Supplemental Digital Content is available in the text
Keywords: claudication, exercice test, oximetry, peripheral artery disease, pulmonary disease, transcutaneous oxygen pressure measurements, walking capacities
Abstract
The prevalence of pulmonary disease in patients with peripheral artery disease (PAD) has not been extensively studied. Recent evidence has shown that ∼20% of the patients have an atypical chest transcutaneous oxygen pressure (TcpO2) pattern during exercise, which suggests walking-induced hypoxemia. The main objectives of this study were to: (1) describe in a retrospective way the characteristics of the patients suffering from claudication, who attended a treadmill testing in our laboratory, (2) assess the prevalence of known or unknown pulmonary disease. The second aim of this study was to evaluate the impact of the therapeutic interventions on the walking capacities, after treatment, of the eventually detected pulmonary disorders.
We retrospectively analyzed 1482 exercise TcpO2 test results. Patients that had no history of pulmonary disease, but either reported severe dyspnea or showed atypical profiles on their chest exercise-TcpO2, were advised to refer to the department of pneumology for additional investigations.
In addition to the 166 patients with a history of pulmonary disease, 158 patients were suspected of unknown pulmonary disease from the result of their TcpO2 test. Many patients (n = 99/158, 62.7%) did not attend a pulmonologist visit. A pulmonary disease was established in 55 (93.2%) of the other 59 patients. Obstructive sleep apnea syndrome (OSAS) was the one and only diagnosis retained in 42/59 patients (71.2%). Among the 47 patients who had a second evaluation of their walking capacity on treadmill, 38 had treatment of the pulmonary disease found, vascular surgery treatment or a severe restricted diet, 9 had no treatment. Only the “treated” group showed a significant improvement in the maximal walking distance on treadmill between the 2 evaluations, 313 ± 251 m to 433 ± 317 m (P = 0.03).
This retrospective pilot study underlines the high prevalence of both known and unknown pulmonary disease in patients whose primary complaint was lower limb claudication. Systematic screening and treatment of pulmonary disease in patients with claudication might be justified, to improve walking ability of such patients and possibly reduce or delay the requirement for revascularization. Prospective studies are required to confirm these preliminary results.
1. Introduction
Walking impairment is a frequent condition in elderly patients. Claudication is a walking impairment resulting in an exercise-induced lower limb pain that, is generally absent at rest, forces the patient to stop and is relieved within a few minutes when walking is stopped. Claudication is one of the first revealing symptoms of lower-limb peripheral artery disease (PAD), limits walking ability, and is frequently associated to other cardiovascular and noncardiovascular affections.[1] Among noncardiovascular diseases, the prevalence of pulmonary disease is high in patients with PAD.[1,2] In some of these patients, exercise-induced hypoxaemia can worsen exercise limitation independently of the presence or absence of exercise-induced dyspnea.[3] Specifically, different studies have shown that obstructive sleep apnea syndrome (OSAS) is responsible for an impaired exercise capacity,[4,5] with possible improvement under treatment with continuous positive airways pressure (CPAP).[6,7]
In the last 10 years, transcutaneous oxygen pressure (TcpO2) at exercise has been used routinely in our laboratory, as an interesting additional tool to the classical ultrasound and pressure measurements in patients with claudication suspected of PAD origin.[8,9] It is currently being developed in France and abroad.[10] Its specific interest is to monitor regional blood flow impairment (by the measurement of local skin oxygen pressure) throughout exercise and recovery in areas that cannot easily be investigated during exercise with classical tools. During exercise-TcpO2, 1 electrode is placed on the chest to monitor systemic arterial changes during walking.[11,12] Indeed, although absolute TcpO2 fairly correlates with arterial oxygen pressure absolute values (PO2a), relative changes over time of PO2a can be accurately detected by the analysis of chest TcpO2-changes.[13] We recently reported that the pattern of chest TcpO2 changes during walking on treadmill in PAD patients with claudication could be classified into 4 different types[13] (Fig. 1). We also showed that, for a defined patient, the classification type was reliable in test–retest procedures.[14] Typically, most patients (∼80%) show an increase in chest TcpO2 as expected from the improvement in ventilation to perfusion ratio and increased total ventilation with walking (type A and B) (Fig. 1). Some patients (∼5%) show a decrease in TcpO2 throughout exercise and a slow postexercise recovery, assumed to result from exercise-induced hypoxemia (type C) (Fig. 1). The remaining patients (∼15%) show another atypical and original pattern described as a walking-induced transient hack (WITH) (Fig. 1). The WITH profile is characterized by an abrupt fall in TcpO2 at exercise onset and a transient overshoot in the early recovery period of the treadmill test.[13] We confirmed with direct iterative arterial samplings that this WITH pattern (type D) did reflect an arterial transient fall of pO2a with an overshoot of pO2a in the recovery from exercise.[15] Pulmonary diseases associated to these C and D type patterns have not been described.
Figure 1.
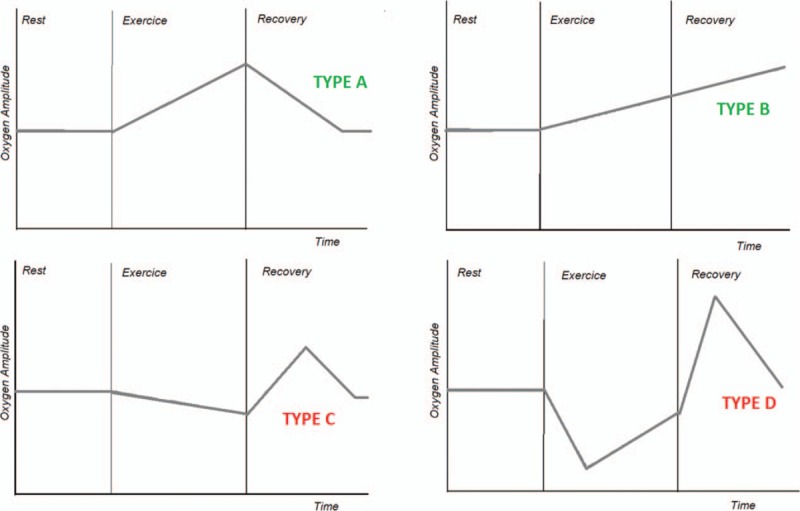
Different thoracic transcutaneous oxygen pressure (TcPO2) patterns. TcpO2 = transcutaneous oxygen pressure.
We report here a retrospective analysis of our routine procedure focusing on patients with claudication. We search for patients with either a history of pulmonary disease or, no history of pulmonary disease but severe dyspnea, or C or D atypical chest TcpO2 patterns during the treadmill test. This has allowed us to identify and report a preliminary observational case series of patients with originally known or unknown pulmonary diseases referred to our department for the investigation of claudication. The specific interest of this series is that most of these patients, primarily complaining of claudication, had no history of pulmonary disease, whereas their exercise testing showed chest-TcpO2 C or D profiles or severe dyspnea.
The main objectives of this study were to describe in a retrospective way the characteristics of the patients suffering from claudication, and to evaluate the pulmonary diseases found in the patients who attended a treadmill testing for claudication. The second aim of this study was to evaluate the impact of the therapeutic interventions on the walking capacities, after treatment of the eventually detected pulmonary disorders.
2. Patients and methods
From December 2009 to December 2013, 1482 different adult patients complaining of claudication were referred for a treadmill test, to the laboratory of vascular investigations at the University Hospital in Angers (France).
As a routine, data collected and recorded at admission before the treadmill testing included: age, gender, stature, body weight, history of chronic pulmonary disease, cardiovascular risk factors (diabetes, dyslipidemia, high blood pressure and smoking), ongoing treatments, self-estimation of maximal walking distance and an evaluation of the symptoms of claudication by the Edinburgh claudication questionnaire. Before the treadmill test, as a routine, we measured the time to walk 10 meters at a usual pace; the ankle to brachial pressure index (ABI) at rest as the lowest ankle systolic pressure divided by the highest arm systolic blood pressure; capillary glycemia and capillary hemoglobin concentration (Hemocue).
Before the treadmill test, chest TcpO2 was measured with TCM4 (TINA, radiometer, DK) after double calibration to air and with respect to a 10–15 minutes skin heating period, to attain stable resting values at 44°C of heat.
Thereafter, all patients underwent an exercise treadmill test up to maximum sustainable pain or to exhaustion at 3.2 km/h and 10% grade, in a 21+/−2°C air-conditioned room and under ECG surveillance. The TcpO2 data were recorded real time at a 1 Hz frequency for 2 minutes at rest, throughout the walking period and for at least 10 minutes in the recovery phase from exercise. Resting chest TcpO2 was the average of the values recorded throughout the resting period and the minimal chest value was the one recorded throughout the whole test (rest, exercise, or recovery). Walking duration on treadmill and maximal walking distance (MWD) were recorded for each patient. At the end of each test, the changes over time of chest values were automatically compared by cross-correlation to 4 mathematically predefined profiles as previously reported (Fig. 1).[13] Each patient was classified in the profile type that showed the highest coefficient with the pre-defined models, provided that the coefficient of correlation was superior to 0.650. Only patients that showed a decrease of TcpO2 from resting values in excess of 3 mmHg could be classified in the type C or D profile groups, to avoid misclassification due to transient artifacts.
After the treadmill test, patients unable to walk 15 minutes on treadmill and that reported severe dyspnea or that showed type C or D profiles on their chest exercise-TcpO2 and had no history of pulmonary disease, were advised that a pulmonary disease was suspected and were advised to refer to the department of pulmonary disease for additional pragmatic pulmonary investigations.
2.1. Pulmonary investigations
Pulmonary investigations were not standardized, but prescribed by the pulmonologist following an initial consultation. For those who attended this consultation, we retrieved all available data including: the Epworth sleepiness scale questionnaire; the Medical Research Council (MRC) score when evaluated, as well as functional respiratory investigations; a 6 minutes walking test (6MWT) according to the standard procedure;[16] x-rays and CT scan results. Lastly, when performed, we retrieved the results of a hospital overnight polysomnography. For the 6MWT, a significant desaturation was defined as a decrease of 4% or more from resting SaO2 over 3 minutes of the test.[17,18] The CT scan and the other pulmonary investigations were double-blind analyzed against the patient's history. Results for polysomnography were analyzed using the apnea/hypopnea index (AHI), mean saturation, and total duration of saturation below 90%.
2.2. Classification of pulmonary diseases
Pulmonary diseases were defined as follows. OSAS was defined and classified according to clinical recommendations,[19] based on the presence of both an AHI ≥ 5 per hour and either a positive A and/or B clinical criterion: OSAS was classified mild for AHI value ranging [5/h–15/h], moderate for an AHI ranging [15/h–30/h]and severe for an AHI of at least 30/h. According to French guidelines continuous positive airway pressure (CPAP) therapy was proposed to patients with either AHI≥30 or AHI≥15 and clinical symptoms of OSAS. Generally, patients with an AHI < 15/h and no clinical symptoms for OSAS were not proposed any treatment. Patients treated for OSAS were systematically offered a control at 3 months to check for exercise and objective CPAP compliance as measured by the average number of hour of use of the CPAP machine per night. Obstructive lung disease was defined by the forced expiratory volume in 1 second (FEV1)/vital capacity (VC) < 70%, chronic bronchitis defined as coughing for >3 months per year and FEV1/VC ≥70%, emphysema and diffuse interstitial lung disease were diagnosed by thoracic CT scan analysis.
2.3. Analysis of results
We retrospectively analyzed our database. As a retrospective study, the present work conforms to the declaration of Helsinki but according to French legislation does not require ethical committee approval. Statistical analysis was performed with SPSS statistics version 17.0 software. Data are expressed in number, percentage, average, and standard deviation. Student's t test was used to compare independent groups for quantitative values. Qualitative values were analyzed with the Pearson's Chi2 test. Comparison of variables before and after CPAP treatment was performed using the nonparametric Wilcoxon test. For all tests a 2-tailed P < 0.05 was used, to define a significant difference.
A video showing the different steps of the procedure is attached, http://links.lww.com/MD/B311.
3. Results
As shown in Fig. 2, among the initial 1482 patients, 324 patients (21.8%) had either a history of known chronic pulmonary disease (n = 166) or were suspected of pulmonary disease from the result of their TcpO2 chest changes (n = 158) or limiting dyspnea. Chest oximetry patterns were divided in the 4 predefined models: Type A: 668 patients (45.1%), Type B: 91 patients (6.1%), Type C: 234 patients (15.8%), Type D: 316 patients (21.3%). Data were missing for 173 patients (11.7%), which meant correlation coefficient <0.650, or nonstandard tests, or data transmission failure.
Figure 2.
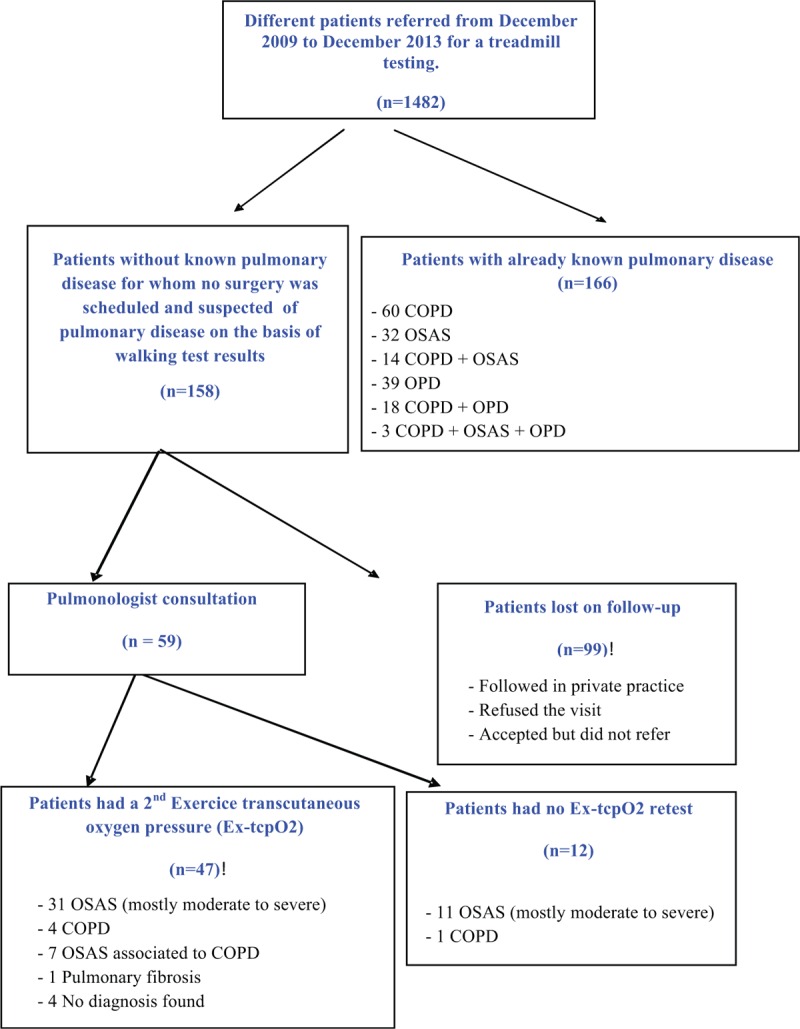
Flowchart of the studied population. COPD = chronic obstructive pulmonary disease, OPD = others pulmonary diseases including pulmonary fibrosis, pulmonary hypertension, infiltrative disease, and emphysema, OSAS = obstructive sleep apnea syndrome.
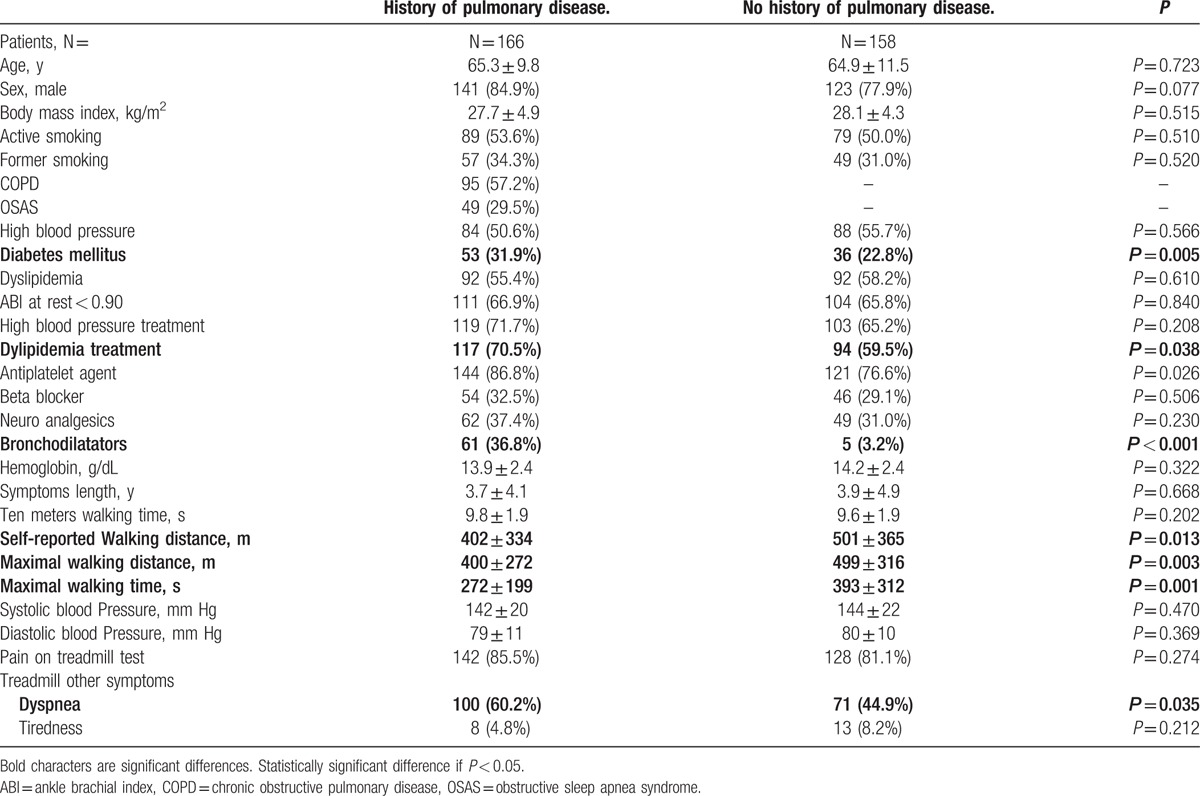
Table 1 presents the characteristics of the 324 patients with previously diagnosed (n = 166) or suspected (n = 158) pulmonary disease. There were a few statistically significant differences between the groups such as the number of patients using bronchodilators (n = 61 [36.8%] vs n = 5 [3.2%], P < 0.001) which was expected and the prevalence of diabetes mellitus which was higher in the group with previously recognized pulmonary disease (n = 53 [31.9%] vs n = 36 [22.8%], P = 0.005). Self-reported walking distance and walking speed over 10 meters were not different between the 2 groups. Patients without known history of pulmonary disease showed a significant higher maximal walking distance on treadmill (400 m ± 272 vs 499 m ± 316, P = 0.003) and a longer maximal walking time (272 s ± 199 vs 393 s ± 312, P = 0.001). As expected, we also observed that a significant proportion of patients had to stop the exercise due to dyspnea in the group of patients with a history of a pulmonary disease (n = 100 [60.2%] vs n = 71 [44.9%], P = 0.035). Notably, 5 patients of the group without self-reported pulmonary disease used broncho-dilatory drugs at the time of exercise referral. In these patients, these prescriptions were apparently made off-label for short durations by their general practitioner, based on recent or transient pulmonary symptoms.
Table 1.
Characteristics of the 324 patients with known or suspected pulmonary disease after the treadmill test.
Patients with no history of pulmonary disease, either reporting severe dyspnea on treadmill or type C or D profiles were all advised to attend a pulmonologist consultation at the Angers University Hospital and their physician received a letter confirming that a pulmonary disease was suspected. Nevertheless, many patients (n = 99, 62.7%) did not follow this recommendation, or were referred to private practitioners from whom we did not have the results of the tests, or who had largely incomplete data (no somnography, no CT scan, no spirometry…). A pulmonary disease was diagnosed in 55 (93.2%) of the 59 patients that attended the hospital pulmonologist consultation (34.8% of the 158 patients with no history of pulmonary disease). Characteristics of the patients that attended or did not attend pulmonary visit are reported online only.
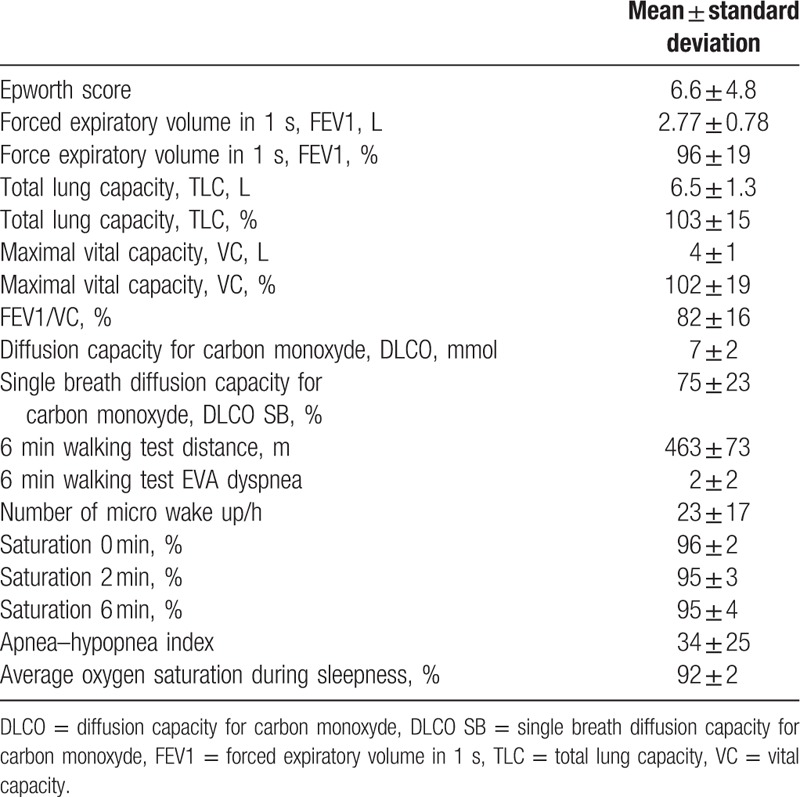
Among the 4 patients that were investigated for pulmonary disease, but for whom no pulmonary disease was found, 1 patient had his severe exercise-related hypoxemia confirmed with iterative arterial blood sampling during exercise and is suspected of right to left shunt, but remains undiagnosed to date. One patient lost 15 kg due to a calorie-restricted diet between our exercise test and the pulmonary investigations, suggesting that the restrictive pulmonary disease or sleep apnea may have disappeared, after the diet was instituted. The third patient is currently investigated for high suspicion of pulmonary arterial hypertension. The fourth patient was diagnosed with an ischemic cardiopathy after cardiac investigations. Among those who were diagnosed with pulmonary disease, OSAS was the one and only diagnosis retained in 42/59 patients (71.2%) and OSAS was associated with chronic obstructive pulmonary disease (COPD) in 7 cases (11.9%). OSAS was classified as severe in 13 patients, moderate in 31 patients, and mild in 5 patients. COPD was diagnosed as the sole disease in 5 patients (8.5%). Lastly, 1 patient was diagnosed with an interstitial lung disease (1.7%). For patients who underwent pulmonologist consultation, the results of the pulmonary investigations are shown in Table 2. The Epworth score was in average of 6.6 which is low in regards to the high prevalence of OSAS in our group. Indeed, in this group the apnea/hypopnea index was very high, with a median of 34 per hour in our study.
Table 2.
Respiratory characteristics of the 59 patients who attended the hospital pulmonologist consultation.
As a standard, patients diagnosed with moderate to severe OSAS are proposed CPAP treatment, whereas patients with COPD are prescribed adequate medications. However, despite these recommendations, 3 patients that had a mild OSAS were proposed a CPAP treatment. Average delay for instauration of CPAP was 2 months. The average observance, measured by the numbers of hours in which the CPAP was worn, was 6 hours per night. Of all patients who were referred to the pulmonary department, 12 were lost on follow up. The other patients (n = 47) had a second evaluation of their walking capacity on treadmill. The characteristics of these 2 populations are presented online only. The results of the first and second TcpO2 evaluation for self-reported walking distance and the maximal walking distance on treadmill, TcpO2 profile classification, hemoglobin concentration, heart rate at rest and at peak maximum are presented in Table 3. Walking speed on 10 meters did not change (10 meters walking time: 9.2 s vs 9.2 s, P = 0.892). Table 3 presents the results for the test duration and MWD of those patients who were “treated” and those who were “not treated” due to the absence of diagnosis (n = 4) or treatment rebuttal or in the case of mild OSAS (n = 5). Among the “treated” patients, 1 patient was treated with a vascular bypass and not for the revealed pulmonary disease, 1 patient went under a severe diet and intensive training program with a result of 10 kg weight loss (15% of initial body weight), which led to a completed disappearance of symptoms, whereas all other patients (n = 36) were treated for their pulmonary problem only (either or both with CPAP and drugs). Among patients with 2 tests, 9 patients (control group) had a second Ex-TcpO2 evaluation but no significant change in treatment or body characteristics.
Table 3.
Comparison of Ex-TcPo2 results between the first and second evaluations.
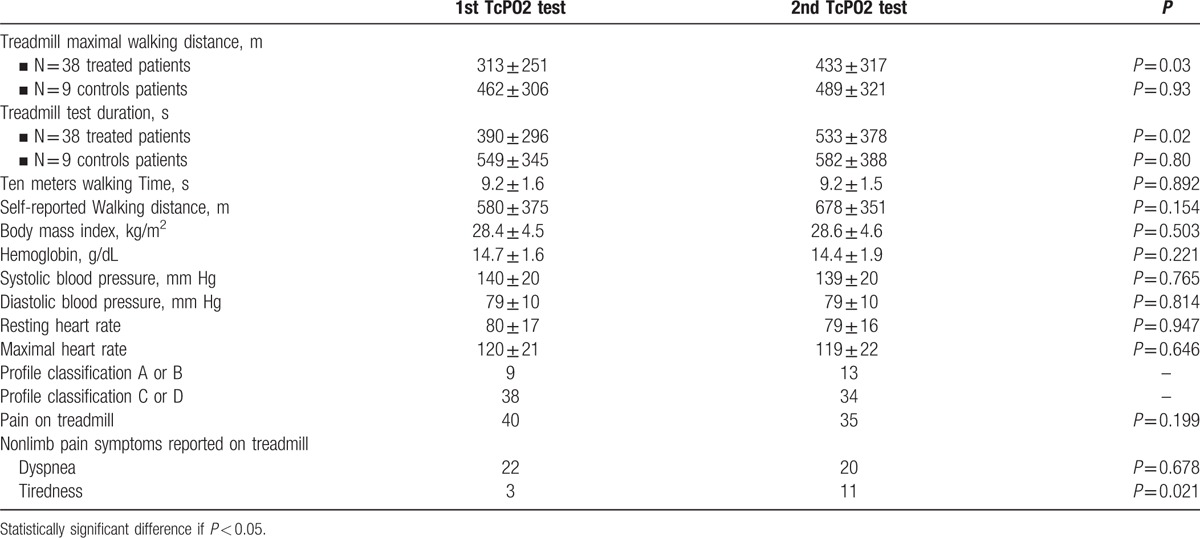
For the 36 treated patients, 1 interesting fact is that the number of patients reporting tiredness as a cause for stopping the treadmill test was increased after treatment from 3 to 11 (P = 0.021). Another interesting observation is that, in 4 patients, the chest TcpO2 pattern (initially type C or D) changed to A or B. Finally, in these treated patients MWD increased from 313 ± 251 m to 433 ± 317 m (P = 0.03), and test duration increased from 390 ± 296 s to 533 ± 378 s (P = 0.02) between test 1 and test 2. Last, we noted that the duration between the start of the CPAP and the second walking test averaged 3 months. In the control (“nontreated”) group, MWD were 462 ± 306 m vs 489 ± 321 m (P = 0.93), whereas test durations were 549 ± 345 s and 582 ± 388 s (P = 0.80), at first and second evaluations, respectively.
4. Discussion
This retrospective pilot study highlights the high prevalence of known but also unknown pulmonary disease in patients, whose primary complaint is lower limb claudication. The literature reports a 15% prevalence of pulmonary disease in patients with peripheral artery disease.[1,2] This seems close to the overall 14.8% prevalence of claudicants of our series finally diagnosed with pulmonary disease from either known respiratory diseases, or discovered pulmonary disease, after referral to pulmonary investigation due to the presence of atypical chest exercise TcpO2 pattern. We believe that the true prevalence is, in fact, much higher. Specifically, it is difficult to extrapolate any conclusions about the 99 patients that did not have pulmonary investigations, despite our advice. Nevertheless, it seems to us reasonable to believe that most of these 99 would have presented a significant prevalence of respiratory diseases. Indeed, characteristics of these patients were comparable to the 59 patients who did perform the recommended tests (see online tables). Further, we have to date no idea about the proportion of the 1158 patients with neither a history of pulmonary disease, nor atypical chest TcpO2 pattern, that may have had unknown pulmonary disease. A prospective study is ongoing to approach this latter question. One remarkable point here is that >90% of the patients that had an atypical chest-TcpO2 profile and did refer for pulmonary investigations had abnormal pulmonary investigations. Another point of interest is that a significant increase in walking distance was observed in those patients that were seen after treatment of their pulmonary disease, suggesting that their pulmonary disease did contribute to their exercise limitation. Nevertheless, because of the lack of a control group a learning effect of the treadmill procedure cannot be excluded.
Controversial results exist about whether maximal VO2 is impaired in patients with OSAS as compared to controls,[20,21] but treatment of OSAS with CPAP seems to improve exercise performance.[20] Specifically, the 3-month period between the start of the CPAP and the second walking test is possibly too short to allow an improvement from this treatment, but in all CPAP-treated patient, there was no arterial revascularization that could otherwise have been a confounding bias. Hargens et al[22] did not find any VO2 max difference but showed that the ventilatory response of normal subjects was lower than for OSAS subjects possibly lowering respiratory fatigue for a defined level of exercise. Whether OSAS treatment in our patients has decreased fatigue and improved walking ability is a fascinating but unproven hypothesis. Another possible mechanism of exercise limitation in OSAS patients is the well-documented endothelial dysfunction observed in OSAS patients, since blood flow regulation is essential to allow muscle vascular flow increase during exercise. In patients, who were treated with CPAP, the walking distance on a treadmill was significantly increased in our group. The effects of CPAP over exercise capacity have been evaluated from 7 days to 8 months,[6,7] but the underlying mechanisms of the improvement under treatment remain largely unknown. An effect on the central nervous system has been suggested and on chemoreceptors as a cause of the beneficial effect of CPAP on heart rate and arterial pressure.[23] A decrease in hemoglobin was previously reported in OSAS-treated patients >65 years old[24] although controversial results are reported likely depending on the inflammatory profile of OSAS patients.[25–28] In our case, the decrease in hemoglobin was not statistically significant.
The high prevalence of OSAS in claudicants is not necessarily surprising as PAD and OSAS share a certain number of common risk factors such as hypertension,[29,30] metabolic syndrome,[31,32] or obesity.[33] The strong prevalence of OSAS in this population referred for claudication can be explained by common patho-physiological mechanisms. Intermittent hypoxia occurring in OSAS increases sympathetic neural system activity toward peripheral blood vessels.[23] Hypoxia–reoxygenation sequences lead to the production of free radicals, systemic inflammation, and to coagulation disorders.[34] These different disorders may contribute to endothelial dysfunction and to atherosclerosis.[35] Lastly, an interesting fact is that the Epworth’ score was relatively low, which was surprising due to the very high apnea/hypopnea index found and which thus illustrates the poor sensitivity of Epworth's test.[36,37]
There are limitations to the present study.
First, we have a sex ratio with a large proportion of males, as in all our previous papers. The exact explanation for this remains unclear, but could rely on the under-diagnosis of PAD in females.
Another limitation of this study is the high number of patient who did not follow our advice to attend a consultation at the pulmonary department. Contrary to what we expected, the patients who did not want to attend a pulmonary specialist visit were surprisingly those that tend to walk slower and suffer more limitation (subject to a statistically significant dispersion).
Last and overall, the lack of control group with patients complaining claudication, but without type C or D TcpO2 profiles, does not allow comparison of the prevalence of OSAS and other pulmonary disease in this population. Whether or not those profiles are specific of underlying diseases, is unknown, to date. Nevertheless, the present study suggests that chest profiles during Ex-TcpO2 tests (and not only the presence of dyspnea) could be helpful to discover undiagnosed pulmonary disease.
5. Conclusion
The prevalence of abnormal (C or D) chest TcpO2 profile or dyspnea is high in patients with claudication but no pulmonary disease by history. Characteristic C or D chest exercise-induced TcpO2 profiles or severe dyspnea seem to be associated with a very high rate of unknown pulmonary diseases. Among these disorders, the high prevalence of moderate to severe OSAS is explained by the great number of risk factors shared in common with PAD. Although retrospective, our study suggests that CPAP treatment could be beneficial in terms of walking capacity in such patients. More studies (and specifically prospective studies) are necessary to confirm these preliminary results. Keeping in mind that as revascularization in patients with claudication is primarily aimed at improving functional capacity in PAD patients, the systematic screening and treatment of pulmonary disease (and specifically OSAS) in patients with claudication may be justified, in order to improve the walking ability of such patients and thus possibly reduce or delay the requirement for revascularization.
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: 6MWT = six minutes walking test, AHI = apnea/hypopnea index, COPD = chronic obstructive pulmonary disease, CPAP = continuous positive airway pressure, DLCO = diffusion capacity for carbon monoxyde, DLCO SB = single breath diffusion capacity for carbon monoxyde, ECG = electrocardiogram, Ex-TcpO2 = exercise transcutaneous oxygen pressure, FEV1 = forced expiratory volume in 1 second, MRC = medical research council, MWD = maximal walking distance, OPD = others pulmonary disease, OSAS = obstructive sleep apnea syndrome, PAD = peripheral arterial disease, PO2a = arterial oxygen pressure, TcpO2 = transcutaneous oxygen pressure, TLC = total lung capacity, VC = vital capacity, VO2 max = maximal oxygen uptake, WITH profile = walking-induced transient hack profile.
Funding: P Abraham benefits the support of Radiometer, Medicap, and Perimed companies for research protocols on TcpO2, but these companies had no access to the design, analysis, results, and discussion of the present study.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- 1.Von Kemp K, Van Den Brande P, Peterson T, et al. Screening for concomitant diseases in peripheral vascular patients. Results of a systematic approach. Int Angiol 1997; 16:114–122. [PubMed] [Google Scholar]
- 2.Heidrich H. Frequency of non-vascular accompanying diseases in patients with peripheral arterial disease. Vasa 2004; 33:155–158. [DOI] [PubMed] [Google Scholar]
- 3.Hamilton AL, Killian KJ, Summers E, et al. Symptom intensity and subjective limitation to exercise in patients with cardiorespiratory disorders. Chest 1996; 110:1255–1263. [DOI] [PubMed] [Google Scholar]
- 4.Oztürk LM, Metin G, Cuhadaroğlu C, et al. Cardiopulmonary responses to exercise in moderate-to-severe obstructive sleep apnea. Tuberk Toraks 2005; 53:10–19. [PubMed] [Google Scholar]
- 5.Lin CC, Hsieh WY, Chou CS, et al. Cardiopulmonary exercise testing in obstructive sleep apnea syndrome. Respir Physiol Neurobiol 2006; 25:27–34. [DOI] [PubMed] [Google Scholar]
- 6.Taguchi O, Hida W, Okabe S, et al. Improvement of exercise performance with short-term nasal continuous positive airway pressure in patients with obstructive sleep apnea. Tohoku J Exp Med 1997; 183:45–53. [DOI] [PubMed] [Google Scholar]
- 7.Maeder MT, Ammann P, Münzer T, et al. Continuous positive airway pressure improves exercise capacity and heart rate recovery in obstructive sleep apnea. Int J Cardiol 2009; 132:75–83. [DOI] [PubMed] [Google Scholar]
- 8.Huch R, Lübbers W, Huch A. Reliability of transcutaneous monitoring of arterial PO2 in newborn infants. Arch Dis Child 1974; 49:213–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Melillo E, Catapano G, Ferrari M, et al. Transcutaneous oxygen tension measurement in patients with chronic arterial obstructive disease: reliability and long-term variability of the method. Angiology 1994; 45:469–475. [DOI] [PubMed] [Google Scholar]
- 10.Mahe G, Kalra M, Abraham P, et al. Application of exercise transcutaneous oxygen pressure measurements for detection of proximal lower extremity arterial disease: a case report. Vasc Med 2015; 20:251–255. [DOI] [PubMed] [Google Scholar]
- 11.Hughes JA, Gray BJ, Hutchison DC. Changes in transcutaneous oxygen tension during exercise in pulmonary emphysema. Thorax 1984; 39:424–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McDowell JW, Thiede WH. Usefulness of the transcutaneous PO2 monitor during exercise testing in adults. Chest 1980; 78:853–855. [DOI] [PubMed] [Google Scholar]
- 13.Ouedraogo N, Feuilloy M, Mahe G, et al. Chest TcpO2 changes during constant-load treadmill walking tests in patients with claudication. Physiol Meas 2011; 32:181–194. [DOI] [PubMed] [Google Scholar]
- 14.Bouyé P, Picquet J, Abraham P, et al. Reproducibility of proximal and distal transcutaneous oxygen pressure measurements during exercise in stage 2 arterial claudication. Int Angiol 2004; 23:114–121. [PubMed] [Google Scholar]
- 15.Abraham P, Picquet J, Vielle J, et al. Transcutaneous oxygen pressure measurements on the buttocks during exercise to detect proximal arterial ischemia: comparison with arteriography. Circulation 2003; 107:1896–1900. [DOI] [PubMed] [Google Scholar]
- 16.Salzman SH. The 6-min walk test: clinical and research role, technique, coding, and reimbursement. Chest 2009; 135:1345–1352. [DOI] [PubMed] [Google Scholar]
- 17.Poulain M, Durand F, Palomba B, et al. 6-minute walk testing is more sensitive than maximal incremental cycle testing for detecting oxygen desaturation in patients with COPD. Chest 2003; 123:1401–1407. [DOI] [PubMed] [Google Scholar]
- 18.Prefaut C, Durand F, Mucci P, et al. Exercise-induced arterial hypoxaemia in athletes: a review. Sports Med 2000; 30:47–61. [DOI] [PubMed] [Google Scholar]
- 19.Elsevier Masson, Recommandations pour la Pratique Clinique. Syndrome d’apnées hypopnées obstructives du sommeil de l’adulte. SPLF-SFRMS. 2010; https://www.ncbi.nlm.nih.gov/pubmed/?term=Sleep+apnea+syndrome%3A+Clinical+practice+guidelines+housset [DOI] [PubMed] [Google Scholar]
- 20.Amann M, Romer LM, Subudhi AW, et al. Severity of arterial hypoxaemia affects the relative contributions of peripheral muscle fatigue to exercise performance in healthy humans. J Physiol 2007; 15:389–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grataloup O, Prieur F. Bussoet al. Effect of hyperoxia on maximal O2 uptake in exercise-induced arterial hypoxaemic subjects. Eur J Appl Physiol 2005; 94:641–645. [DOI] [PubMed] [Google Scholar]
- 22.Hargens TA, Guill SG, Aron A, et al. Altered ventilatory responses to exercise testing in young adult men with obstructive sleep apnea. Respir Med 2009; 103:1063–1069. [DOI] [PubMed] [Google Scholar]
- 23.Narkiewicz K, Kato M, Phillips BG, et al. Nocturnal continuous positive airway pressure decreases daytime sympathetic traffic in obstructive sleep apnea. Circulation 1999; 100:2332–2335. [DOI] [PubMed] [Google Scholar]
- 24.Khan AM, Ashizawa S, Hlebowicz V, et al. Anemia of aging and obstructive sleep apnea. Sleep Breath 2011; 15:29–34. [DOI] [PubMed] [Google Scholar]
- 25.Dyugovskaya L, Lavie P, Lavie L. Increased adhesion molecules expression and production of reactive oxygen species in leukocytes of sleep apnea patients. Am J Respir Crit Care Med 2002; 165:934–939. [DOI] [PubMed] [Google Scholar]
- 26.Fitzpatrick MF, Mackay T, Whyte KF, et al. Nocturnal desaturation and serum erythropoietin: a study in patients with chronic obstructive pulmonary disease and in normal subjects. Clin Sci (Lond) 1993; 84:319–324. [DOI] [PubMed] [Google Scholar]
- 27.Yokoe T, Minoguchi K, Matsuo H, et al. Elevated levels of C-reactive protein and interleukin-6 in patients with obstructive sleep apnea syndrome are decreased by nasal continuous positive airway pressure. Circulation 2003; 107:1129–1134. [DOI] [PubMed] [Google Scholar]
- 28.Faquin WC, Schneider TJ, Goldberg MA. Effect of inflammatory cytokines on hypoxia-induced erythropoietin production. Blood 1992; 79: 1987-1894. [PubMed] [Google Scholar]
- 29.Lavie P, Herer P, Hoffstein V. Obstructive sleep apnoea syndrome as a risk factor for hypertension: population study. BMJ 2000; 320:479–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tamisier R, Pépin JL, Rémy J, et al. 14 nights of intermittent hypoxia elevate daytime blood pressure and sympathetic activity in healthy humans. Eur Respir J 2011; 37:119–128. [DOI] [PubMed] [Google Scholar]
- 31.Grundy SM, Brewer HB, Jr, Cleeman JI, et al. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation 2004; 109:433–438. [DOI] [PubMed] [Google Scholar]
- 32.Gruber A, Horwood F, Sithole J, et al. Obstructive sleep apnoea is independently associated with the metabolic syndrome but not insulin resistance state. Cardiovasc Diabetol 2006; 5:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med 2002; 165:1217–1239. [DOI] [PubMed] [Google Scholar]
- 34.Baguet JP, Nadra M, Barone-Rochette G, et al. 41. Early cardiovascular abnormalities in newly diagnosed obstructive sleep apnea. Vasc Health Risk Manag 2009; 5:1063–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kato M, Roberts-Thomson P, Phillips BG, et al. Impairment of endothelium-dependent vasodilation of resistance vessels in patients with obstructive sleep apnea. Circulation 2000; 102:2607–2610. [DOI] [PubMed] [Google Scholar]
- 36.Johns MW. Daytime sleepiness, snoring, and obstructive sleep apnea. The Epworth Sleepiness Scale. Chest 1993; 103:30–36. [DOI] [PubMed] [Google Scholar]
- 37.Gottlieb DJ, Whitney CW, Bonekat WH, et al. Relation of sleepiness to respiratory disturbance index: the Sleep Heart Health Study. Am J Respir Crit Care Med 1999; 159:502–507. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


