Abstract
Background:
Many clinical studies have demonstrated the survival benefits of oxaliplatin-based chemotherapy for advanced hepatocellular carcinoma patients. Therefore, we aim to evaluate the efficacy and safety of oxaliplatin-based chemotherapy in patients with advanced hepatocellular carcinoma by conducting a meta-analysis of prospective studies.
Methods:
A comprehensive literature search was performed using the PubMed, Cochrane Library, EMBASE, and Web of Science databases from their inception to June 2016. Only prospective studies evaluating oxaliplatin-based chemotherapy in patients with advanced hepatocellular carcinoma were selected. The main outcomes included objective response rate (ORR), progression-free survival (PFS), overall survival (OS), and main adverse events.
Results:
Ten prospective studies involving 525 patients were included. The pooled ORR, 1-year PFS, and OS were 14.4% (95% confidence interval [CI] 9.2–19.6%), 9.3% (95%CI 10–28%), and 35.7% (95%CI 27–44%), respectively, for oxaliplatin-based chemotherapy. The median PFS and OS were 4.7 and 9.4 months, respectively. The incidences of grade 3/4 toxicities of neutropenia, thrombopenia, anemia, neurotoxicity, diarrhea, and nausea/vomiting were 17.2%, 9.2%, 6.0%, 4.8%, 3.1%, and 1.8%, respectively. Subgroup analysis revealed that the pooled ORR was 13.9% (95%CI 6.8–21%) in Asian patients and 12.8% (95%CI 6.8–18.7%) in Western patients. For Asian patients, the median PFS and OS were 4.2 and 9.2 months, and the 1-year PFS and OS were 12.5% and 30.5%, respectively. For Western patients, the median PFS and OS were 4.7 and 9.5 months, and the 1-year PFS and OS were 19.6% and 42.4%, respectively. There were no significant differences in the ORR, 1-year PFS, and OS (P > 0.05) between Asian and Western patients.
Conclusions:
Oxaliplatin-based chemotherapy appears to be effective and safe for the treatment of advanced hepatocellular carcinoma.
Keywords: advanced hepatocellular carcinoma, meta-analysis, oxaliplatin, prospective study, systemic chemotherapy
1. Introduction
Primary hepatic carcinoma is one of the most common malignant digestive system tumors, and hepatocellular carcinoma accounts for 90% of its pathological type. Hepatocellular carcinoma is the sixth most common malignant tumor and the second leading cause of cancer-related mortality worldwide.[1] The prognosis of hepatocellular carcinoma is particularly dismal due to the high degree of malignancy, insidious onset, rapid progress, and the likelihood of invasion and metastasis. In addition, treatment options are often limited because often patients also present with hepatitis and cirrhosis. Most patients have middle- and advanced-stage disease when they are diagnosed with hepatocellular carcinoma, and complete surgical resection is often precluded because of early dissemination of tumor. Potential palliative care includes percutaneous ablation, hepatic artery intervention, and/or systemic chemotherapy in these patients; however, the palliative abilities of these strategies for preserving quality of life and survival are very limited. Local ablation and intervention are only feasible in a considerable portion of patients. Therefore, the development of an effective systemic regimen for hepatocellular carcinoma is still a major challenge.
Currently, the only approved systemic therapy for the treatment of hepatocellular carcinoma is sorafenib according to 2 phase III randomized controlled trials (RCTs).[2,3] These 2 studies have confirmed the survival benefits of sorafenib among patients with advanced hepatocellular carcinoma in different regions, but some patients were not good candidates for this treatment due to the low objective response rate (ORR), unobvious improvement in tumor-related symptoms, unsatisfactory survival benefits, lack of appropriate predictive factors for treatment response, difficulty in selecting appropriate patients, or the high cost of the drug.
Since the early 1950s, many traditional chemotherapy drugs including 5-fluorouracil (5-FU), adriamycin (ADM)/epirubicin (EADM), cisplatin, mitomycin C, and etoposide have been successively applied in the treatment of hepatocellular carcinoma. Previous studies have shown that the ORR is only 0% to 10% when a single cytotoxic agent is used.[4] Moreover, the obvious adverse events often offset their clinical benefits. In addition, the quality and results of studies may be affected by the scientific development of the time and the level of clinical research. Therefore, there is a lack of sufficient and powerful evidence to support the survival benefits of systemic chemotherapy for advanced hepatocellular carcinoma. In recent years, a series of new-generation chemotherapeutic drugs, including oxaliplatin, gemcitabine, and capecitabine, has been widely used. The high efficiency and low toxicity achieved in the treatment of gastric and pancreatic cancer greatly inspired new systemic chemotherapy for advanced hepatocellular carcinoma.[5] The EACH study for the first time proved that oxaliplatin-based systemic chemotherapy is effective and safe in the treatment of Asian patients with advanced hepatocellular carcinoma.[6] Many subsequent studies also demonstrated the survival benefits of oxaliplatin-based chemotherapy for hepatocellular carcinoma patients. However, the baseline characteristics of patients may affect the efficacy and safety of oxaliplatin-based chemotherapy in the treatment of advanced hepatocellular carcinoma.
Here, we carried out a meta-analysis using all available prospective studies to evaluate the efficacy and safety of oxaliplatin-based chemotherapy in the treatment of advanced hepatocellular carcinoma.
2. Methods
2.1. Search strategy
This meta-analysis was conducted according to the criteria of the preferred reporting items for systemic reviews and meta-analyses statement.[7] A comprehensive literature search was carried out using the Pubmed, Cochrane Library, Embase, and Web of Science databases from their inception to June 2016. Potentially relevant studies were retrieved using the following search items: hepatocellular carcinoma OR hepatocellular cancer OR primary liver cancer OR primary hepatic cancer AND oxaliplatin. No language restrictions were applied. In addition, we also manually searched references cited in the original studies or review articles to identify any additional relevant studies.
2.2. Inclusion and exclusion criteria
The following inclusion criteria for retrieved studies were applied: (1) study design: prospective cohort study or RCT; (2) study population: patients with advanced hepatocellular carcinoma confirmed by clinical findings and/or pathological lesions and a life expectancy of 2 months or more; (3) interventions: oxaliplatin-based chemotherapy; chemotherapeutic agents must have been terminated at least 4 weeks before study entry or other antitumor treatments; (4) outcome measures: reporting of ORR and progression-free survival (PFS) or overall survival (OS); and (5) safety outcomes: incidences of grade 3/4 hematological and nonhematological toxicities. The grade of toxicity was assessed according to the Common Toxicity Criteria v2.0 of the National Cancer Institute (http://ctep.cancer.gov/reporting/ctc.html).
Studies were excluded if they: (1) were reviews, case reports, retrospective studies, or repeated publication of data; (2) involved <30 patients; (3) were written in any language but English; and (4) presented incomplete basic data or were of low quality.
2.3. Defining outcomes and follow-up
ORR is defined as the proportion of all treated patients who had a best objective tumor response of either complete response (CR) or partial response (PR) after treatment. PFS is defined as time from randomization to the first occurrence of disease progression or death from any cause of a cancer-related event. OS is defined as the time from randomization to death from any cause, censoring patients who did not die at the date last known alive.
2.4. Data extraction and quality assessment
Two authors (ZYH and HL) independently abstracted the following data from each included study using a standardized form: name of the first author, year of publication, region of study conducted, study design, sample size, median age of the patients, Child-Pugh A, disease stage, chemotherapy regimens, ORR, median/1-year OS, median/1-year PFS, and numbers of main grade 3/4 toxicities (neutropenia, thrombopenia, anemia, neurotoxicity, diarrhea, and nausea/vomiting). Any disagreement in the data extraction was resolved by consultation with the third author.
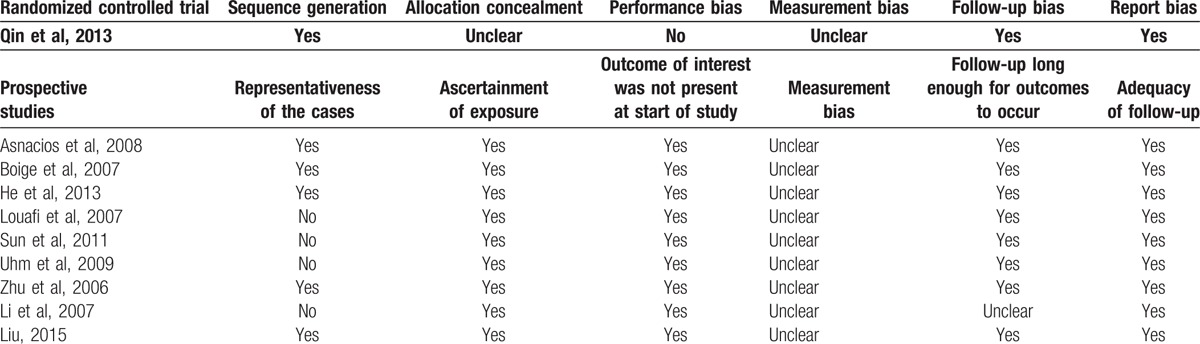
The methodological quality of the included RCTs was evaluated in accordance with the guidelines in the Cochrane reviewers’ handbook.[8] The Newcastle–Ottawa Scale (NOS) was used to assess the quality of a prospective cohort study.[9] The scale consists of 3 items, including patient selection, comparability of the study groups, and outcome assessment. Total NOS scores range from 0 to 9. Studies with a score > 6 points were considered to be of high quality.
2.5. Statistical analyses
All the analyses were conducted using STATA statistical software (version 12.0). Risk ratios and 95% confidence intervals (CIs) were computed for outcome measures as dichotomous outcomes. Hazard ratios were summarized to analyze the time-to-event data as outcomes. The heterogeneity of the pooled effect sizes across studies was tested by the Cochrane Q test and I2-statistic. If P < 0.05 on the Cochrane Q test or I2 was >50%, a random effects model was selected; otherwise, a fixed-effect model was preferred for homogeneous outcomes. Potential publication bias was tested using funnel plots, Begg's rank correlation,[10] and Egger's linear regression test.[11] Subgroup analysis was performed according to the geographical region (Asian vs Western). The t test was used to analyze the differences between the 2 subgroups. A P of <0.05 was judged to indicate statistical significance.
Ethics: Ethical approval was not necessary as this study is a systematic review and meta-analysis.
3. Results
3.1. Search results
Our initial literature search yielded a total of 112 potentially relevant papers. Of these, 62 presenting duplicated records were removed. After screening of the titles and abstracts, 23 papers were excluded. After reviewing the full-text manuscripts, 17 papers were further excluded. Thus, 10 studies were included in the final quantitative analysis.[6,12–20] A flowchart describing the selection process is presented in Fig. 1.
Figure 1.
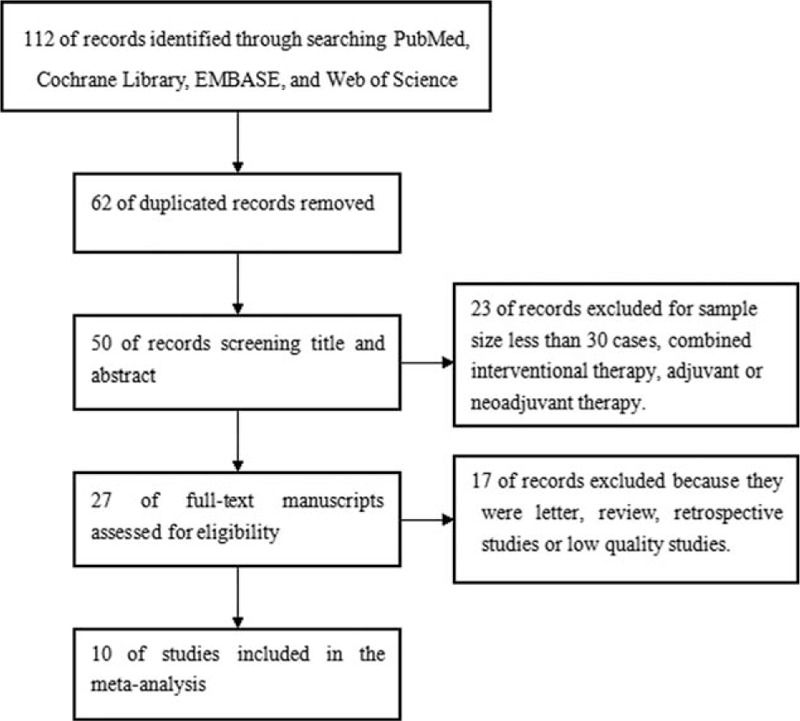
Flowchart of study selection process for meta-analysis.
3.2. Baseline characteristics
The characteristics of the included studies are listed in Table 1. The 10 studies were published between 2006 and 2015. Nine of the included studies had a single-arm design, and 1 study was an RCT.[6] A total of 525 patients with advanced hepatocellular carcinoma received oxaliplatin-based chemotherapy across the 10 studies. The sample sizes in individual studies ranged from 32 to 184 patients. The median age ranged from 49.53 to 68 years. Patients in 9 studies did not receive any systemic chemotherapy,[12–20] whereas 20.65% patients had completed systemic chemotherapy before 4 weeks in 1 study.[6] The chemotherapy regimens included FOLFOX4,[6] GEMOX alone,[15,19] or in combination with cetuximab,[12] bevacizumab,[18] sorafenib,[20] XELOX,[13,14] or in combination with bevacizumab,[16] or oxaliplatin plus doxorubicin.[17] The patients continued sorafenib as maintenance therapy after receiving treatment with GEMOX plus sorafenib in 1 study.[18] The number of cycles ranged from 2 to 8.7. There were 6 studies in Asian patients,[6,14,17–20] and 3 studies in Western patients.[12,13,15] Only 1 study enrolled both Asian and Western patients.[16] The methodological quality of the included studies is described in Table 2. One RCT reported the detailed method of randomization and lost follow-up but none reported allocation concealment.[6] Of the prospective studies, the representativeness of the cases in 4 studies was poor.
Table 1.
Baseline characteristics of the included studies.
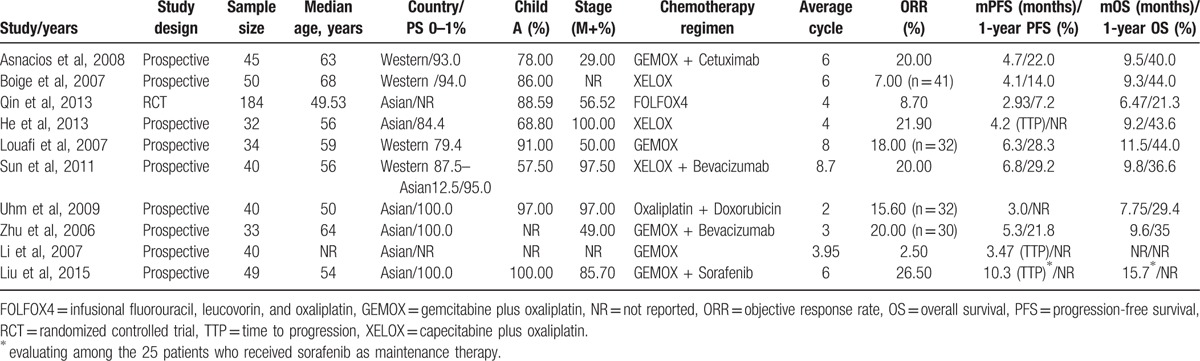
Table 2.
Risk bias of the included studies.
3.3. Pooled ORR
All the included studies reported the ORR, including the complete remission rate and partial remission rate. As shown in Fig. 2A, heterogeneity across 10 studies was significant (I2 = 69.1%, P=0.001), and the pooled ORR was 14.4% (95%CI 9.2–19.6%) in the random effects model. Evidence of publication bias was observed based on asymmetrical funnel plots (Fig. 2B) and Egger's linear regression test (P = 0.002) but not on the Begg's rank correlation test (P = 0.152).
Figure 2.
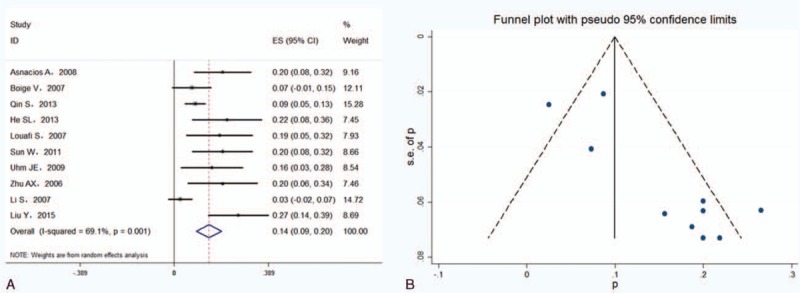
Forest plots showing objective remission rates from eligible studies in a random effects model (A) and funnel plots of objective remission rate (B).
Subgroup analyses indicated that the pooled ORR was 13.9% (95%CI 6.8–21.0%) in Asian patients and 12.8% (95%CI 6.8–18.7%) in Western patients (Fig. 3). The t test indicated that there was no significant difference in this value between the Asian and Western subgroup (P = 0.867). In addition, the pooled ORR was 16.8% (95%CI 5.6–27.9%) with the GEMOX regimen and 15.1% (95%CI 5.1%–25.1%) with the XELOX regimen. There was no significant difference between GEMOX and XELOX regimen subgroup (P > 0.05).
Figure 3.
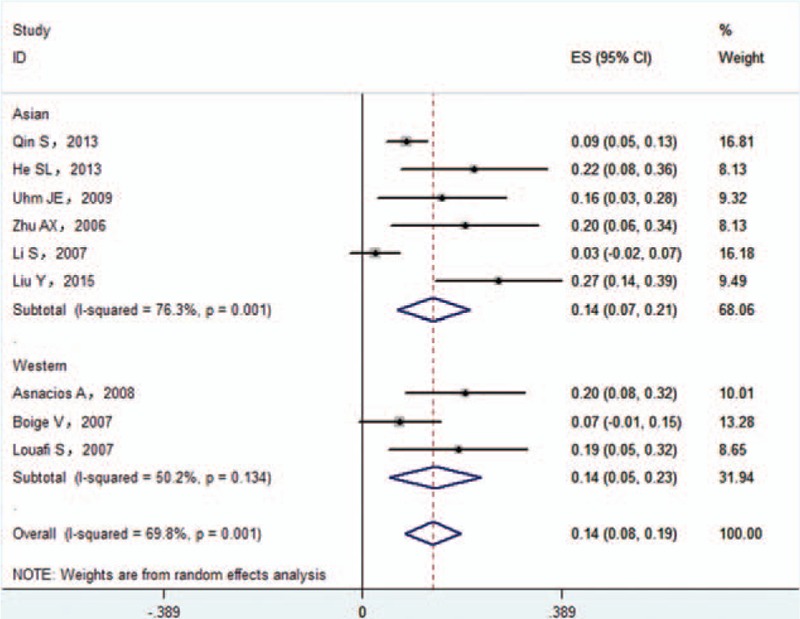
Subgroup analysis of objective remission rate.
3.4. Pooled PFS and OS
Seven studies reported median PFS or 1-year PFS data.[6,12,13,15,16,18] Nine studies reported median OS or 1-year OS data.[6,12–18,20] The pooled median PFS and OS were 4.7 and 9.4 months, respectively. The pooled 1-year PFS was 19.3% (95%CI 10.4–28.1%; I2 = 77.7%, P < 0.001) in a random effects model (Fig. 4A). The pooled 1-year OS was 35.7% (95%CI 27.5–43.9%; I2 = 65.2%, P = 0.005) in a random effects model (Fig. 4B). There was evidence of publication bias for the 1-year PFS and OS according to the symmetrical funnel plots, Begg's rank correlation test, and Egger's linear regression test (data not shown).
Figure 4.
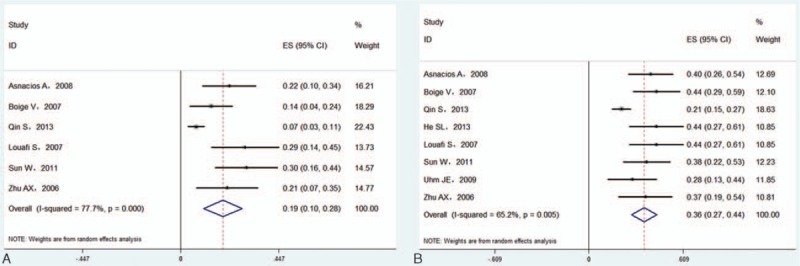
Forest plots showing 1-year progression-free survival (A) and overall survival (B) from eligible studies in a random effects model.
In Asian patients,[6,14,17–20] the pooled median PFS and OS were 4.2 and 9.2 months, respectively. The pooled 1-year PFS was 12.5% (95% CI 0–25.9%) in 2 studies (Fig. 5A),[6,18] and the pooled 1-year OS was 30.5% (95%CI 19.6–41.4%) in 4 studies (Fig. 5B).[6,14,16,18] In Western patients,[12,13,15] the pooled median PFS and OS were 4.7 and 9.5 months, and the pooled 1-year PFS (Fig. 6A) and 1-year OS (Fig. 6B) were 19.6% (95%CI 12.8–26.3%) and 42.4% (95%CI 33.5–51.4%) in a fixed effects model, respectively. There was no significant difference in the 1-year PFS or OS between Asian and Western patients (P > 0.05).
Figure 5.
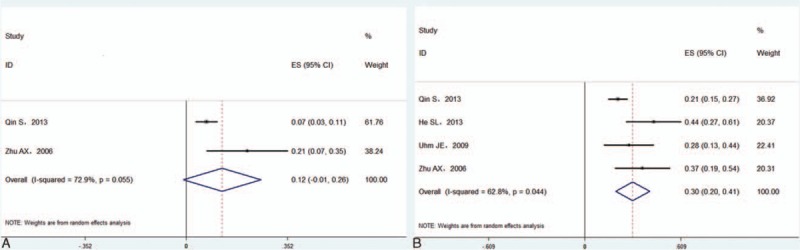
Forest plots showing 1-year progression-free survival (A) and overall survival (B) from eligible studies in Asian patients.
Figure 6.
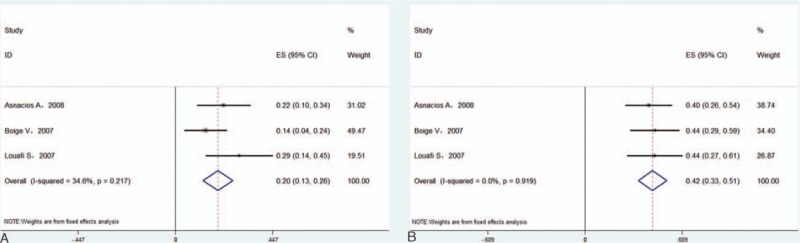
Forest plots showing 1-year progression-free survival (A) and overall survival (B) from eligible studies in Western patients.
3.5. Incidences of main grade 3/4 hematological and nonhematological toxicities
The most often reported grade 3/4 hematological and nonhematological toxicities were neutropenia, thrombopenia, anemia, neurotoxicity, diarrhea, and nausea/vomiting. As shown in Table 3, the pooled incidences of grade 3/4 adverse events were 17.2% (95%CI 10.4–24.1%) for neutropenia, 9.2% (95%CI 6.7–24.1%) for thrombopenia, 6.0% (95%CI 4.0–8.1%) for anemia, 4.8% (95%CI 1.6–7.9%) for neurotoxicity, 3.1% (95%CI 1.6–4.7%) for diarrhea, and 1.8% (95%CI 0.6–3.1%) for nausea/vomiting. Both the Begg's rank correlation and Egger's linear regression test indicated publication bias in pooled grade 3/4 toxicities.
Table 3.
Grade 3/4 hematological and nonhematological toxicities of the included studies.
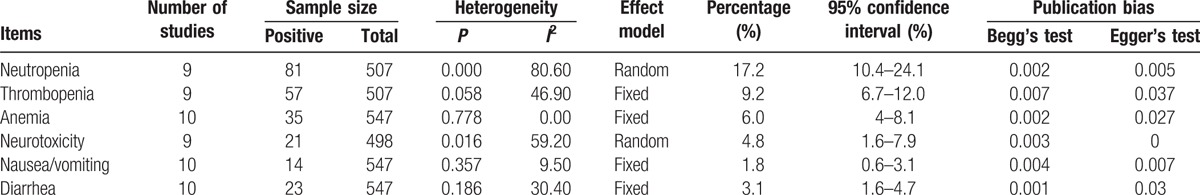
Subgroup analyses indicated that the pooled incidences of grade 3/4 neutropenia, thrombopenia, anemia, neurotoxicity, diarrhea, and nausea/vomiting were 19.2% (95%CI 9.8–28.6%), 7.7% (95%CI 5.0–10.3%), 6.0% (95%CI 4.0–8.1%), 1.0% (95%CI 0–2.0%), 2.7% (95% CI 1.1–4.3%), and 1.6% (95% CI 0.2–3.0%) in Asian patients, whereas the corresponding incidences in Western studies were 14.7% (95%CI 1.1–28.3%), 18.2% (95%CI 11.7–24.8%), 7.2% (95%CI 2.8–11.7%), 8.0% (95%CI 3.3–12.7%), 16.0% (95%CI 6.0–26.0%), and 2.9% (95%CI 0–6.3%), respectively. t Tests showed that there were significant differences in the incidences of thrombopenia, neurotoxicity, and diarrhea (P < 0.05), but not in the incidences of neutropenia, anemia, and nausea/vomiting (P > 0.05).
4. Discussion
We identified 10 prospective studies investigating the efficacy and safety of oxaliplatin-based chemotherapy in 525 patients with advanced hepatocellular carcinoma. The selected studies had obvious clinical heterogeneity mainly due to differences in the countries or ethnic groups of participants, study design, intervention, or statistical methods. We tested the presence and degree of heterogeneity by the Cochrane Q test and I2-statistic. When significant heterogeneity was observed, we adopted a random effects model. Moreover, we also made a subgroup analysis to explore the potential source of heterogeneity in order to ensure the accuracy of statistical analysis.
The major findings of this meta-analysis were that the ORR, median PFS, and OS were 14.4%, 4.7 months, and 9.4 months, respectively. The clinical efficacy was not inferior to that of sorafenib, which demonstrated that oxaliplatin-based chemotherapy was effective in the treatment of advanced hepatocellular carcinoma. Specifically, except for a 17.2% incidence of neutropenia, the incidences of other grade 3/4 toxicities were low, and death events were rare. There may be geographical variation in the distribution of hepatocellular carcinoma in Asian and Western countries. Subgroup analysis showed that there were no significant differences in the efficacy of oxaliplatin for the treatment of hepatocellular carcinoma between the Asian and Western patients. However, we could not determine the best oxaliplatin-based chemotherapy regimen due to the lack of significant differences in the clinical efficacy between the FOLFOX and XELOX regimens. Because evidence of publication bias was detected in some variables analyzed, these results of our findings may be biased.
At present, sorafenib is the only approved standard treatment for medically fit patients with advanced hepatocellular carcinoma, and its use was supported by high level, evidence-based medicine. However, patients who received sorafenib monotherapy only achieved a relatively low ORR and limited survival benefits. Nevertheless, a series of new molecular target drugs including sunitinib, everolimus, erlotinib, axitinib, linifanib, and brivanib have failed to further improve the survival time compared to sorafenib among patients with advanced hepatocellular cancer in randomized phase III trials.[21,22] As for the intrinsic resistance and/or acquired drug resistance of hepatocellular cancer cells, the clinical efficacy of conventional cytotoxic drugs was not satisfactory among patients for systemic chemotherapy.[23–25] A series of combination chemotherapies based on the first-generation platinum drug PDD has been applied in the treatment of hepatocellular carcinoma, but its efficacy was not satisfactory, and severe toxicities in particular were observed.[26–29] Oxaliplatin, a third-generation cisplatin analog, has more broad antitumor activity, based on rapid and stronger inhibition of DNA replication through the formation of DNA-platinum macromolecular adducts in cancer cells.[30,31]
In recent years, a multicenter, open-label, randomized, phase III (EACH) study in an Asian region involved 371 patients who had advanced hepatocellular carcinoma, and the patients were randomly assigned to the FOLFOX4 or doxorubicin group.[6] In the FOLFOX4 group, the median OS, median PFS, and ORR were 6.40 months, 2.93, months, and 8.15%, respectively. However, in the doxorubicin group, the median OS and PFS and ORR were 4.97 and 1.77 months and 2.76%, respectively. Subgroup analyses revealed that FOLFOX4 treatment was associated with greater median OS,[32] median PFS, and ORR compared with doxorubicin in Chinese patients. The EACH study for the first time demonstrated that FOLFOX4 has a certain ORR as well as better survival benefits for the treatment of advanced hepatocellular carcinoma. These findings not only subvert the traditional concept but also change the current situation of the lack of standard systematic chemotherapy for the management of advanced hepatocellular carcinoma.
A well-designed systematic review and pooled analysis summarized the benefits of oxaliplatin-based chemotherapy in advanced hepatocellular carcinoma in patients not exposed to sorafenib.[5] Thirteen prospective or retrospective case series and phase II or III clinical trials that enrolled advanced hepatocellular carcinoma patients treated with first-line oxaliplatin-based chemotherapy were included in this review. The pooled ORR, median PFS and OS were 16.8%, 4.2 and 9.3 months, respectively. The median PFS, OS, and 1-year OS were 4.5 months, 11 months, and 42.3% in Western patients, respectively. Conversely, the median PFS, OS, and 1-year OS were 2.43 months, 6.47 months, and 30.5% in Asiatic patients. This study highlighted that Asian and Western patients had different outcomes when treated with oxaliplatin-based systemic chemotherapy. However, in our meta-analysis, we only included prospective studies in order to reduce selection bias. Additionally, studies that involved <30 patients were also excluded from the analysis. Overall, the pooled ORR, median PFS, and OS were similar to those reported by a previous systematic review. However, there was no significant difference in the clinical efficacy including ORR, PFS, and OS between the Asian and Western patients in our study.
Although oxaliplatin-based chemotherapy achieved a high incidence of ORR and benefits of PFS and OS, a major concern for both patients and physicians is the potential for adverse events caused by the chemotherapy regimen. This meta-analysis suggested that the most often reported grade 3/4 adverse events were neutropenia (17.2%), thrombopenia (9.2%), anemia (6.0%), neurotoxicity (4.8%), diarrhea (3.1%), and nausea/vomiting (1.8%). It should be noted that the toxicity assessment in our meta-analysis was not stable due to obvious publication bias. Liver cirrhosis and hepatic dysfunction obviously increased the risk of adverse events, because several chemotherapeutic drugs are metabolized through the liver. However, oxaliplatin was demonstrated to offer well tolerable hepatic toxicity in patients with hepatic dysfunction.[33]
Several potential limitations of the present study should be recognized. First, most of included studies had a single-arm prospective design because RCTs of oxaliplatin-based chemotherapy for the treatment of advanced hepatocellular carcinoma have not been widely carried out, which might affect the reliability of our findings. Second, the relatively small sample size of the included studies may have been the cause of some selection bias and diminished the statistical power of effect estimates. Third, significant heterogeneity was observed in the main analysis across studies, and the dosage and composition of the chemotherapy regimen may be the sources of heterogeneity. Fourth, our study cannot determine a better or optimal companion agent for an oxaliplatin-based chemotherapy regimen due to the limited number of publications available on the use of oxaliplatin in combination with different chemotherapy agents. Finally, this meta-analysis was based on study-level data; we could not determine the prognostic factors associated with relief and clinical benefits because of a lack of patient-level information.
5. Conclusion
This meta-analysis suggested that oxaliplatin-based chemotherapy may achieve a certain response rate and survival benefits in the treatment of advanced hepatocellular carcinoma in both Asian and Western patients. In addition, oxaliplatin-based chemotherapy appeared to be safe and well-tolerated. Moreover, the efficacy and safety of oxaliplatin-based chemotherapy need to be further verified in direct comparisons with sorafenib or systemic chemotherapy combined sorafenib in RCTs with large sample sizes.
Footnotes
Abbreviations: FOLFOX4 = infusional fluorouracil, leucovorin, and oxaliplatin, GEMOX = gemcitabine plus oxaliplatin, NR = not reported, ORR = objective response rate, OS = overall survival, PFS = progression-free survival, RCT = randomized controlled trial, TTP = time to progression, XELOX = capecitabine plus oxaliplatin.
Funding: This research was supported by grants from the Science and Technology Department of Jiangsu Province (China) through the Science and Technology Support Program (No. BE2012766), the China Postdoctoral Science Foundation (No. 2012T50896), and the Postdoctoral Research Foundation of Jiangsu Province (China) (No. 1102156C). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors have no conflicts of interest to disclose.
References
- 1.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65:87–108. [DOI] [PubMed] [Google Scholar]
- 2.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008; 359:378–390. [DOI] [PubMed] [Google Scholar]
- 3.Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009; 10:25–34. [DOI] [PubMed] [Google Scholar]
- 4.Thomas MB, Zhu AX. Hepatocellular carcinoma: the need for progress. J Clin Oncol 2005; 23:2892–2899. [DOI] [PubMed] [Google Scholar]
- 5.Petrelli F, Coinu A, Borgonovo K, et al. Oxaliplatin-based chemotherapy: a new option in advanced hepatocellular carcinoma. A systematic review and pooled analysis. Clin Oncol (R Coll Radiol) 2014; 26:488–496. [DOI] [PubMed] [Google Scholar]
- 6.Qin S, Bai Y, Lim HY, et al. Randomized, multicenter, open-label study of oxaliplatin plus fluorouracil/leucovorin versus doxorubicin as palliative chemotherapy in patients with advanced hepatocellular carcinoma from Asia. J Clin Oncol 2013; 31:3501–3508. [DOI] [PubMed] [Google Scholar]
- 7.Panic N, Leoncini E, de Belvis G, et al. Evaluation of the endorsement of the preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement on the quality of published systematic review and meta-analyses. PLoS One 2013; 8:e83138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The Cochrane Collaboration, Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. 2011. [Google Scholar]
- 9.Wells G, Shea B, O’Connell D, et al. The Newcastle–Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. [Google Scholar]
- 10.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994; 50:1088–1101. [PubMed] [Google Scholar]
- 11.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Asnacios A, Fartoux L, Romano O, et al. Gemcitabine plus oxaliplatin (GEMOX) combined with cetuximab in patients with progressive advanced stage hepatocellular carcinoma: results of a multicenter phase 2 study. Cancer 2008; 112:2733–2739. [DOI] [PubMed] [Google Scholar]
- 13.Boige V, Raoul JL, Pignon JP, et al. Multicentre phase II trial of capecitabine plus oxaliplatin (XELOX) in patients with advanced hepatocellular carcinoma: FFCD 03-03 trial. Br J Cancer 2007; 97:862–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He SL, Shen J, Sun XJ, et al. Efficacy of capecitabine and oxaliplatin regimen for extrahepatic metastasis of hepatocellular carcinoma following local treatments. World J Gastroenterol 2013; 19:4552–4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Louafi S, Boige V, Ducreux M, et al. Gemcitabine plus oxaliplatin (GEMOX) in patients with advanced hepatocellular carcinoma (HCC): results of a phase II study. Cancer 2007; 109:1384–1390. [DOI] [PubMed] [Google Scholar]
- 16.Sun W, Sohal D, Haller DG, et al. Phase 2 trial of bevacizumab, capecitabine, and oxaliplatin in treatment of advanced hepatocellular carcinoma. Cancer 2011; 117:3187–3192. [DOI] [PubMed] [Google Scholar]
- 17.Uhm JE, Park JO, Lee J, et al. A phase II study of oxaliplatin in combination with doxorubicin as first-line systemic chemotherapy in patients with inoperable hepatocellular carcinoma. Cancer Chemother Pharmacol 2009; 63:929–935. [DOI] [PubMed] [Google Scholar]
- 18.Zhu AX, Blaszkowsky LS, Ryan DP, et al. Phase II study of gemcitabine and oxaliplatin in combination with bevacizumab in patients with advanced hepatocellular carcinoma. J Clin Oncol 2006; 24:1898–1903. [DOI] [PubMed] [Google Scholar]
- 19.Li S, Niu Z, Tian H, et al. Treatment of advanced hepatocellular carcinoma with gemcitabine plus oxaliplatin. Hepatogastroenterology 2007; 54:218–223. [PubMed] [Google Scholar]
- 20.Liu Y, Yue H, Xu S, et al. First-line gemcitabine and oxaliplatin (GEMOX) plus sorafenib, followed by sorafenib as maintenance therapy, for patients with advanced hepatocellular carcinoma: a preliminary study. Int J Clin Oncol 2015; 20:952–959. [DOI] [PubMed] [Google Scholar]
- 21.Johnson PJ, Qin S, Park JW, et al. Brivanib versus sorafenib as first-line therapy in patients with unresectable, advanced hepatocellular carcinoma: results from the randomized phase III BRISK-FL study. J Clin Oncol 2013; 31:3517–3524. [DOI] [PubMed] [Google Scholar]
- 22.Cheng AL, Kang YK, Lin DY, et al. Sunitinib versus sorafenib in advanced hepatocellular cancer: results of a randomized phase III trial. J Clin Oncol 2013; 31:4067–4075. [DOI] [PubMed] [Google Scholar]
- 23.Lai CL, Wu PC, Chan GC, et al. Doxorubicin versus no antitumor therapy in inoperable hepatocellular carcinoma. A prospective randomized trial. Cancer 1988; 62:479–483. [DOI] [PubMed] [Google Scholar]
- 24.Kardinal CG, Moertel CG, Wieand HS, et al. Combined doxorubicin and alpha-interferon therapy of advanced hepatocellular carcinoma. Cancer 1993; 71:2187–2190. [DOI] [PubMed] [Google Scholar]
- 25.Gish RG, Porta C, Lazar L, et al. Phase III randomized controlled trial comparing the survival of patients with unresectable hepatocellular carcinoma treated with nolatrexed or doxorubicin. J Clin Oncol 2007; 25:3069–3075. [DOI] [PubMed] [Google Scholar]
- 26.Yeo W, Mok TS, Zee B, et al. A randomized phase III study of doxorubicin versus cisplatin/interferon alpha-2b/doxorubicin/fluorouracil (PIAF) combination chemotherapy for unresectable hepatocellular carcinoma. J Natl Cancer Inst 2005; 97:1532–1538. [DOI] [PubMed] [Google Scholar]
- 27.Chia WK, Ong S, Toh HC, et al. Phase II trial of gemcitabine in combination with cisplatin in inoperable or advanced hepatocellular carcinoma. Ann Acad Med Singapore 2008; 37:554–558. [PubMed] [Google Scholar]
- 28.Lee JE, Bae SH, Choi JY, et al. Epirubicin, cisplatin, 5-FU combination chemotherapy in sorafenib-refractory metastatic hepatocellular carcinoma. World J Gastroenterol 2014; 20:235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boucher E, Corbinais S, Brissot P, et al. Treatment of hepatocellular carcinoma (HCC) with systemic chemotherapy combining epirubicin, cisplatinum and infusional 5-fluorouracil (ECF regimen). Cancer Chemother Pharmacol 2002; 50:305–308. [DOI] [PubMed] [Google Scholar]
- 30.Faivre S, Chan D, Salinas R, et al. DNA strand breaks and apoptosis induced by oxaliplatin in cancer cells. Biochem Pharmacol 2003; 66:225–237. [DOI] [PubMed] [Google Scholar]
- 31.Petruseva IO, Evdokimov AN, Lavrik OI. Molecular mechanism of global genome nucleotide excision repair. Acta Naturae 2014; 6:23–34. [PMC free article] [PubMed] [Google Scholar]
- 32.Qin S, Cheng Y, Liang J, et al. Efficacy and safety of the FOLFOX4 regimen versus doxorubicin in Chinese patients with advanced hepatocellular carcinoma: a subgroup analysis of the EACH study. Oncologist 2014; 19:1169–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Doroshow JH, Synold TW, Gandara D, et al. Pharmacology of oxaliplatin in solid tumor patients with hepatic dysfunction: a preliminary report of the National Cancer Institute Organ Dysfunction Working Group. Semin Oncol 2003; 30:14–19. [DOI] [PubMed] [Google Scholar]


