Abstract
To date, there have been few reports investigating the relationship between tuberculosis (TB) and gastric cancer.
We conducted a nationwide population-based matched cohort study using data retrieved from Taiwan's National Health Insurance Research Database to determine the incidence of and risk factors for TB in patients diagnosed with gastric cancer. From 2000 to 2011, we identified 36,972 gastric cancer patients and normal subjects from the general population matched for age, sex, and comorbidities at a 1:1 ratio. The data were analyzed using Cox proportional hazards models.
Compared with the matched cohort, gastric cancer patients exhibited a higher risk for TB (adjusted hazard ratio [HR] 2.25, 95% confidence interval [CI] 1.65–3.05, P < 0.001), and those with TB exhibited higher mortality (adjusted HR 1.59, 95% CI 1.41–1.79, P < 0.001). Old age (adjusted HR 2.40, 95% CI 1.92–2.99, P < 0.001), male sex (adjusted HR 2.13, 95% CI 1.76–2.57, P < 0.001), diabetes mellitus (adjusted HR 1.28, 95% CI 1.05–1.56, P = 0.013), and chronic obstructive pulmonary disease (COPD) (adjusted HR 1.44, 95% CI 1.19–1.75, P < 0.001) were identified as independent risk factors for TB in gastric cancer patients. Dyslipidemia was an independent protective factor for both TB (adjusted HR 2.13, 95% CI 1.73–2.62, P < 0.001) and mortality (adjusted HR 1.11, 95% CI 1.08–1.15, P < 0.001) in gastric cancer patients.
Old age, male sex, diabetes mellitus, and COPD were independent risk factors for TB in gastric cancer. High-risk gastric cancer patients, especially those in TB-endemic areas, should be regularly screened for TB.
INTRODUCTION
Tuberculosis (TB) is a widespread infectious disease that represents a major global health problem. According to the 2013 report by the Centers for Disease Control and Prevention (CDC), the incidence of TB in Taiwan was 68 cases per 100,000 people in 2013, and that of TB-related deaths was 2.8 cases per 100,000 people.1 The TB burden in Taiwan is estimated to be relatively higher than that in other developed countries.2 It has been reported that malignancy and malnutrition are risk factors for TB.3–8 Gastric cancer patients who often undergo significant body weight loss might be more likely to suffer from malnutrition than healthy individuals and might therefore be more likely to develop TB.
Gastric cancer is rampant in many countries. Its incidence is much higher in Asia than in other regions, though the global incidence has declined in recent years. However, gastric cancer continues to be ranked as the 5th most common cancer and it accounted for 723,000 deaths worldwide in 2012.9
Gastric cancer is often diagnosed at an advanced stage. Treatment can include surgery, chemotherapy, and radiotherapy. Surgery is the primary treatment for early-stage patients. Complete resection with lymph node dissection is considered a standard goal, whereas chemotherapy and radiotherapy are usually applied in palliative settings.
Few reports to date have mentioned the risk factors for TB in gastric cancer patients.10–12 Our previous study11 reported that compared with the general population, gastric cancer patients who underwent gastrectomy or had previous TB infection were more likely to develop TB infection.
Early gastric cancer patients who received gastrectomy have been reported to have a higher incidence of TB compared with those who received endoscopic mucosal resection.10 However, the number of TB patients among gastric cancer patients in the reported series10–14 was limited due to a low incidence. For gastric cancer patients, whether gastrectomy has an impact on the incidence of TB infection has not been well established.
To clarify this issue, we conducted a nationwide population-based matched cohort study using the National Health Insurance Research Database (NHIRD). We investigated the incidence of TB in patients with newly diagnosed gastric cancer in Taiwan, which exhibits a moderate TB burden, and analyzed the risk factors for the development of TB and mortality in gastric cancer patients.
MATERIALS AND METHODS
Data Sources
Taiwan's National Health Insurance (NHI) program was initiated in 1995. The program supplies comprehensive medical care to all residents of Taiwan, with a coverage rate of more than 99%. The program comprises outpatient, emergency, inpatient, dental, and traditional Chinese herbal medicine, as well as prescription drugs.
Taiwan's National Health Research Institutes (NHRI) manage and publicly release databases collectively known as the NHIRD. We used the NHIRD's Registry for patients with catastrophic illnesses, which provides comprehensive information on NHI enrollment and healthcare resource provision for severely ill patients who receive copayment exemptions under the NHI program. The NHIRD Registry can be integrated with several NHI databases, including claims data, NHI enrollment files, and the prescription registry.
We identified gastric cancer patients from a catastrophic illnesses dataset. The database includes the patients’ registration files and medical claims from 1996 onward and has been validated by the NHRI and released publicly in electronic form. In this cohort database, patients’ identification information is encrypted in a consistent code, linking individual claims within the database. Hence, patients’ prescriptions and diagnoses can be longitudinally followed and reviewed as long as the patients have visited clinics or hospitals in Taiwan. Because the NHIRD consists of de-identified secondary data for research, informed consent can be waived. The Institutional Review Board of Taipei Veterans General Hospital issued a formal written waiver for the need for consent (no. 2014-06-001BE).
Study Population
A retrospective matched cohort study was performed from January 1, 2000 to December 31, 2011. Based on the International Classification of Diseases, 9th revision, and Clinical Modification (ICD-9-CM) codes of gastric cancer (151.0–151.9) from discharge codes and catastrophic illness registration, we identified study cohort during the study period. The diagnosis of TB was achieved by ICD-9-CM codes (010–018). The assurance of diagnosis was accomplished by the prescription definition with at least 2 anti-TB medications for ≥28 days. The exclusion criteria included patients younger than age 20 with a diagnosis of gastric cancer, patients whose diagnosis of TB occurred before enrollment, and patients who were diagnosed as TB within 30 days of the diagnosis of gastric cancer. Diseases related to compromised immunity or lung diseases that may make a patient susceptible to TB were defined as comorbidities; these diseases therefore represent potential confounding factors. The treatment that the patients received, such as major surgery for gastric cancer, chemotherapy, and radiotherapy, was also included for further analysis.
Matched Cohort
The matched cohort was selected from the Longitudinal Health Insurance Database 2000 (LHID2000) and patients with gastric cancer were excluded. LHID2000 is a subset of the NHIRD that includes data from a random sample of approximately 1 million people alive in 2000 to represent the Taiwanese population. Each gastric cancer patient was matched with 1 randomly selected nongastric cancer subject by age, sex, and presence of comorbidities on the same index date as the diagnosis of gastric cancer. The exclusion criteria mentioned above were also used for the matched cohort. The patient selection process is demonstrated in Figure 1.
FIGURE 1.
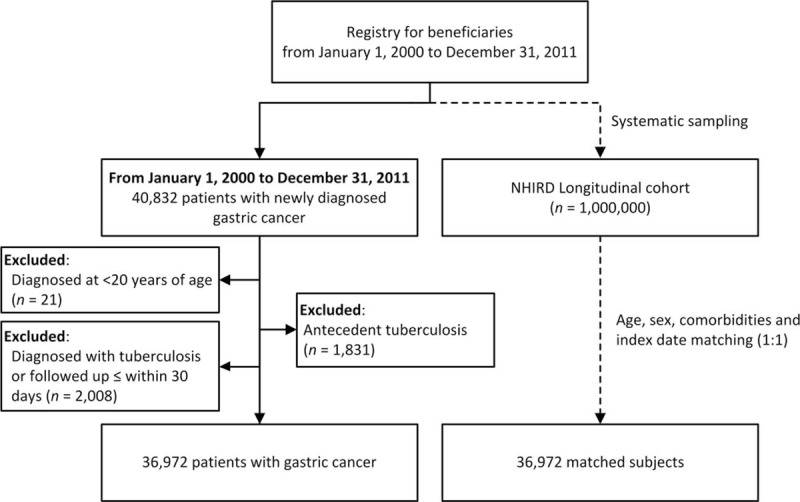
Flow chart for this study. NHIRD = National Health Insurance Research Database.
Statistical Analysis
The development of TB was the key dependent variable for this study. The follow-up of the 2 cohorts was until the date of TB diagnosis, patients’ death, dropout from NHI, or until December 31, 2011. The maximum period of follow-up was 12 years. The chi-squared test was applied for comparing the categorical variables between the 2 groups. The cumulative incidence was analyzed by the Kaplan–Meier method. The hazard ratios (HR) and 95% confidence interval (CI) were calculated using a Cox proportional hazards model. The risk factors for TB among gastric cancer patients after adjusting confounders were performed using a multivariate Cox proportional hazards model. Mortality in the gastric cancer cohort was also analyzed. The variables such as age, sex, comorbidities, and the treatment (such as surgery, chemotherapy, or radiotherapy) were included in this model for analysis. The treatment that patients received after gastric cancer was processed as a time-dependent variable to prevent immortal time bias. Variables with P value <0.1 in the univariate analysis were further included in the model of multivariate analysis. We use the Perl programming language (version 5.12.2) to extract and compute the data. The linkage, processing, and sampling of data were performed using Microsoft SQL Server 2012 (Microsoft Corp., Redmond, WA). The software including SAS (version 9.3; SAS Institute Inc., Cary, NC) or STATA statistical software (version 12.1; StataCorp, College Station, TX) was used for data analysis. A P value <0.05 was defined as statistically significant.
RESULTS
Clinical Characteristics of the Study Population
From January 2000 to December 2011, 36,972 patients were diagnosed with gastric cancer. After excluding patients with antecedent TB (n = 1831), those who were younger than 20 years of age (n = 21), and patients who were diagnosed with TB or who were followed for ≤30 days from the diagnosis of gastric cancer (n = 2008), 36,972 patients were enrolled in the present study. The median age of the study population was 69 years (interquartile range, 56–77 years) with a male to female ratio of 1.69. Chronic pulmonary obstructive disease (32.1%) was the most common comorbidity. Among these gastric cancer patients, 66.9% had received major surgery, 52.2% had received chemotherapy, and 14.3% had received radiotherapy. Table 1 summarizes the demographic characteristics, comorbidities, and treatments of the patients with gastric cancer and the matched cohort. In patients with TB, the median time to the development of TB was 2.05 years for patients with gastric cancer and 2.89 years for the matched cohort.
TABLE 1.
Baseline Patient Characteristics of Patients With Gastric Cancer and the Matched Cohort
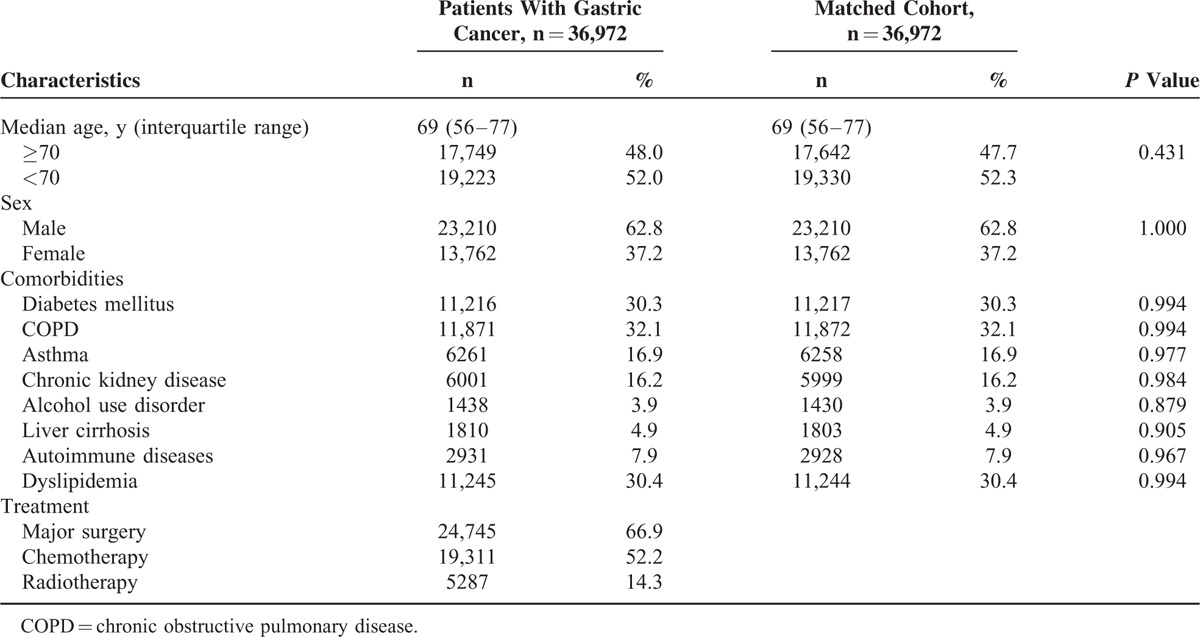
Comparisons of TB Incidence Rates Between Patients With Gastric Cancer and the Matched Cohort
As shown in Figure 2, the cumulative incidence of TB was significantly higher in the patients with gastric cancer than in the matched cohort (P < 0.001). Among the 36,972 gastric cancer patients and the 640 matched controls, TB was diagnosed for 521 patients. During the follow-up of the gastric cancer and the matched cohorts, the incidence rate of TB was 52.3 and 34.4 cases per 10,000 person-years, respectively. As shown in Table 2, after adjusting the variables including age, sex, and the comorbidities, the gastric cancer cohort exhibited a significantly higher overall risk of developing TB (adjusted HR 1.59, 95% CI 1.41–1.79, P < 0.001). The increased risk was observed in both patients ages ≥70 years (adjusted HR 1.42, 95% CI 1.23–1.64, P < 0.001) and those ages <70 years (adjusted HR 2.00, 95% CI 1.63–2.46, P < 0.001). However, the discrepancy was limited to pulmonary TB and was not observed among the extrapulmonary cases.
FIGURE 2.
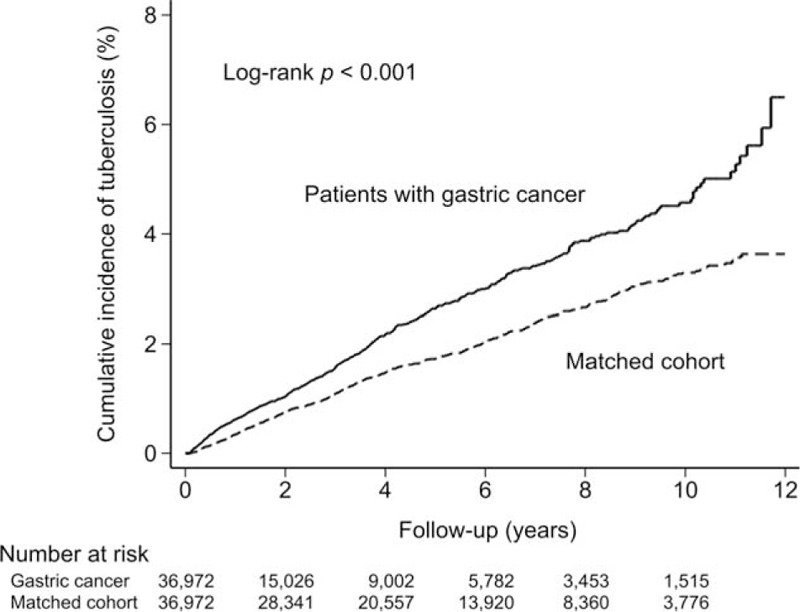
Cumulative incidence of tuberculosis in patients with gastric cancer and the matched cohort.
TABLE 2.
Incidence of Tuberculosis in Patients With Gastric Cancer and the Matched Cohort
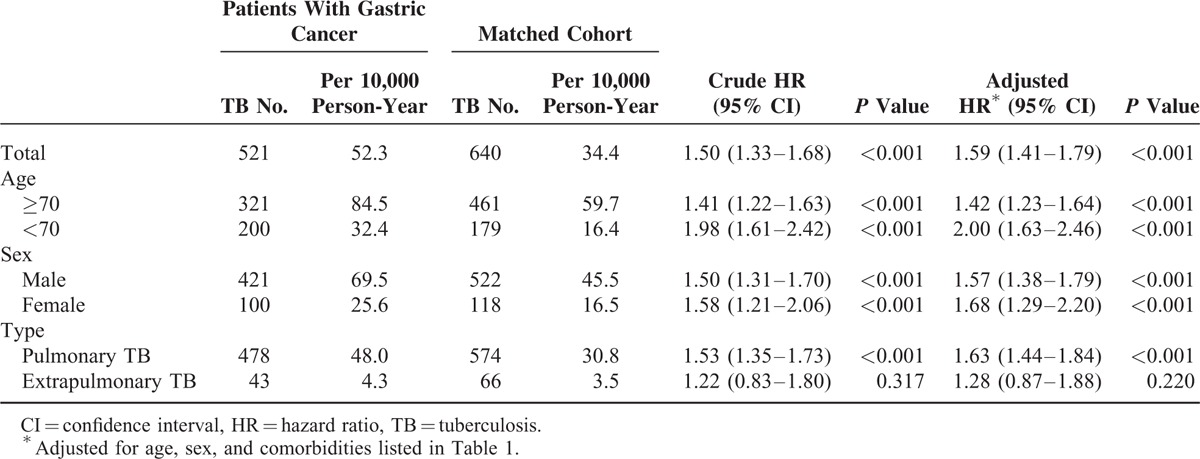
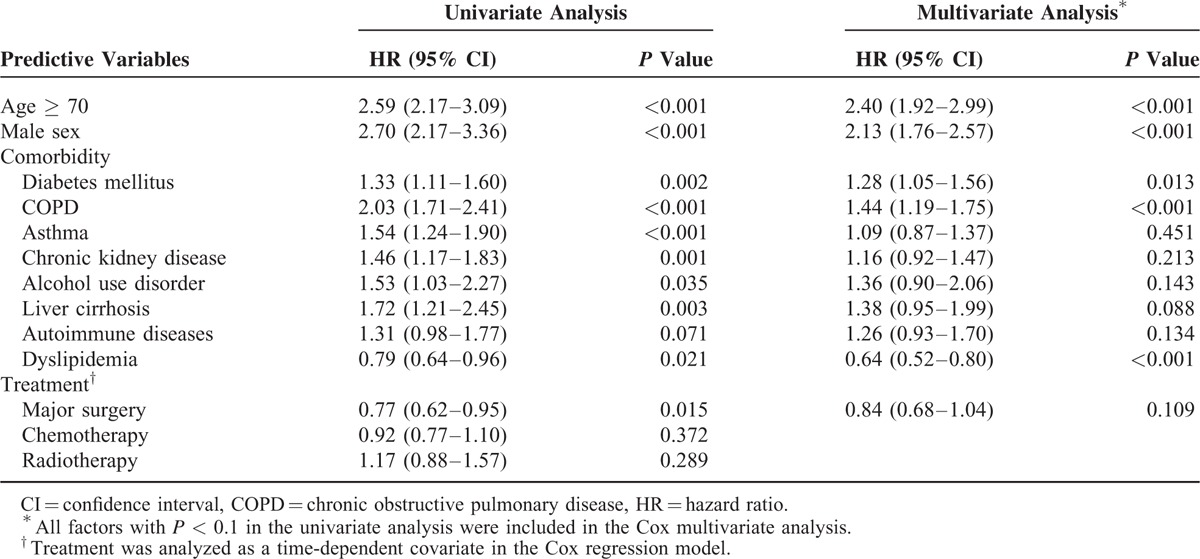
Risk Factors for TB in Patients With Gastric Cancer
We identified 521 cases in which active TB developed in the gastric cancer cohort during the follow-up period. Age, sex, comorbidities, and treatment were included for analysis in a Cox proportional hazards regression model. In addition to age and sex, major comorbidities such as diabetes mellitus, chronic obstructive pulmonary disease (COPD), asthma, chronic kidney disease, alcohol use disorder, liver cirrhosis, autoimmune disease, dyslipidemia, and major surgery were included in the multivariate analysis. Independent risk factors were old age (HR 2.40, 95% CI 1.92–2.99, P < 0.001), male sex (HR 2.13, 95% CI 1.76–2.57, P < 0.001), diabetes mellitus (HR 1.28, 95% CI 1.05–1.56, P = 0.013), and COPD (HR 1.44, 95% CI 1.19–1.75, P < 0.001). However, dyslipidemia (HR 0.64, 95% CI 0.52–0.80, P < 0.001) was identified as an independent protective factor (Table 3).
TABLE 3.
Analyses of Risk Factors for Tuberculosis in Patients With Gastric Cancer
Analysis of the Long-term Outcomes of Gastric Cancer Patients
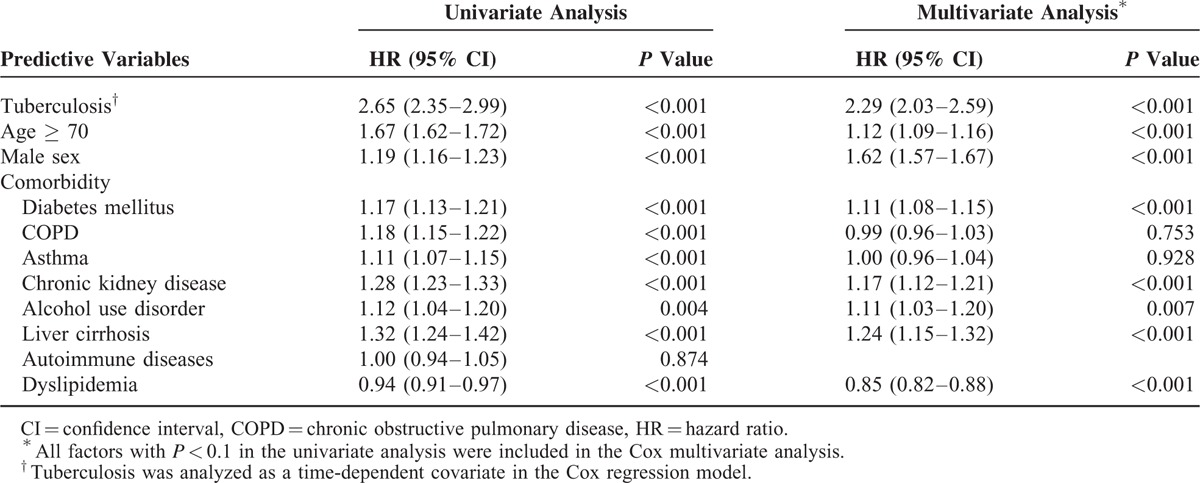
Among the 36,972 patients with gastric cancer, 17,264 (46.7%) died during the follow-up period. A mortality analysis was performed. Among the aforementioned risk factors, TB infection (HR 2.29, 95% CI 2.03–2.59, P < 0.001), age ≥ 70 years (HR 1.12, 95% CI 1.09–1.16, P < 0.001), male sex (HR 1.62, 95% CI 1.57–1.67, P < 0.001), diabetes mellitus (HR 1.11, 95% CI 1.08–1.15, P < 0.001), chronic kidney disease (HR 1.17, 95% CI 1.12–1.21, P < 0.001), alcohol use disorder (HR 1.11, 95% CI 1.03–1.20, P = 0.007), and liver cirrhosis (HR 1.24, 95% CI 1.15–1.32, P < 0.001) were identified as independent risk factors for mortality. Dyslipidemia remained an independent protective factor for mortality in gastric cancer patients (HR 0.85, 95% CI 0.82–0.88, P < 0.001; Table 4).
TABLE 4.
Risk Factors for Mortality in Patients With Gastric Cancer
DISCUSSION
This is the first population-based nationwide study to clearly demonstrate a significantly increased TB risk in patients with gastric cancer. Furthermore, old age, male sex, diabetes mellitus, and COPD were identified as independent risk factors for developing TB among patients diagnosed with gastric cancer. TB is an independent prognostic factor affecting the overall survival of gastric cancer patients. Furthermore, dyslipidemia appears to be an independent protective factor for TB in gastric cancer patients. To the best of our knowledge, this is the largest cohort study in which the correlation between TB and gastric cancer has been investigated.
TB, a notifiable disease in Taiwan, should be reported to Taiwan's CDC within 1 week of diagnosis. Patients who are suspected of having TB should be further diagnosed by TB specialists; this procedure would result in the thorough documentation of diagnosed cases of TB and their listing in the NHIRD.15 NHIRD is well suited for studying the correlation between TB and gastric cancer; therefore, we believe our results provide useful information and reliable evidence.
Old age, male sex, diabetes mellitus, and COPD were identified by multivariate analysis as independent risk factors for TB in gastric cancer patients. A novel finding of our study is that gastrectomy is not an independent risk factor for TB infection in gastric cancer patients. To date, there has been only 1 report demonstrating that in early gastric cancer, gastrectomy rather than endoscopic submucosal resection is a risk factor for TB infection.10 The nutrition status and performance status may differ between early and advanced gastric cancer, which may influence the risk of TB infection. Gastrectomy itself might not be a risk factor for TB in advanced gastric cancer patients; rather, nutrition status and performance status may be more important.
Gastric cancer patients who received gastrectomies might have greater nutrition and performance status compared with those who did not receive surgery or received chemotherapy alone. However, patients who received gastrectomies were reported to have an increased incidence of TB infection, which might be due to vitamin B12 deficiency-induced cellular immunity impairment.16 Because a randomized clinical trial cannot evaluate the effects of gastrectomy and/or chemotherapy on the incidence of TB due to ethical issues, a nationwide population-based study with an extremely large population and comprehensive clinical data might offer the best strategy to investigate this issue.
Nair et al reported that chemotherapy was not associated with the reactivation of TB,14 which is consistent with our results. In our series, the median time to develop TB infection in gastric cancer was 2 years. As a result, patients who receive chemotherapy might have more advanced gastric cancer and might not live long enough to acquire a TB infection.
In the present study, COPD and diabetes mellitus were identified as independent risk factors for developing TB in gastric cancer patients. Moreover, unlike in other series, we analyzed TB by site (pulmonary or extrapulmonary). The increased incidence appears to be limited to cases of pulmonary TB. Understandably, the lungs are the most frequent site of TB infection in gastric cancer patients with COPD, which is reported to be an independent risk factor for TB. Smoking, the most important risk factor for COPD, is known to be associated with pulmonary TB.17–19
The present study identified diabetes mellitus as another risk factors for TB, which has also been reported in previous series.20 Insight into the mechanism underlying this association might be provided by immunological studies. Interferon-gamma, which is crucial for host defense against TB, is expressed at low levels in diabetic mice infected with Mycobacterium tuberculosis.21 Alveolar macrophages are less activated in TB patients with diabetes.22 Consequently, gastric cancer patients with diabetes mellitus are more likely to exhibit decreased immunity and therefore are more likely to be infected with TB. Old age is a risk factor of TB in gastric cancer patients. Elderly patients usually have more comorbidities and worse nutrition and immunity than younger patients, and they might be more likely to develop TB.
The present study demonstrates that dyslipidemia appears to be an independent protective factor both for TB and against mortality in gastric cancer patients, which has not been previously reported. These associations require careful investigation. Madenspacher et al reported that intra-alveolar and circulating immune cells are exposed to divergent environments during dyslipidemia.23 Dyslipidemia primes systemic innate immune responses and attenuates intrapulmonary innate immunity. As a result, dyslipidemia could serve as a protective factor for TB in gastric cancer patients. One possible reason might be that some patients exhibited significant weight loss and poor nutritional status at the time of gastric cancer diagnosis. However, gastric cancer patients with dyslipidemia might exhibit a relatively better nutritional status than those without dyslipidemia and thus have a higher tolerance for gastric cancer treatment.
It has been reported that with the aid of consistent TB control strategies, including early detection of TB and establishing TB treatment under direct observation, the TB mortality rate can be reduced in TB-endemic areas.24 The novel findings in our study identify the risk factors for TB in gastric cancer patients and further confirm that TB is an independent risk factor for death in gastric cancer patients. Consequently, it is necessary to establish a screening program and treatment for TB in high-risk gastric cancer patients to improve overall survival.
This study has some limitations. First, it is possible that some of the observed effects of being male on the development of pulmonary TB were driven by the effects of smoking. However, we could not analyze the correlation between smoking, sex, and TB infection because smoking status data are not available in the NHIRD. Second, the inherent restrictions of the NHIRD allowed us to obtain only administrative data and not data on socioeconomic status, performance status, nutritional status, or other potentially relevant factors, such as family history of TB or the presence of residual scarring lesions in chest X-rays, which contribute to an increased risk of TB. Therefore, this study could not explore the relationships between these factors and the development of TB. Third, we were unable to obtain information on cancer staging from the NHIRD, and therefore could not clarify the association between the incidence of TB and stages of gastric cancer. This is an inevitable limitation of our study. Lastly, this is an observational study, and not all of the factors of interest were included; however, risk factors for TB in gastric cancer patients were identified. These findings provide valuable information and are important for the follow-up of gastric cancer.
CONCLUSIONS
This study demonstrates a higher incidence of TB infection in gastric cancer patients than in the matched cohort. TB infection is associated with worse survival in gastric cancer patients. As a result, early detection and treatment of TB are important for gastric cancer patients, and might decrease TB-related mortality. This study provides empirical support for regularly screening high-risk gastric cancer patients for TB in TB-endemic areas.
Footnotes
Abbreviations: CDC = Centers for Disease Control and Prevention, CI = confidence interval, COPD = chronic obstructive pulmonary disease, HR = hazard ratio, ICD-9-CM = International Classification of Diseases, 9th revision, and Clinical Modification, LHID2000 = Longitudinal Health Insurance Database 2000, NHI = National Health Insurance, NHIRD = National Health Insurance Research Database, NHRI = National Health Research Institutes, TB = tuberculosis.
YC and C-JL have equal contribution to the manuscript.
Those who conceived and designed the experiments are W-LF, C-JL, Y-PH, Y-TL, K-HH, M-HC, YC, S-SL, C-WW, Y-MS, and T-JC; the data were analyzed by C-JL and Y-PH. Contributions to the writing of the manuscript were made by W-LF, Y-PH, and C-JL.
This study was supported by grants from the Taipei Veterans General Hospital (V104E10-001 and V104B-023), the Taiwan Clinical Oncology Research Foundation, and the Szu-Yuan Research Foundation of Internal Medicine (No. 103024). The study is based on data from the NHIRD, provided by the Bureau of National Health Insurance, Department of Health, and managed by the NHRI. The interpretation and conclusions contained herein do not represent those of the Bureau of National Health Insurance, Department of Health, or National Health Research Institutes.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.CDC. Taiwan Tuberculosis Control Report 2013. Taiwan: Centers for Disease Control; 2015. https://www.cdc.gov.tw/uploads/files/201407/103228a0-fadd-47b0-b056-8dedda9fce1d.pdf Accessed June 4, 2015. [Google Scholar]
- 2.Donald PR, van Helden PD. The global burden of tuberculosis: combating drug resistance in difficult times. N Engl J Med 2009; 360:2393–2395. [DOI] [PubMed] [Google Scholar]
- 3.Corbett EL, Churchyard GJ, Clayton TC, et al. HIV infection and silicosis: the impact of two potent risk factors on the incidence of mycobacterial disease in South African miners. AIDS 2000; 14:2759–2768. [DOI] [PubMed] [Google Scholar]
- 4.De La Rosa GR, Jacobson KL, Rolston KV, et al. Mycobacterium tuberculosis at a comprehensive cancer centre: active disease in patients with underlying malignancy during 1990–2000. Clin Microbiol Infect 2004; 10:749–752. [DOI] [PubMed] [Google Scholar]
- 5.Nava-Aguilera E, Andersson N, Harris E, et al. Risk factors associated with recent transmission of tuberculosis: systematic review and meta-analysis. Int J Tuberc Lung Dis 2009; 13:17–26. [PubMed] [Google Scholar]
- 6.Stefan DC, Kruis AL, Schaaf HS, et al. Tuberculosis in oncology patients. Ann Trop Paediatr 2008; 28:111–116. [DOI] [PubMed] [Google Scholar]
- 7.Ibrahim EM, Uwaydah A, Al-Mulhim FA, et al. Tuberculosis in patients with malignant disease. Indian J Cancer 1989; 26:53–57. [PubMed] [Google Scholar]
- 8.Libshitz HI, Pannu HK, Elting LS, et al. Tuberculosis in cancer patients: an update. J Thorac Imaging 1997; 12:41–46. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization: Globocan 2012 Stomach Cancer: Estimated Incidence, Mortality and Prevalence Worldwide in 2012. http://globocan.iarc.fr/old/FactSheets/cancers/stomach-new.asp Accessed October 14, 2014. [Google Scholar]
- 10.Choi IJ, Kim YW, Lee HS, et al. Risk factors for tuberculosis in patients with early gastric cancer: is gastrectomy a significant risk factor for tuberculosis? Chest 2015; 26:774–783. [DOI] [PubMed] [Google Scholar]
- 11.Huang SF, Li CP, Feng JY, et al. Increased risk of tuberculosis after gastrectomy and chemotherapy in gastric cancer: a 7-year cohort study. Gastric Cancer 2011; 14:257–265. [DOI] [PubMed] [Google Scholar]
- 12.Kim CH, Im KH, Yoo SS, et al. Comparison of the incidence between tuberculosis and nontuberculous mycobacterial disease after gastrectomy. Infection 2014; 42:697–704. [DOI] [PubMed] [Google Scholar]
- 13.Kim DK, Lee SW, Yoo CG, et al. Clinical characteristics and treatment responses of tuberculosis in patients with malignancy receiving anticancer chemotherapy. Chest 2005; 128:2218–2222. [DOI] [PubMed] [Google Scholar]
- 14.Nair R, Prabhash K, Sengar M, et al. The effect of short-term intensive chemotherapy on reactivation of tuberculosis. Ann Oncol 2007; 18:1243–1245. [DOI] [PubMed] [Google Scholar]
- 15.Wang CH, Yu CT, Lin HC, et al. Hypodense alveolar macrophages in patients with diabetes mellitus and active pulmonary tuberculosis. Tuber Lung Dis 1999; 79:235–242. [DOI] [PubMed] [Google Scholar]
- 16.Nakasone E, Mato N, Nakayama M, et al. A case of pulmonary tuberculosis with false negative QuantiFERON TB-2G test. Kekkaku 2012; 87:9–13. [PubMed] [Google Scholar]
- 17.Inghammar M, Ekbom A, Engstrom G, et al. COPD and the risk of tuberculosis—a population-based cohort study. PLoS ONE 2010; 5:e10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee CH, Lee MC, Shu CC, et al. Risk factors for pulmonary tuberculosis in patients with chronic obstructive airway disease in Taiwan: a nationwide cohort study. BMC Infect Dis 2013; 13:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin HH, Ezzati M, Chang HY, et al. Association between tobacco smoking and active tuberculosis in Taiwan: prospective cohort study. Am J Respir Crit Care Med 2009; 180:475–480. [DOI] [PubMed] [Google Scholar]
- 20.Shanmuganathan R, Subramaniam ID. Clinical manifestation and risk factors of tuberculosis infection in Malaysia: case study of a community clinic. Glob J Health Sci 2015; 7:42361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alisjahbana B, van Crevel R, Sahiratmadja E, et al. Diabetes mellitus is strongly associated with tuberculosis in Indonesia. Int J Tuberc Lung Dis 2006; 10:696–700. [PubMed] [Google Scholar]
- 22.Yamashiro S, Kawakami K, Uezu K, et al. Lower expression of Th1-related cytokines and inducible nitric oxide synthase in mice with streptozotocin-induced diabetes mellitus infected with Mycobacterium tuberculosis. Clin Exp Immunol 2005; 139:57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Madenspacher JH, Draper DW, Smoak KA, et al. Dyslipidemia induces opposing effects on intrapulmonary and extrapulmonary host defense through divergent TLR response phenotypes. J Immunol 2010; 185:1660–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Didilescu C, Popescu G, Cioran N, et al. Mortality of tuberculosis in Romania: a marker for severity of the endemic. Pneumologia 2012; 61:150–152. [PubMed] [Google Scholar]


