Supplemental Digital Content is available in the text
Abstract
Although hypertension is common among older adults, the optimal blood pressure (BP) for survival in older adults remains unclear. We attempt to use a large cohort to assess the relationship between BP and mortality and to gain insight into what level of BP is required for optimal survival in older adults.
A total of 77,389 community-dwelling adults, aged ≥65 years, were followed between 2006 and 2010. Mortality was determined using matching cohort identifications with national death files. Cox proportional hazards regression models were used to evaluate the relationship of BP with all-cause, cardiovascular disease (CVD), and expanded-CVD mortalities.
The mortality risks of the stage 2–3 hypertension group were substantial (all-cause mortality: hazard ratio [HR]: 1.23; 95% confidence interval [CI]: 1.10–1.37; CVDs mortality: HR: 1.31; 95% CI: 1.05–1.64; expanded-CVDs mortality: HR: 1.40; 95% CI: 1.15–1.71). The cardiovascular and expanded-cardiovascular mortality risks were lowest when systolic blood pressures were 120 to 129 mm Hg, and increased significantly when systolic blood pressures (SBPs) were ≥160 mm Hg or diastolic BPs were ≥90 mm Hg. A J-curve phenomenon for SBP on CVD and expanded-CVD mortality was observed. The impacts of stage 2–3 hypertension on mortality risks were significantly increased among women. The mortality risks of hypertension were not attenuated with older age.
This study provides insight for identifying the optimal BP for survival in older adults, and extends the knowledge of the impacts of hypertension on mortality risks among women and the older adults.
INTRODUCTION
High blood pressure (BP) is one of the most important risk factors for cardiovascular disease (CVD), which is the leading cause of mortality.1,2 Approximately 54% of strokes and 47% of coronary heart diseases, worldwide, are attributable to high BP.3 Hypertension is a common medical condition; its prevalence increases with age,4,5 and is estimated to affect 65% of those ≥60-years-old.6 The global population is aging. By 2030, an estimated 20% of the global population will be ≥65-years-old.7 Therefore, the impact of high BP on mortality among older adults is expected to grow over the coming decades.
Recently, the 2013 European Society of Hypertension/European Society of Cardiology Hypertension Guidelines defined a universal target of <140/90 mm Hg for all patients, except the most elderly population segment (target, <150/90 mm Hg for those ≥80-years-old).8 The American Heart Association, American College of Cardiology, Centers for Disease Control and Prevention,9 American Society of Hypertension, and the International Society of Hypertension10 also supported the treatment goals associated with this guideline. However, based on evidence from randomized controlled trials, the 2014 Evidence-Based Guideline for the Management of High Blood Pressure in Adults (Report from the Panel Members Appointed to the Eighth Joint National Committee (JNC 8)) raised the target to <150/90 mm Hg for adults ≥60-years-old.11 In contrast with the recommendation in the 2014 JNC 8 but in accordance with the 2013 ESH/ESC Hypertension Guidelines, the 2015 Guidelines of the Taiwan Society of Cardiology and the Taiwan Hypertension Society for the Management of Hypertension recommends a target BP of <140/90 mm Hg for those aged 60 to 80 years and a BP target of BP <150/90 mm Hg for those aged ≥80 years.12
Thus, the optimal BP target for older adults has not yet been established. Most guidelines are based on evidence from randomized controlled trials, which are considered the gold standard of evidence for making treatment decisions. However, they often include a select population with limited generalizability. This is particularly relevant for older adults, a population comprised of individuals with heterogeneous health statuses and high prevalences of chronic diseases. Older adults have been underrepresented in clinical trials,13 resulting in treatment decisions being extrapolated from data involving much younger individuals. Therefore, this study might be used to complement randomized trials and extends the knowledge of the association between hypertension and mortality risk among older adults.
The objective of this study was to investigate the associations between BP and all-cause, CVD, and expanded-CVD mortalities among community-dwelling older adults to determine the appropriate BP range with the lowest risk of mortality. Expanded-CVD disease was considered because of the high likelihood of classifying death due to CVD in patients with diabetes as death due to diabetes, whereas, in patients with kidney disease, there is a high likelihood of classifying the death as due to kidney disease.14 Thus, we derived the composite measures of mortality by grouping all deaths due to CVD, diabetes or kidney disease as expanded-CVD mortality, which have been used in several studies.14–17 Furthermore, our large sample size allowed us to perform stratified analyses and investigate the associations among different subgroups.
MATERIALS AND METHODS
Study Population
Data were obtained from the Taipei City Geriatric Health Examination Database. The study cohort comprised 77,389 community-dwelling adults, aged ≥65 years, including 39,280 men and 38,109 women. Between May 1, 2006 and December 31, 2010, the participants were enrolled in an annual physical examination program for older adults managed by the Taipei City Government. Identical screening procedures and protocols were used in all qualified hospitals that contracted with the Taipei City Government Department of Health. Participants voluntarily participated in a routine physical examination program and were encouraged to participate in the program annually. Each year, approximately 40,000 to 46,000 people participate in this program, with approximately a 13% to 16% participation rate in the community. The cohort data were described, in detail, in our previous studies.15,18 For individuals participating in this program more than once, we used the information acquired at their first examination. The evaluations included standardized medical examinations and questionnaires that addressed a variety of health-related topics. Participant identification data were encrypted before being released and analyzed by the researchers. The study complied with the Helsinki Declaration and was approved by the Taipei City Hospital institutional review board (IRB No. TCHIRB-1020417-E).
Definition and Classification of Hypertension and Blood Pressure
Systolic (SBP) and diastolic (DBP) blood pressures were measured during the medical check-ups. BP was measured during the medical check-up using oscillometric sphygmomanometers following a standardized protocol by a trained examiner. The devices had been calibrated and validated regularly. BP was measured in a seated position after at least 5 minutes of rest and no vigorous exercise during the preceding 30 minutes. According to the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7),19 definition and classification of hypertension was classified as normal (SBP, <120 mm Hg and DBP, <80 mm Hg), prehypertension (SBP, 120–139 mm Hg or DBP, 80–89 mm Hg), stage 1 hypertension (SBP, 140–159 mm Hg or DBP, 90–99 mm Hg), and stage 2–3 hypertension (SBP, ≥160 mm Hg or DBP, ≥100 mm Hg).
To investigate the individual associations of SBPs and DBPs with mortalities, we classified the SBPs into 6 groups (<120, 120–129, 130–139, 140–149, 150–159, ≥160 mm Hg) and the DBPs into 4 groups (<80, 80–89, 90–99, ≥100 mm Hg).
Controlled Variables
Baseline data, including age, sex, education level (none, 1–6, 7–12, or >12 years), marital status (single or married/cohabiting), smoking (frequently/occasionally or none), alcohol consumption (frequently/occasionally or none), and regular exercise (none, 1–2, or 3–5 times/week during the past 6 months) were collected using self-administered questionnaires. Blood samples were obtained from fasting persons, and frozen serum was sent for analysis. Laboratory data, including serum hemoglobin, fasting blood sugar, triglyceride, total cholesterol, alanine transaminase, albumin, and creatinine levels, were obtained. Estimated glomerular filtration rates (eGFRs; mL/minute/1.73 m2) were calculated from serum creatinine levels using the Modification of Diet in Renal Disease formula.20 Measurements of height and body weight were obtained, and body mass index (BMI) values were calculated as weight in kilograms divided by height in meter squared. The World Health Organization BMI categories were used for classifying participants as underweight (15–18.4 kg/m2), normal weight (18.5–24.9 kg/m2), overweight (25–29.9 kg/m2), and obese (≥30 kg/m2). The Short Portable Mental Status Questionnaire (SPMSQ), a widely used 10-item cognitive screening instrument, was used to measure cognitive impairment.21 Cognitive status was categorized as no impairment (SPMSQ score, 0–2 errors), mild impairment (SPMSQ score, 3–4 errors), and moderate-to-severe impairment (SPMSQ score, ≥5 errors). Anemia was defined as a hemoglobin level <13 g/dL in men and <12 g/dL in women. Diabetes mellitus was defined as fasting blood sugar levels ≥126 mg/dL, self-reporting of physician-diagnosed diabetes mellitus, or use of hypoglycemic medications. Hyperlipidemia was defined as triglyceride levels ≥200 mg/dL, total cholesterol levels ≥200 mg/dL, self-reporting of physician-diagnosed hyperlipidemia, or use of lipid-lowering medications.
Outcome Variables
The endpoints of this study included mortality data obtained between May 1, 2006 and December 31, 2010. Information on the causes of death was coded according to 2 versions of the International Classification of Diseases (ICD). The 9th version was used for 2006 to 2008 data (ICD-9 codes 001–998), and the 10th version was used for 2009 to 2010 data (ICD-10 codes A00–Z99). Deaths were classified as all-cause, CVD (ICD-9 codes 390–459, ICD-10 codes I00–I99), and expanded-CVD (CVD plus diabetes: ICD-9 code 250, ICD-10 codes E10–E14; CVD plus kidney diseases: ICD-9 580–589; ICD-10 N00–N29). Years of follow-up for each individual were calculated from baseline to the end of the follow-up period or the date of death, whichever was earlier.
Statistical Analyses
For continuous variables, baseline characteristics are presented as means and standard deviations (SDs). Statistical significance was assessed using the t test for continuous variables and the χ2 test for categorical variables. All-cause, CVD, and expanded-CVD mortality rates (expressed per 1000 person-years) were calculated for each stage of hypertension.
In the survival analyses of hypertension and mortality risks, survival curves were estimated using the Kaplan–Meier method, and log-rank tests were used to determine between-group differences. Two Cox proportional hazard regression models were used to evaluate the associations of hypertension stages with mortalities. The first model was adjusted for age and sex, and the second model was fully adjusted for all potential confounding factors (Table 1). To ensure robustness, basic model-fitting techniques were used for variable selection, goodness-of-fit assessment, and regression diagnostics. Variables were entered using stepwise selection, and the significance levels for entry and stay were set at 0.15 or greater using likelihood ratio tests. The assumption of proportional hazards for the Cox models was tested using Schoenfeld residuals. Interaction terms were evaluated, and the possible effect modification was assessed using further stratified analyses to evaluate subgroup mortalities. Spline regression was used to calculate the multivariable adjusted HR for each outcome associated with SBP and DBP modeled as continuous variables. To avoid a reverse causation scenario (preexisting conditions affecting BP), we performed sensitivity analyses that excluded the first and second years of deaths in the Cox model.
TABLE 1.
Characteristics of the Study Population, Stratified by Hypertension Stage
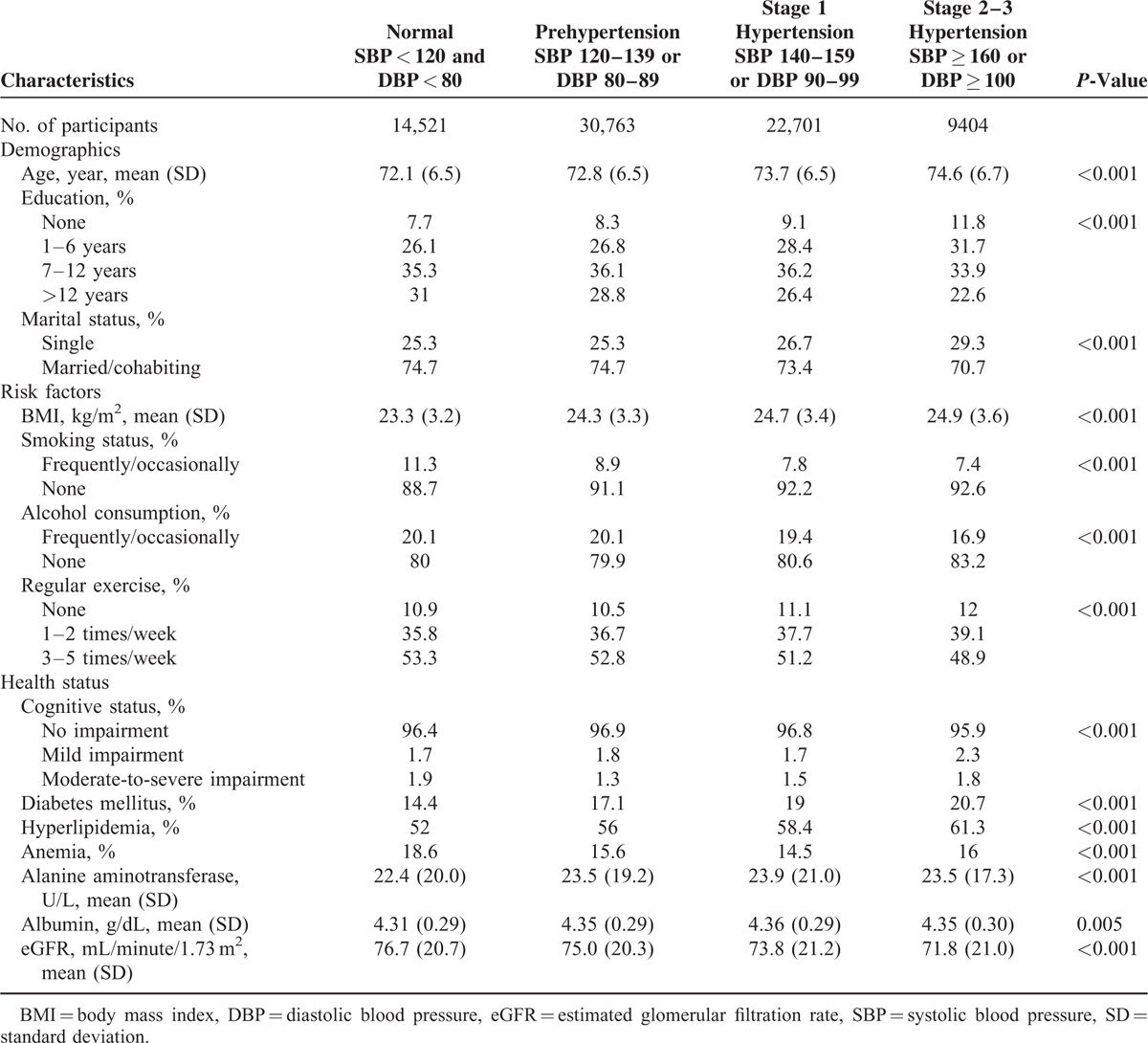
P-values were 2-sided, and values <0.05 were considered statistically significant. All analyses were conducted using SAS, version 9.3 (SAS Institute, Cary, NC), and Stata, version 12.0 (StataCorp, College Station, TX), statistical software packages.
RESULTS
Participant Characteristics
Table 1 shows the characteristics of participants stratified by hypertension stage, according to the JNC 7 definition. Using this standard, 18.8% of participants had normal BP, 39.8% had prehypertension, 29.3% had stage 1 hypertension, and 12.2% of participants had stage 2–3 hypertension. Respectively, the mean SBP and DBP values were 135.0 mm Hg (SD, 19.0) and 76.4 (11.5) among men, 135.7 (20.1) and 75.8 (11.4) among women, 132.0 (18.8) and 77.1 (11.1) among individuals aged 65 to 69, 135.7 (19.1) and 76.6 (11.3) among those aged 70 to 74 years, 137.4 (19.7) and 75.4 (11.5) among those aged 75 to 79 years, 138.7 (20.1) and 74.5 (11.8) among those aged 80 to 84 years, and 139.4 (21.1) and 73.6 (12.2) among those aged ≥85 years. Among them, DBP ≥ 100 mm Hg is unusual in the elderly. In our study, 1670 (2.2%) participants had DBP ≥ 100 mm Hg. Those with higher BPs were significantly older and had higher BMIs. In addition, those with higher BPs involved a larger proportion of individuals who were singles; nonsmokers and nonalcohol consumers; not involved in regular exercise; and who had more cognitive impairment, higher prevalences of diabetes mellitus and hyperlipidemia, lower eGFRs, and lower education levels.
Survival Analysis
The Kaplan–Meier analyses demonstrated a reduction in the expanded-CVD survival probability associated with BP (Fig. 1). The 4-year survival rates for participants with normal BP, prehypertension, stage 1 hypertension, and stage 2–3 hypertension were 98.2%, 98.4%, 98.3%, and 97.3%, respectively. The log-rank tests for equality of survival function were significant across the groups with normal BP and stage 2–3 hypertension (P < 0.001), SBP < 120 mm Hg and SBP 120–129 mm Hg (P = 0.023), SBP < 120 mm Hg and SBP ≥160 mm Hg (P < 0.001), DBP < 80 mm Hg and DBP 80–89 mm Hg (P = 0.005), DBP < 80 mm Hg and DBP ≥ 100 mm Hg (P = 0.048). The relationships of BP to all-cause survival probability (Supplementary Figure 1, http://links.lww.com/MD/A532) were similar to their relationships to expanded-CVD survival probability. There were 14,345 participants aged ≥80, with a survival rate of 87.36%, compared to a survival rate of 96.80% among those aged <80 (P < 0.001).
FIGURE 1.
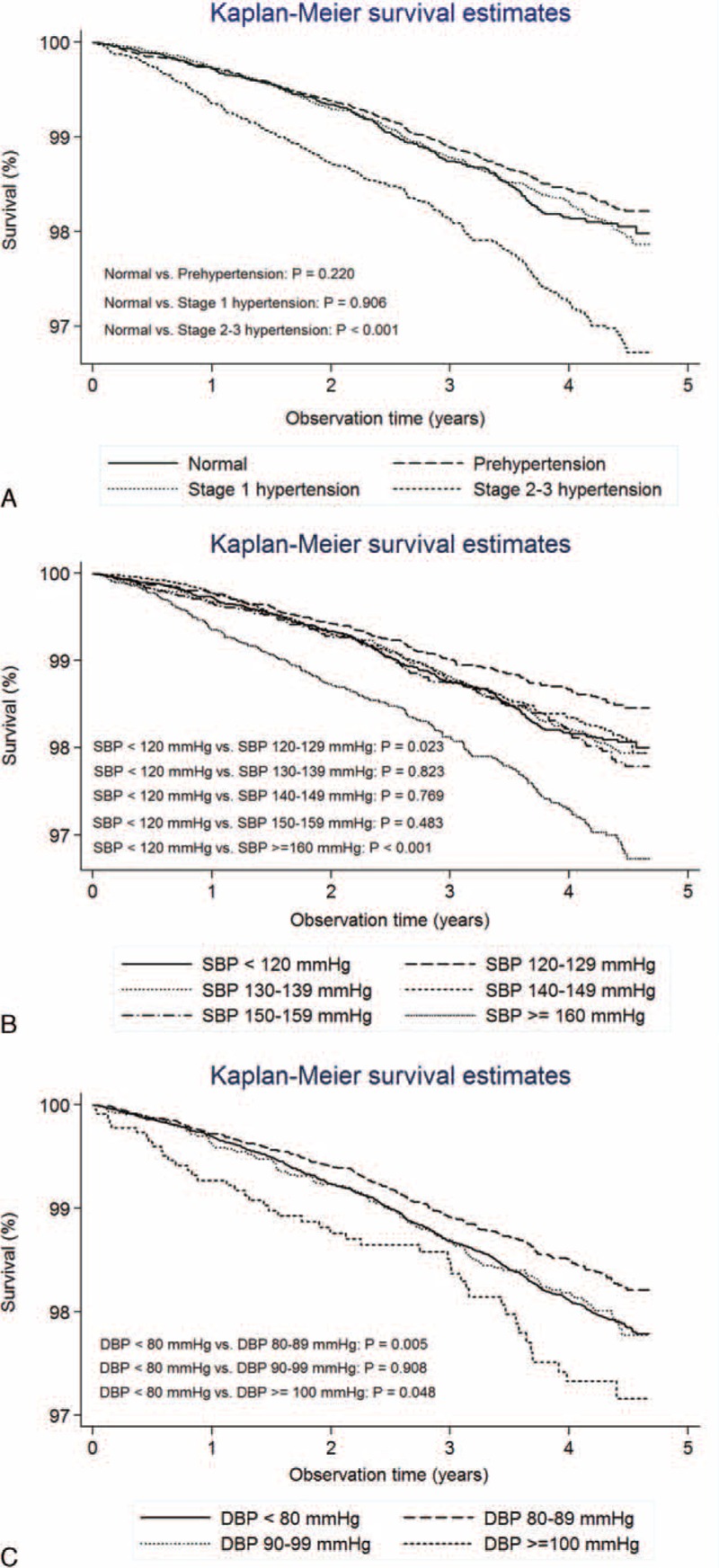
Survival analysis: Expanded-CVD survivals according to (A) stages of hypertension by Seventh Joint National Committee (JNC 7) (B) systolic blood pressures, and (C) diastolic blood pressures.
Hazard Ratios (HRs) for Mortality Risks by Hypertension Stage
During an average of 3.28 (SD, 1.30) years of follow-up, 3830 persons (4.95%) died during the study period. Of the total deaths, 876 (22.9%) were ascribed to CVD and 1115 (29.1%) were expanded-CVD-related. Crude all-cause, CVD, and expanded-CVD mortality rates (of 253,741 person-years) were 15.1 (3830 persons), 3.5 (876 persons), and 4.4 (1115 persons) per 1000 person-years, respectively. The characteristics of the participants with expanded-CVD mortality after stratification by hypertension stage are shown in Supplementary Table 1, http://links.lww.com/MD/A532.
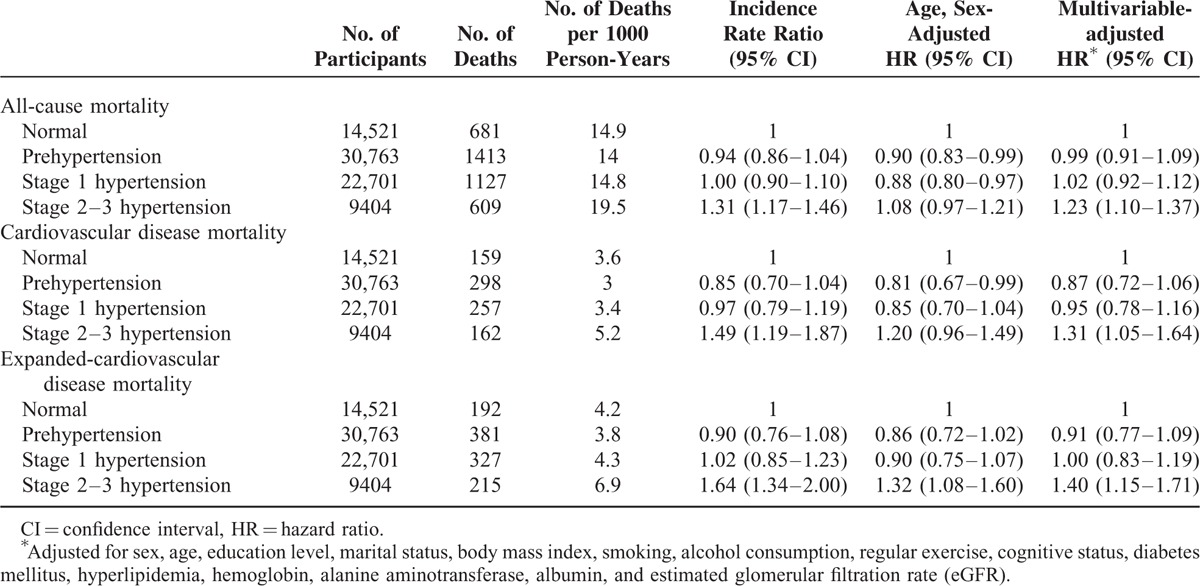
Table 2 shows the incidence rate ratios (IRRs) and HRs of mortalities for different stages of hypertension. The IRRs for mortality risks were significantly higher for the stage 2–3 hypertension group. After adjusting for age and sex, the HRs for expanded-CVD mortality were significantly higher for the stage 2–3 hypertension group. In the fully adjusted model, the mortality risk for the stage 2–3 hypertension group remained substantial (all-cause mortality: HR, 1.23; 95% confidence interval [CI], 1.10–1.37; CVD mortality: HR, 1.31; 95% CI: 1.05–1.64; expanded-CVD mortality: HR, 1.40; 95% CI: 1.15–1.71). In the sensitivity test, after excluding subjects who died within the first and second years of baseline BP measurement, the results remained robust.
TABLE 2.
Association of Hypertension Stages With All-Cause, Cardiovascular disease, and Expanded-Cardiovascular Disease Mortalities
When the data of history of hypertension (48.1%) were used for analysis, compared to those with no hypertension history (51.9%), the CVD and expanded-CVD mortality risks were significantly higher among those with a history of hypertension (HR: 1.06, 95% CI: 0.98–1.13 for all-cause mortality; HR: 1.38, 95% CI: 1.20–1.60 for CVD-mortality; and HR: 1.38, 95% CI: 1.21–1.57 for expanded-CVD mortality). We further classified those with BP ≥ 140/90 mm Hg during physical examination and those with a history of hypertension as the hypertension group (59.6%), and the others as the nonhypertension group (40.4%). Compared to the nonhypertension group, the hypertension group had significantly higher mortality risks (HR: 1.10, 95% CI: 1.02–1.17 for all-cause mortality; HR: 1.42, 95% CI: 1.23–1.65 for CVD mortality; and HR: 1.44, 95% CI: 1.26–1.64 for expanded-CVD mortality).
HRs for Mortality Risks by SBP and DBP
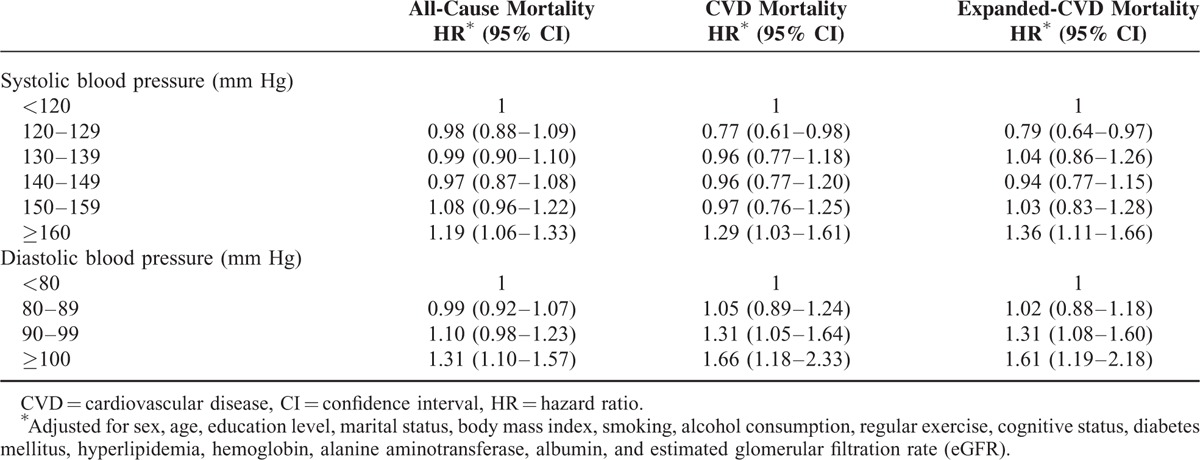
Table 3 shows the associations of mortalities with SBP and DBP. Compared to SBP < 120 mm Hg, those with SBPs of 120–129 mm Hg had significantly lower CVD mortality (HR, 0.77; 95% CI: 0.61–0.98) and lower expanded-CVD mortality (HR, 0.79; 95% CI: 0.64–0.97). The mortality risks increased significantly when the SBP was ≥160 mm Hg (all-cause mortality: HR, 1.19; 95% CI: 1.06–1.33; CVD mortality: HR, 1.29; 95% CI: 1.03–1.61; expanded-CVD mortality: HR, 1.36; 95% CI: 1.11–1.66). The associations of mortality risks increased significantly when DBP was ≥100 mm Hg (all-cause mortality: HR, 1.31; 95% CI: 1.10–1.57; CVD mortality: HR, 1.66; 95% CI: 1.18–2.33; expanded-CVD mortality: HR, 1.61; 95% CI: 1.19–2.18). The associations of CVD and expanded-CVD mortality risks also increased significantly when DBP values were 90 to 99 mm Hg (CVD mortality: HR, 1.31; 95% CI: 1.05–1.64; expanded-CVD mortality: HR, 1.31; 95% CI: 1.08–1.60).
TABLE 3.
Association of Blood Pressure With All-Cause, Cardiovascular Disease, and Expanded-Cardiovascular Disease Mortalities
Spline Regression
When treating SBP and DBP as continuous variables, the relationship of SBP to expanded-CVD mortality was J shaped (Fig. 2A). The lowest risk occurred at an SBP of 121 mm Hg; mortality was greater with both higher and lower BPs. The relationship of DBP to the risk of expanded-CVD mortality was monotonic and positive (Fig. 2B). The relationship was marginal in the lower part of the distribution of DBP, but a steeper increase in risk occurred at DBP above approximately 76 mm Hg. The relationships of SBP and DBP to all-cause mortality were mainly monotonic and positive (Supplementary Figure 2A and B, http://links.lww.com/MD/A532), with little or no relationships seen in the lower parts of the distributions. The relationships of SBP and DBP to CVD mortality (Supplementary Figure 3A and B, http://links.lww.com/MD/A532) were almost identical to their relationships to expanded-CVD mortality.
FIGURE 2.
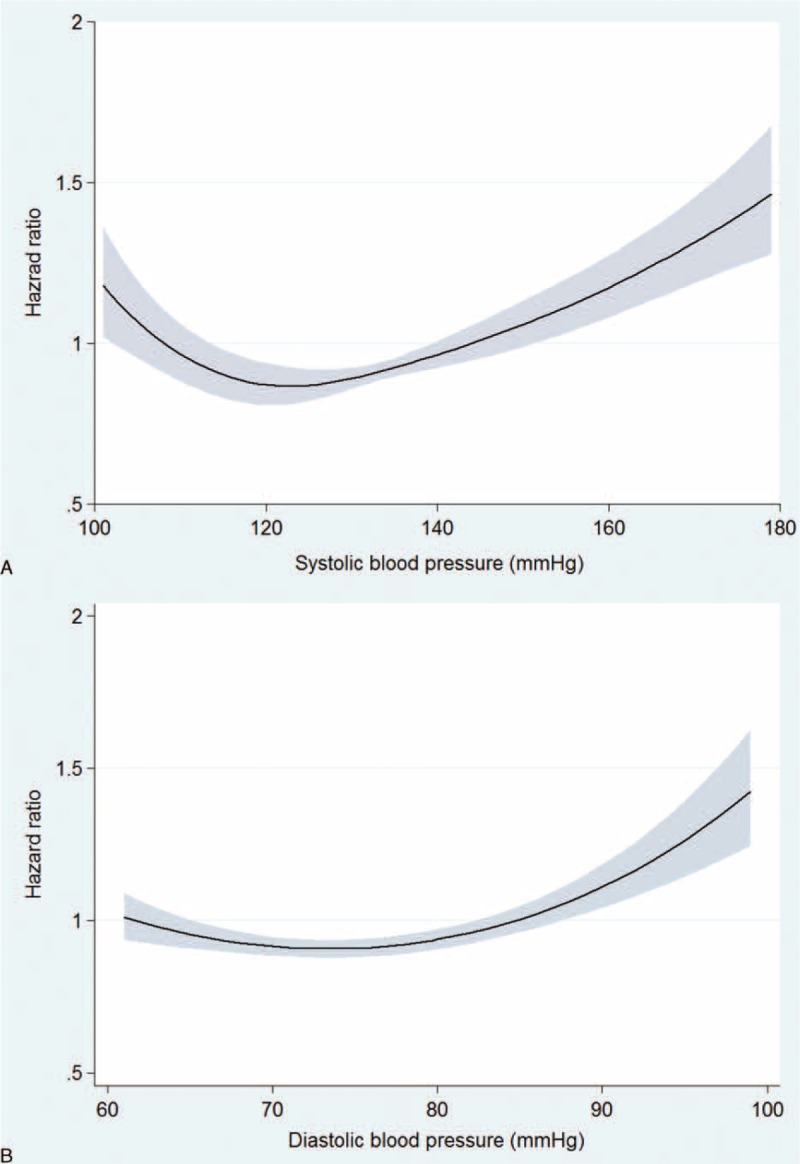
The association between (A) systolic blood pressure (B) diastolic blood pressure and expanded-cardiovascular mortality by spline regression. The line represents the hazard ratio. The gray area represents the 95% confidence interval.
Interaction Terms and Subgroup Analyses
We evaluated the interaction of hypertension with sex, age ≥ 80 years, BMI, existence of DM, hyperlipidemia, and eGFR < 60 mL/minute/1.73 m2. For all-cause mortality, the only significant interaction term was of hypertension with sex (P = 0.006). For CVD and expanded-CVD mortalities, the interaction terms of hypertension with sex (P = 0.01 for CVD; P = 0.006 for expanded-CVD), age ≥ 80 years (P = 0.03 for CVD; P = 0.002 for expanded-CVD), normal weight (P = 0.01 for CVD; P = 0.009 for expanded-CVD), overweight (P = 0.004 for CVD; P = 0.006 for expanded-CVD), and obese (P = 0.01 for CVD; P = 0.01 for expanded-CVD) were significant, whereas the interaction terms of hypertension with comorbidities of diabetes mellitus, hyperlipidemia, and eGFR < 60 mL/minute/1.73 m2 were not significant.
Stratified by sex, age, BMI, regular exercise, presence of anemia, diabetes mellitus or hyperlipidemia, and eGFR, the hypertension mortality risks were further analyzed (Fig. 3). The associations of prehypertension and stage 1 hypertension with all-cause, CVD, and expanded-CVD mortalities were not significant among most subgroups. The associations of stage 2–3 hypertension with these mortalities were significant among the subgroups of women, BMI 18.5 to 24.9, without anemia, without diabetes mellitus, without hyperlipidemia, and eGFR ≥60 mL/minute/1.73 m2.
FIGURE 3.
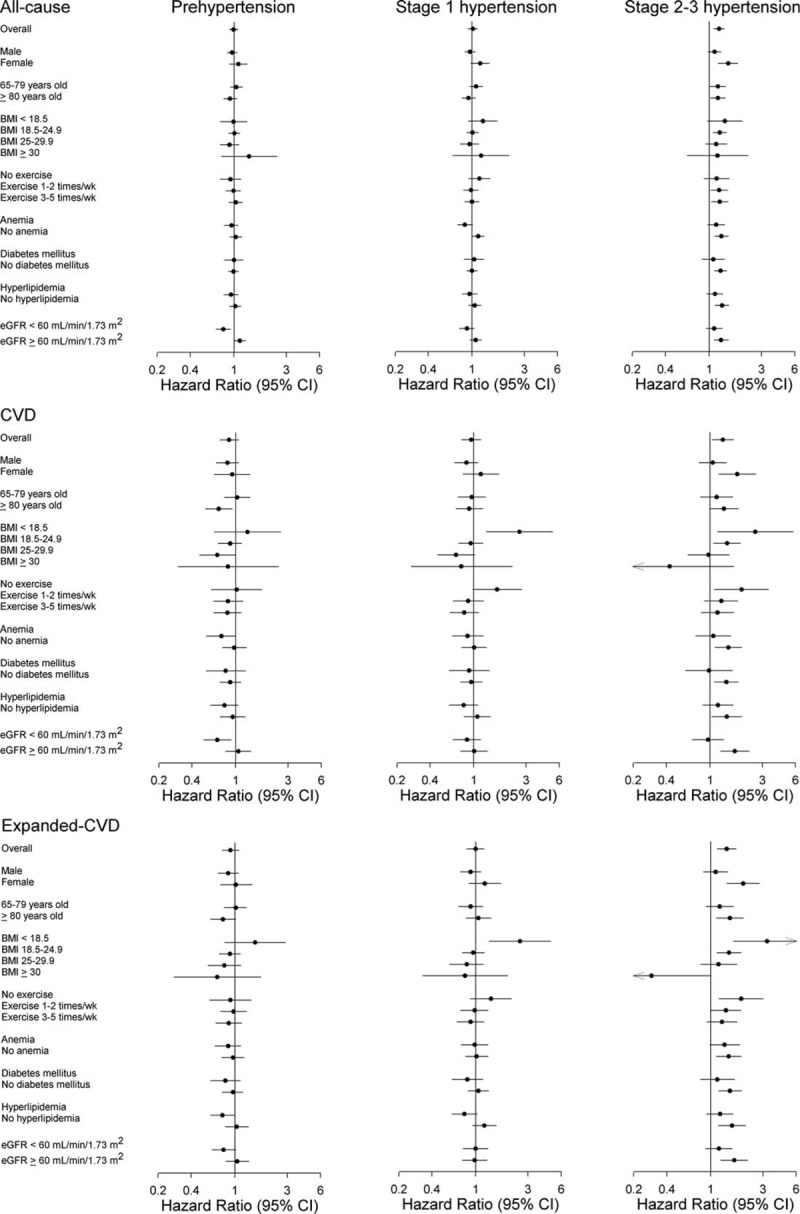
Mortality rate subgroup analysis: The Cox model was adjusted for sex, age, education level, marital status, body mass index, smoking, alcohol consumption, regular exercise, cognitive status, diabetes mellitus, hyperlipidemia, hemoglobin, alanine aminotransferase, albumin, and estimated glomerular filtration rate (eGFR). The effect measurement is presented on a log scale. CI = confidence interval.
We further investigated the association of SBP and DBP with mortalities among each subgroup. Among those participants aged 65 to 79 years with SBP 140 to 150 mm Hg and DBP < 90 mm Hg, compared to those with normal BP, the risk of all-cause, CVD, and expanded-CVD mortalities did not increase (HR: 0.92, 95% CI: 0.78–1.08 for all-cause mortality; HR: 0.88, 95% CI: 0.62–1.26 for CVD mortality; HR 0.77, 95% CI: 0.56–1.06 for expanded-CVD mortality). Among those participants aged 65 to 79 years with BP ≥ 150/90 mm Hg, compared to those with normal BP, the risk of all-cause mortality increased (HR: 1.22, 95% CI: 1.07–1.40); however, the risk of CVD and expanded-CVD mortalities did not increase significantly (HR: 1.10, 95% CI: 0.83–1.46 for CVD mortality; and HR 1.13, 95% CI: 0.88–1.44 for expanded-CVD mortality). Our results support a target of BP < 150/90 mm for patients aged 65 to 79 years, as recommended in JNC 8.
Among women, an SBP ≥ 160 mm Hg was associated with a 45% increased risk for all-cause mortality, a 79% increased risk for CVD mortality, and a 95% increased risk for expanded-CVD mortality; a DBP ≥ 100 mm Hg was associated with a 221% increased risk of CVD mortality, and a 90% increased risk for expanded-CVD mortality. Among those age ≥80-years-old, an SBP ≥ 160 mm Hg was not significantly associated with an increased mortality, whereas a DBP ≥ 100 mm Hg was associated with a 48% increased risk for all-cause mortality, a 95% increased risk for CVD mortality, and an 88% increased risk for expanded-CVD mortality.
DISCUSSION
The present study shows that stage 2–3 hypertension was associated with increased all-cause, CVD, and expanded-CVD mortalities among older adults. When the individual relationships between mortality and SBP and DBP were investigated, the mortality risks increased significantly when the SBP values were ≥160 mm Hg or the DBP values were ≥100 mm Hg. The CVD and expanded-CVD mortality risks were lowest when the SBP values were 120 to 129 mm Hg, compared with SBP values <120 mm Hg, and increased when DBP values were ≥90 mm Hg, compared with DBP values <80 mm Hg. The impact of stage 2–3 hypertension on mortality was more prominent among women, whereas it was insignificant among men. Further, the mortality risks associated with hypertension were not attenuated with increasing age.
In our study, the association of hypertension with expanded-CVD mortality is as strong as the association between hypertension and CVD mortality. The results reflect the fact that that hypertension might complicate and increase the mortality risk among those patients with CVD, diabetes, and renal impairment, which is in accordance with results of previous studies.22,23
The prevalences of stages 1 and 2–3 hypertension were 29.3% and 12.2%, respectively, in our study; these prevalences are quite close to those reported in nationwide survey data from Taiwan.24 Hypertensive women have been reported to be more likely to be treated than men, but less likely to achieve BP control.25,26 There have been limited studies reporting a sex difference in the association of hypertension and mortality.27 In the present study, the association of hypertension with mortality, especially with CVD and expanded-CVD mortalities, is more substantial among women than men. This finding has prompted vigilant BP control among older women.
Many studies have investigated the different effects of BP on mortality in the oldest old group; however, the optimal BP for individuals aged ≥ 80 years remains uncertain.28–31 The randomized clinical trial HYVET demonstrated the benefits of antihypertensive treatment for individuals aged ≥ 80 years with SBP ≥ 160 mm Hg.32 However, a lower BP may be partially related to poor general health in the oldest old group,33 and may be associated with an increased risk of death.29,33–35 Some studies have observed that a higher BP was not associated with increased mortality in the oldest old,28,30,36 and some have even found that a higher BP was associated with better survival.31 Our study included 14,345 participants who were over 80 years old. We did not detect an increased risk of mortality among those with stage 1 hypertension, but did find an increased risk of all-cause and expanded-CVD mortality among those with stage 2–3 hypertension in this older age group. In concordance with previous studies,28–31,33–36 our data support the slightly relaxed BP targets suggested for the oldest old group. Further studies are needed to reconcile the evidence regarding this important target for the growing number of individuals in this oldest age group.
We observed increased CVD and expanded-CVD mortalities among hypertensive older adults who were underweight. These results are slightly different from those reported by Wang et al.37 They reported little difference in cardiovascular mortality in the low BMI group of elderly hypertensive patients. However, as Wang et al mentioned, all of their participants were hospital-based, hypertensive patients undergoing treatment, and the BP levels were nearly identical among the different BMI groups. This may partially explain why there was little difference between the groups, or a trend toward increasing CVD mortality was noted in their study.
The relationship between BP and mortality has been reported to be modified by walking speed in individuals over 65-years-old.38 Higher SBPs were associated with an increased risk of mortality only among elderly adults who had a medium-to-fast walking pace, and there was no association between elevated SBPs and mortality among slower-walking, older adults. However, we observed a tight association between stage 2–3 hypertension and CVD and expanded-CVD mortalities among those older adults not getting regular exercise, which included those with unhealthy lifestyles or who were frailer. Among these hypertensive older adults, there was not a significant increase in CVD and expanded-CVD mortalities if they regularly exercised 3 to 5 times per week.
When hypertension is combined with diabetes and renal impairment, the risk of mortality has been reported to increase significantly with higher BPs.22 The crude IRR of all-cause mortality for those with stage 2–3 hypertension was 1.09 (95% CI: 0.76–1.57) among those with diabetes, 1.35 (95% CI: 1.14–1.60) among those with hyperlipidemia, and 1.08 (95% CI: 0.91–1.28) among those with eGFR < 60 mL/minute/1.73 m2, when compared to those with a normal BP. After adjustment in the Cox models, among those with stage 2–3 hypertension who did not have diabetes mellitus, hyperlipidemia, or eGFR ≥ 60 mL/minute/1.73 m2, we observed a significantly higher risk of mortality. Among those with diabetes mellitus, hyperlipidemia, or eGFR < 60 mL/minute/1.73 m2, hypertension also tended to increase the risk of mortality, but did not reach statistical significance. There was no significant difference regarding the impact of hypertension on mortality between those with and those without comorbidities, and the interaction between hypertension and comorbidity was not significant. Hypertension may have a smaller proportional effect on mortality among those with comorbidities. These findings have also been observed in previous studies.39–41
The strengths of this study include the use of a large population, with prevalent chronic diseases, which provided an unselected population of community-dwelling, older adults. The population in our study is different from that of Lewington et al's study, which did not include adults with previous vascular diseases and did not include patients with BP below 115/75 mm Hg. In contrast to Lewington et al's study, which reported a linear association between BP and CAD, we observed a J-curve phenomenon for SBP on CVD and expanded-CVD mortality. The reliable assessment of the causes of death and the detailed information regarding risk behaviors and health status enabled us to investigate cause-specific mortality while controlling for possible confounders. The follow-up period was short, which likely led to fewer errors that may have otherwise occurred due to BP changes.
Our study has several limitations. First, BP was only measured once; hence, the impact of BP changes on mortality risk during the follow-up could not be investigated. Second, this study analyzed secondary data, and the dataset was based on annual physical examinations of older adults. We did not have access to information regarding the severity of any preexisting diseases. Although our analyses were adjusted for common chronic health conditions and baseline patient characteristics, there is a possibility that subclinical disease or other diseases not measured may have contributed to survival decreases. Third, the questionnaire included information of self-reported physician-diagnosed hypertension or use of BP treatment. However, it was not clear whether the participant received BP treatment when hypertension was noted after the physical examination. Lack of this information precludes us from including BP treatment in the multivariable analysis for adjustment. In Taiwan, there is a mandatory national health insurance program, with an enrollment rate of 99% and good accessibility. Adults ≥65-years-old are reported to have >25 outpatient visits annually,42 suggesting that hypertension could have been treated among our participants. Fourth, a decrease in BP due to antihypertensive treatment is different from having a low BP due to poor general health conditions. To avoid the reverse causality, we performed sensitivity analyses that excluded those who died within the first and second years of follow-up, and the results remained robust. Fifth, this study was conducted during an annual physical examination of the elderly. Because participation in this study was voluntary, the participants may not represent the general population. However, the prevalence of hypertension in our study was quite similar to that reported in previous nationwide survey data.24 Additionally, the observed relative risk may be a reasonable estimate for the general population because the risks were evaluated using internal comparisons. Finally, this study investigated community-dwelling, older adults; the generalizability of this study's findings might not extend to frailer, older adults living in institutions or nursing homes.
CONCLUSION
This study builds on prior work by extending the findings regarding the association of BP to mortality risks among older adults. This study showed that SBPs of 120 to 129 mm Hg were associated with the lowest CVD and expanded-CVD mortality risks, and that stage 2–3 hypertension, SBPs ≥160 mm Hg, or DBPs ≥90 mm Hg significantly increased the CVD and expanded-CVD mortality risk. However, further interventional trials are needed to determine whether consistent BP control, and to which BP targets, can improve mortality among older adults. BP control remains an important public health challenge for the care of this rapidly growing population segment.
Acknowledgments
This study is based on data from the Taipei City Geriatric Health Examination Database provided by the Taipei City Department of Health, and managed by the Taipei Databank for Public Health Analysis. Access to this database is granted for researchers who meet the criteria for access to confidential data. Applicants must follow the Computer-Processed Personal Data Protection Law and Databank for Public Health Analysis regulations, and an agreement must be signed by the applicant and the applicant's supervisor upon submission of the application. All applications are reviewed by the Taipei Databank for Public Health Analysis. We would like to thank the Biostatistics Task Force of Taipei Veterans General Hospital for statistical consultation.
Footnotes
Abbreviations: BMI = body mass index, BP = blood pressure, CI = confidence interval, CVD = cardiovascular disease, DBP = diastolic blood pressure, eGFR = estimated glomerular filtration rate, HR = hazard ratio, ICD = International Classification of Diseases, IRRs = incidence rate ratios, JNC = Joint National Committee, SBPs = systolic blood pressures, SDs = standard deviations, SPMSQ = Short Portable Mental Status Questionnaire.
This study was supported by the Taiwan Ministry of Education through its “Aim for the Top University Plan” and by grants from the Department of Health of the Taipei City Government (10301-62-001), Taipei Veterans General Hospital (V103C-201), and the National Science Council (NSC 98-2314-B-075-029).
The authors have no conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.md-journal.com).
REFERENCES
- 1.He J, Whelton PK. Elevated systolic blood pressure and risk of cardiovascular and renal disease: overview of evidence from observational epidemiologic studies and randomized controlled trials. Am Heart J 1999; 138 (3 Pt 2):211–219. [DOI] [PubMed] [Google Scholar]
- 2.Stamler J, Stamler R, Neaton JD. Blood pressure, systolic and diastolic, and cardiovascular risks. US population data. Arch Intern Med 1993; 153:598–615. [DOI] [PubMed] [Google Scholar]
- 3.Lawes CM, Vander Hoorn S, Rodgers A, et al. Global burden of blood-pressure-related disease, 2001. Lancet 2008; 371:1513–1518. [DOI] [PubMed] [Google Scholar]
- 4.Carson AP, Howard G, Burke GL, et al. Ethnic differences in hypertension incidence among middle-aged and older adults: the multi-ethnic study of atherosclerosis. Hypertension 2011; 57:1101–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. JAMA 2010; 303:2043–2050. [DOI] [PubMed] [Google Scholar]
- 6.Hajjar I, Kotchen TA. Trends in prevalence, awareness, treatment, and control of hypertension in the United States, 1988–2000. JAMA 2003; 290:199–206. [DOI] [PubMed] [Google Scholar]
- 7.Spillman BC, Lubitz J. The effect of longevity on spending for acute and long-term care. N Engl J Med 2000; 342:1409–1415. [DOI] [PubMed] [Google Scholar]
- 8.Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 2013; 31:1281–1357. [DOI] [PubMed] [Google Scholar]
- 9.Go AS, Bauman MA, Coleman King SM, et al. An effective approach to high blood pressure control: a science advisory from the American Heart Association, the American College of Cardiology, and the Centers for Disease Control and Prevention. Hypertension 2014; 63:878–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weber MA, Schiffrin EL, White WB, et al. Clinical practice guidelines for the management of hypertension in the community: a statement by the American Society of Hypertension and the International Society of Hypertension. J Clin Hypertens (Greenwich) 2014; 16:14–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311:507–520. [DOI] [PubMed] [Google Scholar]
- 12.Chiang CE, Wang TD, Ueng KC, et al. 2015 guidelines of the Taiwan Society of Cardiology and the Taiwan Hypertension Society for the management of hypertension. J Chin Med Assoc 2015; 78:1–47. [DOI] [PubMed] [Google Scholar]
- 13.Hall WD. Representation of blacks, women, and the very elderly (aged > or =80) in 28 major randomized clinical trials. Ethn Dis 1999; 9:333–340. [PubMed] [Google Scholar]
- 14.Wen CP, Cheng TY, Tsai MK, et al. All-cause mortality attributable to chronic kidney disease: a prospective cohort study based on 462 293 adults in Taiwan. Lancet 2008; 371:2173–2182. [DOI] [PubMed] [Google Scholar]
- 15.Wu CY, Chou YC, Huang N, et al. Association of body mass index with all-cause and cardiovascular disease mortality in the elderly. PLoS ONE 2014; 9:e102589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yen YF, Hu HY, Lin IF, et al. Associations of metabolic syndrome and its components with mortality in the elderly: a cohort study of 73,547 Taiwanese adults. Medicine (Baltimore) 2015; 94:e956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin CC, Chen CC, Kung PT, et al. Joint relationship between renal function and proteinuria on mortality of patients with type 2 diabetes: the Taichung Diabetes Study. Cardiovasc Diabetol 2012; 11:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu CY, Chou YC, Huang N, et al. Cognitive impairment assessed at annual geriatric health examinations predicts mortality among the elderly. Prev Med 2014; 67:28–34. [DOI] [PubMed] [Google Scholar]
- 19.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289:2560–2572. [DOI] [PubMed] [Google Scholar]
- 20.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999; 130:461–470. [DOI] [PubMed] [Google Scholar]
- 21.Pfeiffer E. A Short Portable Mental Status Questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc 1975; 23:433–441. [DOI] [PubMed] [Google Scholar]
- 22.Afghahi H, Svensson MK, Pirouzifard M, et al. Blood pressure level and risk of major cardiovascular events and all-cause of mortality in patients with type 2 diabetes and renal impairment: an observational study from the Swedish National Diabetes Register. Diabetologia 2015; 58:1203–1211. [DOI] [PubMed] [Google Scholar]
- 23.Cederholm J, Gudbjornsdottir S, Eliasson B, et al. Blood pressure and risk of cardiovascular diseases in type 2 diabetes: further findings from the Swedish National Diabetes Register (NDR-BP II). J Hypertens 2012; 30:2020–2030. [DOI] [PubMed] [Google Scholar]
- 24.Su TC, Bai CH, Chang HY, et al. Evidence for improved control of hypertension in Taiwan: 1993–2002. J Hypertens 2008; 26:600–606. [DOI] [PubMed] [Google Scholar]
- 25.Gee ME, Bienek A, McAlister FA, et al. Factors associated with lack of awareness and uncontrolled high blood pressure among Canadian adults with hypertension. Can J Cardiol 2012; 28:375–382. [DOI] [PubMed] [Google Scholar]
- 26.Gu Q, Burt VL, Paulose-Ram R, et al. Gender differences in hypertension treatment, drug utilization patterns, and blood pressure control among US adults with hypertension: data from the National Health and Nutrition Examination Survey 1999–2004. Am J Hypertens 2008; 21:789–798. [DOI] [PubMed] [Google Scholar]
- 27.Bog-Hansen E, Merlo J, Gullberg B, et al. Survival in patients with hypertension treated in primary care. A population-based follow-up study in the Skaraborg Hypertension and Diabetes Project. Scand J Prim Health Care 2004; 22:222–227. [DOI] [PubMed] [Google Scholar]
- 28.Banach M, Bromfield S, Howard G, et al. Association of systolic blood pressure levels with cardiovascular events and all-cause mortality among older adults taking antihypertensive medication. Int J Cardiol 2014; 176:219–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oates DJ, Berlowitz DR, Glickman ME, et al. Blood pressure and survival in the oldest old. J Am Geriatr Soc 2007; 55:383–388. [DOI] [PubMed] [Google Scholar]
- 30.Jacobs JM, Stessman J, Ein-Mor E, et al. Hypertension and 5-year mortality among 85-year-olds: the Jerusalem Longitudinal Study. J Am Med Dir Assoc 2012; 13:759.e1–759.e6. [DOI] [PubMed] [Google Scholar]
- 31.Satish S, Freeman DH, Jr, Ray L, et al. The relationship between blood pressure and mortality in the oldest old. J Am Geriatr Soc 2001; 49:367–374. [DOI] [PubMed] [Google Scholar]
- 32.Beckett NS, Peters R, Fletcher AE, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008; 358:1887–1898. [DOI] [PubMed] [Google Scholar]
- 33.Rastas S, Pirttila T, Viramo P, et al. Association between blood pressure and survival over 9 years in a general population aged 85 and older. J Am Geriatr Soc 2006; 54:912–918. [DOI] [PubMed] [Google Scholar]
- 34.Mattila K, Haavisto M, Rajala S, et al. Blood pressure and five year survival in the very old. Br Med J (Clin Res Ed) 1988; 296:887–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Langer RD, Ganiats TG, Barrett-Connor E. Paradoxical survival of elderly men with high blood pressure. BMJ 1989; 298:1356–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Bemmel T, Gussekloo J, Westendorp RG, et al. In a population-based prospective study, no association between high blood pressure and mortality after age 85 years. J Hypertens 2006; 24:287–292. [DOI] [PubMed] [Google Scholar]
- 37.Wang Y, Wang Y, Qain Y, et al. Association of body mass index with cause specific deaths in Chinese elderly hypertensive patients: Minhang community study. PLoS ONE 2013; 8:e71223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Odden MC, Peralta CA, Haan MN, et al. Rethinking the association of high blood pressure with mortality in elderly adults: the impact of frailty. Arch Intern Med 2012; 172:1162–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pell S, D’Alonzo CA. Factors associated with long-term survival of diabetics. JAMA 1970; 214:1833–1840. [PubMed] [Google Scholar]
- 40.Hypertension in Diabetes Study (HDS): II. Increased risk of cardiovascular complications in hypertensive type 2 diabetic patients. J Hypertens 1993; 11:319–325. [DOI] [PubMed] [Google Scholar]
- 41.Rosengren A, Welin L, Tsipogianni A, et al. Impact of cardiovascular risk factors on coronary heart disease and mortality among middle aged diabetic men: a general population study. BMJ 1989; 299:1127–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu CY, Hu HY, Li CP, et al. The association between functional disability and acute care utilization among the elderly in Taiwan. Arch Gerontol Geriatr 2013; 57:177–183. [DOI] [PubMed] [Google Scholar]


