Abstract
Background:
Although adenovirus (ADV) infection usually causes self-limiting respiratory disorders in immune competent children; severe and systemic ADV infection in children undergoing chemotherapy for leukemia has been continuously reported. Nevertheless, there has been no consensus on risk factors and treatment strategies for severe ADV infection in children undergoing chemotherapy.
Case summary:
We report a case of a 15-year-old boy with a fatal systemic ADV infection. He had received reinduction chemotherapy for relapsed acute lymphoblastic leukemia under continuing antifungal therapy for previously diagnosed fungal pneumonia. He complained of fever and right shoulder pain 4 days after completing the reinduction chemotherapy. In spite of appropriate antibiotic and antifungal therapy, pneumonia was aggravated and gross hematuria was accompanied. A multiplex polymerase chain reaction test for respiratory viruses was positive for ADV in a blood sample, and a urine culture was positive for ADV. He received oral ribavirin, intravenous immunoglobulin, and intravenous cidofovir therapy; however, he eventually died. Relapsed leukemia, concurrent fungal pneumonia, and delayed cidofovir administration were considered the cause of the grave outcome in this patient.
Conclusion:
ADV may cause severe infections not only in allogeneic hematopoietic cell transplant recipients, but also in patients undergoing chemotherapy for acute leukemia. The risk factors for severe ADV infection in patients undergoing chemotherapy should be determined in the future studies, and early antiviral therapy should be administered to immune compromised patients with systemic ADV infection.
Keywords: adenovirus, case report, child, leukemia
1. Introduction
Adenovirus (ADV) infection usually causes self-limiting respiratory disorders in immune competent children.[1] However, the severity of ADV infection varies according to ADV serotype, and higher severity and poorer prognosis have been observed in immune compromised children compared with immune competent children.[1,2] Among children receiving allogeneic hematopoietic cell transplantation (HCT), ADV viremia occurred in 6% to 28% of cases, and 13% to 50% of patients with ADV infection died.[3] Meanwhile, the incidence of ADV infection is relatively low in children receiving anticancer chemotherapy for acute leukemia. ADV reportedly causes less than 5% of respiratory viral infections in these patients, and few die from ADV infections.[4,5] We saw a case of fatal systemic ADV infection in a child with acute lymphoblastic leukemia (ALL). The patient received reinduction chemotherapy for relapsed ALL under antifungal therapy for previously diagnosed fungal pneumonia, and was infected by ADV during neutropenia following the reinduction chemotherapy. Although the patient did not receive allogeneic HCT, relapsed ALL and concurrent fungal pneumonia in the patient might have led to the grave outcome of his ADV infection. This report was approved by the Institutional Review Board of Seoul St. Mary's Hospital (Approval number: KC16ZISE0626).
2. Case report
A 15-year-old boy complained of fever and right shoulder pain after reinduction chemotherapy for relapsed ALL. He was diagnosed with ALL at another hospital 3 years and 9 months prior, and was transferred to our hospital 2 months prior. At that time, he received reinduction chemotherapy for his second relapse of ALL; however, there was no response, and he received another course of reinduction chemotherapy during this hospitalization.
His fever and right shoulder pain developed 4 days after completing the reinduction chemotherapy. The patient had been diagnosed with possible invasive pulmonary aspergillosis (IPA) during a previous hospitalization and had received oral voriconazole (4 mg/kg twice a day) therapy for 3 weeks following liposomal amphotericin B (5 mg/kg/day) therapy for 2 weeks. On the fever day (FD) 1, blood tests revealed a white blood cell count of 50/mm3 (neutrophils 0%, lymphocytes 0%), hemoglobin concentration of 8.3 g/dL, platelet count of 53000/mm3, erythrocyte sedimentation rate of 34 mm/h, C-reactive protein level of 8.70 mg/dL, and serum galactomannan level of 0.21. Chest X-ray images showed a round consolidation on the right upper lung fields, similar to the finding observed on admission (Fig. 1A and B). Empirical antibiotic therapy with piperacillin/tazobactam and isepamicin was initiated for febrile neutropenia. The dose of voriconazole was increased to 7 mg/kg twice daily because his serum voriconazole level was <0.5 μg/mL. Empirical antibiotics were changed to meropenem and teicoplanin due to persisting fever on FD 3. Although his serum voriconazole level was 8.6 μg/mL and serum galactomannan level was 0.14 on FD 6, the fever persisted. Chest computed tomography (CT) imaging performed on FD 6 showed an aggravation of the pneumonic consolidation on the right upper lobe compared with the chest CT images used for the diagnosis of fungal pneumonia during the previous hospitalization (Fig. 2A and B). The antifungal agent was changed to liposomal amphotericin B (5 mg/kg/day) because we considered that the previously diagnosed possible IPA might actually be mucormycosis. However, his fever persisted, and gross hematuria developed on FD 12. We suspected ADV infection based on the concurrent hematuria and aggravation of the respiratory disease identified on the chest X-ray images (Fig. 1C). A multiplex polymerase chain reaction (PCR) test for respiratory viruses was positive for ADV in a blood sample. The PCR test was qualitative with no report on viral DNA titer, but the cycle threshold was 26.64 for positive PCR results. Urinary BK virus, cytomegalovirus, and other bacteria were not identified; however, a urine culture was later reported to be positive for ADV. On FD 15, oral ribavirin (10 mg/kg 3 times daily) rather than cidofovir was initiated, as the patient had not received allogeneic HCT and his general condition was relatively stable except for his prolonged fever. However, the fever and gross hematuria did not resolve, and chest X-ray images showed an aggravation of the pneumonia without respiratory symptoms. Chest CT performed on FD 21 showed further aggravation of the pneumonic consolidation on the right upper lobe and a new consolidation on the right lower lobe accompanying pleural effusion (Fig. 2C). We considered superimposing ADV pneumonia and proposed cidofovir therapy; however, his parents did not accept this treatment due to its high costs. Intravenous immunoglobulin (IVIG) of 1 g/kg was administered for 3 days instead of cidofovir; however, repeated multiplex PCR of blood was positive for ADV, with a cycle threshold of 14.92 on FD 28, and his fever persisted. Although additional IVIGs (150 mg/kg/day) were administered for 5 days, the fever persisted, his serum creatinine level increased to 2.36 mg/dL and oliguria developed. On FD 33, all antibiotic and antifungal agents were stopped due to acute kidney injury, and bilateral percutaneous nephrostomy was performed. His serum creatinine level began to decrease; however, the fever did not resolve and chest X-ray images showed further aggravation of the pneumonia (Fig. 1D). Although the first dose of cidofovir (5 mg/kg) was administered on FD 36, the patient complained of dyspnea and tachypnea, and required oxygen therapy on FD 38. On FD 40, multiplex PCR of a blood sample was again positive for ADV, with a cycle threshold of 13.41, and liposomal amphotericin B, which was stopped due to acute kidney injury, was readministered. The patient's respiratory symptoms and chest X-ray findings worsened. Mechanical ventilation was applied on FD 45, and he died on FD 48. A bronchial wash fluid culture from a sample collected on FD 46 was reported as positive for Rhizopus spp. and ADV after his death.
Figure 1.
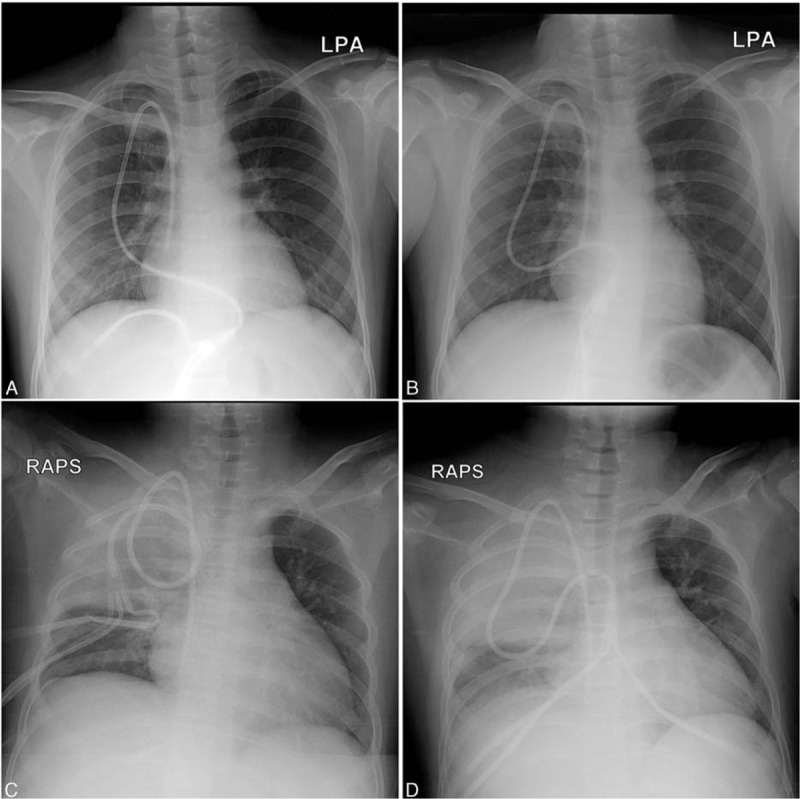
Chest X-ray images showing round consolidations on the right upper lung fields upon admission (A), insignificant change in the consolidation on fever day 1 (B), and aggravation of the pneumonic consolidation on fever days 12 (C) and 35 (D), respectively.
Figure 2.
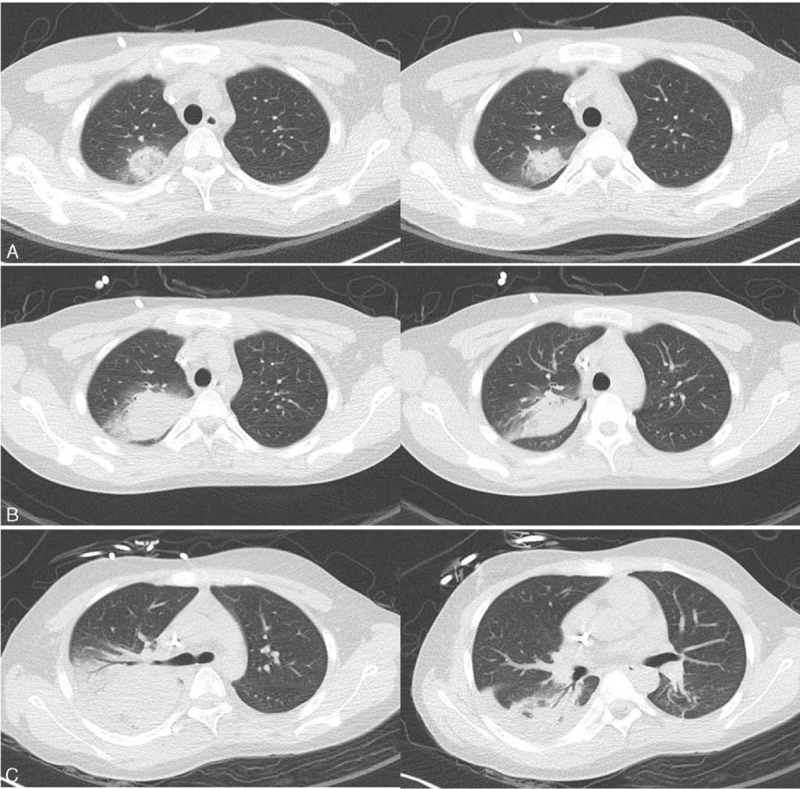
Chest computed tomography showing pneumonic consolidation with surrounding ground glass opacity on the right upper lobe during the previous hospitalization (A), and aggravation of the pneumonic consolidation on fever days 6 (B) and 21 (C), respectively.
3. Discussion
ADV can cause severe infection and fatality in immune compromised patients.[1,2] Weekly monitoring with quantitative PCR for ADV in blood is recommended in allogeneic HCT recipients at risk for ADV infection, and preemptive antiviral therapy with cidofovir is recommended for patients positive for ADV viremia.[3] However, routine monitoring for ADV infection in autologous HCT recipients and leukemia patients receiving chemotherapy is not necessary due to its lower incidence and severity compared with allogeneic HCT recipients.[3] Respiratory viral infections occur in 43% to 59% of children undergoing chemotherapy or those with febrile neutropenia; however, severe complications due to respiratory viral infections are rare and mortality due to respiratory viral infections is only 0% to 2%.[4–6] ADV comprises only 2% to 5% of respiratory viral infections in immune compromised children, less frequent than rhinovirus and respiratory syncytial virus infections: thus, the clinical impact of ADV infection has been underestimated.[4–6] However, severe and systemic ADV infection in children undergoing chemotherapy for leukemia has been continuously reported.[7–10] Children with severe ADV infections tend to have low absolute lymphocyte counts; however, severe ADV infection may occur during any period of the scheduled chemotherapy, including induction, consolidation, and maintenance chemotherapy.[7–10] Nevertheless, there has been no report on risk factors for severe ADV infection in children undergoing chemotherapy. Future studies are required to elucidate the risk factors for severe ADV infection in children undergoing chemotherapy, and appropriate strategies for early diagnosis and antiviral treatment for children most at risk should be established.
Although no specific antiviral therapy has been established for ADV infection, cidofovir is recommended as the first-line agent for ADV infection in allogeneic HCT recipients.[3] Ribavirin is a broad-spectrum antiviral agent; however, its antiviral mechanisms have not been exactly defined.[11] Because a significant efficacy of ribavirin was not observed in allogeneic HCT recipients with systemic ADV infections,[12,13] it is not recommended as a therapeutic agent for ADV infections in allogeneic HCT recipients.[3] However, its clinical effectiveness in allogeneic HCT recipient with hemorrhagic cystitis due to ADV has been reported.[11,14] Systemic ADV infections, which occurred in a patient with myelodysplastic syndrome, who had not received HCT, and in solid organ transplant recipients, responded to ribavirin therapy.[11,15,16] Our patient, who had not received allogeneic HCT, was relatively clinically stable upon diagnosis of ADV viremia, although he complained of fever and gross hematuria. At that time, we administered ribavirin rather than cidofovir because there are currently no guidelines for treatment of ADV infection in children undergoing chemotherapy rather than HCT. In addition, cidofovir may cause adverse effects, such as kidney injury and bone marrow suppression, and increase hospital cost. Although cases showing the IVIG effect on systemic ADV infections in solid organ transplant recipients were reported,[17] its effect on ADV infections of immune compromised patients has not been evaluated in controlled studies. Moreover, IVIG administration cannot guarantee a neutralizing effect against the specific ADV serotype.[11] Therefore, IVIG is not recommended as a therapeutic agent for ADV infections as well as ribavirin.[3,11] Although our patient's clinical status and viremia worsened after ribavirin therapy, cidofovir therapy was delayed for 3 weeks after the diagnosis of ADV viremia due to parental hesitation in deciding to administer cidofovir. Despite administration of cidofovir, 43% to 50% of allogeneic HCT recipients with ADV viremia die: early administration of cidofovir reportedly improves the prognosis of ADV infections.[18,19] Although our patient had not received allogeneic HCT and T-cell-specific immune suppressants, uncontrolled underlying ALL, concurrent fungal pneumonia, and delayed cidofovir administration could have led to the grave outcome. Fatal ADV infections in allogeneic HCT recipients with concurrent bacterial and fungal coinfections have been reported, although the ADV DNA titers from blood samples were low.[20]
In conclusion, ADV may cause severe infections not only in allogeneic HCT recipients, but also in patients undergoing chemotherapy for acute leukemia. ADV infection should be considered in immune compromised patients with severe respiratory tract infections or hemorrhagic cystitis. The risk factors for severe ADV infection in patients undergoing chemotherapy should be determined, and early antiviral therapy should be administered to immune compromised patients with systemic ADV infection.
Footnotes
Abbreviations: ADV = adenovirus, ALL = acute lymphoblastic leukemia, CT = computed tomography, FD = fever day, HCT = hematopoietic cell transplantation, IPA = invasive pulmonary aspergillosis, IVIG = intravenous immunoglobulin, PCR = polymerase chain reaction.
The authors have no conflicts of interest to disclose.
References
- 1.Lynch JP, III, Fishbein M, Echavarria M. Adenovirus. Semin Respir Crit Care Med 2011; 32:494–511. [DOI] [PubMed] [Google Scholar]
- 2.Munoz FM, Piedra PA, Demmler GJ. Disseminated adenovirus disease in immunocompromised and immunocompetent children. Clin Infect Dis 1998; 27:1194–1200. [DOI] [PubMed] [Google Scholar]
- 3.Matthes-Martin S, Feuchtinger T, Shaw PJ, et al. European guidelines for diagnosis and treatment of adenovirus infection in leukemia and stem cell transplantation: summary of ECIL-4 (2011). Transpl Infect Dis 2012; 14:555–563. [DOI] [PubMed] [Google Scholar]
- 4.Hakim H, Dallas R, Zhou Y, et al. Acute respiratory infections in children and adolescents with acute lymphoblastic leukemia. Cancer 2016; 122:798–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koskenvuo M, Möttönen M, Rahiala J, et al. Respiratory viral infections in children with leukemia. Pediatr Infect Dis J 2008; 27:974–980. [DOI] [PubMed] [Google Scholar]
- 6.Torres JP, Labraña Y, Ibañez C, et al. Frequency and clinical outcome of respiratory viral infections and mixed viral-bacterial infections in children with cancer, fever and neutropenia. Pediatr Infect Dis J 2012; 31:889–893. [DOI] [PubMed] [Google Scholar]
- 7.Steiner I, Aebi C, Ridolfi Lüthy A, et al. Fatal adenovirus hepatitis during maintenance therapy for childhood acute lymphoblastic leukemia. Pediatr Blood Cancer 2008; 50:647–649. [DOI] [PubMed] [Google Scholar]
- 8.Caselli D, Tondo A, Tucci F, et al. Adenovirus pneumonia during induction therapy for childhood acute lymphoblastic leukemia. Pediatr Blood Cancer 2013; 60:1390–1391. [DOI] [PubMed] [Google Scholar]
- 9.Hough R, Chetwood A, Sinfield R, et al. Fatal adenovirus hepatitis during standard chemotherapy for childhood acute lymphoblastic leukemia. J Pediatr Hematol Oncol 2005; 27:67–72. [DOI] [PubMed] [Google Scholar]
- 10.Alcamo AM, Pinchasik DE, Mo JQ, et al. Successful treatment of disseminated adenovirus infection in an infant with acute lymphoblastic leukemia. J Pediatr Hematol Oncol 2015; 37:e178–e181. [DOI] [PubMed] [Google Scholar]
- 11.Lenaerts L, De Clercq E, Naesens L. Clinical features and treatment of adenovirus infections. Rev Med Virol 2008; 18:357–374. [DOI] [PubMed] [Google Scholar]
- 12.Omar H, Yun Z, Lewensohn-Fuchs I, et al. Poor outcome of adenovirus infections in adult hematopoietic stem cell transplant patients with sustained adenovirus viremia. Transpl Infect Dis 2010; 12:465–469. [DOI] [PubMed] [Google Scholar]
- 13.Bordigoni P, Carret AS, Venard V, et al. Treatment of adenovirus infections in patients undergoing allogeneic hematopoietic stem cell transplantation. Clin Infect Dis 2001; 32:1290–1297. [DOI] [PubMed] [Google Scholar]
- 14.Abe S, Miyamura K, Oba T, et al. Oral ribavirin for severe adenovirus infection after allogeneic marrow transplantation. Bone Marrow Transplant 2003; 32:1107–1108. [DOI] [PubMed] [Google Scholar]
- 15.Ulrych EE, Dzieciatkowski T, Przybylski M, et al. Disseminated adenovirus disease in immunocompromised patient successfully treated with oral ribavirin: a case report. Arch Immunol Ther Exp 2011; 59:473–477. [DOI] [PubMed] [Google Scholar]
- 16.Park UJ, Hyun SK, Kim HT, et al. Successful treatment of disseminated adenovirus infection with ribavirin and intravenous immunoglobulin in an adult renal transplant recipient: a case report. Transplant Proc 2015; 47:791–793. [DOI] [PubMed] [Google Scholar]
- 17.Dawood US, Nelson A, Wu D, et al. Disseminated adenovirus infection in kidney transplant recipient. Nephrology 2014; 19 suppl 1:10–13. [DOI] [PubMed] [Google Scholar]
- 18.Caruso Brown AE, Cohen MN, Tong S, et al. Pharmacokinetics and safety of intravenous cidofovir for life-threatening viral infections in pediatric hematopoietic stem cell transplant recipients. Antimicrob Agents Chemother 2015; 59:3718–3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lion T, Kosulin K, Landlinger C, et al. Monitoring of adenovirus load in stool by real-time PCR permits early detection of impending invasive infection in patients after allogeneic stem cell transplantation. Leukemia 2010; 24:706–714. [DOI] [PubMed] [Google Scholar]
- 20.Engelmann I, Coiteux V, Heim A, et al. Severe adenovirus pneumonia followed by bacterial septicaemia: relevance of co-infections in allogeneic hematopoietic stem cell transplantation. Infect Disord Drug Targets 2016; 16:69–76. [DOI] [PubMed] [Google Scholar]


