Abstract
The aim of this study was to investigate the relationships between pulmonary function, respiratory muscle strength, perceived dyspnea, degree of fatigue, and activity of daily living with motor function and neurological status in stroke patients with stable congestive heart failure (CHF).
This was a cohort study in a tertiary care medical center. Stroke patients with CHF and exertional dyspnea (New York Heart Association class I–III) were recruited. The baseline characteristics included duration of disease, Brunnstrom stage, spirometry, resting heart rate, resting oxyhemoglobin saturation (SpO2), maximal inspiratory pressure (MIP), maximal expiratory pressure (MEP), Borg scale, fatigue scale, and Barthel index.
A total of 47 stroke patients (24 males, 23 females, mean age 65.9 ± 11.5 years) were included. The average Brunnstrom stages of affected limbs were 3.6 ± 1.3 over the proximal parts and 3.5 ± 1.4 over the distal parts of upper limbs, and 3.9 ± 0.9 over lower limbs. The average forced vital capacity (FVC) was 2.0 ± 0.8 L, with a predicted FVC% of 67.9 ± 18.8%, forced expiratory volume in the first second (FEV1) of 1.6 ± 0.7 L, predicted FEV1% of 70.6 ± 20.1%, FEV1/FVC of 84.2 ± 10.5%, and maximum mid-expiratory flow of 65.4 ± 29.5%. The average MIP and MEP were −52.9 ± 33.3 cmH2O and 60.8 ± 29.0 cmH2O, respectively. The Borg scale was 1.5 ± 0.8. MIP was negatively associated with the average Brunnstrom stage of the proximal (r = −0.318, P < 0.05) and distal (r = −0.391, P < 0.01) parts of the upper extremities and lower extremities (r = −0.288, P < 0.05), FVC (r = −0.471, P < 0.01), predicted FVC% (r = −0.299, P < 0.05), and FEV1 (r = −0.397, P < 0.01). MEP was positively associated with average Brunnstrom stage of the distal area of the upper extremities (r = 0.351, P < 0.05), FVC (r = 0.526, P < 0.01), FEV1 (r = 0.429, P < 0.01), and FEV1/FVC (r = −0.482, P < 0.01). FEV1/FVC was negatively associated with the average Brunnstrom stage over the proximal (r = −0.414, P < 0.01) and distal (r = −0.422, P < 0.01) parts of the upper extremities and lower extremities (r = −0.311, P < 0.05) and Barthel index (r = −0.313, P < 0.05).
Stroke patients with stable CHF and exertional dyspnea had restrictive lung disorder and respiratory muscle weakness, which were associated with the neurological status of the affected limbs. FVC was more strongly associated with MIP and MEP than predicted FVC%. FEV1/FVC may be used as a reference for the pulmonary dysfunction.
Keywords: Barthel index, Borg scale, cerebrovascular accident, congestive heart failure, fatigue scale, maximal expiratory pressure, maximal inspiratory pressure
1. Introduction
Patients with congestive heart failure (CHF) commonly have general weakness, low energy, and dyspnea on exertion, and this can be exacerbated after a cerebrovascular accident. These patients often avoid exertion, thereby leading to a vicious circle of inactivity and poor fitness.
Stroke patients have been reported to have reduced diaphragmatic excursion, higher position of the affected diaphragm in thoracic radiography, and different degrees of pulmonary dysfunction.[1,2,3] Decreases in maximal inspiratory pressure (MIP) and maximal expiratory pressure (MEP), and the predominant contribution of the rib cage during tidal breathing in the supine position have been suggested to be caused by chronic weaknesses of the diaphragm and abdominal muscles in chronic stroke survivors.[4]
Dyspnea and reduced exercise or functional capacity in patients with chronic heart failure can be caused by left ventricular dysfunction and also weakness of the inspiratory muscles.[5–10] Chiappa et al reported that patients with chronic heart failure have weak inspiratory muscles, and that loading of these muscles may result in a marked reduction in blood flow to resting and exercising limbs. They further reported that training inspiratory muscles could improve blood flow to resting and exercising limbs.[5]
Walsh et al reported the significance of a reduction in respiratory muscle endurance in relation to limb muscle strength using the threshold loading technique in patients with mild to moderate CHF (New York Heart Association (NYHA) grade I–III).[11] In addition, inspiratory muscle strength has been reported to be of prognostic value and that it can be used as a therapeutic target and surrogate marker in chronic heart failure.[7]
The Framingham Heart Study criteria had 100% sensitivity and 78% specificity for identifying persons with definite CHF.[12] In addition, the Fatigue Assessment Scale (FAS) has been used to measure fatigue in patients with stroke and in patients with CHF, and the results were similar (15.3 ± 7.6 and 16.5 ± 7.9, respectively; P = 0.44). Furthermore, the fatigue scores of both groups of patients were considerably higher than those of healthy controls.[1]
We hypothesized that patients with both stroke and heart failure will have worse cardiopulmonary function and respiratory muscle strength and poor activity of life than those with only stroke or CHF. To date, few studies have investigated cardiopulmonary function in hemiplegic stroke patients with CHF. The aim of this study, therefore, was to investigate the relationships between pulmonary function, respiratory muscle strength, perceived dyspnea, degree of fatigue, and activity of daily living with motor function and neurological status in stroke patients with stable CHF.
2. Methods and procedures
Stroke patients aged between 20 and 85 years with CHF diagnosed according to the criteria of the Framingham Study[12] with NYHA class I–III were recruited in our hospital from February 2012 to July 2015. Stroke was confirmed by computed tomography or magnetic resonance imaging. The baseline characteristics of each patient, including the duration of disease, neurological level (Brunnstrom stage), and Barthel index, were recorded. Echocardiography, resting Borg scale (range from 0.5 to 10),[13] spirometry, inspiratory and expiratory muscle pressure, fatigue scale or 6-minute walking test, if the patient could walk, were assessed in each participant.
The exclusion criteria included the patients who could not tightly place their lips over a mouthpiece and those in whom air leaked during inhaling or exhaling through the threshold device, a history of recent exacerbation, unstable angina, decompensated CHF, complicated arrhythmias, those at risk or with a history of pneumothorax, large bullae on chest radiography, abnormalities of the vertebral column, marked osteoporosis together with a history of spontaneous rib fractures, a history of recent lung surgery (within the past 12 months), the use of long-term oxygen therapy, and poor cognitive function. The main outcome measurements were spirometry, MIP, MEP, Borg scale at rest, resting heart rate, resting oxyhemoglobin saturation (SpO2), lowest resting SpO2 and Borg scale during a 6-minute walking test, fatigue scale, and Barthel scale.
FAS was used to measure the fatigue level on a 5-point scale (1: never, 2: sometimes; 3: regularly; 4: often, and 5: always). It contains 10 items: 1. I am bothered by fatigue, 2. I get tired very quickly, 3. I don’t do much during the day, 4. I have enough energy for everyday life, 5. Physically, I feel exhausted, 6. I have problems to start things, 7. I have problems to think clearly, 8. I feel no desire to do anything, 9. Mentally, I feel exhausted, 10. When I am doing something, I can concentrate quite well.[1]
This study was approved by the Institutional Review Board of Chang Gung Memorial Hospital, Kaohsiung Medical Center, and all patients provided informed consent before entering the study (IRB number: 99–3663A3; Clinical Trial Identifier: NCT02614001).
3. Data analysis
The results are presented as absolute number (percentage) or mean (standard deviation) and adjusted for age by linear regression analysis. The Kolmogorov–Smirnov Z test was used to determine the normal distribution of data. Pearson correlation analysis was used to determine the relationships among respiratory muscle strength, pulmonary function, and the clinical characteristics of stroke. All statistical analyses were performed using SPSS software version 14.0 (SPSS Inc., Chicago, IL).
4. Results
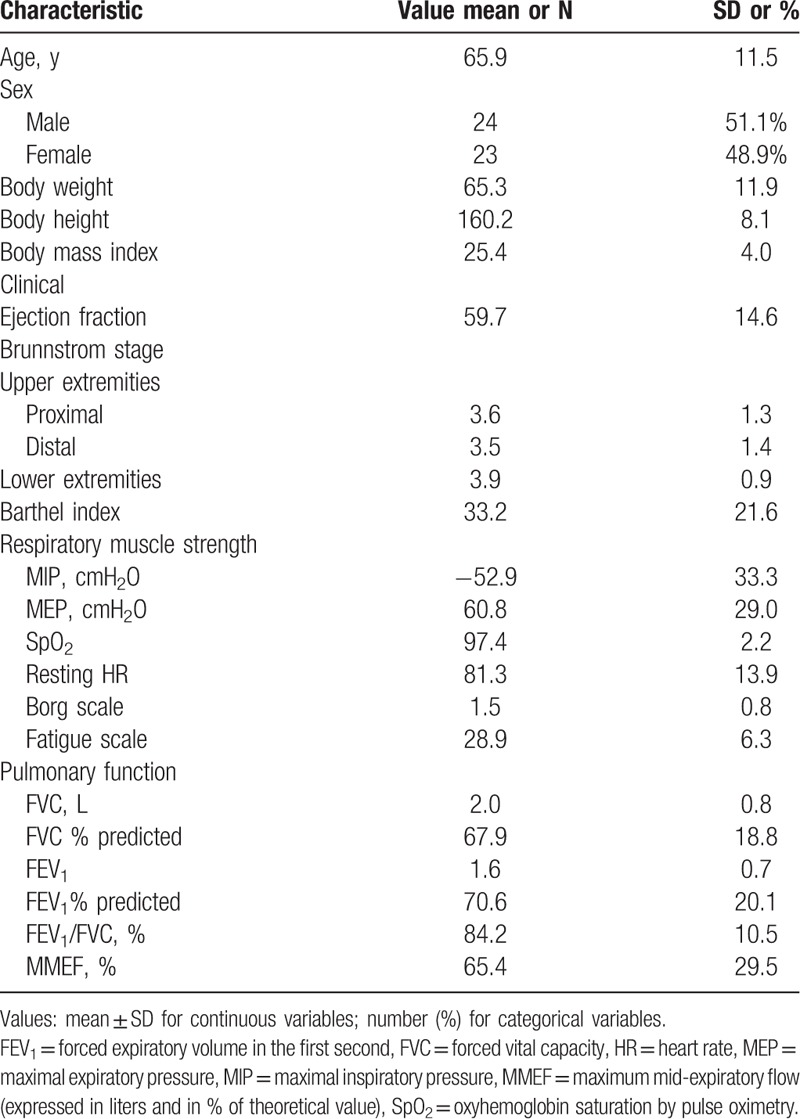
A total of 47 stroke patients (24 males, 23 females, mean age 65.9 ± 11.5 years, mean body height 160.2 ± 8.1 cm, mean body weight 65.3 ± 11.9 kg) with CHF and exertional dyspnea were recruited. Among them, 4 had high MIP and 1 had small airway disease. The fatigue scale score was 28.8 ± 6.3, and the average Brunnstrom stages of the affected upper limbs were 3.6 ± 1.3 over the proximal and 3.5 ± 1.4 over the distal parts, and 3.9 ± 0.9 over the affected lower extremities. The average Borg scale on exertion was 1.5 ± 0.8, and resting SpO2 was 97.4 ± 2.2.
The average forced vital capacity (FVC) was 2.0 ± 0.8 L, with an average predicted FVC% of 67.9 ± 18.8%, average forced expiratory volume in the first second (FEV1) of 1.6 ± 0.7L, average predicted FEV1% of 70.6 ± 20.1%, average FEV1/FVC of 84.2 ± 10.5%, and maximum mid-expiratory flow of 65.4 ± 29.5%. The average MIP and MEP were −52.9 ± 33.0 cmH2O and 60.8 ± 29.0 cmH2O, respectively (Table 1).
Table 1.
Demographic and clinical characteristics of the subjects.
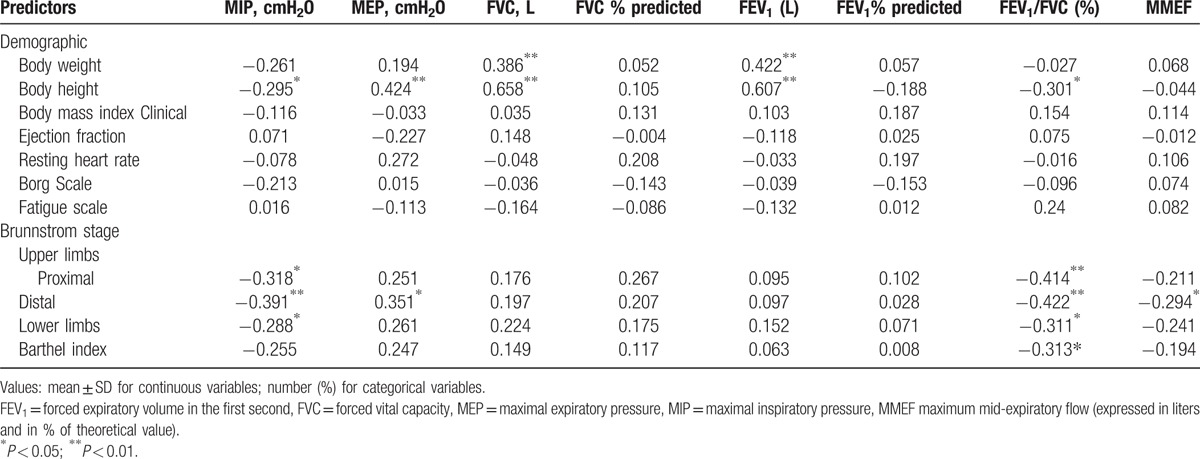
MIP was negatively associated with average Brunnstrom stage of the proximal (r = −0.318, P < 0.05) and distal (r = −0.391, P < 0.01) parts of the affected upper extremities and affected lower extremities (r = −0.288, P < 0.05). MEP was positively associated with average Brunnstrom stage of the distal parts of the affected upper extremities (r = 0.351, P < 0.05). FEV1/FVC was negatively associated with the average Brunnstrom stage over the proximal (r = −0.414, P < 0.01) and distal (r = −0.422, P < 0.01) areas of the affected upper and lower extremities (r = −0.311, P < 0.05) and Barthel index (r = −0.313, P < 0.05). However, MIP was not significantly associated with ejection fraction on echocardiography, resting heart rate, or Borg scale (Table 2).
Table 2.
Pearson analysis between cardiopulmonary function and clinical characteristics.
MIP was negatively associated with FVC (r = −0.471, P < 0.01), predicted FVC% (r = −0.299, P < 0.05), and FEV1 (r = −0.397, P < 0.01). MEP was positively associated with FVC (r = 0.526, P < 0.01), FEV1 (r = 0.429, P < 0.01), and FEV1/FVC (r = −0.482, P < 0.01) (Table 3).
Table 3.
Pearson correlation coefficients for interrelationships between respiratory muscle strength and pulmonary function.

5. Discussion
Most (89.4%) of our patients with stroke and CHF presented with restrictive lung disease with a low average FVC (2.0 ± 0.8 L), low average predicted FVC% (67.9 ± 18.8%), and normal FEV1/FVC. The maximum mid-expiratory flow (MMEF) was 65.4 ± 29.5%. FVC had a stronger relationship with MIP and MEP than predicted FVC%. Among all patients, 4 (8.5%) had high MIP and 1 had small airway disease (2.1%).
The respiratory muscle strength of our patients was weak, with an average MIP of −52.9.6 ± 33.3 cmH2O and average MEP of 60.8 ± 29.0 cmH2O. Our results suggest that the average MIP was significantly positively associated with the motor status of the proximal (P < 0.05) and distal (P < 0.01) areas of the affected upper and affected lower limbs (P < 0.05), and significantly negatively associated with pulmonary function in average FVC (P < 0.01), predicted FVC% (P < 0.05), and FEV1 (P < 0.01). However, MEP was only significantly associated with average Brunnstrom stage of the distal part (P < 0.05) of the affected upper extremities, and FVC (P < 0.05) on the pulmonary function test. This implies that inspiratory muscle strength may be more strongly associated with the motor status of the proximal area of the upper limbs. This may be explained by the closer anatomical and neurological relationship between the diaphragm and proximal area of the upper limbs.
Both MIP and MEP were significantly associated with FVC (both P < 0.01); however, only MIP was significantly correlated with predicted FVC% (P < 0.05). This may be because our stroke patients also had CHF, and therefore a more complex presentation of pulmonary function. In addition, it may be because FVC, MIP, and MEP are absolute values, whereas predicted FVC% is the percentage of the predicted value of healthy subjects with a similar age, weight, and height in the pulmonary function test. Moreover, FEV1/FVC was negatively associated with the average Brunnstrom stage over the proximal (P < 0.01) and distal (P < 0.01) parts of the affected upper (P < 0.05) and lower extremities (P < 0.01). This may be explained by the relatively low FVC in our patients due to their restrictive lung disease, which was associated with both cerebrovascular disease and CHF. In addition, FEV1/FVC was also significantly associated with MEP. This implies that FEV1/FVC may be used as a reference for the pulmonary dysfunction in stroke patients with CHF. It also suggests that expiratory muscle training may also play a role in the restoration or maintenance of pulmonary function in patients post stroke with CHF.
The average Borg scale at rest in the current study was low (1.5 ± 0.8), which is even lower than that in patients with bronchiectasis (2.15 ± 1.14 in the control group and 1.62 ± 0.96 in the inspiratory muscle training group) as reported in our previous study.[14] Clinically, the patients did not report or feel much difficulty during exercise. Sometimes, we noted that some of them had flared noses or a little tachypnea as we talked. This may be because of their lower physical activity or impaired sensation of dyspnea after their cerebrovascular accident. In hemiplegic patients, the unilateral diaphragmatic paralysis is usually asymptomatic due to compensation by the opposite unaffected diaphragm, and it may partially or even spontaneously recover. The average ejection fraction on echocardiography was negatively associated with resting heart rate. However the ejection fraction and Borg scale were not significantly associated with MIP, MEP or pulmonary function.
The average fatigue scale of our patients was 28.8 ± 6.3, which is higher than that reported by Smith et al. In their study, the average fatigue scale of the stroke group was 15.3 ± 7.6 and 16.5 ± 7.9 in the CHF group, and therefore both groups had similar levels of fatigue (P = 0.44), with higher scores than healthy controls.[1]
Inspiratory muscle training has been shown to significantly improve cardiopulmonary function and improve respiratory muscle strength, exertional dyspnea, and exercise capacity in patients with heart failure.[5,9,10,15–17] It also improves blood flow in the extremities during exercise and at rest in patients with chronic heart failure.[5] Inspiratory muscle training has also been shown to improve pulmonary function, maximal voluntary ventilation, peak oxygen consumption, MIP, dyspnea, and quality of life in patients with subacute stroke,[18] and to have short-term effects on inspiratory strength and endurance in patients with chronic stroke.[19]
Therefore, we recommend that screening for pulmonary function and respiratory muscle strength should be performed in post-stroke patients with heart failure. This should then be followed, when necessary, by evaluating the feasibility of respiratory muscle strength and endurance training to improve recovery of cardiopulmonary function.
6. Limitations
The main limitation of the current study is the small sample size, as with previous studies,[18–20] and with somewhat conflicting baseline data. Only 47 stroke patients with CHF who were eligible to participate in this study were identified at our hospital within a 3- to 4-year period. We found that many stroke patients with CHF had cognitive function impairment, poor learning ability, and were unable to follow orders, tightly seal their mouths, were too weak to maintain a sitting position, and had unstable heart conditions. These problems prevented the patients from receiving respiratory muscle strength evaluations such as MIP and MEP, and thus were excluded from this study.
7. Conclusion
Most of the stroke patients with stable CHF and exertional dyspnea had restrictive pulmonary disorder, respiratory muscle weakness, and high fatigue scale but low perception of dyspnea at rest. The abnormal respiratory muscle strength and pulmonary function were related to the neurological and motor status of the affected limbs. FVC was more strongly associated with MIP and MEP than predicted FVC%. FEV1/FVC may be used as a reference for the pulmonary dysfunction. Routine screening for inspiratory muscle weakness is advisable in stroke patients with CHF. However, due to the small sample size in this study, further long-term studies with a larger sample size are needed to validate our findings.
Acknowledgments
We would like to thank Andrew Wei-Hsiang Tiong for his help in this research.
Footnotes
Abbreviations: CHF = congestive heart failure, FEV1 = forced expiratory volume in the first second, FVC = forced vital capacity, MEP = maximal expiratory pressure, MIP = maximal inspiratory pressure, MMEF = maximum mid-expiratory flow, NYHA = New York Heart Association, SpO2 = resting oxyhemoglobin saturation.
The authors report no conflicts of interest.
References
- 1.Smith OR, van den Broek KC, Renkens M, et al. Comparison of fatigue levels in patients with stroke and patients with end-stage heart failure: application of the fatigue assessment scale. J Am Geriatr Soc 2008; 56:1915–1919. [DOI] [PubMed] [Google Scholar]
- 2.Jung KJ, Park JY, Hwang DW, et al. Ultrasonographic diaphragmatic motion analysis and its correlation with pulmonary function in hemiplegic stroke patients. Ann Rehabil Med 2014; 38:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khedr EM, El Shinawy O, Khedr T, et al. Assessment of corticodiaphragmatic pathway and pulmonary function in acute ischemic stroke patients. Eur J Neurol 2000; 7:323–330. [DOI] [PubMed] [Google Scholar]
- 4.Teixeira-Salmela LF, Parreira VF, Britto RR, et al. Respiratory pressures and thoracoabdominal motion in community-dwelling chronic stroke survivors. Arch Phys Med Rehabil 2005; 86:1974–1978. [DOI] [PubMed] [Google Scholar]
- 5.Chiappa GR, Roseguini BT, Vieira PJ, et al. Inspiratory muscle training improves blood flow to resting and exercising limbs in patients with chronic heart failure. J Am Coll Cardiol 2008; 51:1663–1671. [DOI] [PubMed] [Google Scholar]
- 6.Laoutaris I, Dritsas A, Brown MD, et al. Inspiratory muscle training using an incremental endurance test alleviates dyspnea and improves functional status in patients with chronic heart failure. Eur J Cardiovasc Prev Rehabil 2004; 11:489–496. [DOI] [PubMed] [Google Scholar]
- 7.Frankenstein L, Meyer FJ, Sigg C, et al. Is serial determination of inspiratory muscle strength a useful prognostic marker in chronic heart failure? Eur J Cardiovasc Prev Rehabil 2008; 15:156–161. [DOI] [PubMed] [Google Scholar]
- 8.Weiner P, Waizman J, Magadle R, et al. The effect of specific inspiratory muscle training on the sensation of dyspnea and exercise tolerance in patients with congestive heart failure. Clin Cardiol 1999; 22:727–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Padula CA, Yeaw E. Inspiratory muscle training: integrative review of use in conditions other than COPD. Res Theory Nurs Pract 2007; 21:98–118. [DOI] [PubMed] [Google Scholar]
- 10.Ribeiro JP, Chiappa GR, Neder JA, et al. Respiratory muscle function and exercise intolerance in heart failure. Curr Heart Fail Rep 2009; 6:95–101. [DOI] [PubMed] [Google Scholar]
- 11.Walsh JT, Andrews R, Johnson P, et al. Inspiratory muscle endurance in patients with chronic heart failure. Heart 1996; 76:332–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McKee PA, Castelli WP, McNamara PM, et al. The natural history of congestive heart failure: the Framingham study. N Engl J Med 1971; 285:1441–1446. [DOI] [PubMed] [Google Scholar]
- 13.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 1982; 14:377–381. [PubMed] [Google Scholar]
- 14.Liaw MY, Wang YH, Tsai YC, et al. Inspiratory muscle training in bronchiectasis patients: a prospective randomized controlled study. Clin Rehabil 2011; 25:524–536. [DOI] [PubMed] [Google Scholar]
- 15.Padula CA, Yeaw E, Mistry S. A home-based nurse-coached inspiratory muscle training intervention in heart failure. Appl Nurs Res 2009; 22:18–25. [DOI] [PubMed] [Google Scholar]
- 16.Dall’Ago P, Chiappa GR, Guths H, et al. Inspiratory muscle training in patients with heart failure and inspiratory muscle weakness: a randomized trial. J Am Coll Cardiol 2006; 47:757–763. [DOI] [PubMed] [Google Scholar]
- 17.Stein R, Chiappa GR, Guths H, et al. Inspiratory muscle training improves oxygen uptake efficiency slope in patients with chronic heart failure. J Cardiopulm Rehabil Prev 2009; 29:392–395. [DOI] [PubMed] [Google Scholar]
- 18.Sutbeyaz ST, Koseoglu F, Inan L, et al. Respiratory muscle training improves cardiopulmonary function and exercise tolerance in subjects with subacute stroke: a randomized controlled trial. Clin Rehabil 2010; 24:240–250. [DOI] [PubMed] [Google Scholar]
- 19.Britto RR, Rezende NR, Marinho KC, et al. Inspiratory muscular training in chronic stroke survivors: a randomized controlled trial. Arch Phys Med Rehabil 2011; 92:184–190. [DOI] [PubMed] [Google Scholar]
- 20.Martin-Valero R, De La Casa Almeida M, Casuso-Holgado MJ, et al. Systematic review of inspiratory muscle training after cerebrovascular accident. Respir Care 2015; 60:1652–1659. [DOI] [PubMed] [Google Scholar]


