Abstract
Many patients treated with imatinib, used in cancer treatment, are using several other drugs that could interact with imatinib. Our aim was to study all the drug–drug interactions (DDIs) observed in patients treated with imatinib.
We performed 2 observational studies, between the 1st January 2012 and the 31st August 2015 in the Midi-Pyrénées area (South Western France), using the French health insurance reimbursement database and then the French Pharmacovigilance Database (FPVD).
A total of 544 patients received at least 1 reimbursement for imatinib. Among them, 486 (89.3%) had at least 1 drug that could potentially interact with imatinib. Paracetamol was the most frequent drug involved (77.4%). Proton pump inhibitors, dexamethasone and levothyroxine, were found in >10% of patients. In the FPVD, among a total of 25 reports of ADRs with imatinib recorded in the Midi-Pyrénées area, 10 (40%) had potential DDIs with imatinib. Imatinib was most frequently prescribed by hospital physicians and drugs interacting with imatinib, by general practitioners.
Our study showed that at least 40% of the patients treated with imatinib were at risk of DDIs and that all prescribers must be cautious with DDIs in patients treated with imatinib. During imatinib treatment, we particularly recommend to limit the dose of paracetamol at 1300 mg per day, to avoid the use of dexamethasone, and to double the dose of levothyroxine.
Keywords: drug interactions, health insurance reimbursement, imatinib, observational study, pharmacovigilance, protein kinase inhibitors
1. Introduction
Imatinib, the first oral BCR-ABL and c-kit tyrosine kinases inhibitor approved for cancer, is involved in several drug–drug interactions (DDIs). According to the Summary of Product Characteristics (SmPC) of Glivec (Novartis Europharm Limited, Camberley, UK), cytochrome P-450 (CYP450) 3A4 is the main enzyme responsible for imatinib metabolism.[1] The SmPC indicates that “caution should be taken when administering Glivec with inhibitors of the CYP3A4 family” or “concomitant use of rifampicin or other strong CYP3A4 inducers and imatinib should be avoided.” In vitro, imatinib is also a moderate competitive inhibitor of CYP2C9, 2D6, and 3A4/5. This tyrosine kinases inhibitor has also been shown, in vitro and in vivo, to be a substrate for the drug-efflux transporter P-glycoprotein.[2,3] The metabolites of imatinib are eliminated through biliary excretion. Haouala and colleagues[4] reviewed all the DDIs with imatinib reported in the literature or predicted from theoretical considerations. Bowlin and colleagues[5] specifically investigated DDIs with imatinib that could potentially diminish the efficacy or raise the toxicity level of imatinib.
Our aim was to study all the DDIs observed in patients treated with imatinib. Thus, we performed 2 observational studies to identify: (1) drugs most frequently dispensed simultaneously with imatinib through the French health insurance reimbursement database SNIIRAM (Système National d’Information Inter-Régimes de l’Assurance Maladie) and then (2) adverse drug reactions (ADRs) related to DDIs involving imatinib using the French Pharmacovigilance Database (FPVD).
2. Material and methods
SNIIRAM contained data from the general health insurance system that covers almost 90% of the French population. It aimed at better understanding and evaluating beneficiaries’ health care consumption.[6] Data is only available for a period of 3 years plus the current year. This information can also be used, under real-life conditions of patient management, to identify potential DDIs. The database contains information on demographic characteristics of users, the date of dispensing, the quantity of dispensed drug expressed in defined daily doses, and prescribers. Drugs are classified according to the Anatomical Therapeutic Chemical classification system. Only the information on drugs prescribed and reimbursed by the French health system is recorded in SNIIRAM, thus excluding drugs that are not reimbursed or delivered during hospitalizations or sold over-the-counter.
We extracted a sample of patients living in the Midi-Pyrénées area (2,600,000 inhabitants, South Western France) and receiving at least a refund for imatinib between the 1st January 2012 and the 31st August 2015. For practical reasons, we defined periods of exposure to drugs. We considered that a patient was exposed to drugs 30 days after the date of dispensation plus the period for the elimination of these drugs. We considered that elimination was complete after 7 half-lives.[7] A potential DDI with imatinib was defined by superimposing a period of exposure to imatinib with a period of exposure to drugs that could interact with imatinib. We have considered an interruption of treatment when 2 dispensations were spaced at least by 2 months. We considered a potential DDI at each new reimbursement date in a period of exposure to imatinib.
The French adverse drug reactions reporting system was first established in 1973 and the FPVD in 1985 to record spontaneous reporting of ADRs.[8,9] For each report, information about the patient, ADR, and drug exposure is recorded in the FPVD. ADRs are coded according to the Medical Dictionary for Drug Regulatory Activities (MedDRA).[10] For each drug, a causality assessment (“imputability” or “imputation”) is performed, using the validated French Pharmacovigilance System's method.[11] If causality was found between the drug and occurrence of ADR, the drugs were defined as “suspected.”
We first extracted, from the FPVD, all ADRs reported in the Midi-Pyrénées area between the 1st January 2012 and the 31st August 2015 in which imatinib was “suspected” and second, identified potential DDIs with imatinib. The following data were collected: age and gender of the patient, drugs, ADRs.
We used the European SmPC of Glivec and the National Thesaurus recommended by the French Drug Agency (Agence Nationale de Sécurité du Médicament et des produits de santé) to identify the drugs that could interact with imatinib.[1,12] This National Thesaurus has been developed to facilitate clinical management and is regularly updated. The June 2015 version was used for this study.
Statistical analysis was descriptive: we calculated numbers and percentages for qualitative variables, mean ± standard deviations, and ranges for quantitative ones. SAS software version 9.4 (SAS Institute Inc., Cary, NC) was used for analysis.
3. Results
3.1. SNIIRAM cohort
A sample of 544 patients living in the Midi-Pyrénées area with at least 1 reimbursement for imatinib between the 1st January 2012 and the 31st August 2015 was extracted from SNIIRAM. Patients were mainly men (n = 294; 54.1%) and mean age was 62.4 ± 16.9 years old (range: 7–94).
Among the 544 patients exposed to imatinib, 486 (89.3%) had at least 1 drug prescription that could interact with imatinib. The number of potential DDIs ranged from 1 to 9 with a total of 1366 potential DDIs (Table 1).
Table 1.
Distribution of patients according to the number of potential drug–drug interactions with imatinib per patient in the SNIIRAM cohort between 2012 and 2015 in the Midi-Pyrénées area.
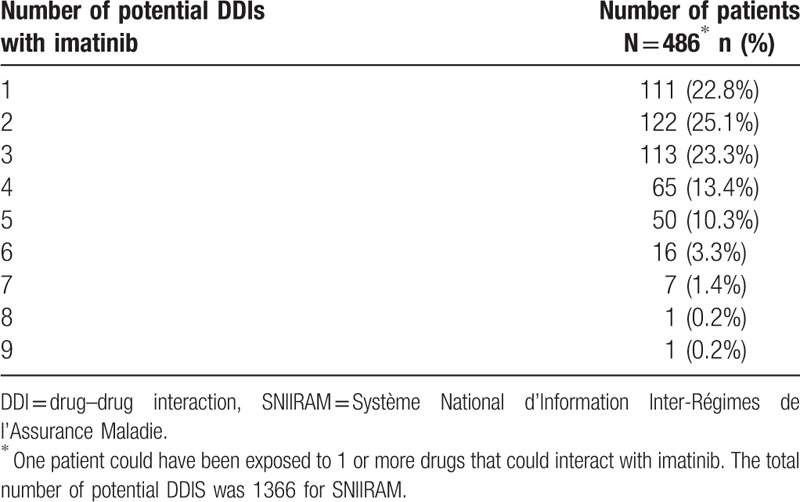
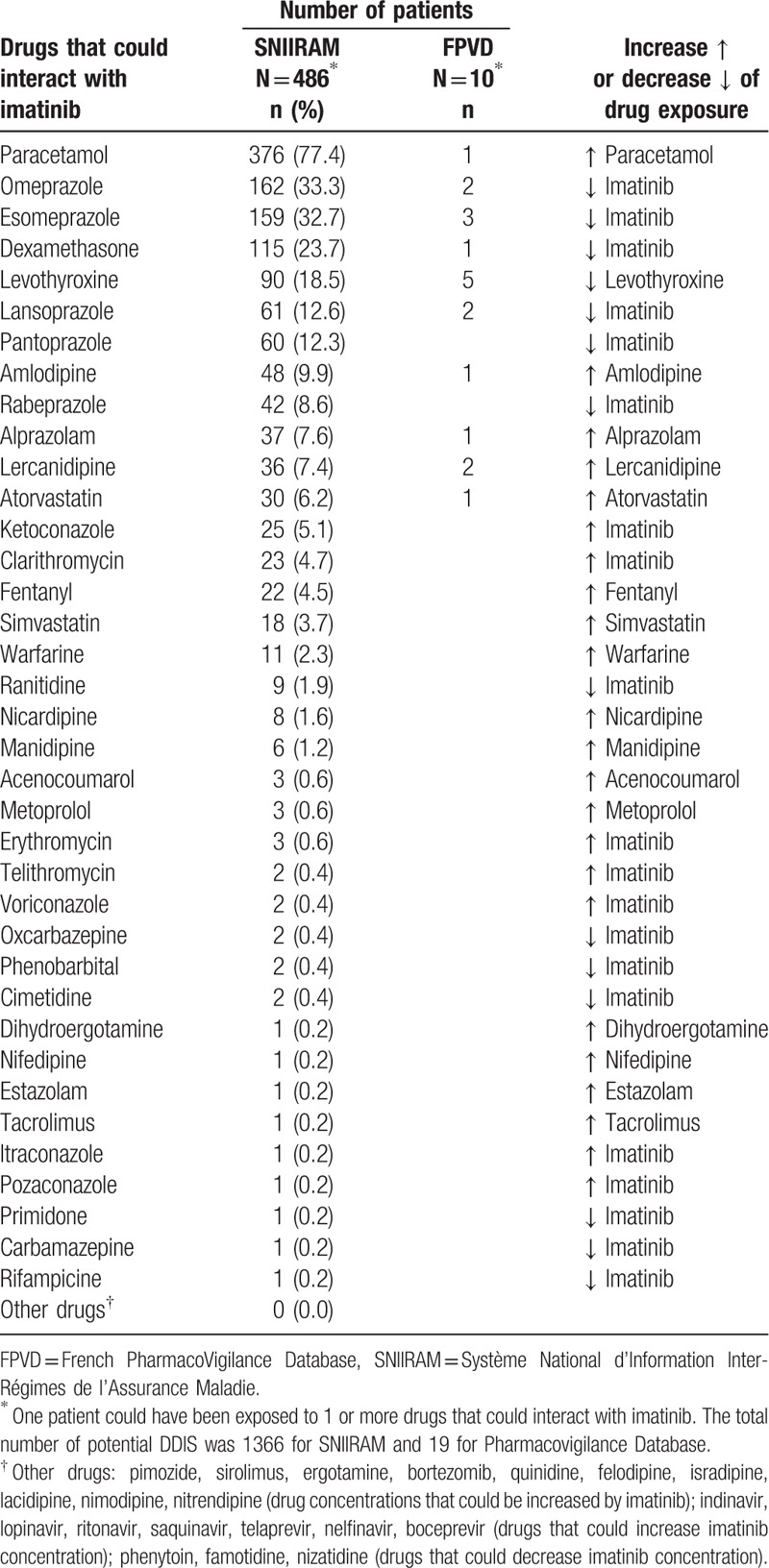
Table 2 shows the distribution of patients according to drugs that could interact with imatinib. The most frequent was paracetamol (77.4%) and its interaction with imatinib could increase paracetamol toxicity. More than 10% of patients also had potential DDIs with proton pump inhibitors (33.3% for omeprazole) or dexamethasone (23.7%) that could decrease imatinib effectiveness and with levothyroxine (18.5%) that could decrease levothyroxine effectiveness. Ketoconazole and clarithromycin were the most frequently used drugs that could increase imatinib toxicity (respectively 5.1% and 4.7%).
Table 2.
Distribution of patients according to drugs that could interact with imatinib in the SNIIRAM cohort and in the FPVD between 2012 and 2015 in the Midi-Pyrénées area.
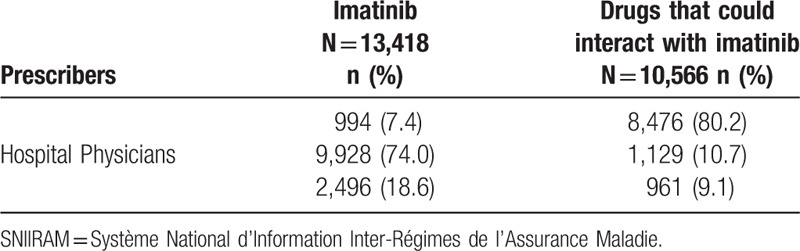
There were 13,418 prescriptions of imatinib and 10,566 prescriptions of drugs that could interact with imatinib. Imatinib was mostly prescribed by hospital physicians (74.0%), and drugs that could interact with imatinib were mostly prescribed by general practitioners (80.2%) (Table 3).
Table 3.
Distribution of the prescriptions of imatinib and drugs that could interact with imatinib according to prescribers in the SNIIRAM cohort between 2012 and 2015 in the Midi-Pyrénées area.
3.2. PharmacoVigilance database
A total of 25 reports of ADRs with imatinib were recorded in the Midi-Pyrénées area from the 1st January 2012 to the 31st August 2015. The mean age was 66.6 ± 9.4 years old (range: 48–90) and there were 13 women (52.0%).
Among the 25 reports of ADRs with imatinib, 10 (40%) had potential DDIs with imatinib (Table 2). Drugs interacting with imatinib and identified in the FPVD were those most frequently found in SNIIRAM. The most frequently involved drugs were proton pump inhibitors (7 cases) and levothyroxine (5 cases). All potential DDIs found in the Midi-Pyrénées PharmacoVigilance. Database were not involved in the ADRs.
4. Discussion
The French health insurance reimbursement database has already been efficiently used in several pharmacoepidemiological studies.[13,14] The use of SNIIRAM has several interests: power and representativeness as the sample size is large.[13] It also covers practically the entire population of the Midi-Pyrénées area (even those whose income is insufficient to pay for their own medication). Although prescription claims data, such as SNIIRAM, is considered by many to be the gold standard for measuring drug exposure, it has some limitations.[15] SNIIRAM does not record drugs not reimbursed, delivered during hospitalization (e.g., docetaxel, alfentanil, midazolam) or sold over-the-counter (e.g., St. John's wort). Therefore, there is the possibility of underestimating the number of drugs taken by the patients in our study. Conversely, there might be an overestimating factor as we are not sure that the patients included in the study really took the prescribed drugs. However, reimbursement data have been found to be highly correlated with drug consumption, especially for chronically used drugs.[16] In addition, we described “potential” DDIs because we did not know if prescribers have taken any precautions to avoid them (measurement of plasma levels, dose adjustment, biological monitoring, and so on).
We showed that up to 89% of patients exposed to imatinib had potential DDIs with imatinib. This percentage is higher than those found by Bowlin and colleagues[5] because we investigated all DDIs with imatinib and not only DDIs that could potentially diminish the efficacy or raise the toxicity level of imatinib (43% and 68% respectively). However, we did not exhaustively study all drugs that could interact with imatinib since we only studied DDIs reported in the European SmPC of Glivec and in the National Thesaurus recommended which are clinically relevant.[1,12] For example, we did not study metformin that is reported to inhibit hOCT1 because the DDI with imatinib has not been showed in clinical studies.[4] We also did not study DDIs with CYP1A2 and CYP2C19 inhibitors that are mentioned in the Food and Drug Administration approved imatinib product label because they play a minor role in the metabolism of imatinib.[17]
In our study, imatinib was most frequently prescribed by oncologists and the drugs interacting with imatinib were most frequently prescribed by general practitioners. These drugs were mainly paracetamol, proton pump inhibitors, dexamethasone, and levothyroxine.
Paracetamol could lead to an increase in paracetamol concentrations.[1]In vitro, imatinib inhibits paracetamol O-glucuronidation. This inhibition was not observed in vivo after administration of 400 mg of imatinib plus 1000 mg of paracetamol. However, higher doses of imatinib and paracetamol have not been studied. A limit of 1300 mg paracetamol per day has been suggested.[18] Liver function tests might be useful to monitor during prolonged treatment.[19] During clinical trials, 1 patient regularly taking paracetamol for a fever, died of acute liver failure 11 days after introduction of imatinib.[20]
Proton pump inhibitors can increase the pH of gastric contents and delay gastric emptying.[12,21,22] They have also been reported to antagonize ATP-binding-cassette transporters, for which imatinib is a known substrate.[23,24] These effects could influence imatinib pharmacokinetics, possibly decrease its absorption and consequently cause imatinib concentrations to fall below therapeutic concentrations.[25,26] However, Oostendorp et al[3] have shown that P-glycoprotein has only a modest effect on the absorption, distribution, metabolism, and excretion of imatinib in comparison to metabolic elimination. Another study indicated that the use of omeprazole does not significantly affect the pharmacokinetics of imatinib.[27]
Dexamethasone is a potent inducer of CYP3A4 and can significantly reduce exposure to imatinib.[1] The SmPC of imatinib, therefore, reasonably recommends caution and suggests that concurrent use with dexamethasone should be avoided. However, we did not find any published case report of DDI with imatinib and dexamethasone.
Hypothyroidism is known in patients treated with imatinib plus levothyroxine.[1] The suspected mechanisms responsible for this phenomenon are an induction by imatinib of nondeiodination clearance or induction by imatinib of uridine diphosphate-glucuronyl transferases.[28,29] A 2-fold increase in levothyroxine substitution therapy during the initiation of imatinib treatment is recommended, along with close monitoring of the thyroid function.[28,29]
We did not identify any report of ADR due to DDI with imatinib in the Midi-Pyrénées PharmacoVigilance Database. Our study underwent some unavoidable methodological drawbacks, as do most pharmacovigilance studies dealing with spontaneous notifications. We were unable to exhaustively describe all cases of ADRs that occurred with imatinib in the Midi-Pyrénées area, but only those which were reported. This phenomenon, called underreporting, is an usual and well-known limitation to all pharmacovigilance surveys.[30] Reporting “serious” or “unlabeled” ADRs to the French regional centers is mandatory for any drug prescriber, physician, dentist, or midwife in France.[9] Then, we can suggest that ADRs related to DDIs with imatinib were mostly “nonserious” or “labeled”’ (listed in the SmPC) and then not reported to the French pharmacovigilance system. We only studied DDIs reported in the European SmPC of Glivec and in the National Thesaurus recommended. Physicians were probably aware of the DDIs and adjusted the dose of drugs to avoid an ADR. Moreover, ADRs related to DDIs could not be reported as imatinib was mostly prescribed by oncologists and drugs that could interact with imatinib were mostly prescribed by general practitioners.
5. Conclusion
In conclusion, this study suggests that at least 40% of patients treated with imatinib are at risk of DDIs. According to the results of the study performed in SNIIRAM, this value may reach 89%. This paper also allows identifying drugs with the highest rate of potential DDI with imatinib: paracetamol, proton pump inhibitors, dexamethasone, or levothyroxine. Recommendations to potentially avoid ADRs related to DDIs with imatinib are: to limit the dose of paracetamol at 1300 mg per day, to avoid the use of dexamethasone and to double the dose of levothyroxine. Concomitant use of imatinib and proton pump inhibitors is possible as there is no evidence that DDI influences imatinib pharmacokinetics. Oncologists as well as general practitioners are concerned by DDIs with imatinib.
Acknowledgments
The French Drug agency (Agence Nationale de Sécurité du Médicament et des produits de santé) allowed us to use the French PharmacoVigilance Database. The authors of this article are solely responsible for its content and conclusions. The content of this article only involved its author and is not validated by the French Drug agency.
Footnotes
Abbreviations: ADR = adverse drug reaction, CYP = cytochrome P, DDI = drug–drug interaction, FPVD = French pharmacovigilance database, MedDRA = Medical Dictionary for Drug Regulatory Activities, SmPC = Summary of Product Characteristics, SNIIRAM = Système National d’Information Inter-Régimes de l’Assurance Maladie.
Authorship: EBG decided the design of the study and participated in all of the steps, IR performed the study and wrote the manuscript, VR prepared the statistical analysis, RB collected data from SNIIRAM, LC collected data from FPVD, MLM, FD and JLM discussed the manuscript.
Funding: This work has been supported by the National Research Agency (ANR: Agence Nationale de la Recherche) for the “investissement d’avenir” (ANR-11-PHUC-001, program CAPTOR).
Footnote: In France, observational studies do not have to be approved by an Ethic Committee or an institutional review board.
The authors have no conflicts of interest to disclose.
References
- 1.European Medicines Agency EPAR Product information on imatinib Glivec. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000406/human_med_000808.jsp&mid=WC0b01ac058001d124 Accessed May 13, 2016. [Google Scholar]
- 2.Hamada A, Miyano H, Watanabe H, et al. Interaction of imatinib mesilate with human P-glycoprotein. J Pharmacol Exp Ther 2003; 307:824–828. [DOI] [PubMed] [Google Scholar]
- 3.Oostendorp RL, Buckle T, Beijnen JH, et al. The effect of P-gp (Mdr1a/1b), BCRP (Bcrp1) and P-gp/BCRP inhibitors on the in vivo absorption, distribution, metabolism and excretion of imatinib. Invest New Drugs 2009; 27:31–40. [DOI] [PubMed] [Google Scholar]
- 4.Haouala A, Widmer N, Duchosal MA, et al. Drug interactions with the tyrosine kinase inhibitors imatinib, dasatinib, and nilotinib. Blood 2011; 117:e75–e87. [DOI] [PubMed] [Google Scholar]
- 5.Bowlin SJ, Xia F, Wang W, et al. Twelve-month frequency of drug-metabolizing enzyme and transporter-based drug-drug interaction potential in patients receiving oral enzyme-targeted kinase inhibitor antineoplastic agents. Mayo Clin Proc 2013; 88:139–148. [DOI] [PubMed] [Google Scholar]
- 6.Tuppin P, de Roquefeuil L, Weill A, et al. French national health insurance information system and the permanent beneficiaries sample. Rev Epidemiol Sante Publique 2010; 58:286–290. [DOI] [PubMed] [Google Scholar]
- 7.Academic Press, Hacker M, II WSM, Bachmann KA. Pharmacology: Principles and Practice. 2009; 607. [Google Scholar]
- 8.Spreux A, Baldin B, Chichmanian RM. Pharmacovigilance in practice. Transfus Clin Biol 1999; 6:254–259. [DOI] [PubMed] [Google Scholar]
- 9.Montastruc J-L, Sommet A, Lacroix I, et al. Pharmacovigilance for evaluating adverse drug reactions: value, organization, and methods. Joint Bone Spine 2006; 73:629–632. [DOI] [PubMed] [Google Scholar]
- 10.Brown EG, Wood L, Wood S. The medical dictionary for regulatory activities (MedDRA). Drug Saf 1999; 20:109–117. [DOI] [PubMed] [Google Scholar]
- 11.Begaud B, Evreux JC, Jouglard J, et al. Imputation of the unexpected or toxic effects of drugs. Actualization of the method used in France. Therapie 1985; 40:111–118. [PubMed] [Google Scholar]
- 12.Agence Nationale de Sécurité du Médicament et des produits de santé - Thesaurus: Référentiel national des interactions médicamenteuses (24/06/2015). http://ansm.sante.fr/Dossiers/Interactions-medicamenteuses/Interactions-medicamenteuses/(offset)/0 Accessed May 13, 2016. [Google Scholar]
- 13.Martin-Latry K, Bégaud B. Pharmacoepidemiological research using French reimbursement databases: yes we can!. Pharmacoepidemiol Drug Saf 2010; 19:256–265. [DOI] [PubMed] [Google Scholar]
- 14.Chaignot C, Weill A, Ricordeau P, et al. Use in France of baclofen for alcohol dependence from 2007 to 2013: cohort study based on the databases SNIIRAM and PMSI. Therapie 2015; 70:443–453. [DOI] [PubMed] [Google Scholar]
- 15.Strom BL, Carson JL, Halpern AC, et al. Using a claims database to investigate drug-induced Stevens–Johnson syndrome. Stat Med 1991; 10:565–576. [DOI] [PubMed] [Google Scholar]
- 16.Noize P, Bazin F, Dufouil C, et al. Comparison of health insurance claims and patient interviews in assessing drug use: data from the Three-City (3C) Study. Pharmacoepidemiol Drug Saf 2009; 18:310–319. [DOI] [PubMed] [Google Scholar]
- 17.Food and Drug Administration prescribing information on imatinib Gleevec. http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/021588s024lbl.pdf. Published April 2011 Accessed May 13, 2016. [Google Scholar]
- 18.Demetri GD, Benjamin RS, Blanke CD, et al. NCCN Task Force report: management of patients with gastrointestinal stromal tumor (GIST)--update of the NCCN clinical practice guidelines. J Natl Compr Canc Netw 2007; 5 suppl 2:S1–S29.quiz S30. [PubMed] [Google Scholar]
- 19.Ridruejo E, Cacchione R, Villamil AG, et al. Imatinib-induced fatal acute liver failure. World J Gastroenterol 2007; 13: 6608-6111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Talpaz M, Silver R, Druker B, et al. A Phase II study of STI571 in adult patients with Philadelphia chromosome-positive chronic myelogenous leukemia on accelerated phase. Blood 2000; 96 suppl 1:469a. [Google Scholar]
- 21.Howden CW. Clinical pharmacology of omeprazole. Clin Pharmacokinet 1991; 20:38–49. [DOI] [PubMed] [Google Scholar]
- 22.Tougas G, Earnest DL, Chen Y, et al. Omeprazole delays gastric emptying in healthy volunteers: an effect prevented by tegaserod. Aliment Pharmacol Ther 2005; 22:59–65. [DOI] [PubMed] [Google Scholar]
- 23.Burger H, van Tol H, Boersma AWM, et al. Imatinib mesylate (STI571) is a substrate for the breast cancer resistance protein (BCRP)/ABCG2 drug pump. Blood 2004; 104:2940–2942. [DOI] [PubMed] [Google Scholar]
- 24.Breedveld P, Pluim D, Cipriani G, et al. The effect of Bcrp1 (Abcg2) on the in vivo pharmacokinetics and brain penetration of imatinib mesylate (Gleevec): implications for the use of breast cancer resistance protein and P-glycoprotein inhibitors to enable the brain penetration of imatinib in patients. Cancer Res 2005; 65:2577–2582. [DOI] [PubMed] [Google Scholar]
- 25.Picard S, Titier K, Etienne G, et al. Trough imatinib plasma levels are associated with both cytogenetic and molecular responses to standard-dose imatinib in chronic myeloid leukemia. Blood 2007; 109:3496–3499. [DOI] [PubMed] [Google Scholar]
- 26.von Mehren M, Widmer N. Correlations between imatinib pharmacokinetics, pharmacodynamics, adherence, and clinical response in advanced metastatic gastrointestinal stromal tumor (GIST): an emerging role for drug blood level testing? Cancer Treat Rev 2011; 37:291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Egorin MJ, Shah DD, Christner SM, et al. Effect of a proton pump inhibitor on the pharmacokinetics of imatinib. Br J Clin Pharmacol 2009; 68:370–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cholongitas E, Pipili C, Katsogridakis K, et al. Dermatitis after suspected imatinib–levothyroxine interaction in a patient with gastrointestinal stromal tumor. Cancer Chemother Pharmacol 2008; 61:1083–1084. [DOI] [PubMed] [Google Scholar]
- 29.de Groot JWB, Zonnenberg BA, Plukker JTM, et al. Imatinib induces hypothyroidism in patients receiving levothyroxine. Clin Pharmacol Ther 2005; 78:433–438. [DOI] [PubMed] [Google Scholar]
- 30.van der Heijden PGM, van Puijenbroek EP, van Buuren S, et al. On the assessment of adverse drug reactions from spontaneous reporting systems: the influence of under-reporting on odds ratios. Stat Med 2002; 21:2027–2044. [DOI] [PubMed] [Google Scholar]


