Supplemental Digital Content is available in the text
Keywords: cold storage, DBD, DCD, ECD, kidney preservation, machine perfusion, warm perfusion
Abstract
Background:
The two main options for renal allograft preservation are static cold storage (CS) and machine perfusion (MP). There has been considerably increased interest in MP preservation of kidneys, however conflicting evidence regarding its efficacy and associated costs have impacted its scale of clinical uptake. Additionally, there is no clear consensus regarding oxygenation, and hypo- or normothermia, in conjunction with MP, and its mechanisms of action are also debated. The primary aims of this article were to elucidate the benefits of MP preservation with and without oxygenation, and/or under normothermic conditions, when compared with CS prior to deceased donor kidney transplantation.
Methods:
Clinical (observational studies and prospective trials) and animal (experimental) articles exploring the use of renal MP were assessed (EMBASE, Medline, and Cochrane databases). Meta-analyses were conducted for the comparisons between hypothermic MP (hypothermic machine perfusion [HMP]) and CS (human studies) and normothermic MP (warm (normothermic) perfusion [WP]) compared with CS or HMP (animal studies). The primary outcome was allograft function. Secondary outcomes included graft and patient survival, acute rejection and parameters of tubular, glomerular and endothelial function. Subgroup analyses were conducted in expanded criteria (ECD) and donation after circulatory (DCD) death donors.
Results:
A total of 101 studies (63 human and 38 animal) were included. There was a lower rate of delayed graft function in recipients with HMP donor grafts compared with CS kidneys (RR 0.77; 95% CI 0.69–0.87). Primary nonfunction (PNF) was reduced in ECD kidneys preserved by HMP (RR 0.28; 95% CI 0.09–0.89). Renal function in animal studies was significantly better in WP kidneys compared with both HMP (standardized mean difference [SMD] of peak creatinine 1.66; 95% CI 3.19 to 0.14) and CS (SMD of peak creatinine 1.72; 95% CI 3.09 to 0.34). MP improves renal preservation through the better maintenance of tubular, glomerular, and endothelial function and integrity.
Conclusions:
HMP improves short-term outcomes after renal transplantation, with a less clear effect in the longer-term. There is considerable room for modification of the process to assess whether superior outcomes can be achieved through oxygenation, perfusion fluid manipulation, and alteration of perfusion temperature. In particular, correlative experimental (animal) data provides strong support for more clinical trials investigating normothermic MP.
1. Introduction
The most optimal long-term treatment option for end-stage renal disease remains kidney transplantation. On a worldwide basis, access and referral for transplantation is limited; in those patients referred for transplantation, there is an imbalance between the supply and demand for suitable organs.[1] In the United States alone, the median time to deceased donor renal transplantation is approximately 3 to 4 years.[2] This organ deficit has prompted the adoption of different strategies to increase the availability of kidneys for transplantation. One approach of considerable importance is the increasing utilization of donation after circulatory death (DCD) and expanded criteria donors (ECD), which must supplement the standard criteria, donation after brain death (DBD) kidneys.[1,3]
The growing demands for DCD and ECD kidneys must be balanced with their perceived suboptimal posttransplant function. There are higher rates of delayed graft function (DGF) for both DCD and ECD kidneys, and higher discard rates and by definition poorer survival in the ECD subset, when compared with standard criteria DBD kidneys.[4–10] Further improvements to the organ procurement and preservation process are therefore essential to improve marginal donor kidney quality.
Although cold static storage (CS) is still the most commonly utilized method for renal preservation, machine perfusion (MP) provides an important alternative. CS largely supplanted MP in the 1980s due to a lack of evidence with regards to improvement in transplantation outcomes and the large associated costs.[11–13] MP has seen a resurgence in the last decade due to the changing donor profile and advancements in perfusion solutions and technology.[14]
Indeed, application of MP is still not widespread, with conflicting evidence even in recent years regarding its utility.[15,16] Furthermore, there is minimal clinical data regarding the utility of evolving modifications to the MP process, and its mechanisms of action are also poorly understood. In particular, the use of warm (normothermic) perfusion (WP), oxygenation, or pharmacotherapies has largely been the subject of experimental (animal) studies.
The aims of this systematic review and meta-analysis were therefore to: describe ways in which MP is currently utilized; provide an updated and comprehensive analysis of the effect of hypothermic MP (HMP) on posttransplant graft function in deceased donor kidney transplantation; and explore experimental (animal) literature to investigate the utility of normothermic (WP) and/or oxygenated MP, and understand the mechanisms of action of MP preservation.
2. Methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) was utilized in the completion of this review (see Table).[17] The review protocol was registered with the PROSPERO International Prospective Register of Systematic Reviews (March, 2016; registration number—CRD42016037100).[18]
2.1. Eligibility
2.1.1. Inclusion criteria
Clinical (human) studies consisted of randomized control trials (RCT) or prospective (nonrandomized) and observational studies, and were included in the presence of MP data. Experimental (animal) studies by their nature are prospective, and were included in the presence of comparative data either between different types of MP, and/or MP and an alternative form of preservation. Both English and non-English articles were considered, utilizing a translator if necessary. Only published works, and not conference abstracts, were included; although there is some evidence to suggest that gray literature exclusion can contribute to publication bias,[19] these abstracts were all assessed and deemed to have either insufficient data or quality for inclusion.
2.1.2. Exclusion criteria
Clinical/human studies were excluded if less than 10 patients were in the MP group, or there was significant data and/or patient overlap between 2 or more published studies, and/or there was insufficient data with regards to delayed graft function, primary nonfunction (PNF), or graft/patient survival. These parameters were chosen as they were the most commonly and uniformly reported in the studies analyzed. For animal studies, an article was excluded if there was no appropriate control group for comparison, and/or there was a lack of a reperfusion period (either ex vivo or in vivo) after MP preservation. All studies prior to 1980 were excluded. This publication year reflects a time after which there was a distinct shift in the type of perfusion machines and perfusion solutions used.
2.2. Search strategy
The EMBASE, Medline, and Cochrane (1980–December 2015) databases were searched using Ovid, with key search terms including “kidney or renal” and “machine perfusion” (see Table, Supplemental Digital Content 2, for complete strategy). In an effort to include all eligible studies, a manual literature search was also conducted using any potential articles’ bibliographies, in addition to reference lists from other reviews.
2.3. Data collection
Data was extracted from each article by 2 independent reviewers utilizing a predetermined template; a third reviewer was consulted if necessary for any disagreements.
2.3.1. Clinical (human) data
Human data was analyzed for the extraction of the following: date of publication and study period; study type (i.e., prospective or retrospective); kidney allocation; study center(s); patients in MP and CS groups; stratification of MP and CS patients by DBD, DCD, and ECD status; MP characteristics, including the use of oxygenation and preservation temperature; perfusion machine(s) used; and the preservation solution(s) used in CS and MP groups. Quantitative data was extracted for—the incidence of DGF and primary nonfunction (PNF), 1-year graft and patient survival in the whole cohort, acute rejection rates, and posttransplant renal function (CrCl in mL/min and serum creatinine in mg/dL). DGF was defined as the need for dialysis in the first week after transplantation.[20] Only 6 studies either utilized an alternate definition of DGF, or did not define DGF.
Hazard ratios (HR) for graft survival were calculated, when possible, using the methods described by Tierney et al.[21]
Although the “ECD” graft description is not as descriptively useful as a high Kidney Donor Profile Index donor kidney, ECD is used in this manuscript as it is the most commonly utilized term in the included literature.
2.3.2. Experimental (animal) data
Study parameters collected for animal data included: date of publication, institution(s) involved, animal/species employed, weight range of animals, experimental procedure(s)/model employed (study groups, DCD or DBD, ex vivo perfusion or transplantation after preservation, experimental period), number of animals in each group, cold/warm ischemic times, perfusion machine and settings used, preservation/perfusion solution(s) used, additives to preservation/perfusion solution(s), temperature of preservation/perfusion, and the use of oxygen. Study outcomes consisted of renal function parameters (peak creatinine in mg/dL, creatinine clearance (CrCl) in mL/min,), renal tubule parameters (fractional excretion of sodium (Na) (FeNa); enzymatic markers of tubular damage), glomerular parameters (proteinuria), endothelial injury parameters, markers of inflammation, oxidative stress markers, microcirculatory tissue perfusion post-preservation, oxygen consumption, histology, and animal survival.
The standardized mean difference (SMD) was calculated between comparator groups for peak creatinine, CrCl, FeNa, and survival using an effects size calculator.[22]
2.4. Bias assessment
2.4.1. Clinical (human) data
Bias assessment of prospective cohort studies included in the meta-analyses was performed using the Newcastle–Ottawa quality assessment scale for cohort studies.[23] RCT study quality was assessed using the Cochrane Collaboration's tool.[24]
2.4.2. Experimental (animal) data
Animal experimental studies have several important differences in comparison to clinical studies. As such, SYRCLE risk of bias tool for animal studies was instead utilized to assess the quality of animal data included in meta-analyses.[25]
2.5. Synthesis and analysis of results
Observational (retrospective) human studies, in conjunction with prospective studies, were collated to systematically summarize the current parameters of MP utilization clinically. Observational studies were not included in subsequent formal quantitative analyses.
Similarly, animal studies comparing HMP and CS were only utilized to explore mechanisms of MP preservation. As there are multiple human studies focusing on the comparison between HMP and CS, animal studies for this comparator group were not formally meta-analyzed to avoid additional heterogeneity.
2.6. Meta-analyses
In general, the HMP or WP groups were considered the intervention group when compared with CS; the intervention group was WP when compared with HMP, and oxygenated HMP when compared with nonoxygenated HMP. In the event of multiple experimental groups and 1 control group, each different experimental group was compared with the control group and analyzed as a separate study.
2.6.1. Human (clinical) data
Only prospective studies were included in meta-analyses. As only 1 study utilized WP[26] it could not be separately analyzed. Therefore, studies comparing HMP to CS were meta-analyzed. Further subgroup analyses for HMP versus CS in DCD and ECD donors were undertaken. In the event that 1 article presented the results from a subgroup of a larger study, the ECD or DCD donor results were only included in subgroup analyses. Forest plots denoting relative risk (RR) were constructed for DGF and PNF; HR was utilized in graft survival plots.
2.6.2. Animal (experimental) data
Meta-analyses were undertaken for studies comparing WP to CS or HMP, and oxygenated HMP to nonoxygenated HMP. All WP studies employed a DCD model so further subgroup analyses could not be undertaken. Forest plots were created for the SMD of relevant quantitative parameters.
Meta-analyses were performed for the above comparator groups using Comprehensive Meta-Analysis Version 2.2 (Biostat Inc, Englewood, NJ). The I2 statistic was used to analyze study heterogeneity, with values ≥50% indicating high levels of heterogeneity. In these cases, a random effects model was used; otherwise, a fixed effects model was employed. Publication bias was assessed using funnel plots. A P value <0.05 denotes statistical significance, and meta-analysis results are presented with 95% confidence intervals (CI).
3. Results
3.1. Summary clinical and experimental study characteristics
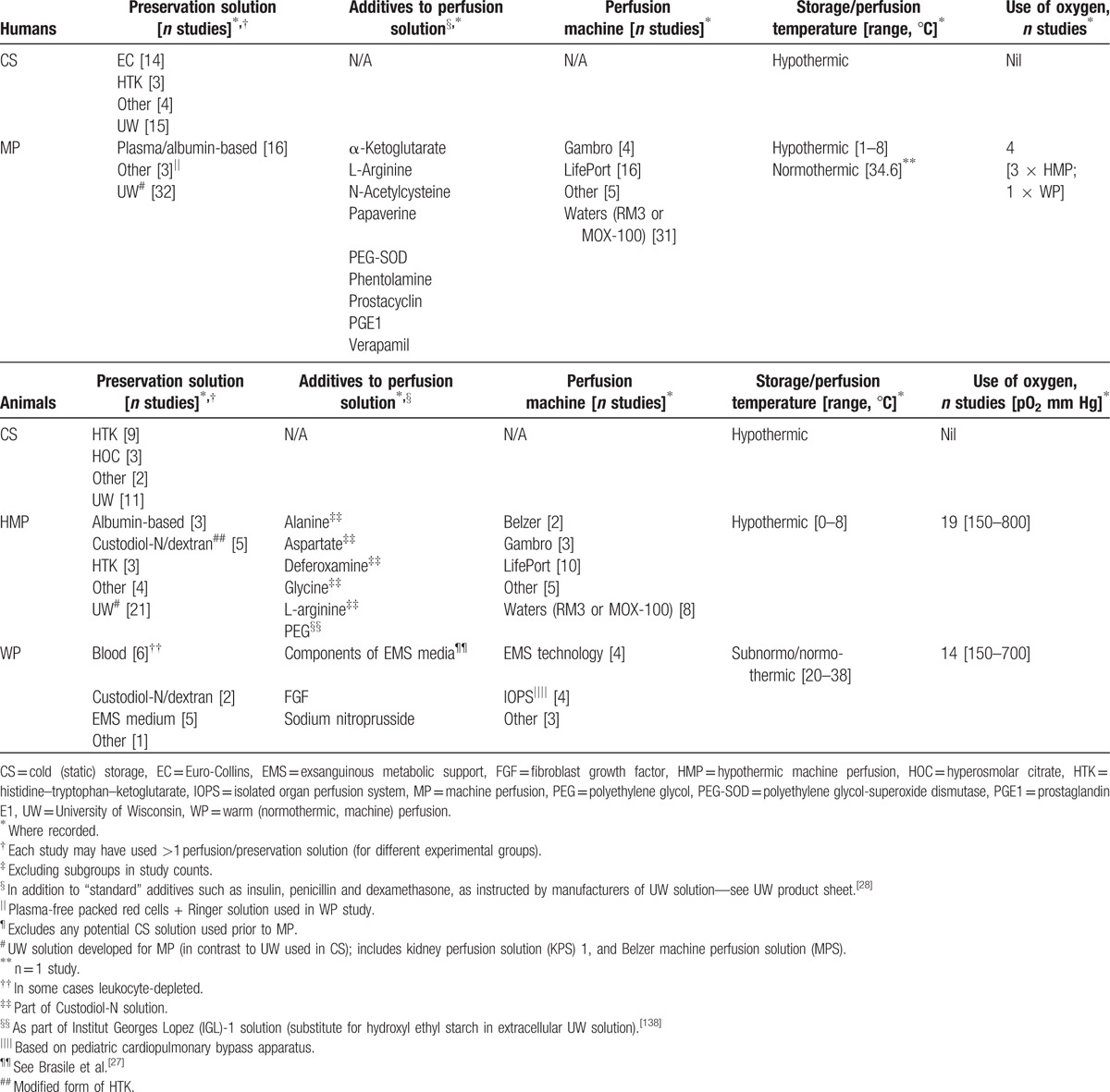
Both human and animal studies were analyzed in the formulation of this systematic review, with human studies used in comparisons between HMP and CS, and animal articles utilized for the analysis of oxygenated HMP, WP, and the mechanisms of MP. In total, 63 human and 38 animal studies met inclusion criteria for which data was extracted for both quantitative and qualitative analyses. Figure 1 outlines the study selection process. Baseline study characteristics are outlined in Supplemental Digital Content 3 and 4 (Tables), while Table 1 summarizes preservation and perfusion parameters for all studies.[27,28]
Figure 1.
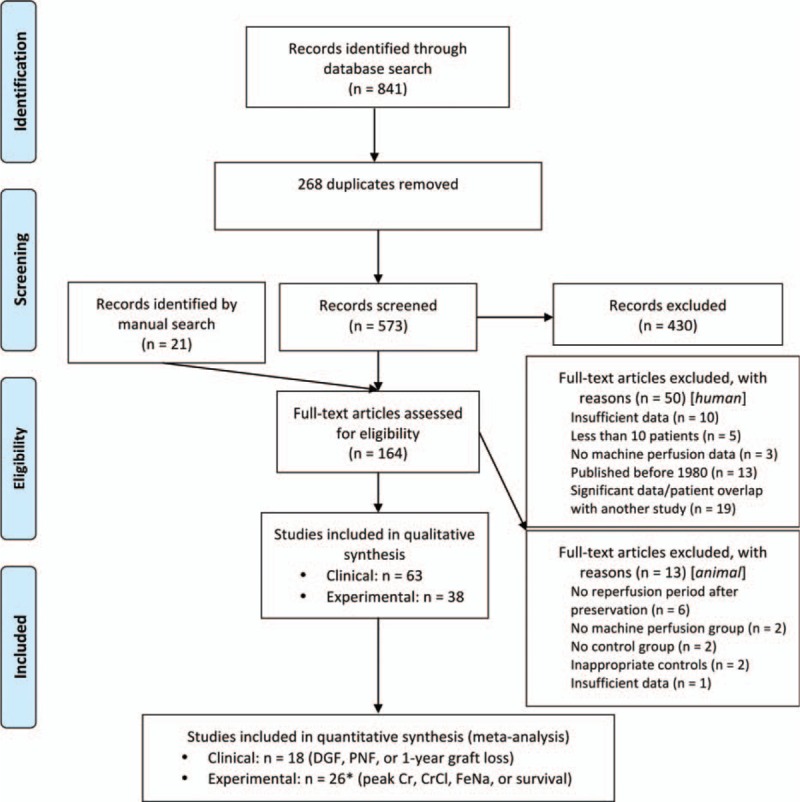
Study selection flow diagram.
Table 1.
Summary human and animal study perfusion and preservation characteristics‡.
3.2. Human (clinical) data
3.2.1. MP parameters for deceased human donor kidney preservation (all studies)
University of Wisconsin (UW)-based MP solutions were the most commonly utilized preservation solutions in human MP (Table 1). Perfusion fluid was pumped through kidneys using Waters or LifePort MP apparatus in most cases (Table 1). Pulsatile perfusion was employed in the vast majority of studies; only 2 (3.2%) articles specified the use of nonpulsatile MP.[29,30] Median perfusion pressure was 50 mm Hg (range 30–60 mm Hg) in HMP articles, while the 1 WP study used pressures of 52 to 70 mm Hg.[26]
Pharmacologic manipulation of the perfusate was minimal, with only 8 (12.7%) human studies entertaining the addition of nonstandard additives (Table 1), and 4 (6.3%) of articles utilizing oxygenated MP. All but 1 human study utilized HMP; in the WP study the perfusate was warmed to a temperature of 32° to 36°C.[26]
The duration and location of placement of kidneys on the machine varied between centers. In particular, 18 of 63 (28.6%) of articles specified the use of CS in conjunction with MP; in these cases, MP was usually commenced upon arrival to the recipient center. Kidneys that underwent MP tended to have greater median CITs compared with CS kidneys (23.4 vs 19.5 hours, respectively) (see Table, Supplemental Digital Content 3), largely reflecting the use of MP as a possible means to extend preservation times.
3.2.2. Meta-analyses (prospective studies)
Eighteen studies were included in the human meta-analysis, out of which 11 (61.1%) articles were RCTs, and 7 (38.9%) studies were prospective but nonrandomized (prospective cohorts). As there was only 1 study comparing WP to CS, WP could not be directly compared with other preservation methods using the human studies.
Forest plots of selected meta-analyses are shown in Figure 2, with all results tabulated in Supplemental Digital Content 5.
Figure 2.
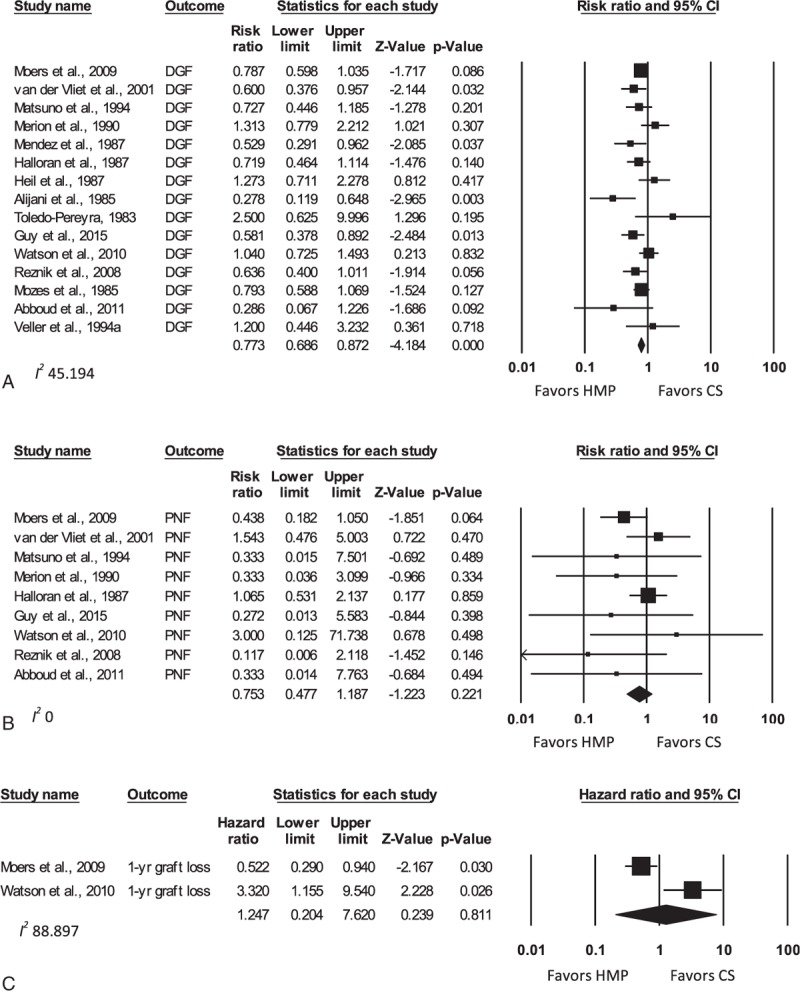
Forest plots comparing DGF (A), PNF (B), and 1-year graft loss (C) for all studies comparing HMP to CS—human studies. Data expressed as RR (for DGF and PNF) and HR (for graft loss) ± 95% CI. Different analyses within the same study are denoted by an alphabetical letter suffix (e.g., “a”). CI = confidence interval, CS = cold (static) storage, DGF = delayed graft function, HMP = hypothermic machine perfusion, HR = hazard ratio, PNF = primary nonfunction.
Human studies displayed the short-term advantages of MP when compared with CS. The RR (unadjusted) of DGF for HMP versus CS studies was 0.77 (95% CI 0.69–0.87; P <0.001). Within the DCD kidney subgroup, the RR of DGF was 0.78 (95% CI 0.66–0.91; P = 0.002), while it was 0.67 for ECD donors (95% CI 0.42–1.08; P = 0.097). It should be noted that only 2 studies were available for the ECD comparison. A significant difference in PNF rates between HMP and CS was only detected in the ECD cohort (RR 0.28, 95% CI 0.09–0.89; P = 0.031).
The medium to long-term effects of MP were less clear. With respect to graft failure rates within the first year, there was no difference between HMP and CS overall (HR 1.25, 95% CI 0.20–7.62; P = 0.81). Insufficient data precluded HR calculations for further subgroup analyses, or for the comparison of patient survival between the HMP and CS groups.
3.2.3. Meta-analysis publication bias and heterogeneity (prospective studies)
Visual assessment of funnel plots displayed no significant asymmetry when comparing HMP to CS for the DGF parameter. There was only mild asymmetry in favor of positive studies for studies comparing PNF (see Figure, Supplemental Digital Content 6, for funnel plots). Study heterogeneity was low for a majority of parameters (see Table, Supplemental Digital Content 5).
3.2.4. Trends in 1-year graft loss and patient survival (prospective studies)
Meta-analyses for graft loss/survival at 1 year could only be conducted in 2 studies. In 1 of these studies by Moers et al, graft loss at 1 year was significantly higher in the CS group compared with HMP (HR 0.52; P = 0.03); this finding was maintained in the ECD (HR 0.35; P = 0.02) but not DCD subgroups (HR 1.29; P = 0.7) in subsequent expansions of the study cohorts.[16,31,32] Graft loss (survival) data for the 1 year time-point were available in 8 further prospective studies. Although there were no statistically significant differences between HMP and CS, there was a trend toward higher survival after HMP in 4 studies, including 1 article investigating ECD kidneys.[33–36] In contrast, although still underpowered to produce statistical significance, 2 studies indicated higher survival in CS kidneys, with 1 of these studies analyzing DCD kidneys.[37,38]
There were 7 prospective studies with results available for patient survival 1 year posttransplant.[15,16,31–34,36] Median survivals were 94.9% (range 80.6–97%) for HMP kidneys, and 96.7% (range 77.7–100%) for CS kidneys. No study reported statistically significant differences between either preservation method.
Nicholson and Hosgood[26] presented the only human study exploring the use of WP for renal preservation. The WP cohort impressively had 100% 1 year graft and patient survival rates, although there were only 18 patients in the WP group.
3.2.5. Graft rejection (prospective studies)
Acute graft rejection rates were not statistically comparable owing to variable definitions and immunosuppression. Rejection rates were no different in the multicenter trial by Moers et al[16] (13.7% for CS vs 13.1% for MP). In contrast, 3 prospective studies showed a strong trend toward lower rates of acute rejection in the HMP group, although this did not reach significance.[15,39,40]
3.2.6. Risk of bias assessment (prospective studies)
The risk of bias assessment of cohort studies is summarized in Supplemental Digital Content 7 (Figure). Six out of 8 domains in the assessment scale were adequately covered in at least 60% of studies. Comparability of cohorts in study design or analysis was less adequately covered, as a proportion of studies did not appropriately account for factors such as organ ischemic times. Supplemental Digital Content 8 (Table) displays the risk of bias assessment for the included RCTs upon utilization of the Cochrane Collaboration bias tool.[24] Across studies, it can be seen that there is a low risk of bias in at least 3 of the domains. Within the domains of blinding and allocation concealment, however, at least half of the studies were at risk of selection and performance bias.
4. Animal (experimental) data
4.1. MP characteristics (all studies)
In stark contrast to human studies, 30 of 38 (78.9%) animal articles utilized oxygenated MP. Furthermore, WP, including subnormothermic MP, was used in 14 (36.8%) of the included animal studies (see Table, Supplemental Digital Content 4). As such, further quantitative analyses regarding oxygenated and/or WP were undertaken in animal studies.
4.2. Meta-analyses (oxygenated HMP and WP studies)
There were 10 distinct animal data-sets utilized in the meta-analyses that compared CS to WP, while 11 studies were included that compared HMP with WP and 5 studies were available for the comparison between oxygenated and nonoxygenated HMP.
Figure 3 displays forest plots of selected meta-analyses, with results tabulated in Supplemental Digital Content 9.
Figure 3.
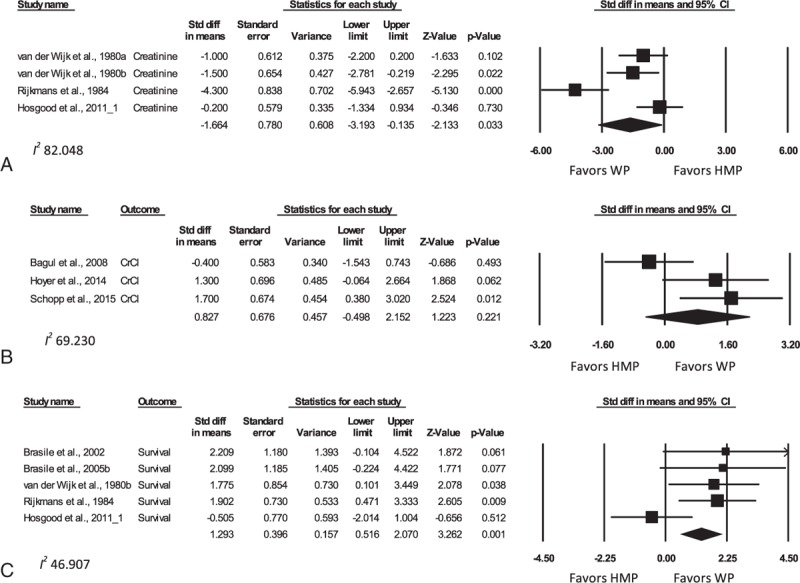
Forest plots comparing peak creatinine (A), peak CrCl (B), and survival (C) for WP compared with HMP—animal studies. Data presented as SMD ± 95% CI. Different analyses within the same study are denoted by an alphabetical letter suffix (e.g., “a”). HMP = hypothermic machine perfusion, SMD = standardized mean difference, WP = warm (normothermic) perfusion.
Postpreservation renal function in animal experiments was assessed using the parameters of peak creatinine, CrCl and FeNa, and animal survival during the experimental period. Peak creatinine values were significantly lower in animal groups utilizing WP (SMD −1.72, 95% CI −3.09 to −0.34; P = 0.014) when compared with CS. The SMD of peak serum creatinine levels in the WP group was also significantly lower when compared with the HMP group (−1.66, 95% CI −3.19 to −0.14; P = 0.033). There was no significant difference however between peak creatinine levels in the oxygenated HMP versus non-oxygenated HMP group (SMD −0.39, 95% CI −1.85 to 1.08; P = 0.60); however, there were only 2 studies eligible for this comparison.[41,42] However, the SMD of peak CrCl between the WP and HMP (0.83, 95% CI −0.50 to 2.15; P = 0.22) and CS (2.08, 95% CI −1.83 to 6.00; P = 0.22) groups was not significantly different.
FeNa could not be compared between WP and other groups due to an insufficient number of studies. Importantly, pooled FeNa was significantly lower in studies comparing oxygenated to nonoxygenated HMP (SMD −1.54; 95% CI −2.54 to −0.54; P = 0.002).
Animal survival in such studies is a reflection of maintenance of renal function as opposed to actual survival per se as the vast majority of deaths reflected euthanasia after manifestation of features of renal failure. Importantly, WP once again demonstrated its superiority over HMP (SMD 1.29; 95% CI 0.52–2.07; P = 0.001). There was not enough data to analyze this parameter for WP compared with CS groups.
4.3. Meta-analysis publication bias and heterogeneity (WP studies)
Analysis of funnel plots did not display significant asymmetry when comparing peak creatinine between WP and the HMP or CS groups (see Figure, Supplemental Digital Content 10, for funnel plots). Study heterogeneity was high for most parameters (see Table, Supplemental Digital Content 9).
4.4. Mechanisms of action of MP—tubules, glomeruli, and endothelium (all studies)
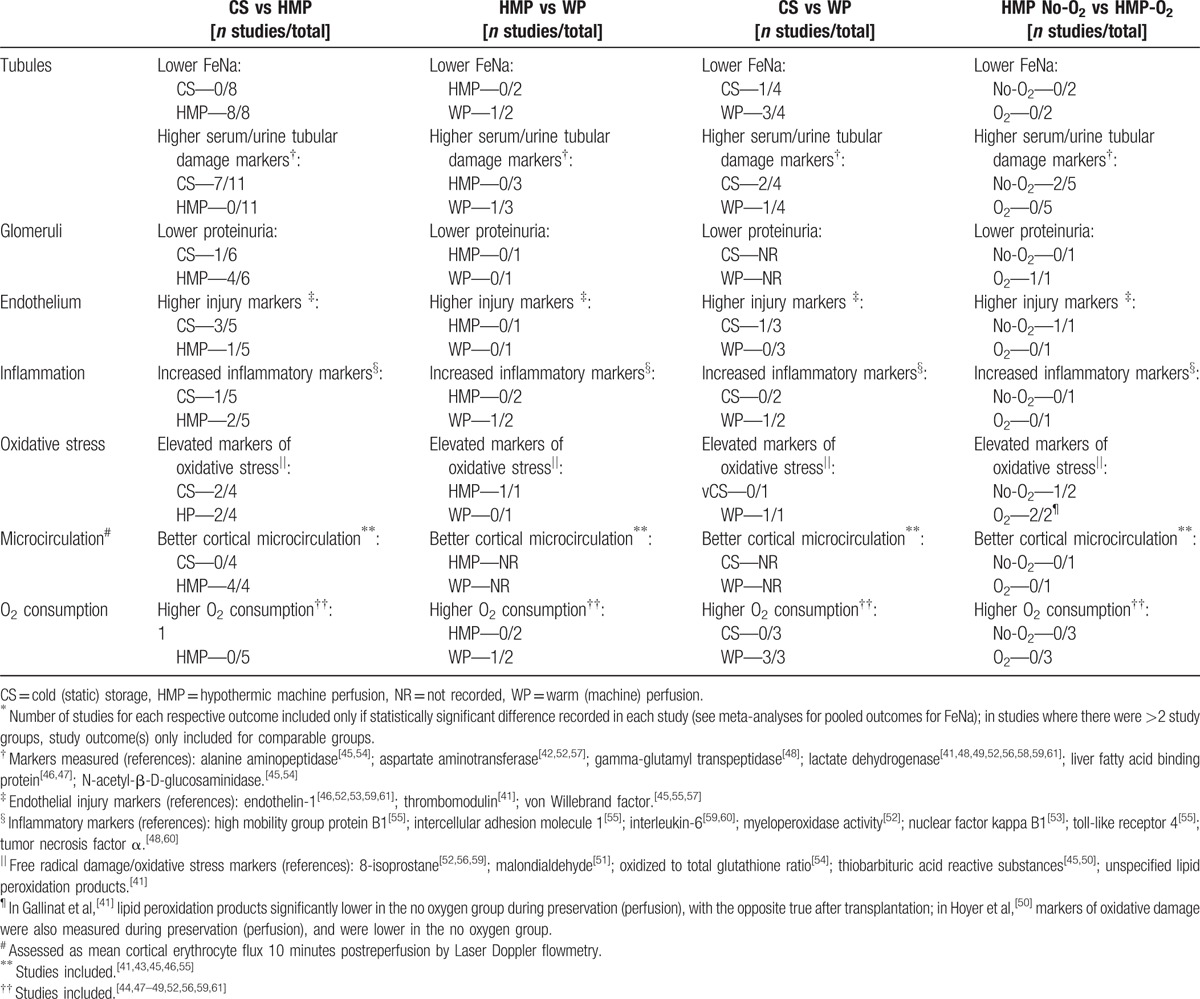
The animal studies outlined comparisons between experimental and control groups for a wide range of parameters that could not be meta-analyzed due to significant variability in reporting between different studies. These functional indicators are displayed in Table 2,[41–61] and can broadly be characterized into those relating to tubular, glomerular, or endothelial function or damage, oxidative stress, levels of inflammation, microcirculatory tissue perfusion, and oxygen consumption. Histology was not included in this analysis due to wide variability in the reporting of histological criteria. Broadly, improved tubular function with a reduction in tubular injury, improved glomerular function, and reduced endothelial injury seemed to be evident after the utilization of HMP compared with CS. Furthermore, HMP appeared to improve renal cortical microcirculation. There was no obvious advantage for any experimental group regarding markers of inflammation or oxidative stress. Furthermore, with the exception of higher oxygen consumption in all 3 studies comparing WP with CS, no clear differences could be elucidated between the other experimental and control groups (Table 2).
Table 2.
Tubular, glomerular, and endothelial function and damage in animal studies∗.
4.5. Risk of bias assessment (all studies)
Animal study bias assessment was performed using SYRCLE assessment tool[25] and is summarized in Supplemental Digital Content 11 (Figure). Overall, there were very few domains in which there was clearly a high risk of bias. In 6 out of the 10 parameters however, bias assessment was largely unclear as the domains could not be analyzed from the available study data.
5. Discussion
This systematic review and meta-analysis provides a comprehensive and up-to-date insight into the current published literature regarding MP preservation of renal grafts prior to transplantation in the clinical setting. Animal data was included to explore modifications to MP that are as yet grossly under-explored in human studies, namely WP and oxygenated MP, in addition to allowing the development of a greater mechanistic understanding of MP.
We show a definite reduction in DGF post-HMP preservation for renal allografts in humans when compared with CS, including in DCD and ECD kidneys. PNF appeared to be reduced in the ECD subset. There was not enough data to give sufficient power to comparisons of 1 year graft survival by meta-analysis, and subgroup analyses could not be conducted for this parameter. One-year patient survival was comparable among the different studies. We obtained mixed results regarding the benefits of oxygenated HMP. Furthermore, although there was only 1 human study that employed WP,[26] multiple animal studies showed its advantages over both CS and HMP kidneys in terms of posttransplantation creatinine levels and animal survival. Animal study results showed mechanisms for improved allograft function in MP kidneys, including better tubular and glomerular function, and less endothelial damage.
Increased demands for donor kidneys have necessitated the use of more marginal organs for transplantation. Indeed, any method such as MP that will increase the pool of usable kidneys can benefit developing and developed countries alike, especially due to the often prohibitively high costs associated with long-term dialysis, and should be explored further.[1] A detailed economic analysis by Wight et al,[62] albeit from 2003, showed that MP is likely to be more effective than CS in the long-term, with an economic benefit more pronounced when MP preservation is applied to DCD kidneys. While Groen et al[63] in 2012 could not make the same conclusion for DCD transplants due to insufficient numbers, these authors found reduced costs after MP in the ECD subset, largely due to a reduced need for post-transplantation dialysis and hospital bed-stays.
Mechanistically, MP reduces preservation-related damage and aids renal recovery through a variety of mechanisms. ATP levels, and thus energy homeostasis, are better preserved in perfused kidneys.[43,44] Tubular and glomerular integrity seems to be aided by MP, an assertion that is supported by the reduction in markers of tubular damage and improved tubular and glomerular function seen after MP as compared with CS (Table 2). Furthermore, MP ensures better reperfusion of grafts as measured by cortical microcirculation; this is likely related to a reduction in endothelial damage and swelling[43,45] (Table 2). The flow cessation itself in CS as compared with MP likely contributes to the increased endothelial dysfunction in CS grafts.[64] The pulsatile aspect of MP likely has an important effect on the maintenance of endothelial integrity, as pulsatile-perfused kidneys compared with nonpulsatile MP have been shown to have higher renal vascular flow, reduced expression of endothelin-1, and increased expression of the vasoprotective kruppel-like factors and nitric oxide.[46] We did not however find significant support for less inflammation and oxidative stress in the HMP group (Table 2), although recent evidence suggests that apoptosis and inflammation may be reduced in HMP through up-regulation of aldehyde dehydrogenase 2 and reduction in expression of nuclear factor-κB and matrix metalloproteinase 9.[65,66]
In congruence with previous systematic reviews[8,67–69] our data shows that DGF is undoubtedly reduced in patients undergoing MP compared with CS. We additionally showed the possibility of reduced PNF after HMP preservation of ECD kidneys. In contrast to Jiao et al[70] however, we could not find statistical evidence for improved graft survival in the ECD cohort, due to a lack of available HR data that could subsequently be pooled. Furthermore, statistical methods in the former study are flawed, with survival analyses conducted using OR instead of HR; in addition, 2 out of the 3 studies in their survival analysis had significant patient overlap.[70] Perhaps most pertinently however, the pivotal large-scale and multicenter RCT performed by Moers et al showed significantly improved graft survival in HMP patients, with this survival advantage still present after 3 years in DBD and especially ECD kidneys, but not in kidneys from DCD donors.[16,71,72]
While Moers et al study provides evidence regarding the efficacy of machine perfusion as it is utilized currently, our analysis of all retrospective and prospective MP studies in humans to date show that it is still employed in a very limited fashion, with considerable room for modification to maximize the potentials of this technique. In particular, temperature modification, oxygenation, and pharmacologic manipulation of perfusion solutions are all in their infancy with regard to human renal preservation via MP.
The inclusion of animal data has allowed this review to capture the possible future of MP, as this experimental work has not yet caught up with application to the clinic. In particular, a reasonable deduction can be made regarding the applicability and potential success of WP, which currently has little human data. WP reverses the pivotal concept of hypothermia in organ preservation, sustaining normal metabolic rates with an oxygenated red blood cell-based perfusate. Compared with CS and HMP kidneys, WP kidneys had significantly lower peak creatinine and better survival (Fig. 3; also see Table, Supplemental Digital Content 9). Nicholson and Hosgood[26] utilized WP in human ECD kidney grafts, and also reported lower rates of DGF compared with CS. WP potentially reduces the possibility of irreversible cold-induced metabolic disruption in addition to reducing ischemia-reperfusion injury upon commencement of normothermic reperfusion in vivo.[27,65,73]
An alternative to WP at body temperature is the concept of subnormothermic MP, successfully utilized here in 2 studies.[47,48] Subnormothermic perfusion helps avoid the injuries induced by cold ischemia without necessitating a significant change in perfusion equipment or solutions.[48] In addition, it guards against the pitfalls inherent to an immediate temperature shift from hypothermia to body temperature upon postanastomotic reperfusion.[47]
The perfusion solution and its additives potentially have a major impact on the effectiveness of kidney preservation. UW or a modified form of UW was the most commonly employed solution for CS and MP in both animal and human studies (Table 1), which is not surprising considering its proven efficacy.[66] Although there is considerable ongoing research into pharmacological manipulation of organ preservation solutions, surprisingly few studies utilized additives to try and change graft outcomes (Table 1). Pathophysiological targets for these additives include free-radical injury, endothelial damage, and vasoconstriction, the complement cascade, and apoptosis.[74–78] These processes were in some cases targeted as part of new perfusion solutions, including Custodiol-N, Vasosol, and Exsanguinous Metabolic Support (EMS) media.[74,76,78,79] It is difficult to ascertain individual effects of each pharmacologic agent, as few studies undertook direct comparisons between them. Guarrera et al[78] compared Vasosol solution, which contains vasodilatory agents such as prostaglandin E1 (PGE1) and nitroglycerin, and the antioxidant N-acetylcysteine, to UW (Belzer MPS), and showed significant lower DGF rates in the Vasosol group. The addition of PGE1 to UW was also shown to be effective in another study.[76] Other pharmacological therapies that may be incorporated into renal preservation are reviewed by Chatauret et al.[80]
Oxygenation is also a pharmacologic intervention that can be applied to HMP. Its use was much more prevalent in animal studies, with comparisons showing significantly lower FeNa in the oxygenated HMP compared to nonoxygenated HMP group (see Table, Supplemental Digital Content 9). The absence of a statistical difference with regards to peak creatinine may be explained by the fact that there were only 2 studies for comparison.[41,42] Active oxygenation of the perfusate may potentially increase the generation of reactive oxygen species (see Table 2), although this was not supported posttransplantation in the study by Gallinat et al.[41] In contrast, the use of oxygen during HMP is purported to restore adequate mitochondrial and cellular homeostasis prior to reperfusion.[49,50] An alternative to oxygenated MP is the use of persufflation, through which oxygen can be delivered to the kidneys directly through its vasculature. Suszynski et al[81] summarize the utility of persufflation for renal preservation; this technique was compared to CS and HMP by Treckmann et al,[51] with persufflated kidneys having significantly lower creatinine levels posttransplantation compared with HMP.
Limitations of this review include the suboptimal comparability of HMP and CS cohorts within the human studies. This was largely due to the fact that CIT for human MP kidneys was higher than that for CS kidneys (see Table, Supplemental Digital Content 3), which is not surprising given that MP is often used as a means to extend the period of preservation. Furthermore, a not insignificant proportion of RCTs suffered from features of selection bias due to poor blinding and allocation concealment. Additionally, it is difficult to tease out the impact of MP solutions on the overall effect of MP, as a variety of solutions were utilized that were usually different to the CS control. Animal studies, although informative, were quite heterogeneous and difficult to formally assess for bias. We attempted to minimize bias by excluding all retrospective studies from the meta-analyses, and in order to account for any study heterogeneity a random effects model was employed to help reduce type I error.
In summary, we have shown distinct short-term advantages in the use of MP over CS for the preservation of renal allografts, especially with regards to the reduction of DGF. ECD graft recipients may benefit further from a reduction in PNF rates. In the medium to long-term, there is likely a survival and cost advantage for ECD kidneys that have undergone MP in this way. Although results from animal studies should be interpreted with more caution, they show some mechanistic advantages to the use of oxygenated MP, and distinct functional improvements upon the use of normothermic perfusion; this should provide a further stimulus for MP oxygenation and WP human trials. We strongly encourage additional exploration and enhancement of the MP preservation technique, through a variety of modifications based on the presented experimental evidence, which may improve its short and long-term efficacy.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: CI = confidence interval, CIT = cold ischemic time, CrCl = creatinine clearance, CS = cold (static) storage, DBD = donation after brain death, DCD = donation after circulatory death, DGF = delayed graft function, ECD = expanded criteria donor, EMS = exsanguinous metabolic support, FeNa = fractional excretion of sodium, HMP = hypothermic machine perfusion, HR = hazard ratio, KPS = kidney perfusion solution, MP = machine perfusion, MPS = machine perfusion solution, PGE1 = prostaglandin E1, PNF = primary nonfunction, PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses, RR = risk ratio (relative risk), SMD = standardized mean difference, UW = University of Wisconsin, WIT = warm ischemic time, WP = warm (normothermic) perfusion.
Funding sources: Australian Government—Australian Postgraduate Award.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- 1.Garcia GG, Harden P, Chapman J. World Kidney Day Steering C. The global role of kidney transplantation. Lancet 2012; 379:e36–e38. [DOI] [PubMed] [Google Scholar]
- 2.United States Renal Data System. USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2015. [Google Scholar]
- 3.Gridelli B, Remuzzi G. Strategies for making more organs available for transplantation. N Engl J Med 2000; 343:404–410. [DOI] [PubMed] [Google Scholar]
- 4.Metzger RA, Delmonico FL, Feng S, et al. Expanded criteria donors for kidney transplantation. Am J Transplant 2003; 3:114–125. [DOI] [PubMed] [Google Scholar]
- 5.Cho YW, Terasaki PI, Cecka JM, et al. Transplantation of kidneys from donors whose hearts have stopped beating. N Engl J Med 1998; 338:221–225. [DOI] [PubMed] [Google Scholar]
- 6.Saidi RF, Elias N, Kawai T, et al. Outcome of kidney transplantation using expanded criteria donors and donation after cardiac death kidneys: realities and costs. Am J Transplant 2007; 7:2769–2774. [DOI] [PubMed] [Google Scholar]
- 7.Rao PS, Ojo A. The alphabet soup of kidney transplantation: SCD, DCD, ECD—fundamentals for the practicing nephrologist. Clin J Am Soc Nephrol 2009; 4:1827–1831. [DOI] [PubMed] [Google Scholar]
- 8.Bathini V, McGregor T, McAlister VC, et al. Renal perfusion pump vs cold storage for donation after cardiac death kidneys: a systematic review. J Urol 2013; 189:2214–2220. [DOI] [PubMed] [Google Scholar]
- 9.Dutkowski P, Schlegel A, de Oliveira M, et al. HOPE for human liver grafts obtained from donors after cardiac death. J Hepatol 2014; 60:765–772. [DOI] [PubMed] [Google Scholar]
- 10.Klein AS, Messersmith EE, Ratner LE, et al. Organ donation and utilization in the United States, 1999–2008. Am J Transplant 2010; 10 (4 Pt 2):973–986. [DOI] [PubMed] [Google Scholar]
- 11.Opelz G, Terasaki PI. Advantage of cold storage over machine perfusion for preservation of cadaver kidneys. Transplantation 1982; 33:64–68. [DOI] [PubMed] [Google Scholar]
- 12.Sheil AG, Drummond JM, Rogers JH, et al. A controlled clinical trial of machine perfusion of cadaveric donor renal allografts. Lancet 1975; 2:287–290. [DOI] [PubMed] [Google Scholar]
- 13.Rosenthal JT, Herman JB, Taylor RJ, et al. Comparison of pulsatile machine perfusion with cold storage for cadaver kidney preservation. Transplantation 1984; 37:425–426. [PubMed] [Google Scholar]
- 14.Jochmans I, O’Callaghan JM, Pirenne J, et al. Hypothermic machine perfusion of kidneys retrieved from standard and high-risk donors. Transpl Int 2015; 28:665–676. [DOI] [PubMed] [Google Scholar]
- 15.Watson CJ, Wells AC, Roberts RJ, et al. Cold machine perfusion versus static cold storage of kidneys donated after cardiac death: a UK multicenter randomized controlled trial. Am J Transplant 2010; 10:1991–1999. [DOI] [PubMed] [Google Scholar]
- 16.Moers C, Smits JM, Maathuis MH, et al. Machine perfusion or cold storage in deceased-donor kidney transplantation. N Engl J Med 2009; 360:7–19. [DOI] [PubMed] [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. Ann Intern Med 2009; 151:264–269. [DOI] [PubMed] [Google Scholar]
- 18.Centre for Reviews and Dissemination. PROSPERO: International prospective register of systematic reviews. 2015. Available at: http://www.crd.york.ac.uk/prospero/prospero.asp Accessed March, 2016. [Google Scholar]
- 19.McAuley L, Pham B, Tugwell P, et al. Does the inclusion of grey literature influence estimates of intervention effectiveness reported in meta-analyses? Lancet 2000; 356:1228–1231. [DOI] [PubMed] [Google Scholar]
- 20.Mallon DH, Summers DM, Bradley JA, et al. Defining delayed graft function after renal transplantation: simplest is best. Transplantation 2013; 96:885–889. [DOI] [PubMed] [Google Scholar]
- 21.Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007; 8:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.D Wilson. Practical Meta-Analysis Effect Size Calculator. 2001. Available at: http://www.campbellcollaboration.org/escalc/html/EffectSizeCalculator-Home.php Accessed February, 2016. [Google Scholar]
- 23.Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale for assessing the quality of nonrandomised studies in meta-analyses. 2014. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp Accessed February, 2016. [Google Scholar]
- 24.Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011; 343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hooijmans CR, Rovers MM, de Vries RB, et al. SYRCLE's risk of bias tool for animal studies. BMC Med Res Methodol 2014; 14:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nicholson ML, Hosgood SA. Renal transplantation after ex vivo normothermic perfusion: the first clinical study. Am J Transplant 2013; 13:1246–1252. [DOI] [PubMed] [Google Scholar]
- 27.Brasile L, Stubenitsky BM, Booster MH, et al. Hypothermia—a limiting factor in using warm ischemically damaged kidneys. Am J Transplant 2001; 1:316–320. [DOI] [PubMed] [Google Scholar]
- 28.Bridge to Life. Belzer UW Cold Storage Solution Instructions. 2016. Available at: http://www.bridgetolife.com/belzer-uw-cold-storage-solution-instructions/. Accessed March, 2016. [Google Scholar]
- 29.Matsuno N, Konno O, Mejit A, et al. Application of machine perfusion preservation as a viability test for marginal kidney graft. Transplantation 2006; 82:1425–1428. [DOI] [PubMed] [Google Scholar]
- 30.Matsuno N, Konno YN, Jyojima Y, et al. Machine perfusion preservation for kidney grafts with a high creatinine from uncontrolled donation after cardiac death. Transplant Proc 2010; 42:155–158. [DOI] [PubMed] [Google Scholar]
- 31.Treckmann J, Moers C, Smits JM, et al. Machine perfusion versus cold storage for preservation of kidneys from expanded criteria donors after brain death. Transpl Int 2011; 24:548–554. [DOI] [PubMed] [Google Scholar]
- 32.Jochmans I, Moers C, Smits JM, et al. Machine perfusion versus cold storage for the preservation of kidneys donated after cardiac death: a multicenter, randomized, controlled trial. Ann Surg 2010; 252:756–764. [DOI] [PubMed] [Google Scholar]
- 33.Mozes M, Finch W, Reckard C, et al. Comparison of cold storage and machine perfusion in the preservation of cadaver kidneys: a prospective, randomized study. Transplant Proc 1985; 17:1474–1477. [Google Scholar]
- 34.Abboud I, Antoine C, Gaudez F, et al. Pulsatile perfusion preservation for expanded-criteria donors kidneys: impact on delayed graft function rate. Int J Artif Organs 2011; 34:513–518. [DOI] [PubMed] [Google Scholar]
- 35.Mendez R, Mendez RG, Koussa N, et al. Preservation effect on oligo-anuria in the cyclosporine era: a prospective trial with 26 paired cadaveric renal allografts. Transplant Proc 1987; 19 (1 Pt 3):2047–2050. [PubMed] [Google Scholar]
- 36.Halloran P, Aprile M. A randomized prospective trial of cold storage versus pulsatile perfusion for cadaver kidney preservation. Transplantation 1987; 43:827–832. [PubMed] [Google Scholar]
- 37.van der Vliet JA, Kievit JK, Hene RJ, et al. Preservation of non-heart-beating donor kidneys: a clinical prospective randomised case-control study of machine perfusion versus cold storage. Transplant Proc 2001; 33:847. [DOI] [PubMed] [Google Scholar]
- 38.Toledo-Pereyra LH. Renal hypothermic storage with a new hyperosmolar colloid solution. Bol Asoc Med P R 1983; 75:347–350. [PubMed] [Google Scholar]
- 39.Matsuno N, Sakurai E, Tamaki I, et al. The effect of machine perfusion preservation versus cold storage on the function of kidneys from non-heart-beating donors. Transplantation 1994; 57:293–294. [PubMed] [Google Scholar]
- 40.Reznik ON, Bagnenko SF, Loginov IV, et al. Machine perfusion as a tool to select kidneys recovered from uncontrolled donors after cardiac death. Transplant Proc 2008; 40:1023–1026. [DOI] [PubMed] [Google Scholar]
- 41.Gallinat A, Paul A, Efferz P, et al. Role of oxygenation in hypothermic machine perfusion of kidneys from heart beating donors. Transplantation 2012; 94:809–813. [DOI] [PubMed] [Google Scholar]
- 42.Thuillier R, Allain G, Celhay O, et al. Benefits of active oxygenation during hypothermic machine perfusion of kidneys in a preclinical model of deceased after cardiac death donors. J Surg Res 2013; 184:1174–1181. [DOI] [PubMed] [Google Scholar]
- 43.Minor T, Sitzia M, Dombrowski F. Kidney transplantation from non-heart-beating donors after oxygenated low-flow machine perfusion preservation with histidine-tryptophan-ketoglutarate solution. Transpl Int 2005; 17:707–712. [DOI] [PubMed] [Google Scholar]
- 44.Yland MJ, Todo S, Zhu Y, et al. An automated and portable low-flow pulsatile perfusion system for organ preservation. Transpl Int 1996; 9:535–540. [DOI] [PubMed] [Google Scholar]
- 45.Maathuis MH, Manekeller S, van der Plaats A, et al. Improved kidney graft function after preservation using a novel hypothermic machine perfusion device. Ann Surg 2007; 246:982–988. [DOI] [PubMed] [Google Scholar]
- 46.Gallinat A, Fox M, Luer B, et al. Role of pulsatility in hypothermic reconditioning of porcine kidney grafts by machine perfusion after cold storage. Transplantation 2013; 96:538–542. [DOI] [PubMed] [Google Scholar]
- 47.Schopp I, Reissberg E, Luer B, et al. Controlled rewarming after hypothermia: adding a new principle to renal preservation. Clin Transl Sci 2015; 8:475–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hoyer DP, Gallinat A, Swoboda S, et al. Subnormothermic machine perfusion for preservation of porcine kidneys in a donation after circulatory death model. Transpl Int 2014; 27:1097–1106. [DOI] [PubMed] [Google Scholar]
- 49.Koetting M, Frotscher C, Minor T. Hypothermic reconditioning after cold storage improves postischemic graft function in isolated porcine kidneys. Transpl Int 2010; 23:538–542. [DOI] [PubMed] [Google Scholar]
- 50.Hoyer DP, Gallinat A, Swoboda S, et al. Influence of oxygen concentration during hypothermic machine perfusion on porcine kidneys from donation after circulatory death. Transplantation 2014; 98:944–950. [DOI] [PubMed] [Google Scholar]
- 51.Treckmann J, Nagelschmidt M, Minor T, et al. Function and quality of kidneys after cold storage, machine perfusion, or retrograde oxygen persufflation: results from a porcine autotransplantation model. Cryobiology 2009; 59:19–23. [DOI] [PubMed] [Google Scholar]
- 52.Hosgood SA, Mohamed IH, Bagul A, et al. Hypothermic machine perfusion after static cold storage does not improve the preservation condition in an experimental porcine kidney model. Br J Surg 2011; 98:943–950. [DOI] [PubMed] [Google Scholar]
- 53.Gallinat A, Efferz P, Paul A, et al. One or 4 h of “in-house” reconditioning by machine perfusion after cold storage improve reperfusion parameters in porcine kidneys. Transpl Int 2014; 27:1214–1219. [DOI] [PubMed] [Google Scholar]
- 54.Codas R, Thuillier R, Hauet T, et al. Renoprotective effect of pulsatile perfusion machine RM3: pathophysiological and kidney injury biomarker characterization in a preclinical model of autotransplanted pig. BJU Int 2012; 109:141–147. [DOI] [PubMed] [Google Scholar]
- 55.Gallinat A, Paul A, Efferz P, et al. Hypothermic reconditioning of porcine kidney grafts by short-term preimplantation machine perfusion. Transplantation 2012; 93:787–793. [DOI] [PubMed] [Google Scholar]
- 56.Hosgood SA, Yang B, Bagul A, et al. A comparison of hypothermic machine perfusion versus static cold storage in an experimental model of renal ischemia reperfusion injury. Transplantation 2010; 89:830–837. [DOI] [PubMed] [Google Scholar]
- 57.Bagul A, Hosgood SA, Kaushik M, et al. Experimental renal preservation by normothermic resuscitation perfusion with autologous blood. Br J Surg 2008; 95:111–118. [DOI] [PubMed] [Google Scholar]
- 58.Stubenitsky BM, Booster MH, Brasile L, et al. Exsanguinous metabolic support perfusion—a new strategy to improve graft function after kidney transplantation. Transplantation 2000; 70:1254–1258. [DOI] [PubMed] [Google Scholar]
- 59.Hosgood SA, Patel M, Nicholson ML. The conditioning effect of ex vivo normothermic perfusion in an experimental kidney model. J Surg Res 2013; 182:153–160. [DOI] [PubMed] [Google Scholar]
- 60.Hosgood SA, Barlow AD, Yates PJ, et al. A pilot study assessing the feasibility of a short period of normothermic preservation in an experimental model of non heart beating donor kidneys. J Surg Res 2011; 171:283–290. [DOI] [PubMed] [Google Scholar]
- 61.Patel M, Hosgood S, Nicholson ML. The effects of arterial pressure during normothermic kidney perfusion. J Surg Res 2014; 191:463–468. [DOI] [PubMed] [Google Scholar]
- 62.Wight J, Chilcott J, Holmes M, et al. The clinical and cost-effectiveness of pulsatile machine perfusion versus cold storage of kidneys for transplantation retrieved from heart-beating and non-heart-beating donors. Health Technol Assess 2003; 7:1–94. [DOI] [PubMed] [Google Scholar]
- 63.Groen H, Moers C, Smits JM, et al. Cost-effectiveness of hypothermic machine preservation versus static cold storage in renal transplantation. Am J Transplant 2012; 12:1824–1830. [DOI] [PubMed] [Google Scholar]
- 64.Gracia-Sancho J, Villarreal G, Jr, Zhang Y, et al. Flow cessation triggers endothelial dysfunction during organ cold storage conditions: strategies for pharmacologic intervention. Transplantation 2010; 90:142–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Salahudeen AK. Cold ischemic injury of transplanted kidneys: new insights from experimental studies. Am J Physiol Renal Physiol 2004; 287:F181–187. [DOI] [PubMed] [Google Scholar]
- 66.James H, Southard MDA, Folkert O, et al. Organ preservation. Annu Rev Med 1995; 46:235–247. [DOI] [PubMed] [Google Scholar]
- 67.Lam VW, Laurence JM, Richardson AJ, et al. Hypothermic machine perfusion in deceased donor kidney transplantation: a systematic review. J Surg Res 2013; 180:176–182. [DOI] [PubMed] [Google Scholar]
- 68.O’Callaghan JM, Morgan RD, Knight SR, et al. Systematic review and meta-analysis of hypothermic machine perfusion versus static cold storage of kidney allografts on transplant outcomes. Br J Surg 2013; 100:991–1001. [DOI] [PubMed] [Google Scholar]
- 69.Wight JP, Chilcott JB, Holmes MW, et al. Pulsatile machine perfusion vs. cold storage of kidneys for transplantation: a rapid and systematic review. Clin Transplant 2003; 17:293–307. [DOI] [PubMed] [Google Scholar]
- 70.Jiao B, Liu S, Liu H, et al. Hypothermic machine perfusion reduces delayed graft function and improves one-year graft survival of kidneys from expanded criteria donors: a meta-analysis. PLoS One 2013; 8:e81826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Moers C, Pirenne J, Paul A, et al. Machine Preservation Trial Study G. Machine perfusion or cold storage in deceased-donor kidney transplantation. N Engl J Med 2012; 366:770–771. [DOI] [PubMed] [Google Scholar]
- 72.Gallinat A, Moers C, Smits JM, et al. Machine perfusion versus static cold storage in expanded criteria donor kidney transplantation: 3-year follow-up data. Transpl Int 2013; 26:E52–53. [DOI] [PubMed] [Google Scholar]
- 73.Stubenitsky BM, Booster MH, Brasile L, et al. Negative effect of cold ischemia on initial renal function. ASAIO J 2000; 46:60–61. [DOI] [PubMed] [Google Scholar]
- 74.Minor T, Paul A, Efferz P, et al. Kidney transplantation after oxygenated machine perfusion preservation with Custodiol-N solution. Transpl Int 2015; 28:1102–1108. [DOI] [PubMed] [Google Scholar]
- 75.Stratta RJ, Moore PS, Farney AC, et al. Influence of pulsatile perfusion preservation on outcomes in kidney transplantation from expanded criteria donors. J Am Coll Surg 2007; 204:873–882. [DOI] [PubMed] [Google Scholar]
- 76.Polyak MM, Arrington BO, Stubenbord WT, et al. The influence of pulsatile preservation on renal transplantation in the 1990 s. Transplantation 2000; 69:249–258. [DOI] [PubMed] [Google Scholar]
- 77.Guarrera JV, Polyak MM, Arrington B, et al. Pushing the envelope in renal preservation; improved results with novel perfusate modifications for pulsatile machine perfusion of cadaver kidneys. Transplant Proc 2004; 36:1257–1260. [DOI] [PubMed] [Google Scholar]
- 78.Guarrera JV, Polyak M, O’Mar Arrington B, et al. Pulsatile machine perfusion with Vasosol solution improves early graft function after cadaveric renal transplantation. Transplantation 2004; 77:1264–1268. [DOI] [PubMed] [Google Scholar]
- 79.Brasile L, Stubenitsky B, Haisch CE, et al. Potential of repairing ischemically damaged kidneys ex vivo. Transplant Proc 2005; 37:375–376. [DOI] [PubMed] [Google Scholar]
- 80.Chatauret N, Thuillier R, Hauet T. Preservation strategies to reduce ischemic injury in kidney transplantation: pharmacological and genetic approaches. Curr Opin Organ Transplant 2011; 16:180–187. [DOI] [PubMed] [Google Scholar]
- 81.Suszynski TM, Rizzari MD, Scott WE, et al. Persufflation (or gaseous oxygen perfusion) as a method of organ preservation. Cryobiology 2012; 64:125–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gallinat A, Moers C, Treckmann J, et al. Machine perfusion versus cold storage for the preservation of kidneys from donors >/ = 65 years allocated in the Eurotransplant Senior Programme. Nephrol Dial Transplant 2012; 27:4458–4463. [DOI] [PubMed] [Google Scholar]
- 83.Gill J, Dong J, Eng M, et al. Pulsatile perfusion reduces the risk of delayed graft function in deceased donor kidney transplants, irrespective of donor type and cold ischemic time. Transplantation 2014; 97:668–674. [DOI] [PubMed] [Google Scholar]
- 84.Opelz G, Dohler B. Multicenter analysis of kidney preservation. Transplantation 2007; 83:247–253. [DOI] [PubMed] [Google Scholar]
- 85.Sung RS, Christensen LL, Leichtman AB, et al. Determinants of discard of expanded criteria donor kidneys: impact of biopsy and machine perfusion. Am J Transplant 2008; 8:783–792. [DOI] [PubMed] [Google Scholar]
- 86.Kosieradzki M, Danielewicz R, Kwiatkowski A, et al. Rejection rate and incidence of acute tubular necrosis after pulsatile perfusion preservation. Transplant Proc 1999; 31:278–279. [DOI] [PubMed] [Google Scholar]
- 87.Gage F, Ali M, Alijani MR, et al. Comparison of static versus pulsatile preservation of matched-paired kidneys. Transplant Proc 1997; 29:3644–3645. [DOI] [PubMed] [Google Scholar]
- 88.Merion RM, Oh HK, Port FK, et al. A prospective controlled trial of cold-storage versus machine-perfusion preservation in cadaveric renal transplantation. Transplantation 1990; 50:230–233. [DOI] [PubMed] [Google Scholar]
- 89.Jaffers GJ, Banowsky LH. The absence of a deleterious effect of mechanical kidney preservation in the era of cyclosporine. Transplantation 1989; 47:734–736. [DOI] [PubMed] [Google Scholar]
- 90.Heil JE, Canafax DM, Sutherland DE, et al. A controlled comparison of kidney preservation by two methods: machine perfusion and cold storage. Transplant Proc 1987; 19 (1 Pt 3):2046. [PubMed] [Google Scholar]
- 91.Alijani MR, Cutler JA, DelValle CJ, et al. Single-donor cold storage versus machine perfusion in cadaver kidney preservation. Transplantation 1985; 40:659–661. [DOI] [PubMed] [Google Scholar]
- 92.Forde JC, Shields WP, Azhar M, et al. Single centre experience of hypothermic machine perfusion of kidneys from extended criteria deceased heart-beating donors: a comparative study. Ir J Med Sci 2016; 185:121–125. [DOI] [PubMed] [Google Scholar]
- 93.Burgos Revilla FJ, Hevia V, Diez V, et al. Machine perfusion: initial results in an expanded criteria donor kidney transplant program. Transplant Proc 2015; 47:19–22. [DOI] [PubMed] [Google Scholar]
- 94.Dion MS, McGregor TB, McAlister VC, et al. Hypothermic machine perfusion improves Doppler ultrasonography resistive indices and long-term allograft function after renal transplantation: a single-centre analysis. BJU Int 2015; 116:932–937. [DOI] [PubMed] [Google Scholar]
- 95.Guy A, McGrogan D, Inston N, et al. Hypothermic machine perfusion permits extended cold ischemia times with improved early graft function. Exp Clin Transplant 2015; 13:130–137. [PubMed] [Google Scholar]
- 96.Wszola M, Kwiatkowski A, Domagala P, et al. Preservation of kidneys by machine perfusion influences gene expression and may limit ischemia/reperfusion injury. Prog Transplant 2014; 24:19–26. [DOI] [PubMed] [Google Scholar]
- 97.Chueh SC, Sankari BR, Lipscomb L, et al. The benefits of pulsatile machine perfusion of standard criteria deceased donor kidneys at a geographically remote transplant center. ASAIO J 2014; 60:76–80. [DOI] [PubMed] [Google Scholar]
- 98.Wszola M, Kwiatkowski A, Diuwe P, et al. One-year results of a prospective, randomized trial comparing two machine perfusion devices used for kidney preservation. Transpl Int 2013; 26:1088–1096. [DOI] [PubMed] [Google Scholar]
- 99.Sedigh A, Tufveson G, Backman L, et al. Initial experience with hypothermic machine perfusion of kidneys from deceased donors in the Uppsala region in Sweden. Transplant Proc 2013; 45:1168–1171. [DOI] [PubMed] [Google Scholar]
- 100.Cannon RM, Brock GN, Garrison RN, et al. To pump or not to pump: a comparison of machine perfusion vs cold storage for deceased donor kidney transplantation. J Am Coll Surg 2013; 216:625–633. [DOI] [PubMed] [Google Scholar]
- 101.Cannon RM, Brock GN, Garrison RN, et al. Machine perfusion: not just for marginal kidney donors. Am Surg 2015; 81:550–556. [PubMed] [Google Scholar]
- 102.Hoogland ER, de Vries EE, Christiaans MH, et al. The value of machine perfusion biomarker concentration in DCD kidney transplantations. Transplantation 2013; 95:603–610. [DOI] [PubMed] [Google Scholar]
- 103.Ciancio G, Gaynor JJ, Sageshima J, et al. Machine perfusion following static cold storage preservation in kidney transplantation: donor-matched pair analysis of the prognostic impact of longer pump time. Transpl Int 2012; 25:34–40. [DOI] [PubMed] [Google Scholar]
- 104.Cantafio AW, Dick AA, Halldorson JB, et al. Risk stratification of kidneys from donation after cardiac death donors and the utility of machine perfusion. Clin Transplant 2011; 25:E530–540. [DOI] [PubMed] [Google Scholar]
- 105.Lodhi SA, Lamb KE, Uddin I, et al. Pulsatile pump decreases risk of delayed graft function in kidneys donated after cardiac death. Am J Transplant 2012; 12:2774–2780. [DOI] [PubMed] [Google Scholar]
- 106.Patel SK, Pankewycz OG, Nader ND, et al. Prognostic utility of hypothermic machine perfusion in deceased donor renal transplantation. Transplant Proc 2012; 44:2207–2212. [DOI] [PubMed] [Google Scholar]
- 107.Ciancio G, Gaynor JJ, Sageshima J, et al. Favorable outcomes with machine perfusion and longer pump times in kidney transplantation: a single-center, observational study. Transplantation 2010; 90:882–890. [DOI] [PubMed] [Google Scholar]
- 108.Kwiatkowski A, Wszola M, Kosieradzki M, et al. The early and long term function and survival of kidney allografts stored before transplantation by hypothermic pulsatile perfusion. A prospective randomized study. Ann Transplant 2009; 14:14–17. [PubMed] [Google Scholar]
- 109.Moustafellos P, Hadjianastassiou V, Roy D, et al. The influence of pulsatile preservation in kidney transplantation from non-heart-beating donors. Transplant Proc 2007; 39:1323–1325. [DOI] [PubMed] [Google Scholar]
- 110.Balupuri S, Mantle D, Mohamed M, et al. Machine perfusion and viability assessment of non-heart-beating donor kidneys-a single-centre result. Transplant Proc 2001; 33:1119–1120. [DOI] [PubMed] [Google Scholar]
- 111.Kumar MS, Samhan M, al Sabawi N, et al. Preservation of cadaveric kidneys longer than 48 hours: comparison between Euro-Collins solution, UW solution, and machine perfusion. Transplant Proc 1991; 23:2392–2393. [PubMed] [Google Scholar]
- 112.Barry JM, Metcalfe JB, Farnsworth MA, et al. Comparison of intracellular flushing and cold storage to machine perfusion for human kidney preservation. J Urol 1980; 123:14–16. [DOI] [PubMed] [Google Scholar]
- 113.Plata-Munoz JJ, Muthusamy A, Quiroga I, et al. Impact of pulsatile perfusion on postoperative outcome of kidneys from controlled donors after cardiac death. Transpl Int 2008; 21:899–907. [DOI] [PubMed] [Google Scholar]
- 114.Matsuoka L, Shah T, Aswad S, et al. Pulsatile perfusion reduces the incidence of delayed graft function in expanded criteria donor kidney transplantation. Am J Transplant 2006; 6:1473–1478. [DOI] [PubMed] [Google Scholar]
- 115.Buchanan PM, Lentine KL, Burroughs TE, et al. Association of lower costs of pulsatile machine perfusion in renal transplantation from expanded criteria donors. Am J Transplant 2008; 8:2391–2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kootstra G, Kievit J, Heineman E. The non heart-beating donor. Br Med Bull 1997; 53:844–853. [DOI] [PubMed] [Google Scholar]
- 117.Sy G, Jr, Toledo-Pereyra LH, Dienst SG, et al. Are there any important predicting factors of renal function during hypothermic pulsatile perfusion for transplantation? Am Surg 1980; 46:340–343. [PubMed] [Google Scholar]
- 118.Kwiatkowski A, Danielewicz R, Kosieradzki M, et al. Six-year experience in continuous hypothermic pulsatile perfusion kidney preservation. Transplant Proc 2001; 33:913–915. [DOI] [PubMed] [Google Scholar]
- 119.Veller MG, Botha JR, Britz RS, et al. Renal allograft preservation: a comparison of University of Wisconsin solution and of hypothermic continuous pulsatile perfusion. Clin Transplant 1994; 8 (2 Pt 1):97–100. [PubMed] [Google Scholar]
- 120.Elec FI, Lucan C, Ghervan L, et al. Ex-vivo perfusion machines in kidney transplantation. The significance of the resistivity index. Clujul Med 2014; 87:27–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Schold JD, Kaplan B, Howard RJ, et al. Are we frozen in time? Analysis of the utilization and efficacy of pulsatile perfusion in renal transplantation. Am J Transplant 2005; 5:1681–1688. [DOI] [PubMed] [Google Scholar]
- 122.Barber WH, Deierhoi MH, Phillips MG, et al. Preservation by pulsatile perfusion improves early renal allograft function. Transplant Proc 1988; 20:865–868. [PubMed] [Google Scholar]
- 123.Sellers MT, Gallichio MH, Hudson SL, et al. Improved outcomes in cadaveric renal allografts with pulsatile preservation. Clin Transplant 2000; 14:543–549. [DOI] [PubMed] [Google Scholar]
- 124.Schreinemachers MC, Doorschodt BM, Florquin S, et al. Pulsatile perfusion preservation of warm ischaemia-damaged experimental kidney grafts. Br J Surg 2010; 97:349–358. [DOI] [PubMed] [Google Scholar]
- 125.La Manna G, Conte D, Cappuccilli ML, et al. An in vivo autotransplant model of renal preservation: cold storage versus machine perfusion in the prevention of ischemia/reperfusion injury. Artif Organs 2009; 33:565–570. [DOI] [PubMed] [Google Scholar]
- 126.Manekeller S, Leuvenink H, Sitzia M, et al. Oxygenated machine perfusion preservation of predamaged kidneys with HTK and Belzer machine perfusion solution: an experimental study in pigs. Transplant Proc 2005; 37:3274–3275. [DOI] [PubMed] [Google Scholar]
- 127.Lindell SL, Compagnon P, Mangino MJ, et al. UW solution for hypothermic machine perfusion of warm ischemic kidneys. Transplantation 2005; 79:1358–1361. [DOI] [PubMed] [Google Scholar]
- 128.Nicholson ML, Hosgood SA, Metcalfe MS, et al. A comparison of renal preservation by cold storage and machine perfusion using a porcine autotransplant model. Transplantation 2004; 78:333–337. [DOI] [PubMed] [Google Scholar]
- 129.Hansen TN, D’Alessandro A, Southard JH. Reduced renal vascular injury following warm ischemia and preservation by hypothermic machine perfusion. Transplant Proc 1997; 29:3577–3579. [DOI] [PubMed] [Google Scholar]
- 130.Booster MH, Wijnen RM, Yin M, et al. Enhanced resistance to the effects of normothermic ischemia in kidneys using pulsatile machine perfusion. Transplant Proc 1993; 25:3006–3011. [PubMed] [Google Scholar]
- 131.McAnulty JF, Ploeg RJ, Southard JH, et al. Successful five-day perfusion preservation of the canine kidney. Transplantation 1989; 47:37–41. [DOI] [PubMed] [Google Scholar]
- 132.Brasile L, Stubenitsky BM, Booster MH, et al. The potential of repairing organs ex vivo. Transplant Proc 2002; 34:2625. [DOI] [PubMed] [Google Scholar]
- 133.Brasile L, Stubenitsky BM, Booster MH, et al. Overcoming severe renal ischemia: the role of ex vivo warm perfusion. Transplantation 2002; 73:897–901. [DOI] [PubMed] [Google Scholar]
- 134.van der Wijk J, Slooff MJ, Rijkmans BG, et al. Successful 96- and 144-hour experimental kidney preservation: a combination of standard machine preservation and newly developed normothermic ex vivo perfusion. Cryobiology 1980; 17:473–477. [DOI] [PubMed] [Google Scholar]
- 135.Rijkmans BG, Buurman WA, Kootstra G. Six-day canine kidney preservation. Hypothermic perfusion combined with isolated blood perfusion. Transplantation 1984; 37:130–134. [DOI] [PubMed] [Google Scholar]
- 136.Metcalfe MS, Waller JR, Hosgood SA, et al. A paired study comparing the efficacy of renal preservation by normothermic autologous blood perfusion and hypothermic pulsatile perfusion. Transplant Proc 2002; 34:1473–1474. [DOI] [PubMed] [Google Scholar]
- 137.Lindell SL, Muir H, Brassil J, et al. Hypothermic machine perfusion preservation of the DCD kidney: machine effects. J Transplant 2013; 2013:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Badet L, Petruzzo P, Lefrancois N, et al. Kidney preservation with IGL-1 solution: a preliminary report. Transplant Proc 2005; 37:308–311. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


