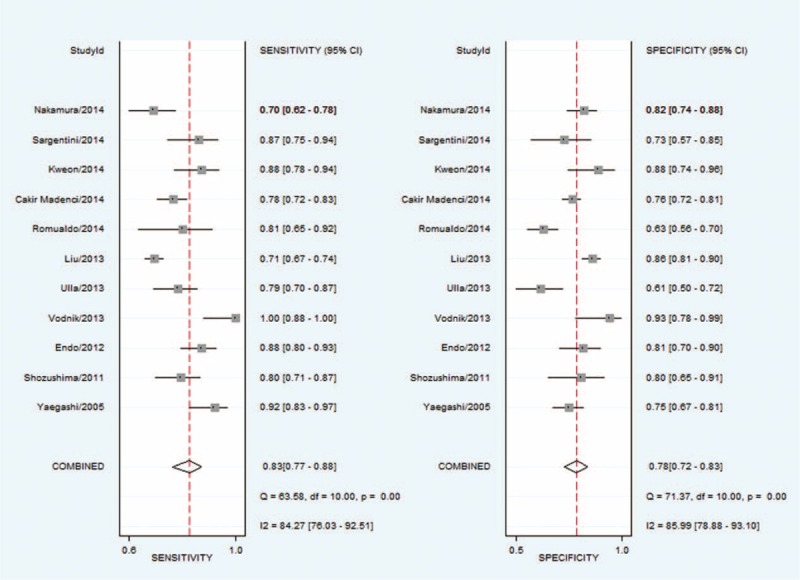
Sensitivity and specificity of presepsin for the diagnosis of sepsis assessed by Forest plots.
The sROC AUC of presepsin for sepsis was 0.88 (95% CI: 0.84–0.90; Figure 3). According to the Fagan nomogram of the present meta-analysis (Fig. 4), the pretest probability of the presence of sepsis was 0.56 among all subjects (ie, a prevalence of 56%). When presepsin was introduced as a diagnostic test for sepsis, the posttest probabilities of sepsis was 0.81 for a positive result and 0.19 for a negative. Taken together, these results indicate that presepsin was a useful biomarker for sepsis diagnosis.
FIGURE 3.
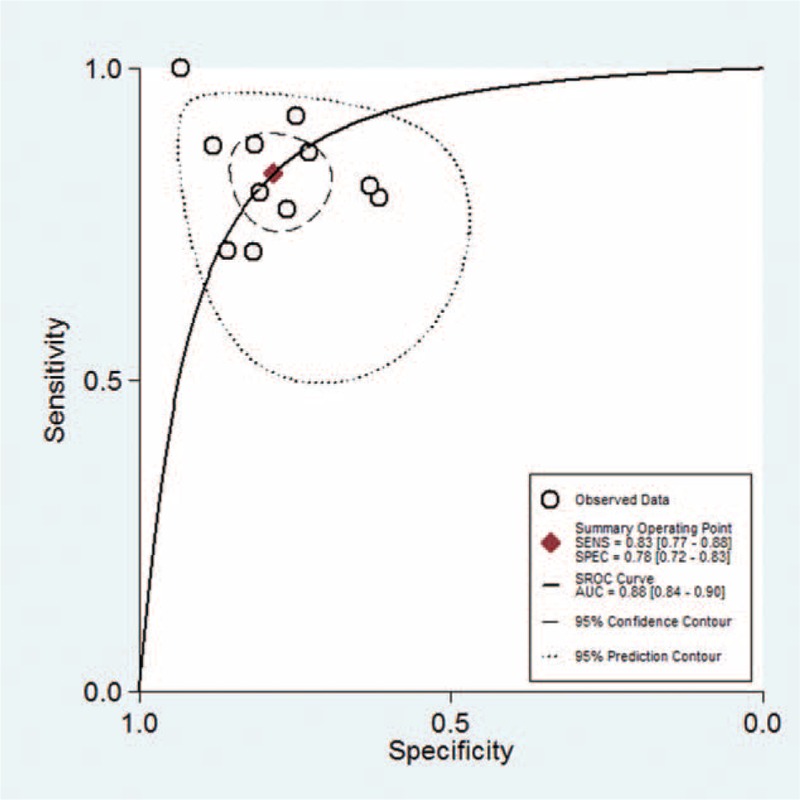
The AUC of presepsin for the diagnosis of sepsis. The overall diagnostic efficiency is summarized by the regression curve.
FIGURE 4.
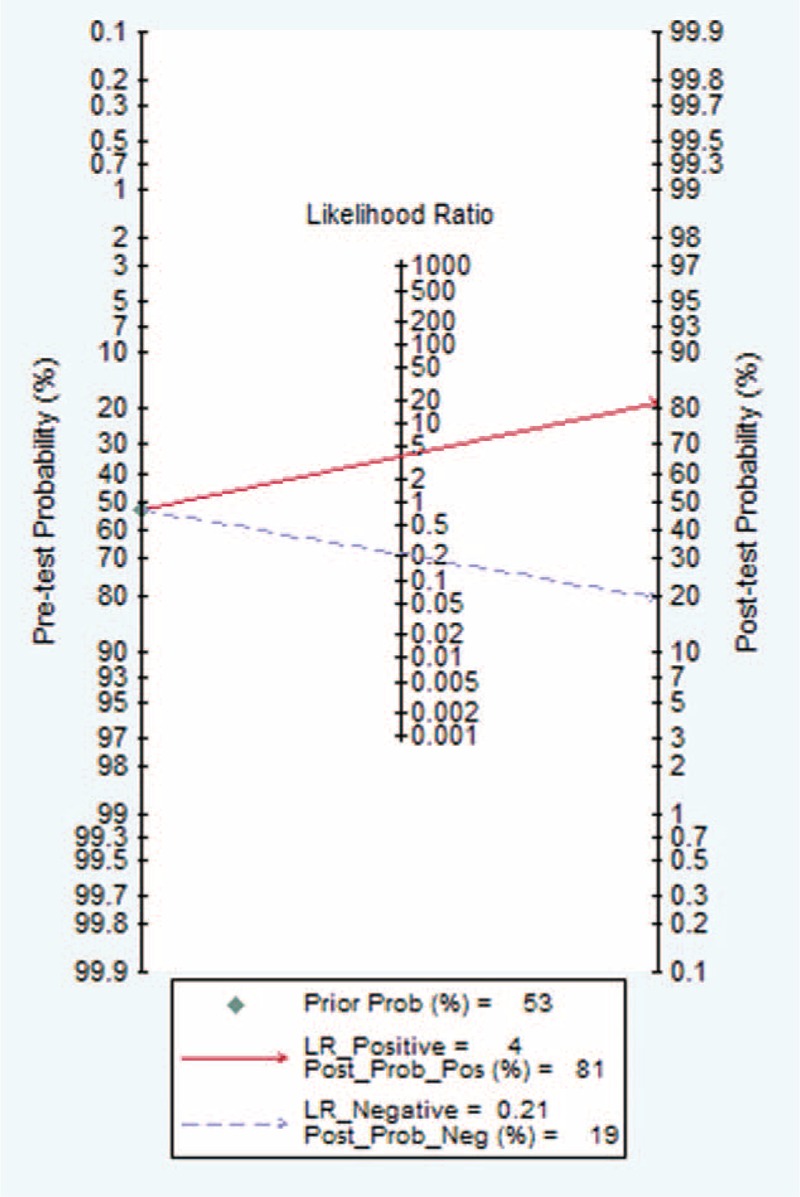
Fagan nomogram used to illustrate the posttest probabilities of presepsin.
Subgroup Analysis and Meta-Regression
The goal of a meta-analysis is not limited to pooling the results of available studies, but an exploration of the sources for heterogeneity is also important. In the present study, we found that the I2 statistic for the present meta-analysis was 0.94 (95% CI: 0.90–0.99), indicating that great heterogeneity existed across all the studies. However, the proportion of heterogeneity that was likely due to the threshold effect was only 0.02, implying that nonthreshold effect factors accounted for the high heterogeneity. We hypothesized that the potential sources of heterogeneity were the study design or participant characteristics. Such factors might be the type of data collection (prospective or not), country of origin (Europe or not), sample size (> 200 or not), control group (whether healthy controls were included), reference for criteria (ACCP/SCCM or not), or the site of subject enrollment (emergency department or not). Therefore, subgroup analyses and univariate meta-regression were performed to explore these potential sources of heterogeneity (Fig. 5).
FIGURE 5.
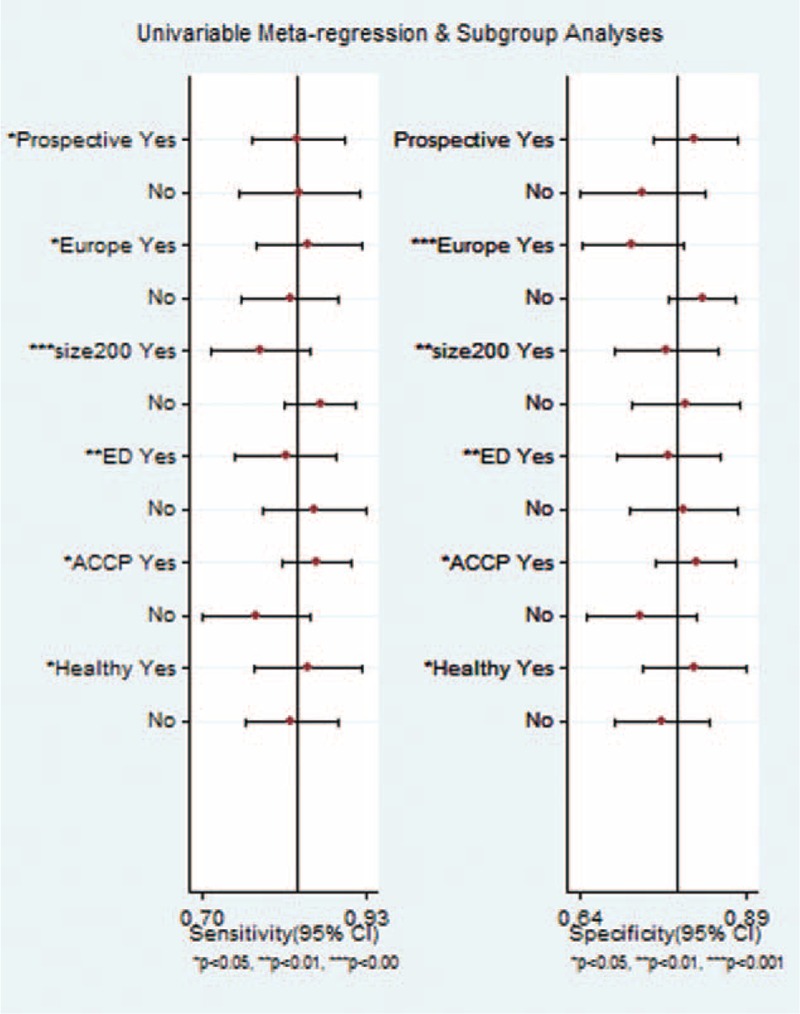
Subgroup analysis of presepsin for sepsis diagnosis.
Generally, the factors mentioned above could influence the sensitivity and specificity of presepsin as a diagnostic test. Knowing that the threshold effect was not taken into consideration in the subgroup analysis, we further explored the sources of heterogeneity via meta-regression. In the joint model, only the reference for criteria proved to be the source of heterogeneity across all studies (P = 0.03). Thus, the source of heterogeneity was whether the ACCP/SCCM guideline was used as the criteria reference, and a study based on the ACCP/SCCM guideline shad high diagnostic accuracy.
Publication Bias
Deek funnel plot test showed that publication bias was not statistically significant (P = 0.12; Figure 6).
FIGURE 6.
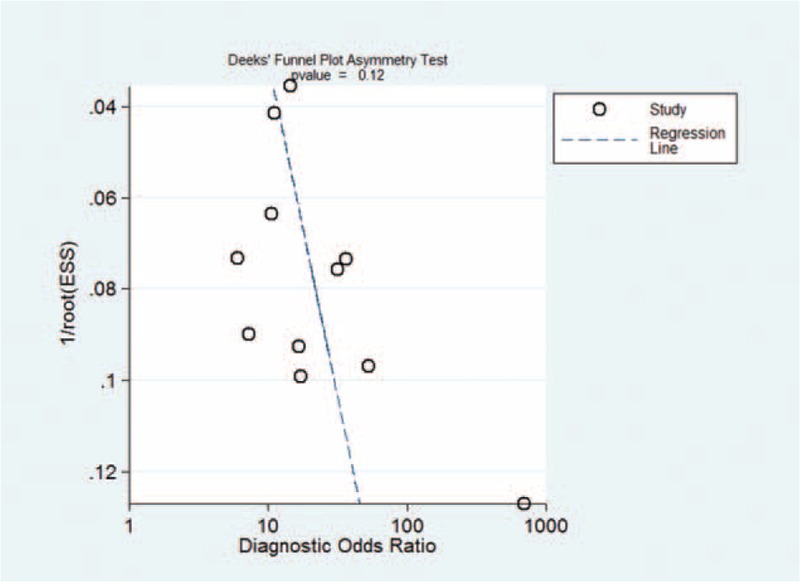
Funnel plot for the assessment of publication bias.
DISCUSSION
The present systematic review included 11 published studies that investigated the value of circulatory presepsin as a biomarker for sepsis. The meta-analysis revealed that presepsin was an effective diagnostic marker for sepsis, with an overall sensitivity and specificity of 0.83 and 0.78, respectively. Some of the eligible studies had design defects, such as not enrolling subjects consecutively or not using a prespecified threshold. The publication bias across all the studies was negative.
Generally, there is a trade-off between sensitivity and specificity in a test of diagnostic accuracy. That is, an increase in sensitivity (the true positive rate) is usually accompanied by a reduction in specificity (the true negative rate). Therefore, sensitivity and specificity alone may not be the best estimation of diagnostic accuracy. Alternatively, the area under the sROC, or AUC, may be a better index. The AUC ranges between 0.50 and 1.00, and correlates with overall diagnostic accuracy. The present study found that the AUC for sROC was 0.88, indicating that presepsin was a useful diagnostic marker for sepsis.
Although the AUC is a well-established index to estimate the overall diagnostic accuracy of an index test, it is not easy to interpret and use in clinical practice. By contrast, the positive and negative likelihood ratios are considered more clinically meaningful.38 The present meta-analysis found that the positive and negative likelihood ratios of presepsin for revealing sepsis were 3.9 and 0.21, respectively. This indicates that the chance that a patient with sepsis has elevated presepsin is almost 4-fold that of a patient without sepsis, while a patient testing negative for presepsin is only 21% likely to have sepsis. Generally, a positive likelihood ratio higher than 10 indicates that the test under consideration is sufficient to confirm the diagnosis, while a negative likelihood ratio less than 0.10 means that the negative index test is sufficient to rule out the target disease.39 Therefore, we conclude that presepsin cannot be used to confirm or rule out sepsis when used alone, and its results should be interpreted within the clinical context. According to the Fagan nomogram, the posttest probability for presepsin indicating sepsis was 0.81 for a positive test result and 0.19 for a negative test result. This further confirmed our conclusion that presepsin, when used alone, is not sufficient to confirm or rule out sepsis.
We note that there was great heterogeneity across the 11 studies. A subgroup analysis revealed that the only verifiable source of heterogeneity was the reference that was used for diagnostic criteria. However, it should be noted that the possibility of other sources of heterogeneity should not be discounted, due to the small number of studies. In particular, characteristics of the enrolled subjects may be a potential source of heterogeneity, but because the inclusion and exclusion criteria of these studies varied and the number of studies in each subgroup was small, a subgroup analysis and meta-regression was not performed to resolve this question. This issue needs to be addressed in future studies.
Procalcitonin is an acknowledged biomarker for sepsis.40 It would be valuable to address whether the combined use of procalcitonin and presepsin could improve the diagnostic accuracy of sepsis in a cost-effective manner. However, none of the eligible studies discussed this issue. Further studies are needed to settle this, using rigorous statistical methods such as a logistic regression model, net reclassification, and integrated discrimination.41–44
Flaws in the design of these studies should be considered when interpreting the results of the present systematic review. Some studies included healthy individuals in the control group, which may lead to a great overestimation of the diagnostic value of presepsin.45 Some of the studies were not in accordance with the Standards for Reporting of Diagnostic Accuracy.46 For example, it is sometimes not clear how the investigators selected the subjects (consecutively, randomly, or neither).16,47 If the subjects were not recruited either consecutively or randomly, this may introduce selection bias. The thresholds used in the studies were not always prespecified, which may also introduce bias.48 In addition, the stability of presepsin in storage should be accounted for. According to the package insert of the PATHFAST Presepsin kit, plasma samples are stable for only 3 days at 2 to 8°C and 9 months at −20°C or lower. Four23–25,32 of the studies used frozen samples for analysis, but they did not report the period between sample collection and analysis. If the sample storage period was more than 9 months, the test result may not be reliable and a bias may be introduced.
Some of the studies reviewed here also reported that presepsin correlated with procalcitonin,14,22,24 C-reactive protein,14 or scores for Sequential Organ Failure Assessment,14 Mortality in Emergency Department Sepsis,24 and Acute Physiology and Chronic Health Evaluation II.20,22,24 Since all these indices are well-established prognostic factors in sepsis, it could be concluded that presepsin is also a potential prognostic biomarker. Indeed, 2 studies reported that presepsin was a useful predictor of 28-day24 or 60-day23 mortality. These results warrant further investigation into the prognostic value of presepsin for the diagnosis of sepsis.
CONCLUSIONS
The present systematic review indicates that presepsin is an effective biomarker for the diagnosis of sepsis, and may prove comparable to procalcitonin. However, when used as the only diagnostic test, presepsin is insufficient to rule out or confirm sepsis, and its diagnostic value should be interpreted with consideration given to the clinical context.
Acknowledgment
We thank Medjaden Bioscience Limited, Hong Kong, China, for assisting in the preparation of this manuscript.
Footnotes
Abbreviations: ABA = American Burn Association, ACCP = American College of Chest Physicians, AUC = area under curve, ELISA = enzyme-linked immunosorbent assay, ICU = intensive care unit, IPSCG = International Pediatric Sepsis Consensus Guidelines, PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses, QUADAS = Quality Assessment for Studies of Diagnostic Accuracy, ROC = receiver operating characteristic, SCCM = Society of Critical Care Medicine, SIRS = systemic inflammatory response syndrome, SSIDCM = Spanish Society of Infectious Diseases and Clinical Microbiology.
JZ and Z-D H contributed equally to this work.
This study was supported by the National Natural Science Foundation of China (grant no. 81302541).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 2001; 29:1303–1310. [DOI] [PubMed] [Google Scholar]
- 2.Martin GS, Mannino DM, Eaton S, et al. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med 2003; 348:1546–1554. [DOI] [PubMed] [Google Scholar]
- 3.Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 2006; 34:1589–1596. [DOI] [PubMed] [Google Scholar]
- 4.Hall KK, Lyman JA. Updated review of blood culture contamination. Clin Microbiol Rev 2006; 19:788–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cho SY, Choi JH. Biomarkers of sepsis. Infect Chemother 2014; 46:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Faix JD. Biomarkers of sepsis. Crit Rev Clin Lab Sci 2013; 50:23–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zanoni I, Granucci F. Role of CD14 in host protection against infections and in metabolism regulation. Front Cell Infect Microbiol 2013; 3:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wright SD, Ramos RA, Tobias PS, et al. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science 1990; 249:1431–1433. [DOI] [PubMed] [Google Scholar]
- 9.Schumann RR. Function of lipopolysaccharide (LPS)-binding protein (LBP) and CD14, the receptor for LPS/LBP complexes: a short review. Res Immunol 1992; 143:11–15. [DOI] [PubMed] [Google Scholar]
- 10.Qi Z, Wei W, Zhang XC. Presepsin as a novel sepsis biomarker. World J Emerg Med 2014; 5:16–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meuleman P, Steyaert S, Libbrecht L, et al. Human hepatocytes secrete soluble CD14, a process not directly influenced by HBV and HCV infection. Clin Chim Acta 2006; 366:156–162. [DOI] [PubMed] [Google Scholar]
- 12.Su GL, Dorko K, Strom SC, et al. CD14 expression and production by human hepatocytes. J Hepatol 1999; 31:435–442. [DOI] [PubMed] [Google Scholar]
- 13.Mussap M, Noto A, Fravega M, et al. Soluble CD14 subtype presepsin (sCD14-ST) and lipopolysaccharide binding protein (LBP) in neonatal sepsis: new clinical and analytical perspectives for two old biomarkers. J Matern Fetal Neonatal Med 2011; 24 suppl 2:12–14. [DOI] [PubMed] [Google Scholar]
- 14.Yaegashi Y, Shirakawa K, Sato N, et al. Evaluation of a newly identified soluble CD14 subtype as a marker for sepsis. J Infect Chemother 2005; 11:234–238. [DOI] [PubMed] [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009; 151:264–269.W264. [DOI] [PubMed] [Google Scholar]
- 16.Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011; 155:529–536. [DOI] [PubMed] [Google Scholar]
- 17.Reitsma JB, Glas AS, Rutjes AW, et al. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol 2005; 58:982–990. [DOI] [PubMed] [Google Scholar]
- 18.Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol 2005; 58:882–893. [DOI] [PubMed] [Google Scholar]
- 19.Hu ZD, Wei TT, Yang M, et al. Diagnostic value of osteopontin in ovarian cancer: A meta-analysis and systematic review. PLoS One 2015; 10:e0126444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shozushima T, Takahashi G, Matsumoto N, et al. Usefulness of presepsin (sCD14-ST) measurements as a marker for the diagnosis and severity of sepsis that satisfied diagnostic criteria of systemic inflammatory response syndrome. J Infect Chemother 2011; 17:764–769. [DOI] [PubMed] [Google Scholar]
- 21.Endo S, Suzuki Y, Takahashi G, et al. Usefulness of presepsin in the diagnosis of sepsis in a multicenter prospective study. J Infect Chemother 2012; 18:891–897. [DOI] [PubMed] [Google Scholar]
- 22.Vodnik T, Kaljevic G, Tadic T, et al. Presepsin (sCD14-ST) in preoperative diagnosis of abdominal sepsis. Clin Chem Lab Med 2013; 51:2053–2062. [DOI] [PubMed] [Google Scholar]
- 23.Ulla M, Pizzolato E, Lucchiari M, et al. Diagnostic and prognostic value of presepsin in the management of sepsis in the emergency department: a multicenter prospective study. Crit Care 2013; 17:R168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu B, Chen YX, Yin Q, et al. Diagnostic value and prognostic evaluation of presepsin for sepsis in an emergency department. Crit Care 2013; 17:R244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Romualdo LG, Torrella PE, Gonzalez MV, et al. Diagnostic accuracy of presepsin (soluble CD14 subtype) for prediction of bacteremia in patients with systemic inflammatory response syndrome in the Emergency Department. Clin Biochem 2014; 47:505–508. [DOI] [PubMed] [Google Scholar]
- 26.Cakir Madenci O, Yakupoglu S, Benzonana N, et al. Evaluation of soluble CD14 subtype (presepsin) in burn sepsis. Burns 2014; 40:664–669. [DOI] [PubMed] [Google Scholar]
- 27.Kweon OJ, Choi JH, Park SK, et al. Usefulness of presepsin (sCD14 subtype) measurements as a new marker for the diagnosis and prediction of disease severity of sepsis in the Korean population. J Crit Care 2014; 29:965–970. [DOI] [PubMed] [Google Scholar]
- 28.Sargentini V, Ceccarelli G, D’Alessandro M, et al. Presepsin as a potential marker for bacterial infection relapse in critical care patients. A preliminary study. Clin Chem Lab Med 2015; 53:567–573. [DOI] [PubMed] [Google Scholar]
- 29.Nakamura Y, Ishikura H, Nishida T, et al. Usefulness of presepsin in the diagnosis of sepsis in patients with or without acute kidney injury. BMC Anesthesiol 2014; 14:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 1992; 101:1644–1655. [DOI] [PubMed] [Google Scholar]
- 31.Goldstein B, Giroir B, Randolph A, et al. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med 2005; 6:2–8. [DOI] [PubMed] [Google Scholar]
- 32.Urbonas V, Eidukaite A, Tamuliene I. The predictive value of soluble biomarkers (CD14 subtype, interleukin-2 receptor, human leucocyte antigen-G) and procalcitonin in the detection of bacteremia and sepsis in pediatric oncology patients with chemotherapy-induced febrile neutropenia. Cytokine 2013; 62:34–37. [DOI] [PubMed] [Google Scholar]
- 33.Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med 2003; 29:530–538. [DOI] [PubMed] [Google Scholar]
- 34.Dellinger RP, Levy MM, Rhodes A, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med 2013; 39:165–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 2013; 41:580–637. [DOI] [PubMed] [Google Scholar]
- 36.Greenhalgh DG, Saffle JR, Holmes JHt, et al. American Burn Association consensus conference to define sepsis and infection in burns. J Burn Care Res 2007; 28:776–790. [DOI] [PubMed] [Google Scholar]
- 37.Okamura Y, Yokoi H. Development of a point-of-care assay system for measurement of presepsin (sCD14-ST). Clin Chim Acta 2011; 412:2157–2161. [DOI] [PubMed] [Google Scholar]
- 38.Linnet K, Bossuyt PM, Moons KG, et al. Quantifying the accuracy of a diagnostic test or marker. Clin Chem 2012; 58:1292–1301. [DOI] [PubMed] [Google Scholar]
- 39.Deeks JJ, Altman DG. Diagnostic tests 4: likelihood ratios. BMJ 2004; 329:168–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wacker C, Prkno A, Brunkhorst FM, et al. Procalcitonin as a diagnostic marker for sepsis: a systematic review and meta-analysis. Lancet Infect Dis 2013; 13:426–435. [DOI] [PubMed] [Google Scholar]
- 41.Levinson SS. Letter to the editor reply: statistical methods for assessment of added usefulness of new biomarkers. Clin Chem Lab Med 2011; 49:1241–1242. [DOI] [PubMed] [Google Scholar]
- 42.Pencina MJ, D’Agostino RB, Vasan RS. Statistical methods for assessment of added usefulness of new biomarkers. Clin Chem Lab Med 2010; 48:1703–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pencina MJ, D’Agostino RB, Sr, D’Agostino RB, Jr, et al. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med 2008; 27:157–172. [DOI] [PubMed] [Google Scholar]
- 44.Moons KG, de Groot JA, Linnet K, et al. Quantifying the added value of a diagnostic test or marker. Clin Chem 2012; 58:1408–1417. [DOI] [PubMed] [Google Scholar]
- 45.Whiting P, Rutjes AW, Reitsma JB, et al. Sources of variation and bias in studies of diagnostic accuracy: a systematic review. Ann Intern Med 2004; 140:189–202. [DOI] [PubMed] [Google Scholar]
- 46.Bossuyt PM, Reitsma JB, Bruns DE, et al. The STARD statement for reporting studies of diagnostic accuracy: explanation and elaboration. Clin Chem 2003; 49:7–18. [DOI] [PubMed] [Google Scholar]
- 47.Whiting P, Rutjes AW, Reitsma JB, et al. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 2003; 3:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leeflang MM, Moons KG, Reitsma JB, et al. Bias in sensitivity and specificity caused by data-driven selection of optimal cutoff values: mechanisms, magnitude, and solutions. Clin Chem 2008; 54:729–737. [DOI] [PubMed] [Google Scholar]


