Abstract
Background:
The potential association between endometriosis and physical activity (PA) has been suggested in several epidemiological studies.
We aimed to establish whether PA influences endometriosis risk.
Methods:
MEDLINE and EMBASE were searched using “physical activity” OR “exercise” combined with “endometriosis,” in Medical Subject Headings and free text. We selected original articles in English, published up to April 2016, evaluating the association between endometriosis and recent or past PA (case–control or cohort studies). References of retrieved papers were reviewed. We computed summary odds ratios (ORs) of endometriosis for recent and past PA.
Results:
Six case–control and 3 cohort studies included 3355 cases for recent PA and 4600 cases for past PA. The summary OR for endometriosis according to PA level, calculated by the random-effect model, was 0.85 [95% confidence interval (CI) 0.67–1.07] for any recent versus no PA. As compared to no recent PA, ORs for low and moderate/high PA were 1.00 (95% CI: 0.68–1.28) and 0.75 (95% CI: 0.53–1.07), respectively.
Conclusions:
Though it suggests that PA may reduce the risk of endometriosis, this meta-analysis does not conclusively support the hypothesis. Whether our findings are really explained by the benefit of exercise at molecular and endocrine level, or related to confounding mechanisms, such as study design, choice of controls, and PA potentially improving pain, needs to be further investigated.
Keywords: endometriosis, gender medicine, meta-analysis, physical activity, risk factors, systematic review
1. Introduction
Endometriosis is an estrogen-dependent chronic inflammatory condition that affects women in their reproductive period, and is associated with pelvic pain and infertility.[1–3] This gynecologic condition has a prevalence of ∼5%, and a peak between 25 and 35 years of age. A 0.1% annual incidence has been reported among women aged 15 to 49 years. Despite the high morbidity and healthcare costs associated with this condition, the exact cause of endometriosis remains unknown, although many theories have been developed regarding the pathophysiology.[4] As estrogens fuel ectopic endometrial growth, and alterations of estrogen signaling have been associated with the disease, one of the proposed mechanism may include the influence in levels and availability of sex hormones.[5–7]
It has been hypothesized that participation in recreational or occupational physical activity (PA) may decrease estrogen levels and, with extreme exercise, reduce the frequency of ovulation. Further, PA may increase levels of sex hormone binding globulin (SHBG), which would reduce bioavailable estrogens.[8–10] Regular PA also reduces insulin resistance and hyperinsulinemia. Hyperinsulinemia may increase concentrations of estrogens through decreasing concentrations of SHBG, and may increase concentrations of insulin-like growth factor-1 (IGF-1, that can stimulate endometrial cell proliferation) through decreasing concentrations of insulin-like growth factor binding protein (IGFBP)-1.[8] Finally, regular PA seems to have protective effects on diseases that involve inflammatory processes and oxidative stress, as it increases systemic levels of antiinflammatory cytokines.[9,10]
On the basis of these biological hypotheses, in the last years several studies have analyzed the relation between PA and the risk of endometriosis.[11–19] Most studies suggested that adult PA decreases endometriosis risk, but up to date, to the best of our knowledge, no meta-analysis has been performed to critically evaluate and statistically combine the results of comparable studies.
To summarize the currently available information and to provide the estimates of the possible effect size of this association, we conducted a systematic review and a meta-analysis of epidemiological data from case–control and cohort studies, on the relation between PA and risk of endometriosis.
2. Methods
This study was undertaken in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. Ethical approval and written informed consent from patients were not necessary because our study was based on summaries and analyses of results of published studies.
2.1. Study eligibility
We carried out a literature search of all case–control and cohort studies published as original articles in English up to April 2016.
2.2. Data sources
We searched the electronic databases MEDLINE (1966 to 2016/04/12) and EMBASE (1985 to 2016/04/12) using the Medical Subject Heading (MeSH) term “physical activity” OR “exercise” OR “walking” combined with “endometriosis.” The research was run again using the same terms in free text.
2.3. Study selection
Limits for human studies were activated, as well as for English language. Furthermore, we reviewed reference lists of retrieved articles to search for other pertinent studies.
Two authors reviewed the papers and independently selected the articles eligible for the systematic review. Studies were selected for the review if they met all of the following criteria: case–control or cohort study reporting original data; diagnosis of endometriosis was clinically and/or histologically based; number or percentage of subject with and without endometriosis according to PA were provided; full-length articles, published in English.
If multiple published reports from a same study were available, we included only the one with the most detailed information.
2.4. Data abstraction
Data were extracted independently by 2 investigators and discrepancies were resolved by discussion. For each study, the following information was collected on a standard form: first author's last name; year of publication; country of origin; study design; number of subjects; age, if available; category of PA, if available; relative risks (RR) or odds ratios (OR) of endometriosis and corresponding 95% confidence intervals (CI); covariates adjusted for in the statistical analysis.
2.5. Risk of bias assessment
Study quality was independently evaluated by 2 reviewers, using the Newcastle-Ottawa quality assessment scale for case–control (selection, comparability, exposure) and cohort studies (selection, comparability, outcome). Any disagreement between 2 reviewers was resolved by the judgment of a third person.
2.6. Data synthesis
We combined the OR and RR estimates from each study, using the adjusted estimates as published. To account for adjustments in the estimates, we used log OR transformation (and corresponding standard error). We computed unadjusted ORs from the exposure distributions of cases and controls as reported in the publications if the adjusted OR was unavailable.
The inverse variance method was used to pool the relative risks. ORs and 95% CIs were calculated by using both fixed-effect and random-effect models. Since the studies included in the meta-analysis were different in many aspects, first of all the design and the population included, we chose to present the results of the random-effect model.[20] The I2 statistic was used as a measure of heterogeneity, categorized, as suggested by Higgins et al,[21] in low (25%), moderate (50%), and high (75%).
The funnel plot was used to detect small study effect.
In these studies, cases and controls could be fertile, infertile, or unselected for fertility. When available, the estimates included in the pooled analyses came from the comparison between cases and controls with the same characteristic (infertile/infertile, fertile/fertile, unselected/unselected). If unavailable, we included the estimates from the comparison with fertile controls. To avoid duplication of data, if a study had 2 control groups and did not report an overall estimate, we chose to include the fertile control group in the main analysis.
Main comparison was PA yes versus no. When the overall result was not published, we calculated the OR from the published numbers of cases and controls (crude OR). As we were authors of one of the papers,[13] original data were available and we could calculate and include the adjusted OR for PA versus not PA. Since in the cohort studies the prevalence of endometriosis was comparatively low, we calculated the ORs as estimates of RRs. A dose–effect analysis was also planned: as the cut-off values were different among studies, we compared the highest category as reported in each paper versus no PA; if a moderate level of PA was present, we included it in the low–moderate versus no PA analysis, else we included the lowest level of PA as reported. In a paper reporting several levels of PA,[19] we calculated the crude RR of endometriosis for low–moderate PA adding subjects under and in the intermediate level, and for high PA adding those over the intermediate level.
The results of the meta-analysis were also presented through cumulative meta-analysis over time: studies were added one a time according to publication year, and results summarized as each new study was added.
In some cases, the study-specific 95% CIs might slightly differ from those published in the original publications because of rounding.
2.7. Subgroup analyses
We performed an analysis in subgroups by type of controls (fertile, infertile, both or not specified). As some studies included both types of controls, we did not compute an overall estimate, to avoid duplication.
A subgroup analysis by type of observational study was initially planned, but as we retrieved just one cohort study[19] reporting current PA, we calculated the pooled estimate including and excluding this study, to evaluate if the result was significantly different. Other 2 cohort studies were retrieved,[15,17] but they only considered past PA.
Review Manager (RevMan; computer program, version 5.3; The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark, 2014) was used to analyze the data.
3. Results
Figure 1 shows the flowchart of the selection of publications. From the literature search, combining EMBASE and MEDLINE results we found 109 papers. Activating English, Human, and “not Review” as filters, we identified 60 studies. After reading abstracts, we excluded 8 case reports, 11 studies without controls, 10 reviews/guidelines, 3 papers on malignancies, and 17 on other subjects (endometriosis and infertility, endometriosis as reason for surgery, prevalence of endometriosis, bone health, etc.). Eleven papers were extracted and extensively read: 2 were excluded because they were analyses on the same group of women, 1 because information was just recorded on energetic PA during menstruation.
Figure 1.
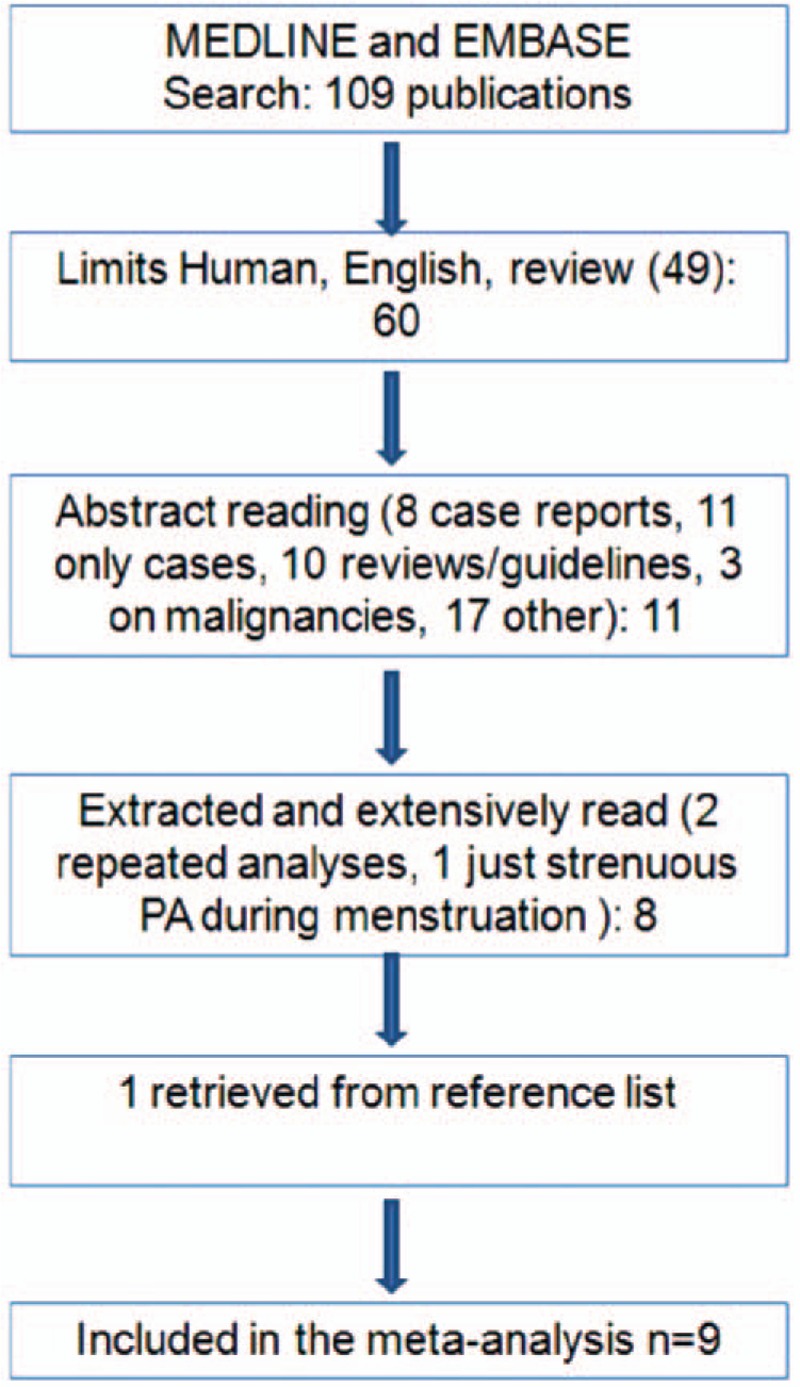
Flow chart of the selection of studies on physical activity (PA) and risk of endometriosis included in the meta-analysis.
Then, we identified 1 additional publication by reviewing the reference lists of the retrieved papers. Thus, in the present systematic review and meta-analysis, we combined data from 9 studies.[11–19] Two studies were on the same sample of women,[18,19] but they separately analyzed current[18] and past[19] PA.
Table 1 shows the main characteristics of the studies included in the present meta-analysis. Of these, 4 studies were from Europe and 5 from the United States.
Table 1.
Description of studies included in the meta-analysis of endometriosis and recreational physical activity.
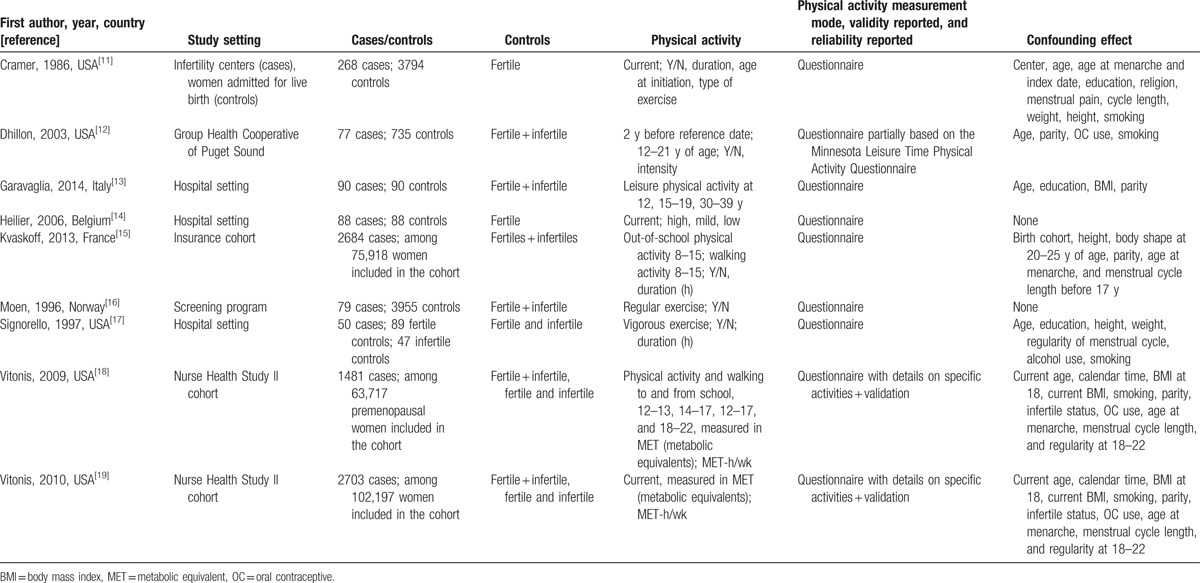
Seven studies investigated recent PA, whereas 5 also or exclusively asked information about PA during adolescence. We performed two separate meta-analyses, including a total of 3355 cases for recent exposure and 4600 cases for past exposure. PA was evaluated in several different ways: hours of PA per week,[11,13,15,17] metabolic equivalents (MET)-h/week,[18,19] author-defined low or high intensity activity.[12,14]
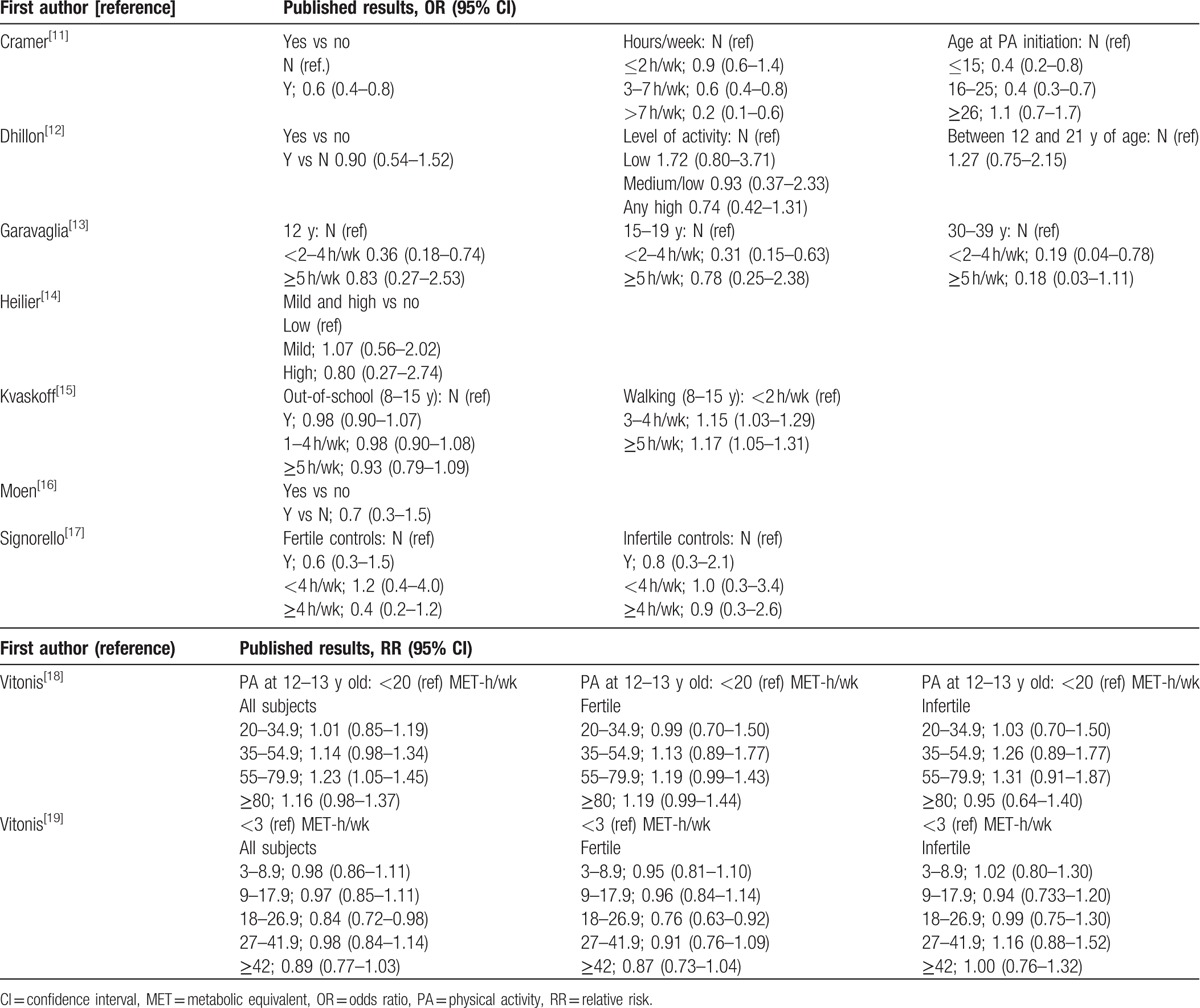
The main results of the published studies are reported in Table 2. Authors who provided adjusted estimates of OR[11–13,17] found that current PA was protective against endometriosis: results from Cramer et al[11] and Garavaglia et al[13] were statistically significant. The cohort study of current PA provided, besides overall estimates, RRs according to fertile status: a slight effect of PA was observed in women with no past or concurrent infertility, but no association emerged when considering infertile women.
Table 2.
Main results of studies included in the meta-analysis of endometriosis and recreational physical activity.
Study quality evaluation is shown in Table 3. The main limitation of all studies regarded the choice of controls, that were not representative of general population, but were extracted by hospital controls,[13,17] or infertility clinics,[11] or included in cohorts selected for profession.[15,18,19] Our search strategy did not exclude studies where cases had clinically confirmed endometriosis, but most selected papers enrolled only women with laparoscopically or surgically confirmed endometriosis as cases. The only exception was the paper by Kvaskoff et al,[15] reporting data on past PA, that included only women reporting surgically confirmed diagnosis.
Table 3.
Quality of studies according to Newcastle-Ottawa Scale.
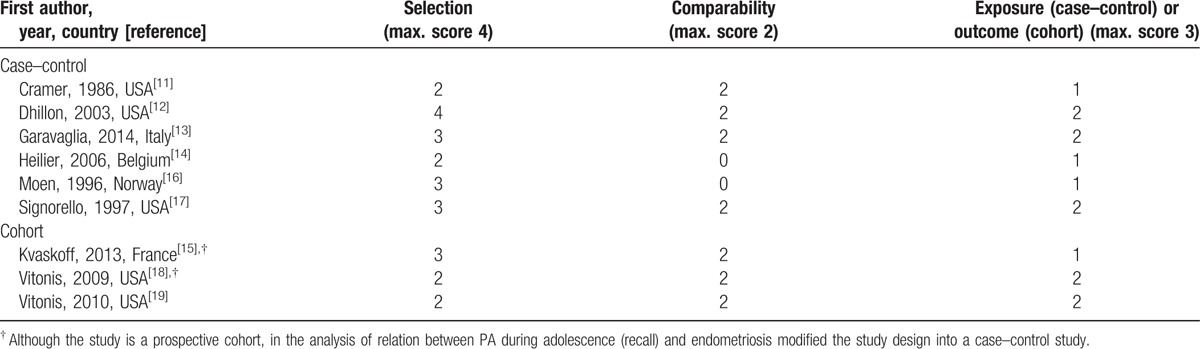
Figure 2 shows the study-specific and summary ORs of endometriosis for ever versus never PA. The summary OR was 0.85 (95% CI: 0.67–1.07) (heterogeneity Chi-square between studies = 11.12, P = 0.08). Excluding the cohort study reporting current PA, the summary OR was 0.73 (95% CI: 0.58–0.92, heterogeneity Chi-square = 3.21, P = 0.67). Rerunning the analysis, only including adjusted estimates,[11–13,17] we found an overall OR of 0.69 (95% CI: 0.53–0.89, heterogeneity Chi-square 1.69, P = 0.64). Considering I2 statistic, moderate heterogeneity was present in the overall analysis of current PA (I2 = 46%), whereas the pooled estimates both from case–control studies and adjusted ORs showed low heterogeneity (I2 = 0%).
Figure 2.
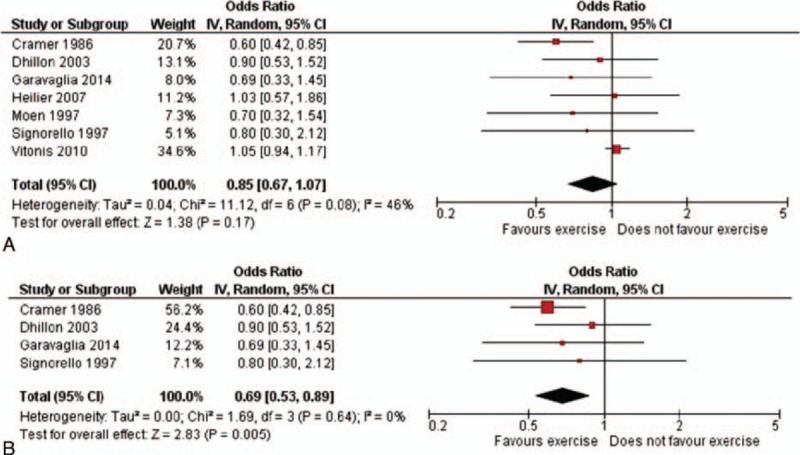
Study-specific and summary odds ratios of endometriosis for any versus no physical activity (95% confidence interval in brackets): (A) all studies; (B) only adjusted estimates.
Excluding each study in turn, the highest estimate was observed excluding Vitonis et al[19] (OR 0.73, 95% CI: 0.58–0.92), the lowest excluding Cramer et al[11] (1.02, 95% CI: 0.93–1.14). A cumulative meta-analysis (Fig. 3) showed a steadily increasing estimate over years, from 0.60 (95% CI: 0.42–0.85, estimate of Cramer et al,[11] 1986) to 0.85 (95% CI: 0.67–1.07) including the last published study.[13]
Figure 3.
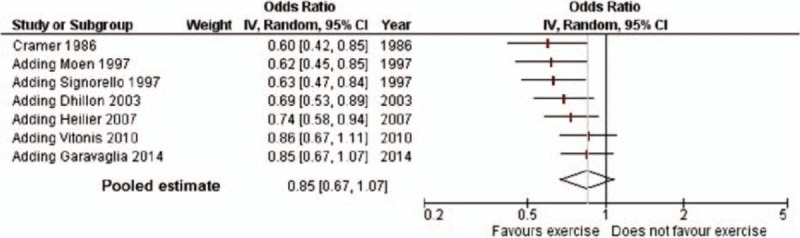
Cumulative meta-analysis of endometriosis for any versus no physical activity (95% confidence interval in brackets). Odds ratios are shown (with the corresponding 95% CI) by year of publication of subsequent reports.
Figure 4 shows the study-specific and summary ORs of low–moderate current PA (A) and high current PA (B), 1.00 (95% CI: 0.78–1.28) and 0.75 (95% CI: 0.53–1.07), respectively.
Figure 4.
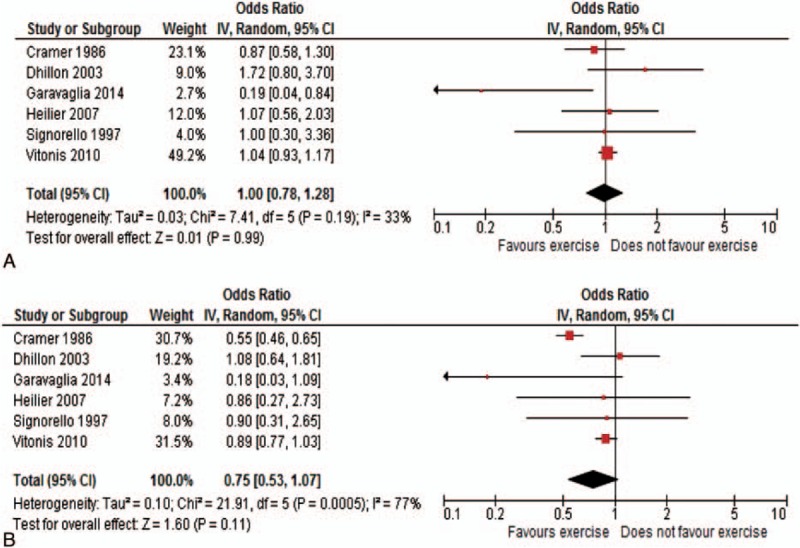
Study-specific and summary odds ratios of endometriosis for low level (A) and moderate/high level (B) versus no physical activity (95% confidence interval in brackets).
We also analyzed our data according to selection of cases. In fact, in some studies cases were selected for infertility,[11,17] or a subanalysis for infertility was provided.[18,19] Since all cases were laparoscopically or surgically confirmed, it is likely that women without infertility would undergo these procedures only if pelvic pain was severe: thus, they are different from women who underwent the evaluation for infertility problems. We summarized the information regarding type of cases in Fig. 5: the overall estimate was not calculated, since 1 study[19] was considered in both groups. We found that the OR for endometriosis in infertile women was 0.76 (95% CI: 0.51–1.12) and in fertile ones, more probably undergoing examination for pelvic pain, 1.03 (95% CI: 0.93–1.14).
Figure 5.
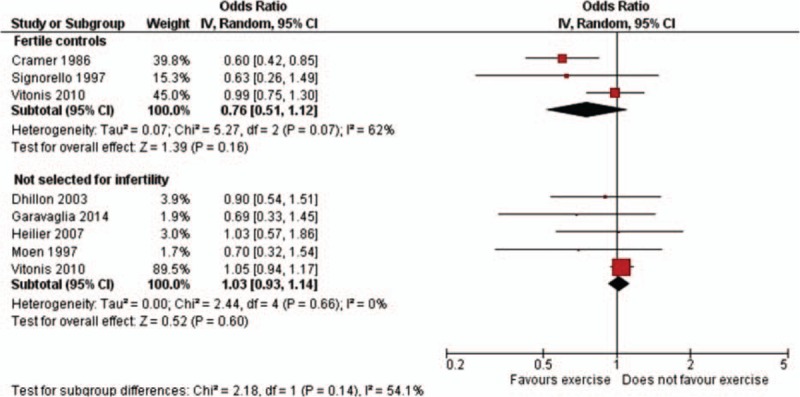
Study-specific and summary odds ratios of endometriosis for any versus no physical activity, by selection of cases (95% confidence interval in brackets).
The choice of controls affected the results as well. When the cases were infertile and controls were fertile women,[11,17] PA appeared more protective (OR 0.60, 95% CI: 0.44–0.83), whereas when controls were women not selected for fertility,[12,13,16,19] the result was not significant (OR 1.03, 95% CI: 0.93–1.14) (figure not shown).
Information about past PA was collected for different age ranges. However, since the information included at least part of adolescence years (Fig. 6), we tried to summarize it. In this analysis, the heterogeneity was significant (Chi-square 18.99, P = 0.0008); the pooled estimate was 0.81 (95% CI: 0.64–1.04): the study by Cramer et al[11] indicated a significant inverse association between PA, if started at less than 16 years, and endometriosis risk, and a similar finding emerged in Garavaglia et al,[13] whereas none of the other studies indicated such a significant effect, the study by Dhillon and Holt[12] even suggesting the opposite.
Figure 6.
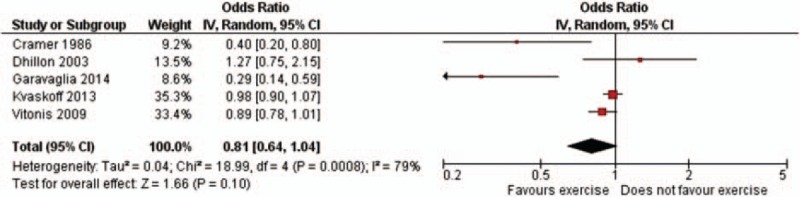
Study-specific and summary odds ratios of endometriosis for any versus no physical activity during adolescent years (95% confidence interval in brackets).
Figure 7 shows the funnel plots for current PA versus no current PA (A) and past PA versus no past PA (B). There was no evidence for small study effect.
Figure 7.
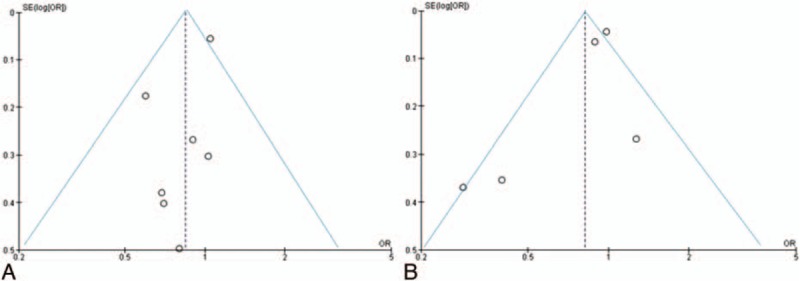
Funnel plot of studies on physical activity, current (A) and past (B), and risk of endometriosis. OR = odds ratio, SE = standard error.
4. Discussion
4.1. Main findings
The general results of this analysis suggest that PA may reduce the risk of endometriosis, but does not conclusively support the hypothesis. Although the pooled estimate of adjusted ORs[11–13,17] shows a significant protective effect of recent PA on endometriosis risk, the overall estimate was not statistically significant when including all retrieved articles. Studies on the association between PA during adolescence and endometriosis are inconsistent, and our meta-analysis cannot reach conclusive findings.
A relationship between PA and endometriosis risk is biologically plausible for both the inflammatory and estrogen-dependent nature of the disease.[1] Inflammation is a typical feature of endometriosis, as the presence of ectopic tissue in the peritoneal cavity is associated with overproduction of prostaglandins, cytokines, and chemokines.[1] Endometriosis is associated with statistically significantly increased levels of peritoneal interleukin (IL)-6 and IL-8.[22] The idea of exercise as a potential antiinflammatory tool is a relatively new concept.[23] Disease-related excessive production of proinflammatory cytokines might predispose to endothelial dysfunction, accelerated atherosclerosis, and metabolic disorders such as insulin resistance.[24] Conversely, myokines secreted by skeletal muscle include various cytokines such as leukemia inhibitory factor, IL-7, irisin, able to induce an antiinflammatory response.[22] It has been shown[25,26] that fewer inflammatory markers are detectable after long-term behavioral changes involving both reduced energy intake and increased physical exercise.
Moreover, PA reduces insulin resistance, thus avoiding stimulation possibly linked to hyperglycemia and hyperinsulinemia. Hyperinsulinemia may increase levels of estrogens through decreasing concentrations of SHBG, and may increase levels of IGF-1 through decreasing levels of IGFBP-1. Both estrogens and IGF-1 stimulates endometrial cell proliferation.[6,8] Finally, regular physical exercise is associated with a cumulative effect of reduction of menstrual flow and of estrogen action.[27]
In 2014, a review on endometriosis and PA[28] addressed the issue of the effects of PA on women with endometriosis, in terms of prevalence and possible therapeutic effects. The authors of this review[28] noted that the few existing studies are observational, with little or no statistical significance, but the indication of an inverse relationship between the practice of physical exercise and the risk of endometriosis may be due to the discomfort experienced by women, that prevent the practice of physical exercise. As a narrative review, among 6 reviewed papers, it included 2 that did not meet our criteria. One of them found that strenuous exercise during menstruation—but not in other periods—was associated with a 2-fold risk of endometriosis[29]; the other paper focused on effects of PA on endometriosis related pain and interaction between painkillers and PA,[30] concluding that taking painkillers might be less effective among endometriosis patients performing regular daily sport activities, and, thus, it might impose them to an unnecessary burden of possible side-effects. The data considered in that review are inconclusive regarding the benefits of physical exercise as a risk factor for the disease. The authors also noted that no data existed about the potential impact of exercise on the course of the endometriosis.
Only recently, a study[31] evaluated the role of PA in women diagnosed with endometriosis. Though it focused on sleep quality and pain threshold, Nunes et al[31] considered that performing PA regularly can contribute toward relieving sleep disorders and consequently may improve the quality of sleep; therefore, patients with endometriosis should be encouraged to exercise during treatment. Even if it considers PA in women with endometriosis, this study just addresses a general effect on women's well being, rather than the role of regular exercise in relieving pelvic pain and affecting the disease course.
4.2. Strengths and limitations
In the interpretation of the association, potential confounding factors should be considered. Pelvic pain is a common reason for diagnosis of endometriosis. The protective effect of high-level PA on risk of endometriosis may be at least partially explained by the fact that women affected by pain related to endometriosis may reduce their level of activity. Otherwise, it has been showed that PA lowers pain symptoms.[32] Several mechanisms primed with aerobic exercise can lead to an important pain decrease. Through numerous neuroendocrine modifications and a modulating action on the central and peripheral nervous systems, regular activity has a large series of possible ways of interacting with the course of pain symptoms. The exercise-induced endogenous analgesia is presumed to be due to the release of endogenous opioids and growth factors and activation of (supra)spinal nociceptive inhibitory mechanisms orchestrated by the brain.[32,33] This factor may represent one of the causes of delayed endometriosis diagnosis.
Further, PA may keep the pain symptoms of endometriosis at bay. The symptoms, in fact, may be reduced by PA to an extent that the women may not present them; thus, in these women the endometriosis remains largely undiagnosed. Along this line, a previous review on endometriosis and PA[28] reported an article on beneficial effects of PA and interaction between painkillers and PA in women with endometriosis[30]: the authors concluded that taking painkillers might be less effective among endometriosis patients performing regular daily sport activities. This diagnostic bias is of course a potential major limitation of epidemiological studies on the association between PA and endometriosis risk. However, up to date, information about the effectiveness of physical exercise to reduce endometriosis-related pain is scanty, and further research is needed. Moreover, since elective endometriosis drug treatment, that is hormones, can lead to depression, investigating whether PA can control these symptoms may be an interesting line of research.
Even if PA may prevent the current symptoms (mainly pain) of endometriosis, we are unable to conclude that it prevents the development and progression of the disease. In order to take into account this point, we have analyzed the role of PA during adolescence, thus, probably, before the onset of the disease. Unfortunately, studies on the association between PA during adolescence and endometriosis are inconsistent, and our meta-analysis cannot reach conclusive findings.
Further, we have considered separately the role of PA on the risk of endometriosis in women with pain or infertility. Also in this case, the analysis was not conclusive, since studies which have analyzed the role of PA in women with pain and endometriosis are few.
We can assume that cases with no infertility, who underwent a laparoscopic evaluation, had experienced pelvic pain, a condition potentially preventing them from regular exercise. To test this hypothesis, Vitonis et al[19] performed an analysis on the level of activity 4 years before diagnosis: they observed a moderately protective association between PA and endometriosis, similar to that seen in the main analysis (activity levels 2 years before diagnosis). Aiming to account for this potential confounder, findings by Cramer et al[11] were adjusted for menstrual pain. On the contrary, Dhillon and Holt[12] did not consider menstrual pain for inclusion as a confounder, because it could be either in the causal pathway between PA and endometrioma, or a marker of disease. Information on pelvic pain was collected by some authors,[14,16,17] but not used to adjust OR estimates. Kvaskoff et al,[15] Garavaglia et al,[13] and Vitonis et al[18,19] did not report pain (chronic or menstrual).
Another potential limitation is that information on PA duration and intensity was not homogeneously collected. Cramer et al[11] reported the age at which regular exercise began, so that duration depended on woman's age at enrolment, and if she continued to exercise regularly (at least 1–2 h/wk) over years. If protection was due to PA duration or initiation at younger age is debatable. Dhillon and Holt[12] included women in the category of current PA = yes if they were exercising during the 2 years before the reference date, or according to PA between 12 and 21 years of age, for past PA: in this study, exercise was defined regular if performed at least 24 times per year. Information on level of PA at different ages was given in Garavaglia et al,[13] but no minimum duration was required to define PA as a “regular activity.”
Other authors[14,16,17] just recorded information on current PA and did not provide details about duration or type. Conversely, Kvaskoff et al[15] focused on past PA: walking to school or out-of-school activity, in terms of hours per week between 8 and 15 years. Finally, Vitonis et al[19] ascertained regular exercise as that performed in the last 2 years; moreover, they also analyzed cumulative PA, to estimate long-term PA effect.
Among other confounding factors, we have to consider socioeconomic status and body mass. The diagnosis of endometriosis has been shown to be more frequent among higher social class and more educated women,[2] that are also more frequently involved in leisure activities. Closer attention to health may favor the diagnosis of endometriosis, thus producing an incorrect estimation of the real association with PA. Further, PA is associated with body weight, which in turn is inversely associated with endometriosis.[6] A few studies included adjustment for these covariates,[11–13,15,17,18] whereas others[14,16] did not. Thus, we cannot totally exclude the possibility that confounding may play some role in the observed association.
Further, there are problems of reliability and validity in the evaluation of PA. Most studies used a subjective score with no quantification of total energy expenditure. Any misclassification should, however, tend to reduce the estimated association, though in a validity study[34] it has been noted a strong correlation between heavy activities reported but weak or no associations for light or moderate activities. Then, high-level PA should be consistently and reliably reported, though the categories of PA are not homogeneously classified through studies.
Lastly, a limitation of this meta-analysis was that we only searched 2 databases. With reference to other sources of bias, the funnel plots did not show any evidence of small study effect, providing further indication of the robustness of our results.
Two main methodological problems in the epidemiology of endometriosis are the selection of cases and the choice of controls. In this study, comparison between cases not selected for infertility did not show any effect of recent PA, whereas an inverse association between PA and risk of endometriosis was observed in studies comparing infertile cases and fertile or unselected controls. Controls should be representative of the source population from which the cases derive. If cases are infertile, the use of fertile controls may have led to bias. For instance, in controls with a laparoscopic evaluation and no infertility, if the possible symptoms influenced the activity levels, then the protective effect may be due not to biological mechanisms but to impairment.[18] On the other hand, the underlying causes of infertility in controls might influence the results. Moreover, the only published cohort study showed opposite results as compared to case–control ones. However, it must be considered that we have included crude results in the analysis of PA versus no PA, whereas in the original paper the adjusted RRs showed a modestly protective effect of PA on endometriosis risk.[18,19]
Endometriosis is a chronic, long-lasting condition. Thus, it is of interest to understand the role of PA in the adolescents (i.e., probably before the onset of endometriosis) or later in life. Few studies have analyzed the association between PA early in life and subsequent endometriosis risk. The large previously reported French cohort study has suggested that walking 5 or more hours a week at age 8 to 15 years slightly increases the risk of endometriosis later in life. In that study, however, no association emerged with out-of-school PA.[15] The Nurses’ Health Study II found an increased risk of endometriosis among women reporting strenuous PA at 12 to 13 years of age.[18] However, also in this study, other types of activity and activity at any other adolescent age were unrelated to the risk. Finally, no association emerged between any PA and risk of endometrioma at 12 to 21 years of age in a case–control study including 77 cases of endometriosis, conducted in the United States; in this study even high PA was not related with endometriosis risk.[12] Conversely, in an old study conducted by Cramer et al,[11] strenuous exercises started at age ≤15 years decreased endometriosis risk. A recent study[13] showed a protective effect of PA at 15 to 19 years, but it did not suggest a dose–effect relation between hours per week and risk reduction. In our estimate, PA in adolescent years was inversely related to endometriosis risk, though the CI includes 1.
Whereas single studies showed inconsistent results, in our analysis PA level showed a modest dose effect relation, with no risk reduction for low–moderate level of PA and 20% reduction (although not significant) for high-level PA. An inverse dose–effect relation was retrieved in some studies[11,12,14,17] (in the comparison with fertile controls), but in the Nurses’ Health Study II,[19] the RRs of endometriosis declined with increasing PA and then showed an irregular trend for the 2 highest levels: similar trends emerged in the comparison with fertile and infertile controls.
It has been suggested that the risk factors (as well as the pathogenesis) of pelvic and deep endometriosis may differ. We could not analyze separately the effect of PA on the risk of endometriosis in different sites, given the small number of studies reporting this information. However, in the study by Heilier et al,[14] peritoneal endometriosis and deep endometriotic nodules were separately analyzed: the authors concluded that both forms of the disease share similar patterns of risk and protective factors.
5. Conclusions
In general, curative treatment alone is not enough to promote health. Supporting healthy behavior is the main goal of health promotion, and healthy behavior is a result of a multidimensional approach that is influenced by several factors.[35] Healthy behavior is not only PA, but also a construct of mental, educational, and environmental situations that enables us to live a healthy life. In the situation of endometriosis and pain, or perception of pain, social factors and environment play an important role; thus, the social and psychological support might be of further relevance. Sedentary behavior and physical inactivity are risk factors for cancer, diabetes, and ischemic coronary heart disease. On the contrary, PA has been described as the real polypill[36]: regular exercise reduces the risk of cardiovascular events and all-cause mortality, cardiovascular disease, type II diabetes, stroke, metabolic syndrome. Regular exercise has been compared to drug intervention (hypoglycemic, lipid-lowering, antihypertensive, antithrombotic drugs), concluding that the overall risk reduction due to PA goes beyond reducing traditional cardiovascular risk factors. Moreover, exercise, and especially the contracting muscle, is a source of several drug-like molecules with beneficial effects across all ages.[32,33] In general, regular PA has preventive/therapeutic effects against most prevalent chronic diseases, and a dose-dependent effect is usually observed in the general population.
As regards endometriosis, though the present meta-analysis suggests that PA may reduce the risk, it does not conclusively support the hypothesis. Exercise probably has pleiotropic positive effects in almost every organ system potentially having myokine-mediated direct and indirect antiinflammatory effects in chronic inflammatory diseases. However, the results of this analysis seem also to depend on the study design and the choice of controls. Whether these findings are really explained by the benefit of exercise at molecular and endocrine level or related to confounding mechanisms, including the fact that PA may improve pain, needs to be further investigated. In consideration of the fact that if we wish to analyze the role of PA in the development of the endometriosis, the exposure (i.e., moderate or intense PA) should act in the early phases of the disease, interventional studies on endometriosis prevention are substantially not feasible. Case–control studies on risk factors for endometriosis should focus on collecting a detailed history of PA at all ages, and investigating potential changes of lifestyle habits due to pelvic pain. On the contrary, randomized studies identifying whether regular exercise prevents the progression of the endometriosis are feasible. Multicountry studies are further requested, in consideration of the fact that occupational or leisure PA is strongly related with other determinants of endometriosis, such as economic status, reproductive pattern, and menstrual pattern.
Footnotes
Abbreviations: CI = confidence interval, IGF = insulin growth factor, IL = interleukin, MET = metabolic equivalent, OR = odds ratio, PA = physical activity, RR = risk ratio, SHBG = sex hormone binding globulin.
Funding: This work was partially funded by Fondazione IRCCS Ca’ Granda, Ospedale Maggiore Policlinico, in the framework of the activities of PRIN (Progetti di Ricerca di Interesse Nazionale).
Authors’ contributions: PV and FP conceived the study; ER and PV led the writing of the manuscript. GR and FC participated in the article selection and data extraction; SC and ER analyzed the data; SB participated to the writing of the Discussion. All authors contributed to the study design, writing of the manuscript, and approved the final version.
Data sharing statement: Technical appendix and statistical code available from the corresponding author.
The authors have no conflicts of interest to disclose.
References
- 1.Vercellini P, Viganò P, Somigliana E, et al. Endometriosis: pathogenesis and treatment. Nat Rev Endocrinol 2014; 10:261–275. [DOI] [PubMed] [Google Scholar]
- 2.Parazzini F, Vercellini P, Pelucchi C. Giudice LC, Evers JLH, Healy DL. Endometriosis: epidemiology, and etiological factors. Endometriosis: Science and Practice. Oxford, UK: Wiley-Blackwell; 2012. 19–26. [Google Scholar]
- 3.Gylfason JT, Kristjansson KA, Sverrisdottir G, et al. Pelvic endometriosis diagnosed in an entire nation over 20 years. Am J Epidemiol 2013; 172:237–243. [DOI] [PubMed] [Google Scholar]
- 4.Viganò P, Somigliana E, Panina P, et al. Principles of phenomics in endometriosis. Hum Reprod Update 2012; 18:248–259. [DOI] [PubMed] [Google Scholar]
- 5.Giudice LC. Clinical practice. Endometriosis. N Engl J Med 2010; 362:2389–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parazzini F, Viganò P, Candiani M, et al. Diet and endometriosis risk: a literature review. Reprod Biomed Online 2013; 26:323–336. [DOI] [PubMed] [Google Scholar]
- 7.Burney RO, Talbi S, Hamilton AE, et al. Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology 2007; 148:3814–3826. [DOI] [PubMed] [Google Scholar]
- 8.Friberg E, Wallin A, Wolk A. Sucrose, high-sugar foods, and risk of endometrial cancer—a population-based cohort study. Cancer Epidemiol Biomark Prev 2011; 20:1831–1837. [DOI] [PubMed] [Google Scholar]
- 9.Colditz GA, Cannuscio CC, Frazier AL. Physical activity and reduced risk of colon cancer: implications for prevention. Cancer Cause Control 1997; 8:649–667. [DOI] [PubMed] [Google Scholar]
- 10.Wu MH, Shoji Y, Chuang PC, et al. Endometriosis: disease pathophysiology and the role of prostaglandins. Expert Rev Mol Med 2007; 9:1–20. [DOI] [PubMed] [Google Scholar]
- 11.Cramer DW, Wilson E, Stillman RJ, et al. The relation of endometriosis to menstrual characteristics, smoking, and exercise. JAMA 1986; 255:1904–1908. [PubMed] [Google Scholar]
- 12.Dhillon PK, Holt VL. Recreational physical activity and endometrioma risk. Am J Epidemiol 2003; 158:156–164. [DOI] [PubMed] [Google Scholar]
- 13.Garavaglia E, Ricci E, Chiaffarino F, et al. Leisure and occupational physical activity at different ages and risk of endometriosis. Eur J Obstet Gynecol Reprod Biol 2014; 183:104–108. [DOI] [PubMed] [Google Scholar]
- 14.Heilier JF, Donnez J, Nackers F, et al. Environmental and host-associated risk factors in endometriosis and deep endometriotic nodules: a matched case-control study. Environ Res 2007; 103:121–129. [DOI] [PubMed] [Google Scholar]
- 15.Kvaskoff M, Bijon A, Clavel-Chapelon F, et al. Childhood and adolescent exposures and the risk of endometriosis. Epidemiology 2013; 24:261–269. [DOI] [PubMed] [Google Scholar]
- 16.Moen MH, Schei B. Epidemiology of endometriosis in a Norwegian county. Acta Obstet Gynecol Scand 1997; 76:559–562. [DOI] [PubMed] [Google Scholar]
- 17.Signorello LB, Harlow BL, Cramer DW, et al. Epidemiologic determinants of endometriosis: a hospital-based case-control study. Ann Epidemiol 1997; 7:267–274. [DOI] [PubMed] [Google Scholar]
- 18.Vitonis AF, Maruti SS, Hankinson SE, et al. Adolescent physical activity and endometriosis risk. J Endometr 2009; 1:157–163. [PMC free article] [PubMed] [Google Scholar]
- 19.Vitonis AF, Hankinson SE, Hornstein MD, et al. Adult physical activity and endometriosis risk. Epidemiology 2010; 21:16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borenstein M, Hedges LV, Higgins JPT, et al. Meta-analysis. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods 2010; 1:97–111. [DOI] [PubMed] [Google Scholar]
- 21.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barcz E, Milewski Ł, Dziunycz P, et al. Peritoneal cytokines and adhesion formation in endometriosis: an inverse association with vascular endothelial growth factor concentration. Fertil Steril 2012; 97:1380.e1–1386.e1. [DOI] [PubMed] [Google Scholar]
- 23.Benatti FB, Pedersen BK. Exercise as an anti-inflammatory therapy for rheumatic diseases-myokine regulation. Nat Rev Rheumatol 2015; 11:86–97. [DOI] [PubMed] [Google Scholar]
- 24.Santoro L, D’Onofrio F, Campo S, et al. Endothelial dysfunction but not increased carotid intima-media thickness in young European women with endometriosis. Hum Reprod 2012; 27:1320–1326. [DOI] [PubMed] [Google Scholar]
- 25.Hamer M, Sabia S, Batty GD, et al. Physical activity and inflammatory markers over 10 years follow up in men and women from the Whitehall II cohort study. Circulation 2012; 126:928–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beavers KM, Hsu FC, Isom S, et al. Long-term physical activity and inflammatory biomarkers in older adults. Med Sci Sports Exerc 2010; 42:2189–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Warren MP, Perlroth NE. The effects of intense exercise on the female reproductive system. J Endocrinol 2001; 170:3–11. [DOI] [PubMed] [Google Scholar]
- 28.Bonocher CM, Montenegro ML, Rosa E, et al. Endometriosis and physical exercises: a systematic review. Reprod Biol Endocrinol 2014; 12:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han M, Pan L, Wu B, et al. A case control epidemiologic study of endometriosis. Chin Med Sci J 1994; 9:114–118. [PubMed] [Google Scholar]
- 30.Koppan A, Hamori J, Vranics I, et al. Pelvic pain in endometriosis: painkillers or sport to alleviate symptoms? Acta Physiol Hung 2010; 97:234–239. [DOI] [PubMed] [Google Scholar]
- 31.Nunes FR, Ferreira JM, Bahamondes L. Pain threshold and sleep quality in women with endometriosis. Eur J Pain 2015; 19:15–20. [DOI] [PubMed] [Google Scholar]
- 32.Nijs J, Kosek E, Van Oosterwijck J, et al. Dysfunctional endogenous analgesia during exercise in patients with chronic pain: to exercise or not to exercise? Pain Physician 2012; 15 (3 suppl):ES205–ES213. [PubMed] [Google Scholar]
- 33.Stratton P, Berkley KJ. Chronic pelvic pain and endometriosis: translational evidence of the relationship and implications. Hum Reprod Update 2011; 17:327–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Richardson MT, Leon AS, Jacobs DR, Jr, et al. Comprehensive evaluation of the Minnesota Leisure Time Physical Activity Questionnaire. J Clin Epidemiol 1994; 47:271–281. [DOI] [PubMed] [Google Scholar]
- 35.Leischik R, Dworrak B, Strauss M, et al. Plasticity of health. German J Med 2016; 1:1–17. [Google Scholar]
- 36.Fiuza-Luces C, Garatachea N, Berger NA, et al. Exercise is the real polypill. Physiology 2013; 28:330–358. [DOI] [PubMed] [Google Scholar]


