Abstract
Colorectal cancer (CRC) is a major public health problem, and its incidence is rising in developing countries. However, studies characterizing CRC clinicopathological features in cases from developing countries are still lacking. The goal of this study was to evaluate clinicopathological and demographic features in one of the largest CRC studies in Latin America.
The study involved over 1525 CRC cases recruited in a multicenter study in Colombia between 2005 and 2014 as part of ongoing genetic and epidemiological studies. We gathered clinicopathological data such as age at diagnosis, sex, body mass index, tobacco and alcohol consumption, family history of cancer, and tumor features including location, histological type, and stage. Statistical analyses were performed to test the association between age of onset, sex, and clinical manifestations.
The average age at CRC diagnosis was 57.4 years, with 26.5% of cases having early-onset CRC (diagnosed by age 50 years). Most cases were women (53.2%; P = 0.009), 49.2% were overweight or obese, 49.1% were regular alcohol drinkers, 52% were smokers/former smokers, and 12.2% reported relatives with cancer. Most tumors in the study were located in the rectum (42.7%), were adenocarcinomas (91.5%), and had advanced stage (T3–T4, 79.8%). Comparisons by sex found that male cases were more likely to be obese (36.5% vs 31.1%; P = 0.001), less likely to have a family history of cancer (9.7% vs 15.3%; P = 0.016), and more likely to have advanced-stage tumors (83.9% vs 76.1%; P = 0.036). Comparisons by age of onset found that early-onset cases were more likely to be women (59.3% vs 51.0%; P = 0.005) and report a family history of cancer (17.4% vs 10.2%; P = 0.001).
To our knowledge, our study is the largest report of clinicopathological characterization of Hispanic CRC cases, and we suggest that further studies are needed to understand CRC etiology in diverse Hispanic populations.
Keywords: clinicopathological features, colorectal cancer (CRC), Hispanic population
1. Introduction
Colorectal cancer (CRC) is a global public health concern, with an increasing incidence and mortality rates in the developing countries.[1] Various estimates indicate that CRC incidence is increasing in countries with limited healthcare resources, particularly in Latin America and Eastern Europe.[2] Worldwide, CRC is the third leading cause of cancer mortality in men and the second in women.[3] Certain demographic features associated with the disease differ between world regions such as distribution by sex, age, and race. For example, CRC is more commonly diagnosed in older individuals and in men in most of the developed countries, its incidence is higher in African Americans, and higher CRC mortality is often associated with lower socioeconomic status.[4–6] Environmental exposures, and personal and family history of colorectal polyps[7–11] and cancer[12] are both known risk factors for CRC development. CRC outcomes depend primarily on the distribution and spread of the disease, and also early diagnosis and intervention.[13]
In Colombia, an upper-middle income country with the third largest population in Latin America, CRC is the fourth most common cause of cancer incidence and mortality with age-standardized rates (ASRs) for incidence 13.4 and 12.5 per 100,000 among men and women, respectively, and mortality ASR 7.6 and 7.0 per 100,000 for men and women, respectively.[14] These rates reflect a significant increase in the CRC incidence in Colombia when compared with previous years.[15] Furthermore, mortality trends in this country show a significant increase for both sexes.[16] Despite the associated burden, scarce data are available on clinical characteristics of CRC in Colombia and Hispanic populations, which have unique admixed American Indian, African, and European origin.[17–20] Therefore, the goal of this multicenter study was to describe the clinicopathological characteristics of a large cohort of Colombian CRC cases.[21–23] The data gathered in our study, which, to our knowledge, are the largest in Hispanics from Latin America or the United States, will be useful to support future research and to inform prevention and screening programs in these populations.
2. Materials and methods
2.1. Study population
A total of 1525 CRC-incident cases diagnosed between 2006 and 2014 were enrolled as part of ongoing CRC genetic studies in Colombia.[21–23] Cases were recruited through large cancer hospitals in 10 of largest Colombian cities (Cartagena, Santa Marta, Barranquilla, Cali, Pasto, Medellin, Bucaramanga, Bogota, Ibague, Neiva), and hence the study population is representative of most major geographical regions in the country and where most of Colombians are settled. The study adhered to the Helsinki Declaration and received IRB approvals from University of Tolima and Instituto Nacional de Cancerologia (Colombian National Cancer Institute), and also from ethic committees at the clinical centers when required (University of Tolima, Hospital Federico Lleras Acosta, Hospital Hernando Moncaleano Perdomo, Hospital Pablo Tobon Uribe, and multiple private oncology centers).
2.2. Demographic and clinical data
After providing informed consent, patients were interviewed in person by trained research nurses. A questionnaire was completed and information was collected about sociodemographic characteristics, height, weight, smoking and drinking habits, and family history of cancer. Using the questionnaire and histopathology reports, we obtained information about sex, age at diagnosis (in years), family history of cancer in first and second-degree relatives, histological subtype, degree of differentiation, tumor location (see below), presence of synchronous or metachronous colorectal and extracolorectal tumors, type of resection (right, left, rectum), number of positive lymph nodes, and the tumor, lymph node, metastasis (TNM) stage. The histopathological re-classification was performed according to the latest recommendations of the World Health Organization.[24] Tumor location was determined as proximal (cecum, appendix, ascending colon, hepatic flexure, and transverse colon), distal (splenic flexure, descending colon, and sigmoid colon), or rectal.
2.3. Statistical analyses
We stratified patients by age of diagnosis or by sex. Those with CRC by age of 50 years were classified as early-onset cases and those diagnosed with CRC >50 years were classified as having late-onset disease. Chi-square tests were used to explore associations between age of onset or sex and clinical variables. For cases with histopathology reports from surgical resections (n = 543 cases), we also explored associations between age of onset, and sex and histological variables such as tumor stage, presence or absence of lymph node metastasis, and TNM. Data were analyzed using the R software.[25]
3. Results
3.1. Demographic and clinical features of the study cohort
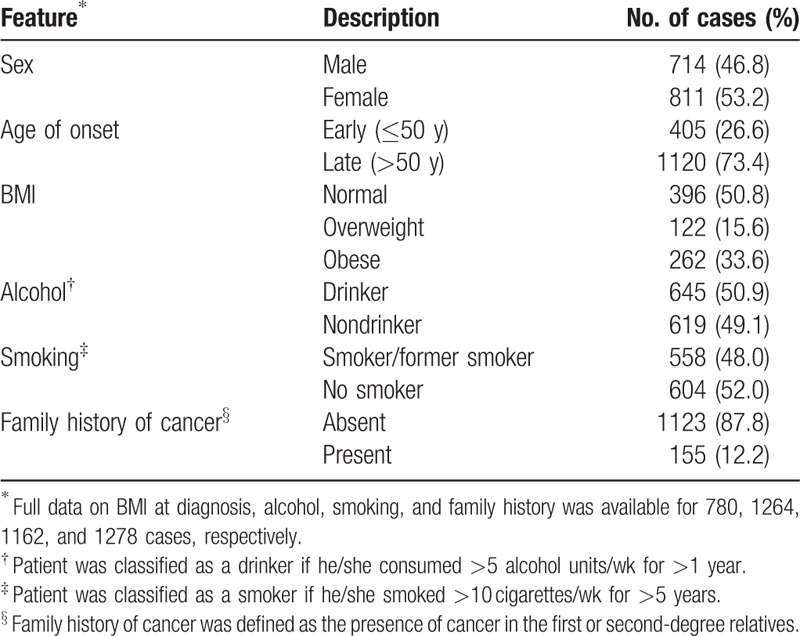
Table 1 shows the main clinical and histological characteristics of the 1525 CRC cases analyzed in our study. We found a significantly higher number of female cases than that of male cases in our study (811 female cases and 714 male cases; P = 0.009; Table 1). The average age at CRC diagnosis in the cohort was 57.4 years (range 19–75 years), with men and women having similar average age at diagnosis (57 and 58 years, respectively; data not shown). Four hundred five cases had early-onset disease and 1120 were late-onset cases. Of the late-onset cases, 37% were diagnosed between ages 51 and 60 years, and 63% >60 years (data not shown). At the time of diagnosis, 50.8% of these cases had normal body mass index (BMI, range 20–25), whereas 15.6% were overweight and 33.6% were obese. Analysis of alcohol consumption indicated that 51% regularly consumed alcohol and 49% were nondrinkers. Similarly, analysis of smoking habits indicated that 48% of these cases were smokers/former smokers, whereas 52% were nonsmokers. Of all cases, 87.8% did not report relatives with cancer, whereas 12.2% reported first-degree relatives with cancer. Out of 155 patients with cancer family history, 55 cases fulfilled the criteria for Lynch syndrome and 7 cases fulfilled the criteria for familial adenomatous polyposis[26] (data not shown).
Table 1.
Demographic and risk factor data in 1525 Colombian colorectal cancer cases.
3.2. Histological features and TNM staging
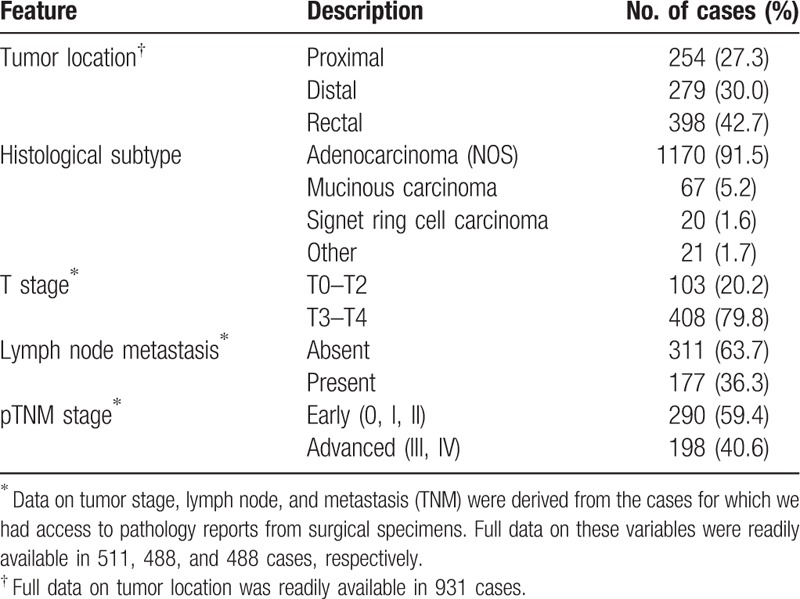
Out of all cases, information about tumor location was readily available in 931 cases and information about tumor stage in 511 cases. For 735 cases, the source of histopathological information (histopathology reports) was biopsies, whereas for 543 cases reports were available from surgical resections. Therefore, we were able to analyze TNM staging only in a subset of 488 patients with complete histopathological reports from surgical specimens. Table 2 summarizes the main clinical and histological characteristics of the tumors belonging to the participant cases in our study. Tumors in our Colombian cases occurred more frequently in the rectum (42.7% of the cases had rectal tumors compared with 30% with distal and 27.3% with tumors at the proximal location). The most common tumor type was the adenocarcinoma, diagnosed in 91.5% of the cases, followed by mucinous carcinoma in 5.2% cases and carcinoma with signet ring cells histology in 1.6% of cases. Other histological subtypes included squamous cell carcinoma and neuroendocrine tumors, which accounted for 1.7% of the cases (data not shown). We also found that 79.8% of the cases had advanced stages and 20.2% had early-stage tumors. The analysis of the lymph node metastasis, however, indicated that 63.7% reported no lymph node involvement, whereas 36.3% included involvement of 1 or more nodes. We found an average of 10.7 lymph nodes were examined per case (data not shown), in contrast to recommended examination of a minimum of 12 lymph nodes. However, for advanced rectal cancer, the number of cases with lymph nodes examined in this cohort was 42.7% and such cases were mostly treated with preoperative radiotherapy. We acknowledge that this approach can diminish the amount of lymph nodes dissected at the surgical specimen, and lymph node clearance was not done as a rule in the surgical specimen study. Table 2 also shows that, based on TNM classification, advanced tumors were diagnosed in more than 40% of all cases.
Table 2.
Clinical and histological characteristics of the Colombian cases included in the study.
3.3. Differences in clinical manifestations by the sex and age of onset
Intrigued by a higher proportion of female cases in our study, we decided to analyze the clinicopathological characteristics, stratified by sex. Table 3 shows the results of these comparisons. We found that female cases, when compared with male cases, were more likely to be diagnosed with early-onset CRC (30% vs 23%; P = 0.005), to have a normal weight at time of CRC diagnosis (55% vs 46%; P = 0.001), to report a family history of cancer (15% vs 10%; P = 0.016), and to be diagnosed with localized tumors (24% vs 16%; P = 0.036). Table 4 shows the results when analyses were stratified by age of onset (early onset vs late onset). As expected, most early-onset cases were women, who represented 59% of these cases. Early-onset cases, compared with older cases, reported more often family history of cancer (17% vs 10%; P = 0.001), had more rectal tumors (49% vs 40%; P = 0.012), were less likely to be diagnosed with proximal tumors (21% vs 30%), and had a higher fraction of mucinous or signet ring cell tumors (14% vs 5%; P < 0.001).
Table 3.
Clinical and demographic characteristics stratified by the sex of the case.
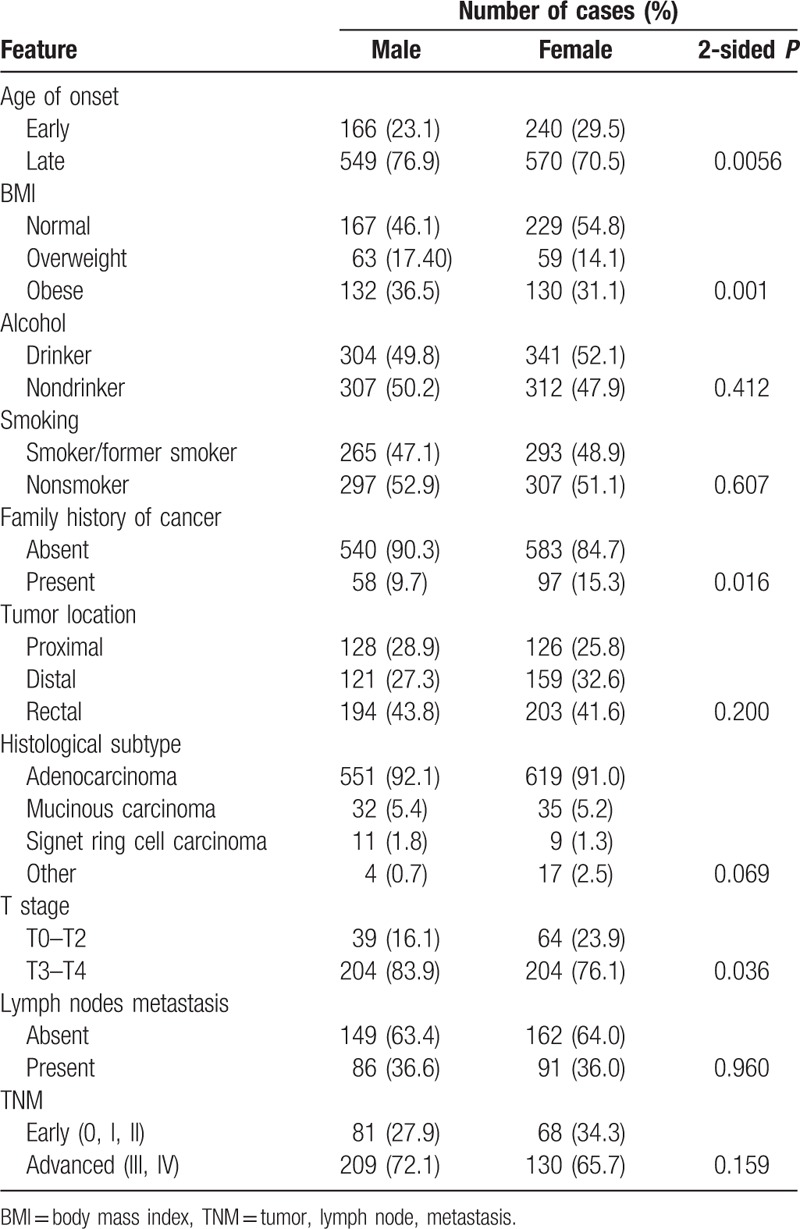
Table 4.
Clinical and demographic characteristics, stratified by the age of onset of the case.

4. Discussion
In this study, we carried out a comprehensive evaluation of the clinicopathological features in, what is, to our knowledge, the largest existing cohort of Hispanic CRC cases. The study was conducted by sampling more than 1500 incident cases from several Colombian regions, in which we collected and analyzed several demographic, clinical, and pathological variables. Our study uncovered important key features of Colombian CRC cases such as a significant proportion of individuals (26.5%) had early-onset disease and a large fraction of cases were female, had rectal tumors, and advance disease.
One of the most striking finding of the study was the presence of a higher fraction female CRC cases (53.2% women vs 46.8% men). This is in contrast to reports from developed countries, including reports on US Hispanics, where men are more often diagnosed with CRC.[3,14,27–29] Our findings are, however, consistent with previous reports suggesting a higher CRC incidence in Colombian women.[5,14] Interestingly, when we carried sex-stratified analysis, we found that compared with men, a statistically significant higher proportion of female cases had normal BMI at CRC diagnosis (55% vs 46%), diagnosed at younger age (30% female cases had early-onset CRC, compared with 23% males), and reported higher family history of cancer (15% vs 10%). Our findings of a higher proportion of female CRC cases having normal BMI are consistent with previous studies that failed to detect consistent association between obesity and CRC cases in females, in contrast to males.[30] Various genetic and environmental factors such as diet, hormonal exposure, reproductive history, and willingness to seek medical help may contribute to these sex-related disparities. However, case-control and cohort studies are clearly needed to understand these sex differences in Hispanic CRC cases from Latin America.[31,32]
Our Colombian cases were also unique in terms of age at CRC onset. The average age of CRC diagnosis in our cohort was 57.4 years, with 26.5% of cases diagnosed with early-onset disease. These findings are in contrast to cases from the developed countries where the average age at CRC diagnosis ranges from 64 to 72 years.[33] However, our findings echo the recent evidence that suggests rise in early-onset CRC cases, specifically in US minorities like Hispanics.[34,35] Interestingly, early-onset cases in our study reported a family history of cancer more often than older cases (17% vs 10%), indicating a possible role of a genetic predisposition. Indeed, we have carried small-scale genetic studies in some of these individuals and have identified carriers of mutations in highly penetrant genes.[21,23] Early-onset cases were also enriched for tumors of mucinous and signet ring subtypes (14% vs 5%), tumor types that are known to be more aggressive and have poor prognosis.[36–38] A higher fraction of early-onset cases in Colombia could partly reflect the demography of the country, which has a larger fraction of younger individuals than countries in the developed world.[39] Nonetheless, other factors such as environment, heredity, lifestyle, and ancestry, which could play a role in early-onset disease, need to be further studied in this population.[40] Our findings of a high fraction of early-onset cases and their aggressive tumor types strongly support the need to modify screening strategies to include younger Colombian individuals.[35,41] Additionally, molecular testing to screen for mutations in CRC predisposition genes could also benefit better understanding of the etiology and management of early-onset cases.
A third interesting finding of our study was the large fraction of cases with rectal tumors (42.7%). Similar to previous studies, early-onset cases were more commonly diagnosed with rectal tumors (49%).[34,42] Patients with distal and rectal CRC, in addition to presenting symptoms earlier and differences in prognosis, show different embryological origin, digestive function, and gene expression, when compared with those tumors at other locations.[43,44] As the cases in this study were not identified as a result of screening, it is likely that the high proportion of rectal and distal cases could be explained by the presence of symptoms such as bleeding and changes in bowel habits, which cause patients to consult a physician earlier.[45] The majority of our cases also had advanced stages of tumor (T3–T4, 79.8%) that could explain the high rate of mortality in countries with limited resources like Colombia.[46,47]
We also acknowledge the limitations of the study, including the fact that full data collection was not possible due to incomplete clinical records in the hospitals from this limited-resource country. Furthermore, even though all patients were in person interviewed by trained nurses, an important fraction of such interviews occurred during hospital visits when the patients could not readily provide accurate information due to time limitations for the interview or poor/difficult state of the patient. We also acknowledge that future studies should carry out a population-based approach, but given the limited number of cancer registries existing in the county, such studies are likely to be very difficult. Because our study was carried out in large cancer hospitals from the main cities, we are confident that it reflects the most common characteristics of CRC cases in Colombia. Furthermore, because medicine in Colombia is socialized, we are confident our results are representative of the main clinical characteristics of CRC cases from the entire Colombian population. Despite some of these limitations, we believe that our large study uncovered many interesting and unique clinicopathological characteristics of Colombian CRC patients that are worth following up in future clinical and epidemiological studies.
In summary, our findings, in a large sample of Hispanic patients from a multicenter study, highlight some of the key clinicopathological features of CRC cases from a developing country such as Colombia that will be helpful in further understanding the causation and designing better strategies for CRC diagnosis and management. More extensive epidemiological and molecular studies are needed to allow a better understanding of CRC etiology in this Hispanic population.
Acknowledgments
We are grateful to all of the individuals who participated in the study and to the many research nurses, study coordinators, and clinicians who helped during patient recruitment and data collection. During the preparation of this manuscript, Dr Rodrigo Prieto, our friend, colleague, and co-author passed away. This study is dedicated to his memory.
Footnotes
Abbreviations: ASR = age-standardized rates, BMI = body mass index, CRC = colorectal cancer, TNM = tumor, lymph node, metastasis.
Rodrigo Prieto, In memoriam.
Funding disclosures: The University of Tolima, Cancer Research UK, and The European Union FP7 Programme provided principal funding for the study (Grants to LGCC, ME, and IT). LGCC, MB, RP, and ME receive funding from Colciencias and the Research Office of Universidad del Tolima (Projects 50308 and 40113). LGCC receives funding from the V Foundation from Cancer Research and the National Cancer Institute (K12CA138464, R21CA199631) of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflicts of interest: The authors declare that they have no conflict of interest.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136:E359–386. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011; 61:69–90. [DOI] [PubMed] [Google Scholar]
- 3.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65:87–108. [DOI] [PubMed] [Google Scholar]
- 4.Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin 2014; 64:104–117. [DOI] [PubMed] [Google Scholar]
- 5.Cortés AB, Luis Eduardo, García Luz Stella, et al. Incidencia, mortalidad y supervivencia por cáncer colorrectal en Cali, Colombia, 1962–2012. Salud pública México 2014; 56:8. [PubMed] [Google Scholar]
- 6.Jemal A, Siegel RL, Ma J, et al. Inequalities in premature death from colorectal cancer by state. J Clin Oncol 2015; 33:829–835. [DOI] [PubMed] [Google Scholar]
- 7.Carvajal-Carmona LG, Howarth KM, Lockett M, et al. Molecular classification and genetic pathways in hyperplastic polyposis syndrome. J Pathol 2007; 212:378–385. [DOI] [PubMed] [Google Scholar]
- 8.Carvajal-Carmona LG, Zauber AG, Jones AM, et al. Much of the genetic risk of colorectal cancer is likely to be mediated through susceptibility to adenomas. Gastroenterology 2013; 144:53–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Montazeri Z, Theodoratou E, Nyiraneza C, et al. Systematic meta-analyses and field synopsis of genetic association studies in colorectal adenomas. Int J Epidemiol 2016; 45:186–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang J, Carvajal-Carmona LG, Chu JH, et al. Germline variants and advanced colorectal adenomas: adenoma prevention with celecoxib trial genome-wide association study. Clin Cancer Res 2013; 19:6430–6437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Will O, Carvajal-Carmona LG, Gorman P, et al. Homozygous PMS2 deletion causes a severe colorectal cancer and multiple adenoma phenotype without extraintestinal cancer. Gastroenterology 2007; 132:527–530. [DOI] [PubMed] [Google Scholar]
- 12.Carvajal Carmona LG, Tomlinson I. The hunting of the snark: whither genome-wide association studies for colorectal cancer? Gastroenterology 2016; 150:1528–1530. [DOI] [PubMed] [Google Scholar]
- 13.Manne U, Shanmugam C, Katkoori VR, et al. Development and progression of colorectal neoplasia. Cancer Biomark 2010; 9:235–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization. International Agency for Research on Cancer. GLOBOCAN 2012: Estimated cancer incidence, mortality and prevalence worldwide in 2012. Available at: http://globocan.iarc.fr/Pages/fact_sheets_population.aspx. Published 2012 Accessed January 2016. [Google Scholar]
- 15.Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010; 127:2893–2917. [DOI] [PubMed] [Google Scholar]
- 16.Pineros M, Gamboa O, Hernandez-Suarez G, et al. Patterns and trends in cancer mortality in Colombia 1984–2008. Cancer Epidemiol 2013; 37:233–239. [DOI] [PubMed] [Google Scholar]
- 17.Bedoya G, Montoya P, Garcia J, et al. Admixture dynamics in Hispanics: a shift in the nuclear genetic ancestry of a South American population isolate. Proc Natl Acad Sci U S A 2006; 103:7234–7239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carvajal-Carmona LG, Ophoff R, Service S, et al. Genetic demography of Antioquia (Colombia) and the Central Valley of Costa Rica. Hum Genet 2003; 112:534–541. [DOI] [PubMed] [Google Scholar]
- 19.Carvajal-Carmona LG, Soto ID, Pineda N, et al. Strong Amerind/white sex bias and a possible Sephardic contribution among the founders of a population in northwest Colombia. Am J Hum Genet 2000; 67:1287–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bravo LEC Tito, Collazos Paola, García Luz Stella, et al. Trends of cancer incidence and mortality in Cali, Colombia. 50 years experience. Colombia Médica 2012; 43:9. [PMC free article] [PubMed] [Google Scholar]
- 21.Alonso-Espinaco V, Giraldez MD, Trujillo C, et al. Novel MLH1 duplication identified in Colombian families with Lynch syndrome. Genet Med 2011; 13:155–160. [DOI] [PubMed] [Google Scholar]
- 22.Florez-Delgado N, Bohorquez ME, Mateus G, et al. Caracterización de los hallazgos histopatológicos de tumores colorrectales en pacientes del Tolima, Colombia. Revista Colombiana de Gastroenterología 2012; 27:88–95. [Google Scholar]
- 23.Velez A, Gaitan MH, Marquez JR, et al. Two novel LKB1 mutations in Colombian Peutz-Jeghers syndrome patients. Clin Genet 2009; 75:304–306. [DOI] [PubMed] [Google Scholar]
- 24.Obrocea FL, Sajin M, Marinescu EC, et al. Colorectal cancer and the 7th revision of the TNM staging system: review of changes and suggestions for uniform pathologic reporting. Rom J Morphol Embryol 2011; 52:537–544. [PubMed] [Google Scholar]
- 25.Belonogova NM, Svishcheva GR, Axenovich TI. FREGAT: an R package for region-based association analysis. Bioinformatics 2016; 32:2392–2393. [DOI] [PubMed] [Google Scholar]
- 26.Carvajal Carmona LG, Silver A, Tomlinson I. Rodriguez-Bigas MA, Cutait R, Lynch PM, et al. Molecular genetics of familial adenomatous polyposis. Hereditare Colorectal Cancer. New York: Springer; 2010. 45–66. [Google Scholar]
- 27.Siegel RL, Fedewa SA, Miller KD, et al. Cancer statistics for Hispanics/Latinos, 2015. CA: a cancer journal for clinicians 2015; 65:457–480. [DOI] [PubMed] [Google Scholar]
- 28.McPhail S, Johnson S, Greenberg D, et al. Stage at diagnosis and early mortality from cancer in England. Br J Cancer 2015; 112 suppl 1:S108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murphy G, Devesa SS, Cross AJ, et al. Sex disparities in colorectal cancer incidence by anatomic subsite, race and age. Int J Cancer 2011; 128:1668–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bardou M, Barkun AN, Martel M. Obesity and colorectal cancer. Gut 2013; 62:933–947. [DOI] [PubMed] [Google Scholar]
- 31.Kim SE, Paik HY, Yoon H, et al. Sex- and gender-specific disparities in colorectal cancer risk. World J Gastroenterol 2015; 21:5167–5175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ritvo P, Myers RE, Paszat L, et al. Gender differences in attitudes impeding colorectal cancer screening. BMC Public Health 2013; 13:500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet 2014; 383:1490–1502. [DOI] [PubMed] [Google Scholar]
- 34.Siegel RL, Jemal A, Ward EM. Increase in incidence of colorectal cancer among young men and women in the United States. Cancer Epidemiol Biomarkers Prev 2009; 18:1695–1698. [DOI] [PubMed] [Google Scholar]
- 35.Rahman R, Schmaltz C, Jackson CS, et al. Increased risk for colorectal cancer under age 50 in racial and ethnic minorities living in the United States. Cancer Med 2015; 4:1863–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hyngstrom JR, Hu CY, Xing Y, et al. Clinicopathology and outcomes for mucinous and signet ring colorectal adenocarcinoma: analysis from the National Cancer Data Base. Ann Surg Oncol 2012; 19:2814–2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park JS, Huh JW, Park YA, et al. Prognostic comparison between mucinous and nonmucinous adenocarcinoma in colorectal cancer. Medicine (Baltimore) 2015; 94:e658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Foda AA, El-Hawary AK, Aziz AA. Colorectal adenocarcinoma with mucinous component: relation of MMP-13, EGFR, and E-cadherin expressions to clinicopathological features and prognosis. APMIS 2015; 123:502–508. [DOI] [PubMed] [Google Scholar]
- 39.Ministerio de Salud y Protección Social. Envejecimiento demográfico. Colombia 1951–2020 Dinámica demográfica y estructuras poblacionales. Available at: https://http://www.minsalud.gov.co/sites/rid/Lists/BibliotecaDigital/RIDE/DE/PS/Envejecimiento-demografico-Colombia-1951–2020.pdf [Published April 2013] Accessed January 2016. [Google Scholar]
- 40.Hernandez-Suarez G, Sanabria MC, Serrano M, et al. Genetic ancestry is associated with colorectal adenomas and adenocarcinomas in Latino populations. Eur J Hum Genet 2014; 22:1208–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Inra JA, Syngal S. Colorectal cancer in young adults. Dig Dis Sci 2015; 60:722–733. [DOI] [PubMed] [Google Scholar]
- 42.Meyer JE, Narang T, Schnoll-Sussman FH, et al. Increasing incidence of rectal cancer in patients aged younger than 40 years: an analysis of the surveillance, epidemiology, and end results database. Cancer 2010; 116:4354–4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Silla IO, Rueda D, Rodriguez Y, et al. Early-onset colorectal cancer: a separate subset of colorectal cancer. World J Gastroenterol 2014; 20:17288–17296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tamas K, Walenkamp AM, de Vries EG, et al. Rectal and colon cancer: not just a different anatomic site. Cancer Treat Rev 2015; 41:671–679. [DOI] [PubMed] [Google Scholar]
- 45.Del Giudice ME, Vella ET, Hey A, et al. Systematic review of clinical features of suspected colorectal cancer in primary care. Can Fam Physician 2014; 60:e405–415. [PMC free article] [PubMed] [Google Scholar]
- 46.Instituto Nacional de Cancerología-ESE. Cancer en cifras. 2015. Available at: http://www.cancer.gov.co/content/estadisticas. Published 2015 Accessed January 2016. [Google Scholar]
- 47.Zarate AJ, Alonso FT, Garmendia ML, et al. Increasing crude and adjusted mortality rates for colorectal cancer in a developing South American country. Colorect Dis 2013; 15:47–51. [DOI] [PubMed] [Google Scholar]


