Abstract
The preoperative neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) are known to be prognostic factors in several cancers. However, no previous investigation has been performed to evaluate the significance of the NLR and PLR in medullary thyroid carcinoma (MTC).
The aim of this study was to identify the ability of the preoperative NLR or PLR to predict lymph node metastasis and recurrence in patients with MTC. Data from all patients with MTC who had undergone surgery at our institution from May 2009 to May 2016 were retrospectively evaluated. Receiver operating characteristic (ROC) analysis was performed to identify optimal NLR and PLR cutoff points, and we assessed independent predictors of lymph node metastasis and recurrence using univariate and multivariate analyses.
Based on the inclusion and exclusion criteria, a total of 70 patients were enrolled in this study. The ideal cutoff points for predicting lymph node involvement were 2.7 for the NLR and 105.3 for the PLR. The optimal cutoff points of the NLR and PLR for predicting recurrence were 2.8 and 129.8, respectively. Using the cutoff values, we found that PLR>105.3 (odds ratio [OR] 4.782, 95% confidence interval [CI] 1.4–16.7) was an independent predictor of lymph node metastasis and that PLR>129.8 (OR 3.838, 95% CI 1.1–13.5) was an independent predictor of recurrence.
Our study suggests that the preoperative PLR, but not NLR, was significantly associated with lymph node metastasis and recurrence in patients with MTC.
Keywords: lymph node metastasis, medullary thyroid carcinoma, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, recurrence
1. Introduction
Medullary thyroid carcinoma, originating from the neural crest-derived parafollicular C cells, occurs in either a sporadic or a hereditary form as a component of multiple endocrine neoplasia type 2A (MEN2A), multiple endocrine neoplasia type 2B (MEN2B), and familial MTC syndrome (FMTC). All forms of MTC have a propensity for lymphatic metastasis early in the disease course. More than 50% of patients with MTC have cervical lymph node metastasis at the time of diagnosis, and up to 5% have distant metastasis.[1] Furthermore, there is an inverse relationship between cervical lymph node metastasis and survival.[2] Although MTC comprises only 3% to 5% of thyroid cancer diagnoses, it causes 15% of thyroid cancer-related deaths due to its aggressiveness.[3] Due to a lack of good treatment options other than surgery, complete surgical resection consisting of at least total thyroidectomy with central lymph node dissection is the only modality that is able to cure patients with MTC. The overall 10-year survival rate of patients with MTC is ∼75% to 85% for the entire population, compared with a 10-year survival rate of over 90% for patients with well-differentiated thyroid cancer (WDTC).[4–7]
Although calcitonin is an important clinical marker for diagnosis, outcome evaluation, and prognostic stratification of patients with MTC, there are many limitations in the use of this marker.[8] The levels of calcitonin change notably during the day, and calcitonin has a concentration-dependent biphasic half-life. In addition, calcitonin can be rapidly degraded by serum proteases.[9] All these limitations compromise the wider utility of calcitonin.
The tumor microenvironment and systemic inflammatory response have recently been documented to play several important roles in many human cancers, with potential mechanisms including the promotion of tumor cell proliferation, angiogenesis, invasion, and metastasis.[10,11] Many systemic inflammatory markers, including the neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR), have been shown to be independent and reliable prognostic biomarkers in lung, ovarian, gastric, hepatocellular, colorectal, esophageal, and pancreatic cancers.[12–19] These markers can be measured easily, reproducibly, and inexpensively, enhancing the evaluation of prognosis in a variety of human cancers. Unlike other types of cancer, only a few studies have examined the significance of the NLR and PLR in thyroid cancer.[20–24] Furthermore, most of these studies focused on differentiated thyroid cancer. To the best of our knowledge, there have been no studies investigating the NLR and PLR in patients with medullary thyroid cancer (MTC).
In this analysis, we evaluated the association of the preoperative NLR and PLR with clinicopathological characteristics and prognosis in patients with MTC.
2. Patients and methods
We retrospectively reviewed the electronic medical records of 120 patients with MTC in the Department of Thyroid and Parathyroid Surgery Center at West China Hospital between May 2009 and May 2016. To avoid possible confounders, patients who had undergone initial surgical treatment at another hospital (n = 43), patients with unresectable tumors at the time of referral (n = 3), minor patients (n = 2), patients with other malignancies or who had undergone cancer treatment within the past 12 months (n = 1), and patients with acute myocardial infarction/coronary revascularization within the past 6 months (n = 1) were excluded. Finally, a total of 70 MTC patients were eligible for analysis. The present study protocol was approved by the Institutional Review Board of West China Hospital of Sichuan University, and written informed consent was obtained from all of the study participants. Complete blood counts with automated differential counts were performed on all patients 1 to 2 days before the operation. The NLR was calculated by dividing the absolute neutrophil count by the absolute lymphocyte count; similarly, the PLR was calculated by dividing the absolute platelet count by the absolute lymphocyte count. To evaluate the association between NLR/PLR and other tumor clinicopathological features and outcomes, patients were assigned to categories based on the preoperative NLR/PLR values. In addition, the patients’ demographics, preoperative ultrasonographic tumor characteristics, surgical details, postoperative pathological details, postoperative outcome, and tumor recurrence were also obtained.
All patients underwent preoperative fine needle aspiration (FNA) cytology to confirm the diagnosis. Three surgeons with at least 15 years of thyroid and parathyroid surgery experience performed all of the operations, and the histopathological evaluation of thyroid specimens was performed by 2 pathologists at our institution. Both the thyroid gland and nodal tissue were evaluated from 1-mm-thick anatomical slices. Total thyroidectomy with complete central neck dissection (TT-CND) was performed in all patients, and unilateral or bilateral lateral neck dissection (LND) was performed depending on serum calcitonin levels, imaging studies, or intraoperative morphological appearance. We then evaluated the number of resected lymph nodes (LNs) and the number of LN metastases for each LN neck compartment. We also calculated the LN ratio (LNR), which is defined as the ratio of the number of metastatic LNs to the total number of LNs removed in the central and lateral compartments.
Postoperative serum calcitonin was assessed and neck ultrasonography (US) was performed every 3 months during the first year after surgery and every 6 months thereafter. Patients underwent additional imaging studies, such as computed tomography (CT), magnetic resonance imaging (MRI), and 18F-deoxyglucose positron emission tomography (FDG-PET), when indicated by a rise in calcitonin level and/or evidence of persistent/recurrent disease. For the analyses, recurrence was defined as increased serum calcitonin levels, locoregional recurrence based on radiological evidence, and/or distant metastasis based on histological evidence.[25,26]
The clinical characteristics considered in the univariate and multivariate analysis of MTC recurrence were age at diagnosis (≤45 years vs >45 years), gender (male vs female), body mass index (BMI), NLR, PLR, greatest tumor diameter, number of tumors, TNM stage (I/II vs III/IV), capsular invasion (yes vs no), tumor bilaterality (yes vs no), coexisting nodular goiter (yes vs no), coexisting autoimmune thyroid disease (yes vs no), and lateral node involvement (yes vs no).
3. Statistical analysis
Continuous variables were reported as means ± standard deviations (SD) and were compared using the Mann–Whitney U test. Categorical variables were presented as counts (percentages) and were analyzed using the chi-square and Fisher's exact test (2-tailed) if necessary. Receiver operating characteristic (ROC) curves were used to determine the optimal cutoff values for the NLR and PLR. Then, univariate and multivariate analyses were performed to determine the independent prognostic factors, and variables that were significant in the univariate analysis were entered into the multivariate analysis. A P-value of < 0.05 was considered statistically significant. SPSS software (SPSS 22; SPSS Inc., Chicago, IL) was used for the statistical analysis.
4. Results
From May 2009 to May 2016, a total of 70 consecutive patients underwent surgery for a previously untreated MTC in our center were enrolled. The patients’ baseline characteristics are listed in Table 1. The mean NLR in our cohort was 2.1 ± 0.9, and the mean PLR was 112.7 ± 49.4. LN metastasis occurred in 43 (61.4%) patients (N1), 31 (44.3%) of whom had lateral compartment LN metastasis (N1b). In addition, 35 (50.0%) patients had advanced-stage MTC (T3 or T4) according to the TNM staging system. After a mean follow-up of 39.4 ± 25.6 months, recurrence occurred in 16 patients. Of these patients, 15 had locoregional recurrence and 1 had locoregional and distant recurrence. The mean duration to first recurrence was 31.4 ± 19.4 months, and the 5-year recurrence rate was 21.4%.
Table 1.
Baseline characteristics of patients.
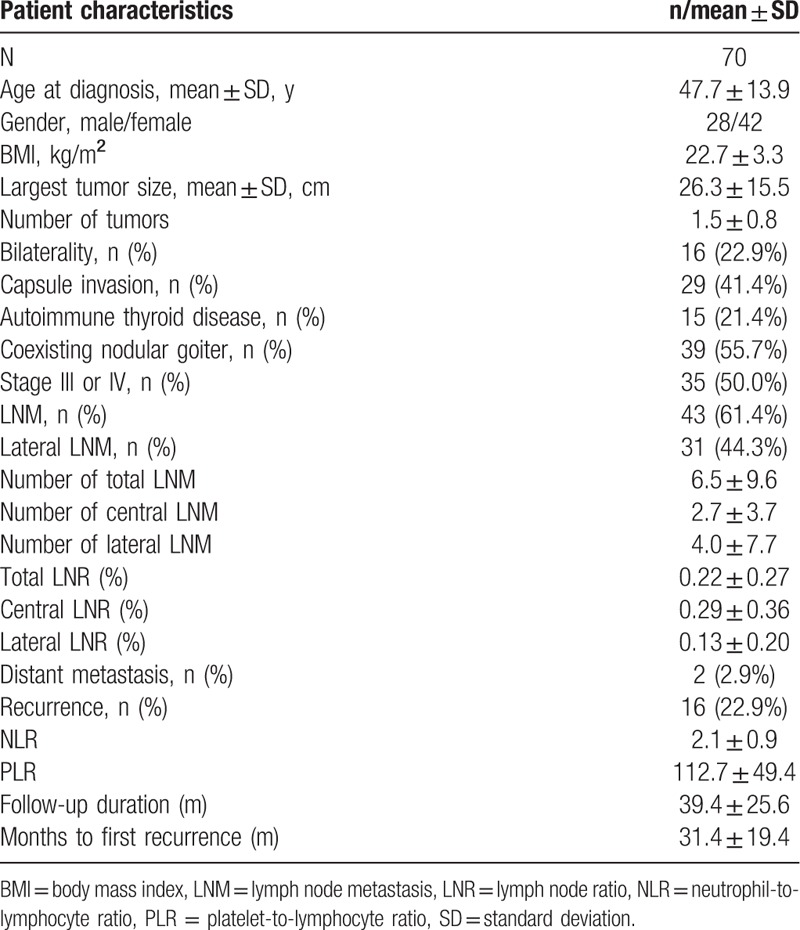
Patients were stratified into 2 groups according to the median of the preoperative NLR and PLR values. Patients with NLR lower than the median value were classified as the first half group, and the other patients were classified as the second half group. This grouping method is also applicable to PLR. The prevalence of prognostic factors was then compared (Tables 2 and 3). Table 2 shows the relationships between clinicopathological factors and NLR groups. The group with the higher NLR values had significantly more multifocal (14.2 vs 54.3%, P < 0.001) and bilateral (5.7 vs 40.0%, P = 0.001) tumors than the group with the lower NLR values. Significant differences were also found between the NLR and largest tumor size (22.0 vs 30.5, P = 0.037). Table 3 shows a comparison of clinicopathological features between PLR groups. The greatest tumor diameter was significantly smaller in the low PLR group (20.2 vs 27.8, P = 0.031) compared with the high PLR group. The number of metastatic LNs (6.3 vs 1.7, P = 0.030) and LN ratio (0.18 vs 0.08, P = 0.048) were significantly higher in the high PLR group in the lateral compartments. Furthermore, these values were also significantly higher in the ipsilateral lateral compartments (6.06 vs 1.69, P = 0.032, 0.20 vs 0.08, P = 0.034, respectively). However, there was no significant difference in the central metastatic LN number (2.14 vs 3.17, P = 0.133) or central LN ratio (0.25 vs 0.34, P = 0.276) between the 2 PLR groups.
Table 2.
Clinicopathological characteristics of patients with MTC according to the NLR group.
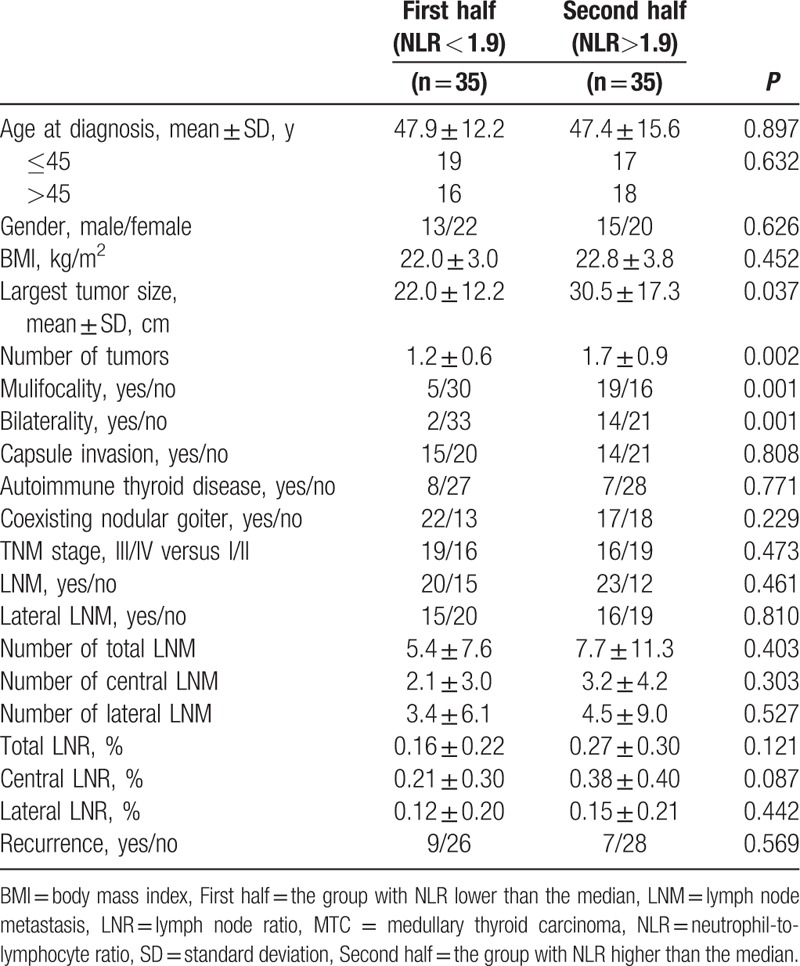
Table 3.
Clinicopathological characteristics of patients with MTC according to the PLR group.
ROC curve analysis was performed to determine the optimal cutoff value of the NLR and PLR for predicting LN metastasis and recurrence. The recommended cutoff value was based on the most prominent point on the ROC curve for sensitivity and specificity. Figure 1 shows the ROC analysis for total LN metastasis, central LN metastasis, lateral LN metastasis, and recurrence. ROC analysis demonstrated that the sensitivity and specificity were highest when the PLR was 105.3 for total LN metastasis and central LN metastasis and 142.1 for lateral LN metastasis. The ROC curve also revealed that the recommended cutoff value of the NLR was 2.7 for LN metastasis. The optimal cutoff values of the NLR and PLR for recurrence were 2.8 and 129.8, respectively (Table 4).
Figure 1.
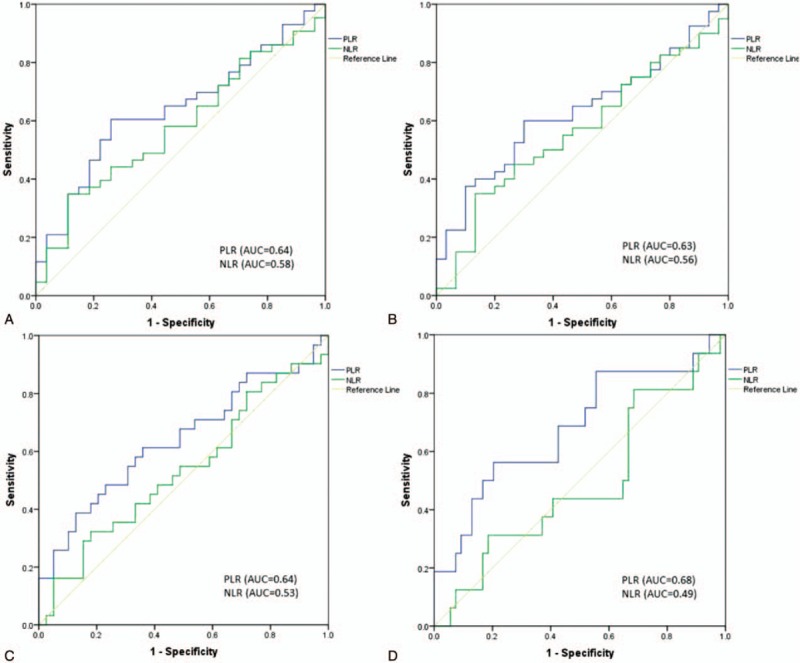
Receiver operating characteristic curve for preoperative NLR and PLR to predict (A) LN metastasis, (B) central LN metastasis, (C) lateral LN metastasis, and (D) recurrence in MTC patients. LN = lymph node, NLR = neutrophil-to-lymphocyte ratio, PLR = platelet-to-lymphocyte ratio, MTC = medullary thyroid carcinoma.
Table 4.
The cutoff values of preoperative NLR and PLR to predict the LN metastasis, central LN metastasis, lateral LN metastasis and recurrence of MTC patients.

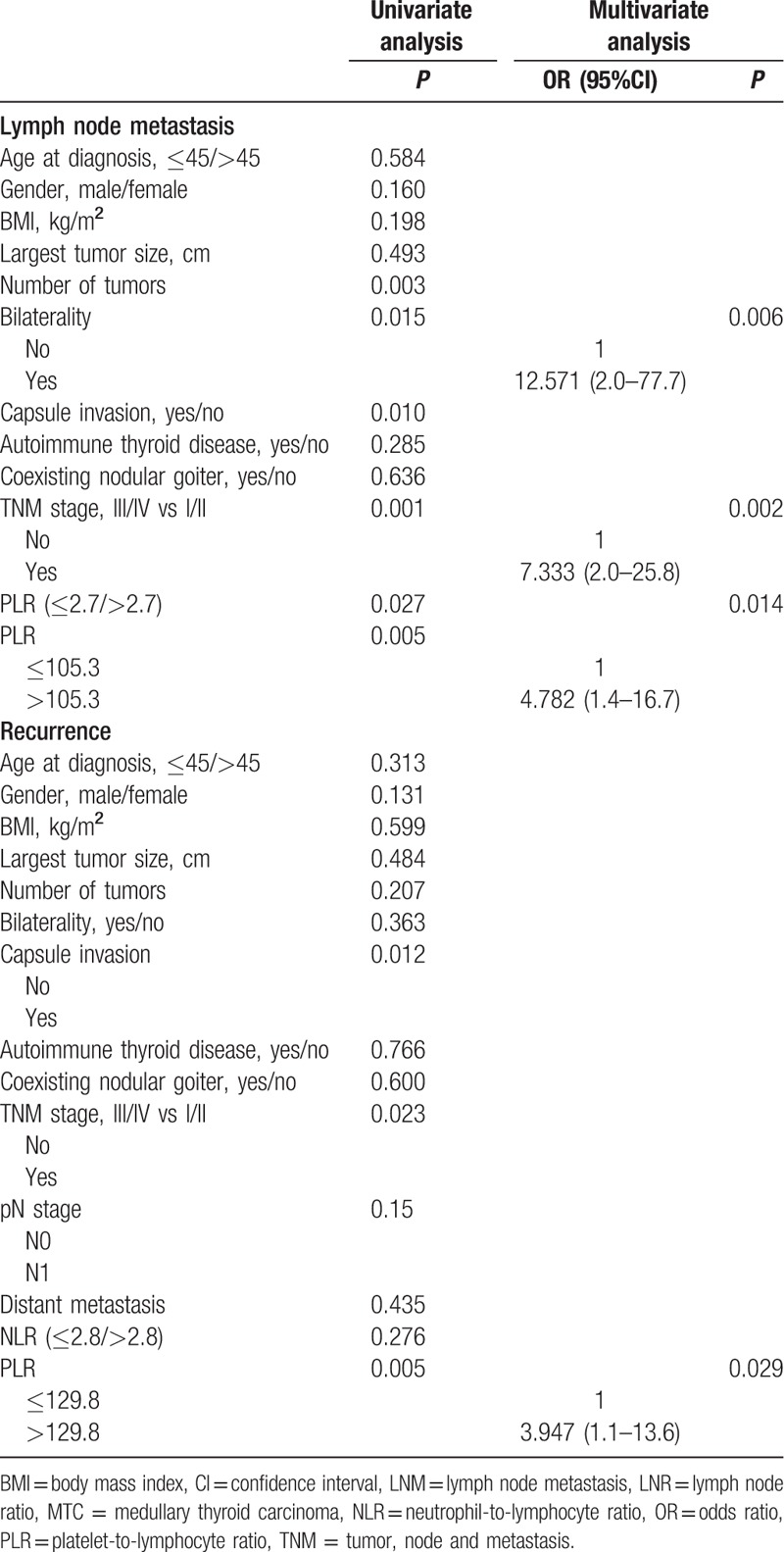
To determine the independent prognostic factors, univariate and multivariate analyses were performed (Table 5). Central compartment LN metastasis (N1a) occurred in 36 of the 70 patients (51.4%), and lateral compartment LN metastasis (N1b) occurred in 31 patients (44%). Using the NLR and PLR cutoff points determined by ROC analysis, we found that a PLR>105.3 (OR 4.782, 95% CI 1.4–16.7), a primary tumor stage of III or IV (OR 7.333, 95% CI 2.0–25.8), and the presence of a bilateral tumor (OR 12.571, 95% CI 2.0–77.7) were independent predictors of LN metastasis. Furthermore, we observed the independent predictors of central and lateral compartment LN metastasis. The multivariate analysis of central compartment LN metastasis revealed that a PLR>105.3 (OR 3.408, 95% CI 1.1–11.0), a primary tumor stage of III or IV (OR 7.397, 95% CI 2.2–24.6), and the presence of a bilateral tumor (OR 8.180, 95% CI 1.6–40.8) were independent predictors. In addition, the multivariate analysis of lateral compartment LN metastasis revealed that a PLR>142.1 (OR 3.452, 95% CI 1.0–11.8) and primary tumor stage of III or IV (OR 3.156, 95% CI 1.1–8.9) were independent predictors. The multivariate analysis of recurrence revealed that a PLR>129.8 (OR 3.947, 95% CI 1.1–13.6) was the only significant prognostic factor.
Table 5.
Univariate and multivariate analyses of clinicopathological features for the prediction of lymph node metastasis and recurrence in patients with MTC.
5. Discussion
To our knowledge, the present study is the first attempt to assess the value of the preoperative NLR and PLR as predictive markers of lymph node metastasis and recurrence in patients with MTC. In the current study, we found that the pretreatment PLR, a regularly used blood-based parameter, was independently associated with clinical outcome in MTC patients, as patients with elevated PLR values showed significantly higher LNRs, more lymph node metastasis (LNM), and postoperative recurrence.
Using the optimal cutoff points determined by ROC analysis, we identified a PLR of >105.3 as an independent predictor of cervical node involvement and a PLR>142.1 as an independent predictor of lateral lymph node metastasis. Furthermore, a PLR>129.8 was identified as a significant prognostic factor for recurrence. All of these results suggest that PLR may be a complementary marker to the TNM classification and serum calcitonin for selecting high-risk patients with MTC who should be considered for more intensive therapies such as unilateral or bilateral LND.
There is increasing evidence that systemic inflammation plays important roles in tumor progression and recurrence in a variety of solid tumors. The inflammatory response is triggered by circulating cytokines and chemokines, which are released by malignant cells.[27] As a result, systematic alterations, such as neutrophilia, thrombocytosis, and lymphocytopenia, occur. Various investigations have revealed that platelets not only support the growth of primary tumor cells via angiogenesis but also have an obvious association with tumor metastasis through evasion of the immune system.[28–30] In addition, platelets can also hamper the lysis of tumor cells by natural killer cells.[31] Similar to platelets, neutrophils have also been shown to be closely associated with cancer survival via enhancing angiogenesis, primary tumor progression, and metastasis.[32–34] Lymphocytopenia has been observed as a biomarker for poor survival in various cancers. Lymphocytes play an important role in cell-mediated immune response activation and tumor cell demolition.[35,36]
In view of the effects of neutrophils, platelets, and lymphocytes mentioned above, the NLR and PLR could potentially be effective biomarkers for tumor prognosis. Recently, an increasing number of investigations have revealed that NLR and PLR indeed act as significant prognostic factors in a variety of cancer types. Several studies have been carried out to investigate the association of the NLR and PLR with thyroid malignancies. Kocer et al[21] demonstrated that the NLR was significantly higher in patients with PTC compared with patients with benign diseases, such as multinodular goiter and lymphocytic thyroiditis, and that a cutoff point of 1.91 may be optimal for distinguishing between them. Liu et al[22] reported that a higher NLR was associated with a larger tumor size and a higher risk of recurrence. We observed a similar result in terms of the association between the NLR and primary tumor size in the present study, but no significant association was found between the NLR and recurrence. In addition, we observed that the high NLR group had more lymph node involvement and a higher LN ratio, but the results were not significant. Kim et al[24] concluded that a higher preoperative PLR, but not NLR, could be a significant predictor of lateral node involvement in patients with PTC. Although there was a trend, no significance was found between the PLR and N1b proportion in the present study. However, we demonstrated that a higher PLR was significantly associated with more lateral LNM and a higher lateral LNR in patients with MTC. Furthermore, a higher preoperative PLR was also found to be an independent predictor of lateral node involvement and recurrence in the multivariate analysis.
Although a few studies have reported an association between preoperative NLR/PLR and thyroid malignancies, there have been no investigations in MTC patients. Therefore, the present study is important because we not only identified PLR as an independent predictor of lymph node metastasis and recurrence in MTC patients for the first time, but also because we calculated optimal cutoff points. Thus, the preoperative PLR could be a complementary marker in addition to TNM staging and serum calcitonin with which to determine appropriate treatment protocols.
There are some limitations to this study. First, selection biases may exist because this study was retrospective. Second, this was a single-center analysis, and the sample size of MTC patients was limited due to the low incidence of MTC. Finally, preoperative serum calcitonin and CEA were not assessed in the analysis because these 2 biochemical parameters were not determined in all patients before the operation. More studies are needed to verify our findings.
In conclusion, surgery is the only effective curative treatment for MTC patients. Using the calculated cutoff points mentioned above, the preoperative PLR may be helpful for stratifying high-risk patients to enable appropriate measurements to be performed.
Footnotes
Abbreviations: FMTC = familial MTC syndrome, FNA = fine needle aspiration, LND = lateral neck dissection, LNR = LN ratio, LNs = lymph nodes, MEN2A = multiple endocrine neoplasia type 2A, MEN2B = multiple endocrine neoplasia type 2B, MTC = medullary thyroid carcinoma, NLR = neutrophil-to-lymphocyte ratio, ORs = odds ratios, PLR = platelet-to-lymphocyte ratio, PTC = papillary thyroid carcinoma, SD = standard deviation, SPSS = Statistical Package for Social Sciences, TNM = tumor, node and metastasis, TT-CND = total thyroidectomy with complete central neck dissection, WDTC = well-differentiated thyroid cancer.
JK and LJ contributed equally to this study and should be treated as co-first authors.
Authorship: JK and LJY performed research and wrote the first draft. All authors contributed to the design and interpretation of the study and to further drafts. Zhu JQ is the guarantor.
Funding: This study was supported by grants from The Sciences and Technology Project of Sichuan Province, China (2013SZ0041).
Competing interest: No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
The authors have no conflicts of interest to disclose.
References
- 1.Saad MF, Ordonez NG, Rashid RK, et al. Medullary carcinoma of the thyroid. A study of the clinical features and prognostic factors in 161 patients. Medicine 1984; 63:319–342. [PubMed] [Google Scholar]
- 2.Esfandiari NH, Hughes DT, Yin H, et al. The effect of extent of surgery and number of lymph node metastases on overall survival in patients with medullary thyroid cancer. J Clin Endocrinol Metab 2014; 99:448–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmed SR, Ball DW. Clinical review: incidentally discovered medullary thyroid cancer: diagnostic strategies and treatment. J Clin Endocrinol Metab 2011; 96:1237–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pelizzo MR, Boschin IM, Bernante P, et al. Natural history, diagnosis, treatment and outcome of medullary thyroid cancer: 37 years experience on 157 patients. Eur J Surg Oncol 2007; 33:493–497. [DOI] [PubMed] [Google Scholar]
- 5.Roy M, Chen H, Sippel RS. Current understanding and management of medullary thyroid cancer. Oncologist 2013; 18:1093–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee YM, Sung TY, Kim WB, et al. Risk factors for recurrence in patients with papillary thyroid carcinoma undergoing modified radical neck dissection. British J Surg 2016; 103:1020–1025. [DOI] [PubMed] [Google Scholar]
- 7.Nixon IJ, Wang LY, Ganly I, et al. Outcomes for patients with papillary thyroid cancer who do not undergo prophylactic central neck dissection. Br J Surg 2016; 103:218–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Machens A, Dralle H. Biomarker-based risk stratification for previously untreated medullary thyroid cancer. J Clin Endocrinol Metab 2010; 95:2655–2663. [DOI] [PubMed] [Google Scholar]
- 9.Machens A, Lorenz K, Dralle H. Utility of serum procalcitonin for screening and risk stratification of medullary thyroid cancer. J Clin Endocrinol Metab 2014; 99:2986–2994. [DOI] [PubMed] [Google Scholar]
- 10.Wang DS, Ren C, Qiu MZ, et al. Comparison of the prognostic value of various preoperative inflammation-based factors in patients with stage III gastric cancer. Tumour Biol 2012; 33:749–756. [DOI] [PubMed] [Google Scholar]
- 11.Roxburgh CS, McMillan DC. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol (London, England) 2010; 6:149–163. [DOI] [PubMed] [Google Scholar]
- 12.Takahashi Y, Kawamura M, Hato T, et al. Neutrophil-lymphocyte ratio as a prognostic marker for lung adenocarcinoma after complete resection. World J Surg 2016; 40:365–372. [DOI] [PubMed] [Google Scholar]
- 13.Zhang WW, Liu KJ, Hu GL, et al. Preoperative platelet/lymphocyte ratio is a superior prognostic factor compared to other systemic inflammatory response markers in ovarian cancer patients. Tumour Biol 2015; 36:8831–8837. [DOI] [PubMed] [Google Scholar]
- 14.Wang SC, Chou JF, Strong VE, et al. Pretreatment neutrophil to lymphocyte ratio independently predicts disease-specific survival in resectable gastroesophageal junction and gastric adenocarcinoma. Ann Surg 2016; 263:292–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiao WK, Chen D, Li SQ, et al. Prognostic significance of neutrophil-lymphocyte ratio in hepatocellular carcinoma: a meta-analysis. BMC Cancer 2014; 14:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen ZY, Raghav K, Lieu CH, et al. Cytokine profile and prognostic significance of high neutrophil-lymphocyte ratio in colorectal cancer. Br J Cancer 2015; 112:1088–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duan H, Zhang X, Wang FX, et al. Prognostic role of neutrophil-lymphocyte ratio in operable esophageal squamous cell carcinoma. World J Gastroenterol 2015; 21:5591–5597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Templeton AJ, Ace O, McNamara MG, et al. Prognostic role of platelet to lymphocyte ratio in solid tumors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev 2014; 23:1204–1212. [DOI] [PubMed] [Google Scholar]
- 19.Stotz M, Gerger A, Eisner F, et al. Increased neutrophil-lymphocyte ratio is a poor prognostic factor in patients with primary operable and inoperable pancreatic cancer. Br J Cancer 2013; 109:416–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lang BH, Ng CP, Au KB, et al. Does preoperative neutrophil lymphocyte ratio predict risk of recurrence and occult central nodal metastasis in papillary thyroid carcinoma? World J Surg 2014; 38:2605–2612. [DOI] [PubMed] [Google Scholar]
- 21.Kocer D, Karakukcu C, Karaman H, et al. May the neutrophil/lymphocyte ratio be a predictor in the differentiation of different thyroid disorders? Asian Pac J Cancer Prev 2015; 16:3875–3879. [DOI] [PubMed] [Google Scholar]
- 22.Liu J, Du J, Fan J, et al. The neutrophil-to-lymphocyte ratio correlates with age in patients with papillary thyroid carcinoma. ORL 2015; 77:109–116. [DOI] [PubMed] [Google Scholar]
- 23.Kim JY, Park T, Jeong SH, et al. Prognostic importance of baseline neutrophil to lymphocyte ratio in patients with advanced papillary thyroid carcinomas. Endocrine 2014; 46:526–531. [DOI] [PubMed] [Google Scholar]
- 24.Kim SM, Kim EH, Kim BH, et al. Association of the preoperative neutrophil-to-lymphocyte count ratio and platelet-to-lymphocyte count ratio with clinicopathological characteristics in patients with papillary thyroid cancer. Endocrinol Metab 2015; 30:494–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Crea C, Raffaelli M, Milano V, et al. Intraoperative high-dose calcium stimulation test in patients with sporadic medullary thyroid carcinoma is highly accurate in predicting lateral neck metastases. Surgery 2016; 159:70–76. [DOI] [PubMed] [Google Scholar]
- 26.Chandeze MM, Noullet S, Faron M, et al. Can we predict the lateral compartment lymph node involvement in RET-negative patients with medullary thyroid carcinoma? Ann Surg Oncol 2016; 23:3653–3659. [DOI] [PubMed] [Google Scholar]
- 27.Salazar-Onfray F, Lopez MN, Mendoza-Naranjo A. Paradoxical effects of cytokines in tumor immune surveillance and tumor immune escape. Cytokine Growth Factor Rev 2007; 18:171–182. [DOI] [PubMed] [Google Scholar]
- 28.Kim PY. Platelets: connecting clotting and lysis. Blood 2015; 125:2459. [DOI] [PubMed] [Google Scholar]
- 29.Jain S, Harris J, Ware J. Platelets: linking hemostasis and cancer. Arterioscler Thromb Vasc Biol 2010; 30:2362–2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garraud O, Cognasse F. Are platelets cells? And if yes, are they immune cells? Front Immunol 2015; 6:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nieswandt B, Hafner M, Echtenacher B, et al. Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Cancer Res 1999; 59:1295–1300. [PubMed] [Google Scholar]
- 32.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet (London, England) 2001; 357:539–545. [DOI] [PubMed] [Google Scholar]
- 33.Tavares-Murta BM, Mendonça MAO, Duarte NL, et al. Systemic leukocyte alterations are associated with invasive uterine cervical cancer. Int J Gynecol Cancer 2010; 20:1154–1159. [DOI] [PubMed] [Google Scholar]
- 34.Pichler M, Hutterer GC, Stoeckigt C, et al. Validation of the pre-treatment neutrophil-lymphocyte ratio as a prognostic factor in a large European cohort of renal cell carcinoma patients. Br J Cancer 2013; 108:901–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mehrazin R, Uzzo RG, Kutikov A, et al. Lymphopenia is an independent predictor of inferior outcome in papillary renal cell carcinoma. Urol Oncol 2015; 33:388.e319-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu ES, Oduyebo T, Cobb LP, et al. Lymphopenia and its association with survival in patients with locally advanced cervical cancer. Gynecol Oncol 2016; 140:76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]


