Abstract
Spot 14 (S14) is a protein involved in fatty acid synthesis and was shown to be induced by thyroid hormone in rat liver. However, the presence of S14 in human serum and its relations with thyroid function status have not been investigated.
The objectives of this study were to compare serum S14 concentrations in patients with hyperthyroidism or euthyroidism and to evaluate the associations between serum S14 and free thyroxine (fT4) or thyroid-stimulating hormone (TSH) levels.
We set up an immunoassay for human serum S14 concentrations and compared its levels between hyperthyroid and euthyroid subjects. Twenty-six hyperthyroid patients and 29 euthyroid individuals were recruited. Data of all patients were pooled for the analysis of the associations between the levels of S14 and fT4, TSH, or quartile of TSH.
The hyperthyroid patients had significantly higher serum S14 levels than the euthyroid subjects (median [Q1, Q3]: 975 [669, 1612] ng/mL vs 436 [347, 638] ng/mL, P < 0.001). In univariate linear regression, the log-transformed S14 level (logS14) was positively associated with fT4 but negatively associated with creatinine (Cre), total cholesterol (T-C), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), and TSH. The positive associations between logS14 and fT4 and the negative associations between logS14 and Cre, TG, T-C, or TSH remained significant after adjustment with sex and age. These associations were prominent in females but not in males. The logS14 levels were negatively associated with the TSH levels grouped by quartile (ß = −0.3020, P < 0.001). The association between logS14 and TSH quartile persisted after adjustment with sex and age (ß = −0.2828, P = 0.001). In stepwise multivariate regression analysis, only TSH grouped by quartile remained significantly associated with logS14 level.
We developed an ELISA to measure serum S14 levels in human. Female patients with hyperthyroidism had higher serum S14 levels than the female subjects with euthyroidism. The serum logS14 concentrations were negatively associated with TSH levels. Changes of serum S14 level in the whole thyroid function spectrum deserve further investigation.
Keywords: euthyroidism, free thyroxine, hyperthyroidism, spot 14, thyroid-stimulating hormone
1. Introduction
Spot 14 (S14, also called thyroid hormone responsive protein, THRSP) was so named because it is the 14th spot up-regulated in the rat liver by thyroid hormone revealed on two-dimensional gels.[1] Further studies showed that there was a marked reduction of S14 mRNA in the liver of hypothyroid rats, compared with the euthyroids. Moreover, S14 mRNA was increased in a dose-dependent manner with triiodothyronine (T3) treatment in these hypothyroid animals.[2] The experiments with the hypothyroid rats injected with T3 demonstrated a swift induction of hepatic S14 mRNA within 20 minutes of treatment.[3] This is the most rapid effect of T3 on hepatic gene expression documented to date.[4] Identification of several thyroid hormone response elements far upstream of the transcription start site of S14 gene confirmed the direct role of T3 in regulating its transcription.[5] S14 can be tremendously induced not only by T3 but also by high-carbohydrate diet and insulin.[4,6–9]
S14 gene is mainly expressed in lipid-producing tissues, primarily in the liver, adipose tissue, and lactating mammary glands,[10] where it was shown to play a role in fatty acid synthesis.[11,12] In a previous report, hepatocytes were transfected with S14 antisense oligonucleotides. Compared with the controls, lipogenesis as well as lipogenic enzymes, such as fatty acid synthase (FAS), were reduced in antisense oligonucleotide transfected cells, implying its roles in lipogenesis.[11]
Animal experiments also showed that deletion of S14 dramatically reduced fatty acid synthesis in mammary glands, but surprisingly it was not altered in the liver, suggesting the existence of a redundant pathway in the liver.[13,14] Later, a ubiquitously expressed paralogous protein S14-related (S14-R) protein was identified. It is abundant in the liver. In contrast, the levels of S14-R are low in the mammary glands, leading to the speculation that the reduced fatty acid synthesis in mammary gland in S14-deletion mice was secondary to the lack of S14-R.[13] In addition, the S14-deletion mice also had the other metabolic phenotypes in the adult animals. The S14 knockout mice gained significantly less weight than wild-type mice.[14,15] The deletion of S14 also resulted in resistance to diet-induced obesity and favorable insulin sensitivity and glucose tolerance.[14]
However, very limited information is available regarding the function of S14 in human subjects. One human study showed that S14 mRNA in adipose tissues was abnormally regulated in obese subjects.[16] The obese subjects had higher basal S14 mRNA expressions than the controls. However, the obese subjects showed less downregulation of S14 mRNA after a 48-hour fast compared with the nonobese individuals. Contradictory to the above findings, the other study showed that the levels of S14 mRNA in overweight and obese subjects were lower than normal subjects.[17] Therefore, the exact role of S14 in the maintenance of adiposity in humans is still not clear. So far, no study in humans has assayed the serum levels of S14 and tried to relate its blood levels to any human physiological or pathological conditions. In this report, we developed an in-house enzyme-linked immunosorbent assay (ELISA) to investigate the relationship of S14 and thyroid function in human subjects.
2. Materials and methods
2.1. Human subjects
This is a cross-sectional observation study. Human subjects were recruited from the National Taiwan University Hospital at their first visit to our Endocrinology clinics between year 2010 and 2011. Subjects, who had recurrent thyroid conditions or had other comorbidities or were under any medication, were excluded. At the end, 26 subjects with newly diagnosed hyperthyroidism and 29 with euthyroidism were included for the analysis. All subjects with hyperthyroidism had typical clinical manifestations of thyrotoxicosis, elevated free thyroxine (fT4), lower thyroid-stimulating hormone (TSH), and positive TSH receptor antibody. Written informed consent was obtained from each subject. In accordance with the Declaration of Helsinki and the approved guidelines, the research ethics committee of the National Taiwan University Hospital approved the study including the protocol, the informed consent form, and applicable recruiting materials.
2.2. Data collection, thyroid function status, and biochemical assay
The basic characteristics, such as age, sex, body height (BH), and body weight (BW) were collected. Body mass index (BMI) was calculated as BW in kilograms (kg) divided by the square of BH in meters (m2).
Blood samples were drawn from antecubital vein after overnight fast for around 12 hours. Biochemical data including alanine transaminase (ALT), aspartate transaminase (AST), creatinine (Cre), fasting plasma glucose (FPG), fT4, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), total cholesterol (T-C), triglyceride (TG), and TSH were assayed. Levels of ALT and AST were determined by Hitachi 7080 biochemical analyzer (Hitachi, Japan). Levels of FPG were measured using the Olympus AU series 680 with hexokinase method (Beckman Coulter, Nyon, Switzerland). Serum T-C, TG, HDL-C, and LDL-C were measured using the Olympus AU series 5800 with CHOD-PAP method, GPO-PAP method, accelerator selective detergent, and liquid selective detergent, respectively (Beckman Coulter, Nyon, Switzerland). TSH and fT4 were measured by Siemens DPC Immulite 2000 (Siemens, Erlangen, Germany). TSH-receptor antibody (TRAb) levels were determined by using the radioimmunoassay method (TSH receptor autoantibody coated tube kit, RSR, Cardiff, United Kingdom). A percentage inhibition of TSH binding < 10% was recorded as negative, 10% to 15% as borderline positive, and > 15% as positive. All these assays were performed following the manufacturers’ instructions.
The normal reference of fT4 and TSH used in our hospital were 0.6 to 1.75 ng/dL and 0.1 to 4.5 μIU/mL, respectively. When the values were way outside the laboratory measurement range (fT4 level > 5.4 ng/dL or TSH level < 0.004 μIU/mL), they were recorded as an fT4 level = 5.4 ng/dL or a TSH level = 0.004 μIU/mL, respectively. In addition to the clinical history and physical findings, the laboratory definition of hyperthyroidism was an fT4 level > 1.75 ng/dL and a TSH level < 0.1 μIU/mL, whereas euthyroidism was defined as both fT4 and TSH levels within their normal reference ranges.
2.3. Thyroid ultrasound
All of the participants received a thyroid ultrasonographic examination at baseline. An endocrine specialist performed the sonographic examination with the use of the Toshiba Aplio XG SSA-790A Ultrasound System (Toshiba Medical Systems Co, Ltd, Tochigi, Japan) with a PLT-805AT probe. Aspiration cytological examination was performed as clinically indicated. None of the recruited patients had lesions suspicious for malignancy.
2.4. S14 immunoassay
A competitive ELISA for S14 was developed. Different reagents and working dilutions of antigen or antibody were tested. Polystyrene MaxiSorp 96-well plates (Nunc A/S, Roskilde, Denmark) were coated with 100 μL human recombinant S14 proteins (100ng/mL, diluted in PBS; cat no. ag3721, ProteinTech, Chicago, IL) per well. The coated plates were sealed and incubated on an orbital shaker (at 100 rpm; OS701, KS, Taiwan) at 4°C overnight. The solutions were then discarded and the plates were washed in washing buffer (PBS-Tween (PBS-T), 0.05% Tween 20), and were pad-dried on a paper towel. Then the plates were blocked with 100 μL blocking buffer (PBST with 1% BSA) each well, and incubated at 4°C overnight at 100 rpm on the orbital shaker. After 3 times of washing with PBS-T, 50 μL serum samples were added in each well and incubated for 1 hour at room temperature (RT) on a rotor at 150 rpm. Subsequently, 50 μL rabbit anti-S14 polyclonal antibody (diluted by 1:10000 in blocking buffer; catalog no. 13054–1-AP, ProteinTech, Chicago, IL) were added and incubated for 2 hours at RT and shaken at 150 rpm. After 3 times of washing with PBS-T, 100 μL of horseradish peroxidase-conjugated goat anti-rabbit IgG polyclonal antibody (diluted in blocking buffer by 1:10000; GTX213110–01, Irvine, CA) was added to each well and shaken (150 rpm) for 1 hour at RT. Following 5 times of washing with PBS-T, color was developed using the 100 μL 3, 3’, 5, 5’-tetramethylbenzidine (TMB) solution (KPL, Gaithersburg, MD) each well. After 10-minute incubation, the reaction was stopped by addition of 100 μL 2.0 M H2SO4 per well. Immediately, the optical density of each well was read at 450 nm using microplate reader (VERSA max, Munich, Germany). Four-parameter logistic model was used to draw the standard curve.
The assay was validated by the determination of assay sensitivity, intra- and interassay variability. For the sensitivity, the minimum detection limit was 10 ng/mL. For the intra-assay variability, the coefficient of variance (CV) of 6 replicate sets of one serum sample was 7.5%. For the interassay variability, the CV of 6 independent assays of one serum sample was 9.5%.
2.5. Statistical analysis
This study enrolled 26 subjects with hyperthyroidism and 29 with euthyroidism. We presented the numerical variables as median values (Q1, Q3) and used Mann–Whitney U test to compare the numerical variables between the hyperthyroidism and euthyroidism groups. Categorical data were presented as percentage. Fisher exact test was used for comparison of categorical variables. P values < 0.05 were considered as statically significant.
The data of the patients with hyperthyroidism or euthyroidism were pooled together for analyzing the possible associations between logS14 levels and other variables. We used Anderson–Darling or Cramer Von–Mises test to examine normality. Thus, we made log transformation of S14 levels for further analysis. The effects of demographic, anthropometric, or laboratory parameters (sex, age, BH, BW, BMI, FPG, Cre, AST, ALT, T-C, TG, HDL-C, LDL-C, and levels of fT4, TSH) for logS14 were evaluated by performing a linear regression analysis. Those having statistical significance were further tested by adjustment with sex and age. To minimize the possible bias that might be induced by applying fixed values of fT4 or TSH when their levels exceed the range of the commercial kits, we divided the TSH into quartiles (Group 1: TSH ≦ 0.004; Group 2: 0.004 ≤ TSH ≦ 0.422; Group 3: 0.422 < TSH ≦ 1.17; Group 4: 1.17 < TSH). The logS14 levels in different TSH quartiles were compared. The effect of TSH quartile group for logS14 was also calculated by regression analysis.
The effects of demographic, anthropometric, or laboratory parameters for logS14 concentrations were further tested by performing stepwise forward multivariate regression. In a stepwise forward multivariate regression, variables with P < 0.10 remained in the model. Only variables with P values <0.05 were considered as statistically significant. All the analyses were performed by using the SAS version 9.1 statistical package for Windows (SAS, Cary, NC). The normality of all the models was assessed using Anderson–Darling or Cramer Von–Mises tests, and none of the models violated the normality assumption.
3. Results
Twenty-six patients were diagnosed with hyperthyroidism (HY group). All of them had positive TRAb. Sonograms of the HY group patients revealed characteristics (hypoechoic and diffuse enlargement) compatible with autoimmune thyroiditis. Twenty-nine patients were classified as euthyroid (EU group). They all had negative examination results for TRAb.
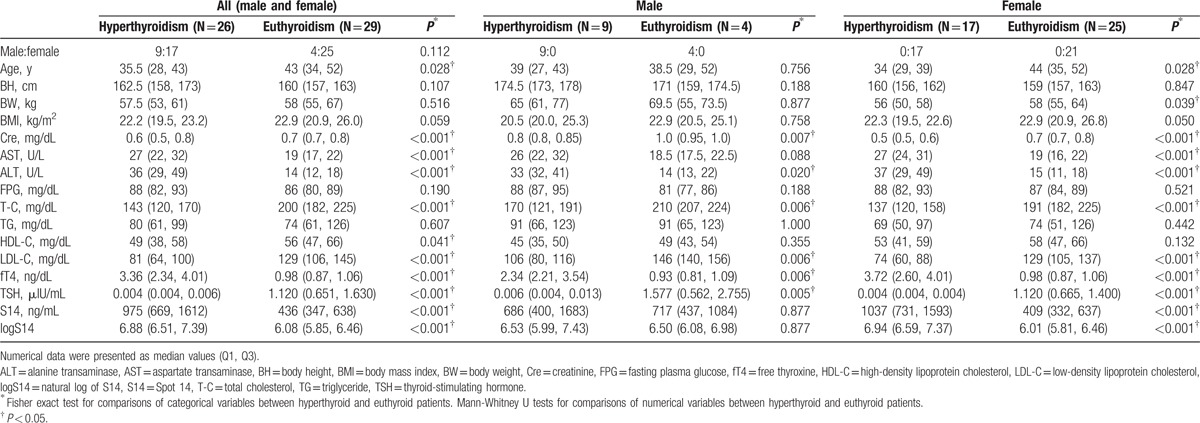
The anthropometric characteristics and laboratory data of the hyperthyroid and euthyroid patients are shown in Table 1. The hyperthyroid patients were younger and had higher fT4, AST, and ALT levels but lower TSH, Cre, T-C, HDL-C, and LDL-C level than the euthyroid patients (Table 1). The hyperthyroid patients apparently had higher S14 levels than the euthyroid subjects (975 [669, 1612] ng/mL vs 436 [347, 638] ng/mL, p < 0.001) (Table 1). The difference of S14 levels among HY group or EU group was significant in females, but not in males (Table 1).
Table 1.
Characteristics of subjects with hyperthyroidism or euthyroidism.
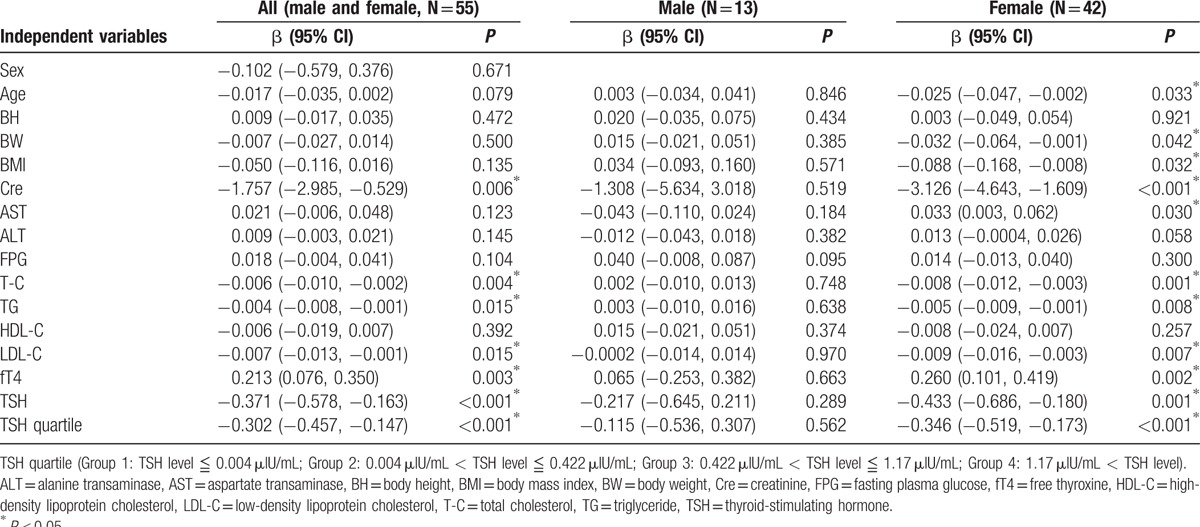
The effects of demographic, anthropometric, or laboratory parameters (age, sex, and concentrations of fT4, TSH, BH, BW, BMI, Cre, AST, ALT, FPG, T-C, TG, HDL-C, and LDL-C) for levels of logS14 were evaluated by performing a linear regression analysis. In all patients (both males and females), the univariate linear regression analysis revealed that logS14 levels were positively associated with fT4 (β = 0.213, P = 0.003), but negatively associated with Cre (β = −1.757, P = 0.006), T-C (β = −0.006, P = 0.004), TG (β = −0.004, P = 0.015), LDL-C (β = −0.007, P = 0.015), and TSH (β = −0.371, P < 0.001) (Table 2). The variables with statistical significance were further analyzed by adjustment with age and sex. The associations between logS14 and Cre, T-C, TG, fT4, TSH remained significant (β = −2.681, P < 0.001; β = −0.006, P = 0.023; β = −0.004, P = 0.029; β = 0.192, P = 0.010; and β = −0.340, P = 0.003, respectively). However, the association between LDL-C with logS14 became insignificant when adjusted with sex and age (data not shown). The linear regression analysis revealed that the associations between logS14 and age, BW, BMI, Cre, AST, T-C, TG, LDL-C, fT4, and TSH were prominent in females but not in males (Table 2).
Table 2.
Univariate regression model with logS14 as dependent variable, and demographic, anthropometric, and laboratory parameters as independent variables.
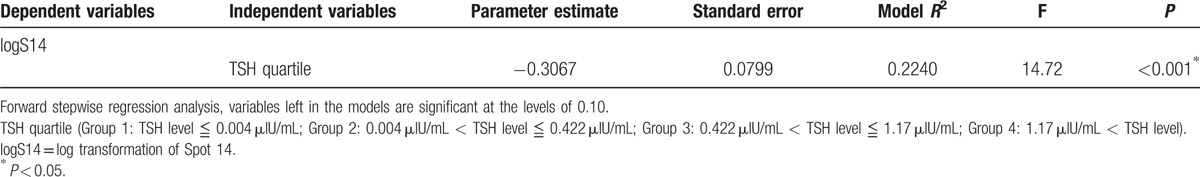
The linear regression analysis revealed that TSH as a categorical variable grouped in quartiles was negatively associated with logS14 level (β = −0.302, P < 0.001) (Fig. 1 and Table 2). The negative association between TSH quartile and logS14 level persisted when adjusted with age and sex (β = −0.283, P = 0.004) (data not shown). Only TSH quartile group, but not fT4 or TSH, remained as the significant parameter related to the serum logS14 level in stepwise multivariate regression analysis (Table 3).
Figure 1.
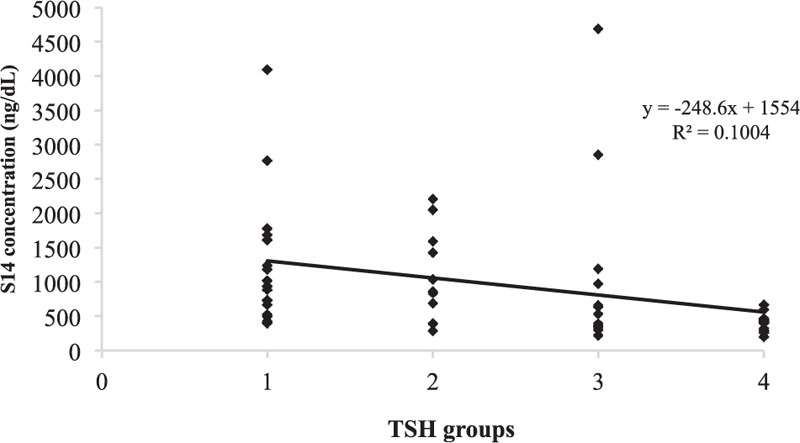
A negative relationship between logS14 and TSH levels in which the subjects were divided into 4 groups based on their TSH levels. logS14 = log transformation of Spot 14, TSH = thyroid-stimulating hormone.
Table 3.
Forward stepwise regression models in all subjects (N = 55) with levels of logS14 as dependent variables, and sex, age, anthropometric, and laboratory parameters as independent variables.
4. Discussion
S14 is a 17-kDa protein mainly expressed in lipogenic tissues and is postulated to play a role in lipogenesis stimulated by thyroid hormone.[10] Thyroid hormone was demonstrated to upregulate S14 gene expression in the rat liver.[1,2] In this study, we successfully developed a competitive ELISA system to measure serum S14 levels in human subjects. Our study is the first to investigate the serum S14 levels in humans and the effect of thyroid function status on serum S14 level. Our analysis revealed that the patients with hyperthyroidism had significantly higher serum S14 levels than the subjects with euthyroidism. Previous studies demonstrated a tremendous induction of S14 by thyroid hormone in animal.[1,2,6] Our results suggested the effects of thyroid hormone on serum S14 levels in human.
Thyroid dysfunction may affect adipocyte function and lipid metabolism.[18,19] With the stimulation of thyroid hormones, the triglycerides stored in adipose tissue will be utilized. Patients with hyperthyroidism usually have decreased levels of lipid,[18] but increased concentrations of plasma fatty acids.[19] However, the mechanism of thyroid hormone action on lipolysis remains unclear. In our study, the patients with hyperthyroidism had lower T-C, LDL-C, and HDL-C levels. These findings are comparable to those reported by previous studies.[20,21] The changes in lipid profile confirmed the effects of thyroid function on lipid metabolism. S14 has been reported to play a role in fatty acid synthesis.[11,12] Our study revealed a decrease in lipid levels despite a rise in serum S14 in patients with hyperthyroidism. This finding suggests a direct effect of thyroid hormone on lipid metabolism rather than an indirect mechanism via S14.
It has been reported that patients with hyperthyroidism usually have increased glomerular filtration rate and reduced serum Cre levels.[22,23] In this study, the patients with hyperthyroidism had lower serum Cre levels than the subjects with euthyroidism. Our analysis revealed that serum Cre was negatively associated with level of logS14. The association between Cre and logS14 remained significant after adjustment with sex and age, but it became insignificant in multivariate stepwise regression. S14 gene is mainly expressed in lipid-producing tissues such as liver, adipose tissue, and lactating mammary glands,[10] but less in other tissues. MacDougald et al suggested different mechanisms of S14 transcription in liver or kidney.[24] The relationship between S14 and Cre, especially in different thyroid function statuses, remains to be clarified.
Thyroid hormones may regulate the basal metabolic rate of hepatocytes and affect hepatic function.[25,26] Around 15% to 76% patients with hyperthyroidism presented abnormal liver function test.[25,27–29] S14 has been reported to be highly expressed in the liver and involved in fatty acid synthesis.[10,11] Wu et al suggested that S14 may play a role in the pathogenesis of nonalcoholic fatty liver disease (NAFLD).[30] However, there is no direct evidence supporting the impact of S14 expression level on liver function yet. In this study, patients with hyperthyroidism had higher AST and ALT levels than the subjects with euthyroidism. The linear regression in this study revealed no association between serum level of logS14 with AST or ALT.
The association between S14 and insulin resistance has been discussed in animal studies.[14,15] S14 mRNA in the rat liver can be increased with insulin or carbohydrate diet treatment.[4,7–9] However, there is no study to show whether this is also true in human subjects. Therefore, our development of this S14 ELISA will be helpful to delineate the roles of S14 in obesity, metabolic syndrome, and type 2 diabetes mellitus. Changes of thyroid function may affect glucose and insulin metabolism.[31] S14 expression in the rat liver could be affected by thyroid hormone.[3,4] Our study revealed that patients with hyperthyroidism has higher serum S14 than subjects with euthyroidism. The negative association between TSH quartile groups with levels of logS14 persisted after adjustment with sex and age, and remained significant in forward stepwise logistic regression. The impact of different thyroid function statuses on the association between S14 and insulin resistance deserves further investigation.
S14 gene is expressed in lactating mammary glands.[10] Deletion of S14 dramatically reduced fatty acid synthesis in mammary glands.[12,13] It was speculated that the reduced fatty acid synthesis in mammary gland in S14-deletion mice was secondary to the lack of S14-R.[13] Most breast cancers rely on fatty acid synthesis for growth and survival.[32,33] Using tumor tissue blocks from breast cancer patients for immunohistochemistry, it was found that S14 protein was detectable in all mammary samples, and was highly expressed in malignant breast cancers.[34] Using S14 siRNA to knockdown the S14 gene expression in lipogenic T47D breast cancer cells and nonlipogenic MCF10a mammary epithelial cells, Wells et al found that the cell growth was inhibited only in T47D cells.[34] These studies suggest that S14 may play a role in the tumorigenesis of lipogenic breast cancers.[34–36] Therefore, our in-house ELISA may also be helpful for breast cancer research.
The known biological functions of S14 are strictly intracellular and mainly in the liver and adipose tissues.[10–14] Whether the circulating S14 may have a distinct biological activity beyond simply a biomarker is unclear. We boldly speculate that elevated circulating S14 may be taken up by cells which require enhanced fatty acid synthesis.
In our study, logS14 was positively correlated with fT4 and negatively correlated with TSH. Those correlations persisted after adjustment with sex and age. However, only TSH quartile remained significant in the stepwise multivariate regression model. We applied fixed values of fT4 or TSH to those levels exceeding the ranges of the commercial kits, the significance of the correlations between fT4 or TSH with levels of S14 thus could be biased. The circulating concentrations of TSH and thyroxine are tightly regulated in healthy individuals.[37,38] It was reported that the relationship between TSH and fT4 is complex and nonlinear.[37,38] Small changes in thyroxine levels may result in relatively large changes in TSH.[37,38] Our study clearly demonstrated that patients with hyperthyroidism had higher serum levels of S14 than subjects with euthyroidism. Whether TSH has a direct influence on S14 levels warrants future study.
Although this is the first human study to investigate human serum S14 levels, there were still several limitations in our study. First, when the levels of fT4 or TSH exceed the linear range of the commercial kits used in our hospital, fixed values were assigned. Furthermore, fT3 level was not measured in our study. We were unable to directly correlate S14 with fT3. Therefore, it may be difficult to determine a precise linear relationship between thyroid function and serum S14 levels. Second, our case numbers were small. More subjects should be recruited in future study. Nevertheless, a significant relation between thyroid function and serum S14 levels was observed with this small number. Third, patients with hypothyroidism were not recruited in this study. Whether the negative association between TSH and serum S14 persisted in the whole thyroid function spectrum remained to be studied. Fourth, the study was performed in a medical center. The generalizability is limited. Fifth, the S14-R protein is 32% homologous to S14 in amino acid sequences. Since a polyclonal antibody against S14 was used, our ELISA may detect S14-R as well. However, S14-R was known not to be influenced by thyroid hormone treatment.[13] Therefore, the negative relation between the serum S14 and thyroid function cannot be ascribed to S14-R. Specific monoclonal antibodies should be generated in the future to solve this problem. Sixth, changes of serum S14 levels after treatment for thyroid dysfunctions would provide more information concerning the correlations between S14 and fT4 or TSH. However, we had no posttreatment data for the hyperthyroid patients in this study. Seventh, our analysis revealed that the differences of S14 concentrations among hyperthyroid or euthyroid patients were prominent in females but not in males. We had small male patient number. The sex effect on the changes of serum S14 levels in hyperthyroidism or euthyroidism remained to be investigated. Eighth, glucose may influence the levels of S14; there were 2 subjects in hyperthyroid group and 1 in euthyroid group with elevated fasting glucose. However, there was no difference in FPG levels between patients with hyperthyroidism and euthyroidism. In addition, we did not have information of their dietary content, including carbohydrate intake.
In conclusion, we developed an ELISA to measure serum level of S14. We presented the first study to evaluate the serum S14 level in hyperthyroidism or euthyroidism in human. Our study revealed that patients with hyperthyroidism had higher serum level of S14 than subjects with euthyroidism. The difference of S14 levels among hyperthyroid or euthyroid patients were prominent in females but not in males. The TSH quartile group had negative association with serum logS14. The S14 ELISA could be applied to study the impact of S14 in various conditions in human, such as lipid metabolism, insulin resistance, obesity, or breast cancer. The negative association between S14 and TSH should be further investigated in the whole thyroid function spectrum. A more comprehensive follow-up study is required to investigate whether S14 can be a biomarker to monitor thyroid function and treatment response.
Acknowledgments
We would like to express our sincere appreciation for Prof Shu-Hui Chang, Dr Deng-Huang Su, and Dr Chih-Hao Chang for statistic consultations.
Footnotes
Abbreviations: ALT = alanine transaminase, AST = aspartate transaminase, BH = body height, BMI = body mass index, BW = body weight, Cre = creatinine, ELISA = enzyme-linked immunosorbent assay, FPG = fasting plasma glucose, fT4 = free thyroxine, HDL-C = high-density lipoprotein cholesterol, LDL-C = low-density lipoprotein cholesterol, logS14 = log transformation of Spot 14, S14 = Spot 14, T-C = total cholesterol, TG = triglycerides, THRSP = thyroid hormone responsive protein, TSH = thyroid-stimulating hormone.
Authors’ contribution: YTC wrote the main manuscript text, provided figures, and established the ELISA of S14. FYT and PLC provided the source of human subjects. YCC and DSH advised and facilitated the assay. WSY acquired the grant, supervised the project, and edited the manuscript. All authors reviewed the manuscript.
Funding/support: This work was supported by research grants (MOST 104–2314-B-002–157) from the National Research Program for Biopharmaceuticals (NRPB), Ministry of Science & Technology of Taiwan.
Authors have no competing interests as defined by Nature Publishing Group, or other interests that might be perceived to influence the results and/or discussion reported in this paper.
References
- 1.Seelig S, Liaw C, Towle HC, et al. Thyroid hormone attenuates and augments hepatic gene expression at a pretranslational level. Proc Natl Acad Sci U S A 1981; 78:4733–4737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Franklyn JA, King S, Ahlquist JA, et al. Effect of hypothyroidism and thyroid hormone treatment of the rat on hepatic Spot 14 and thyroxine binding prealbumin mRNAs. Acta Endocrinol (Copenh) 1989; 121:383–388. [DOI] [PubMed] [Google Scholar]
- 3.Jump DB, Narayan P, Towle H, et al. Rapid effects of triiodothyronine on hepatic gene expression. Hybridization analysis of tissue-specific triiodothyronine regulation of mRNAS14. J Biol Chem 1984; 259:2789–2797. [PubMed] [Google Scholar]
- 4.Mariash CN, Seelig S, Schwartz HL, et al. Rapid synergistic interaction between thyroid hormone and carbohydrate on mRNAS14 induction. J Biol Chem 1986; 261:9583–9586. [PubMed] [Google Scholar]
- 5.Liu HC, Towle HC. Functional synergism between multiple thyroid hormone response elements regulates hepatic expression of the rat S14 gene. Mol Endocrinol 1994; 8:1021–1037. [DOI] [PubMed] [Google Scholar]
- 6.Brown SB, Maloney M, Kinlaw WB. Spot 14 protein functions at the pretranslational level in the regulation of hepatic metabolism by thyroid hormone and glucose. J Biol Chem 1997; 272:2163–2166. [PubMed] [Google Scholar]
- 7.Jump DB, Bell A, Lepar G, et al. Insulin rapidly induces rat liver S14 gene transcription. Mol Endocrinol 1990; 4:1655–1660. [DOI] [PubMed] [Google Scholar]
- 8.Jump DB, Bell A, Santiago V. Thyroid hormone and dietary carbohydrate interact to regulate rat liver S14 gene transcription and chromatin structure. J Biol Chem 1990; 265:3474–3478. [PubMed] [Google Scholar]
- 9.Koo SH, Towle HC. Glucose regulation of mouse S(14) gene expression in hepatocytes. Involvement of a novel transcription factor complex. J Biol Chem 2000; 275:5200–5207. [DOI] [PubMed] [Google Scholar]
- 10.Jump DB, Oppenheimer JH. High basal expression and 3,5,3′-triiodothyronine regulation of messenger ribonucleic acid S14 in lipogenic tissues. Endocrinology 1985; 117:2259–2266. [DOI] [PubMed] [Google Scholar]
- 11.Kinlaw WB, Church JL, Harmon J, et al. Direct evidence for a role of the “spot 14” protein in the regulation of lipid synthesis. J Biol Chem 1995; 270:16615–16618. [DOI] [PubMed] [Google Scholar]
- 12.Zhu Q, Anderson GW, Mucha GT, et al. The Spot 14 protein is required for de novo lipid synthesis in the lactating mammary gland. Endocrinology 2005; 146:3343–3350. [DOI] [PubMed] [Google Scholar]
- 13.Aipoalani DL, O’Callaghan BL, Mashek DG, et al. Overlapping roles of the glucose-responsive genes, S14 and S14R, in hepatic lipogenesis. Endocrinology 2010; 151:2071–2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson GW, Zhu Q, Metkowski J, et al. The Thrsp null mouse (Thrsp(tm1cnm)) and diet-induced obesity. Mol Cell Endocrinol 2009; 302:99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.LaFave LT, Augustin LB, Mariash CN. S14: insights from knockout mice. Endocrinology 2006; 147:4044–4047. [DOI] [PubMed] [Google Scholar]
- 16.Kirschner LS, Mariash CN. Adipose S14 mRNA is abnormally regulated in obese subjects. Thyroid 1999; 9:143–148. [DOI] [PubMed] [Google Scholar]
- 17.Ortega FJ, Vazquez-Martin A, Moreno-Navarrete JM, et al. Thyroid hormone responsive Spot 14 increases during differentiation of human adipocytes and its expression is down-regulated in obese subjects. Int J Obes (Lond) 2010; 34:487–499. [DOI] [PubMed] [Google Scholar]
- 18.Pontikides N, Krassas GE. Basic endocrine products of adipose tissue in states of thyroid dysfunction. Thyroid 2007; 17:421–431. [DOI] [PubMed] [Google Scholar]
- 19.Silva JE. The thermogenic effect of thyroid hormone and its clinical implications. Ann Intern Med 2003; 139:205–213. [PubMed] [Google Scholar]
- 20.Canaris GJ, Manowitz NR, Mayor G, et al. The Colorado thyroid disease prevalence study. Arch Intern Med 2000; 160:526–534. [DOI] [PubMed] [Google Scholar]
- 21.Knudsen N, Laurberg P, Rasmussen LB, et al. Small differences in thyroid function may be important for body mass index and the occurrence of obesity in the population. J Clin Endocrinol Metab 2005; 90:4019–4024. [DOI] [PubMed] [Google Scholar]
- 22.Mariani LH, Berns JS. The renal manifestations of thyroid disease. J Am Soc Nephrol 2012; 23:22–26. [DOI] [PubMed] [Google Scholar]
- 23.Verhelst J, Berwaerts J, Marescau B, et al. Serum creatine, creatinine, and other guanidino compounds in patients with thyroid dysfunction. Metabolism 1997; 46:1063–1067. [DOI] [PubMed] [Google Scholar]
- 24.MacDougald OA, Clarke SD, Jump DB. Tissue specificity of S14 and fatty acid synthase in vitro transcription. Biochem Biophys Res Commun 1992; 182:631–637. [DOI] [PubMed] [Google Scholar]
- 25.Khemichian S, Fong TL. Hepatic dysfunction in hyperthyroidism. Gastroenterol Hepatol (N Y) 2011; 7:337–339. [PMC free article] [PubMed] [Google Scholar]
- 26.Malik R, Hodgson H. The relationship between the thyroid gland and the liver. QJM 2002; 95:559–569. [DOI] [PubMed] [Google Scholar]
- 27.Biscoveanu M, Hasinski S. Abnormal results of liver function tests in patients with Graves’ disease. Endocr Pract 2000; 6:367–369. [DOI] [PubMed] [Google Scholar]
- 28.Gurlek A, Cobankara V, Bayraktar M. Liver tests in hyperthyroidism: effect of antithyroid therapy. J Clin Gastroenterol 1997; 24:180–183. [DOI] [PubMed] [Google Scholar]
- 29.Kubota S, Amino N, Matsumoto Y, et al. Serial changes in liver function tests in patients with thyrotoxicosis induced by Graves’ disease and painless thyroiditis. Thyroid 2008; 18:283–287. [DOI] [PubMed] [Google Scholar]
- 30.Wu J, Wang C, Li S, et al. Thyroid hormone-responsive SPOT 14 homolog promotes hepatic lipogenesis, and its expression is regulated by liver X receptor alpha through a sterol regulatory element-binding protein 1c-dependent mechanism in mice. Hepatology 2013; 58:617–628. [DOI] [PubMed] [Google Scholar]
- 31.Maratou E, Hadjidakis DJ, Peppa M, et al. Studies of insulin resistance in patients with clinical and subclinical hyperthyroidism. Eur J Endocrinol 2010; 163:625–630. [DOI] [PubMed] [Google Scholar]
- 32.Flavin R, Peluso S, Nguyen PL, et al. Fatty acid synthase as a potential therapeutic target in cancer. Future Oncol 2010; 6:551–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zaidi N, Lupien L, Kuemmerle NB, et al. Lipogenesis and lipolysis: the pathways exploited by the cancer cells to acquire fatty acids. Prog Lipid Res 2013; 52:585–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wells WA, Schwartz GN, Morganelli PM, et al. Expression of “Spot 14” (THRSP) predicts disease free survival in invasive breast cancer: immunohistochemical analysis of a new molecular marker. Breast Cancer Res Treat 2006; 98:231–240. [DOI] [PubMed] [Google Scholar]
- 35.Kinlaw WB, Quinn JL, Wells WA, et al. Spot 14: a marker of aggressive breast cancer and a potential therapeutic target. Endocrinology 2006; 147:4048–4055. [DOI] [PubMed] [Google Scholar]
- 36.Martel PM, Bingham CM, McGraw CJ, et al. S14 protein in breast cancer cells: direct evidence of regulation by SREBP-1c, superinduction with progestin, and effects on cell growth. Exp Cell Res 2006; 312:278–288. [DOI] [PubMed] [Google Scholar]
- 37.Hadlow NC, Rothacker KM, Wardrop R, et al. The relationship between TSH and free T(4) in a large population is complex and nonlinear and differs by age and sex. J Clin Endocrinol Metab 2013; 98:2936–2943. [DOI] [PubMed] [Google Scholar]
- 38.Hoermann R, Eckl W, Hoermann C, et al. Complex relationship between free thyroxine and TSH in the regulation of thyroid function. Eur J Endocrinol 2010; 162:1123–1129. [DOI] [PubMed] [Google Scholar]


