Supplemental Digital Content is available in the text
Keywords: ADAM28, metalloproteinase, migration, proliferation, prostate cancer
Abstract
Prostate cancer is one of the most prevalent cancers in men. It is critical to identify and characterize oncogenes that drive the pathogenesis of human prostate cancer. The current study builds upon previous research showing that a disintegrin and metallproteinase (ADAM)28 is involved in the pathogenesis of numerous cancers. Our novel study used overexpression, pharmacological, and molecular approaches to investigate the biological function of ADAM28 in human prostate cancer cells, with a focus on cell proliferation and migration. The results of this study provide important insights into the role of metalloproteinases in human prostate cancer.
The expression of ADAM28 protein levels was assessed within human prostate tumors and normal adjacent tissue by immunohistochemistry. Immunocytochemistry and western blotting were used to assess ADAM28 protein expression in human prostate cancer cell lines. Functional assays were conducted to assess proliferation and migration in human prostate cancer cells in which ADAM28 protein expression or activity had been altered by overexpression, pharmacological inhibition, or by siRNA gene knockdown.
The membrane bound ADAM28 was increased in human tumor biopsies and prostate cancer cell lines. Pharmacological inhibition of ADAM28 activity and/or knockdown of ADAM28 significantly reduced proliferation and migration of human prostate cancer cells, while overexpression of ADAM28 significantly increased proliferation and migration.
ADAM28 is overexpressed in primary human prostate tumor biopsies, and it promotes human prostate cancer cell proliferation and migration. This study supports the notion that inhibition of ADAM28 may be a potential novel therapeutic strategy for human prostate cancer.
1. INTRODUCTION
Prostate cancer is the 2nd most common cancer in men, and the 5th most common cause of cancer-related deaths in men worldwide.[1] The age-adjusted incidence of prostate cancer has risen in line with an increase in the number of men being tested and improvements in widespread diagnostic testing.[1] Current therapies for prostate cancer such as androgen ablation result in a reduction in symptoms seen in around 70% to 80% of patients with advanced prostate cancer.[2] However, tumors can relapse within 2 years and transform into an incurable androgen-independent state,[3] and there are other disadvantages.[4,5] It is therefore important to develop alternative treatment options, which aim to reduce the proliferation and progression of prostate cancer cells.
A disintegrin and metalloproteinases (ADAMs) are a gene family of transmembrane and/or secreted proteins which regulate cell phenotype via effects on cell adhesion, migration, proteolysis, and signaling.[6–9] Numerous ADAM family members have previously been linked with the malignant progression of human prostate cancer. Fritzsche et al[10] report that ADAM8 expression is associated with increased Gleason scores and positive node status. ADAM9 expression is significantly higher in prostate cancer tissue than normal prostate tissue[11] and inhibition of ADAM9 expression in prostate cancer enhanced prostate cancer sensitivity to chemotherapy and radiation.[12] Knockdown of ADAM10 reduced proliferation of prostate cancer cells, suggesting that ADAM10 may contribute to the progression of prostate cancer by increasing proliferation.[13] McCulloch et al[14] report that ADAM12 expression is significantly higher in prostate cancer tissues than normal prostate tissue. ADAM15 has been shown to contribute to the metastatic progression of human prostate cancer through the binding of its disintegrin domain to various integrins.[15] Finally, Xiao et al[16] demonstrated that ADAM17 increased the invasive capacity of prostate cancer cells by targeting matrix metalloproteinases (MMPs) 2 and 9. Interestingly, in contrast to other ADAM family members, our team have elucidated that ADAM19 is a protective biomarker in human prostate cancer.[17] Herein, we advance current knowledge by focusing on the potential role of the metalloproteinase ADAM28 in human prostate cancer to determine if it could be a new target for intervention.
ADAM28 exists in 2 isoforms: a secreted soluble form (ADAM28s) and a membrane bound form (ADAM28m).[18] Both forms contain the metalloproteinase and disintegrin domains, which function in proteolysis and cellular adhesion, respectively. ADAM28 is expressed and synthesized in its precursor form (zymogen form-proADAM28) by lymphocytes and some cancer cells.[18] Studies have suggested that the prodomain is required to maintain the latency of metalloproteinases,[19] and proADAM28 is activated by matrix metalloproteinases-7 via the removal of its prodomain.[18] The metalloprotease domain facilitates the degradation of several substrates, including tumor necrosis factor-α[20] and others that are discussed in more detail below. ADAM28's disintegrin domain is reported to bind the integrins α4β1, α4β7, and α9β1 on lymphocytes in an activation-dependent manner. The leukocyte integrin interaction with ADAM28 proposes a potential attachment and cleavage role for ADAM28 in inflammation and immunity.[21,22]
ADAM28 cleaves substrates known to be involved in metastasis including insulin-like growth factor (IGF) binding protein-3 (IGFBP-3),[23] connective tissue growth factor (CTGF),[24] and von Willebrand factor (VWF).[25] High levels of the mitogen IGF-I and low levels of IGFBP-3 are associated with a higher risk of prostate cancer in men.[26] Previous studies have indicated that IGF-IR blockade reduces the invasive activity of PC-3[27] and DU145[28] human prostate cancer cells. IGFBP-3 degradation by ADAM28 may play vital roles in carcinoma cell proliferation and metastasis.[9]We have previously demonstrated that ADAM28 cleaves VWF[25] and high levels of ADAM28 expression are implicated in the inactivation of proapoptotic VWF. Studies from our team have also demonstrated that overexpression of ADAM28 in human breast carcinoma cells was positively associated with enhanced expression of both vascular endothelial growth factor (VEGF) and CTGF.[24]
ADAM28 is suggested to be implicated in proliferation, migration, and invasion in nonsmall cell lung cancer,[29] breast cancer tissue,[23] bladder cancer,[30] head and neck cancer,[31] and B-cell acute lymphoblastic leukemia.[32] Given that ADAM28 has an oncogenic effect in other cancers and cleaves substrates with known association with cancer metastases, these findings provide a strong foundation for the investigation of ADAM28 in the pathogenesis of human prostate cancer. Our study aimed to determine the importance of ADAM28 in human prostate cancer, and whether it could be further progressed as a potential therapeutic target.
2. METHODS
2.1. Clinical samples
Human prostate tumor biopsies (n = 8) and paired adjacent benign prostate tissue samples (n = 8) were obtained from Dr Ronald Cohen (Uropath, Perth, Western Australia). Tissue samples were obtained with consent (institutional human ethics application number EC 2008/118). Informed consent was obtained from patients and a high standard of ethics was applied in carrying out the investigations. Full face sections of prostate cancer tissue were immunostained for ADAM28.
2.2. Immunohistochemistry
Tissue was fixed in glutaraldehyde (2.5%) and paraffin embedded. Sections were deparaffinized through xylene, rehydrated through graded alcohols to distilled water, and subjected to antigen retrieval in EDTA pH 8.0 under pressure. After blocking endogenous peroxide activity with hydrogen peroxide, the mouse antihuman ADAM28 antibody (297-2F3)[23] was applied at 1:500 for 60 minutes. A horse-radish peroxidase labeled polymer conjugated with rabbit/mouse secondary antibodies (DAKO envision+) was then incubated for 30 minutes. Sections were visualized with diaminobenzidine (DAKO) followed by a light counterstain with hematoxylin.
2.3. Cell culture
All cells were purchased from the American Type Culture Collection (Manassas, VA). LNCaP and DU145 cells were cultured in a 6 well cell bind plate (Costar) containing Roswell Park Memorial Institute (RPMI)-1640 media (Sigma-Aldrich, Steinheim, Germany) with 10% FCS and 2% penicillin/streptomycin (Invitrogen, USA). They were maintained and grown at 37 °C, in 95% O2/5% CO2. RWPE1 cells were cultured in a 6 well cell bind plate containing Keratinocyte-serum-free medium (Life Technologies) with 2% penicillin/streptomycin (Invitrogen).
2.4. Determination of protein expression
Cell harvesting and western blots were conducted as previously described.[20]
2.5. Antibodies
The primary antibodies used for western blot analysis were as follows: anti-ADAM28 mouse monoclonal antibody (297-2F3) specific to the ADAM28 metalloproteinase domain[29]; anti-ADAM28 rabbit polyclonal antibody specific to the intracytoplasmic domain (#ab39875, Abcam, Cambridge, USA); mouse IgG isotype control (#sc-2025, Santa Cruz, Texas, USA); mouse anti-β-actin antibody (Millenium), and rabbit anti-prostate specific antigen polyclonal antibody (#A0562, DakoCytomation). The secondary antibodies, antimouse IRDYE 800 and antimouse IRDYE 680 were purchased from Millennium.
2.6. Transfection of plasmids into cells
Transfection was conducted as previously described.[17] Transfected cells were used for immunocytochemistry to evaluate ADAM28 overexpression, proliferation, and migration studies. The plasmid vectors utilized were pCMV Tag4A ADAM28 and pCMV Tag4A empty vector.[33]
2.7. Immunocytochemistry
Immunocytochemistry was conducted on transfected cells as previously described.[17]
2.8. Enzyme-linked immunosorbent assay (ELISA)
Human IGF-I and interleukin-6 (IL-6) were measured in cell free culture supernatants (collected from cells transfected for 48 hours) using commercially available ELISA kits as per manufacturer instructions (ELISAkit.com).
2.9. ADAM28 knockdown utilizing short-interfering RNA (siRNA)
In all siRNA experiments, cells were seeded into 12 well cell culture dishes, and transfections were conducted using X-tremeGENE HP DNA transfection reagent (Roche). ADAM28 was knocked down using Silencer Select siRNA A28 siRNA 2 (#s21323, Applied Biosystems). The sequences are as follows:
Sense: CGACUAUUCUUGCAAGUG/Antisense: ACACUUGCAAGAAUAGUCG
siRNA (10 nM) was added to 0.25 × 105 cells/mL suspended in 100 μL of RPMI-1640 growth media and incubated for 48 hours (cells were free of streptomycin/penicillin for 4 hours). A Cy3 labeled double-stranded transfection control (scrambled siRNA; IDT) was used at 10 nM as a negative control.
2.10. Real time PCR analysis
Cells grown in 12 well plates were directly lysed with 200 μL/well of TRIzol Reagent (Invitrogen). RNA was isolated from the cells in accordance to manufacturer's instructions. RNA samples were DNase treated using the RQ1 RNase-Free DNase (Cat# M6101, Promega). DNase-treated RNA was mixed with a master mix prepared from the TaqMan Reverse Transcription Reagents kit (Applied Biosystems, Branchburg, NJ). The samples were then reverse transcribed in the Gene Amp PCR system 9700 Thermocycler (Applied Biosystems).
To quantitate the relative expression of genes of interest from extracted RNA samples, the cDNA samples were subjected to real time PCR analysis. PCR reactions were composed of cDNA sample, TaqMan master mix, DEPC-treated water, and 20X Taqman assay from Life Technologies (HPRT [Hs02800695_m1] or human ADAM28 [Hs00248020_m1]). The cDNA samples were amplified using the Rotor Gene-2000 real-time PCR thermal cycler (Corbett Research). Real time PCR data were analyzed using the comparative critical threshold (also known as threshold cycle; CT) method.
2.11. Pharmacological inhibition of ADAM28
Cells were seeded into 2 separate treatments of either the dimethyl sulfoxide (DMSO) or the ADAM28 inhibitor KB-R7785 (1–5 μM) diluted in DMSO.[23] Cells were then used for proliferation and migration assays.
2.12. DHT treatment of prostate cancer cells
Prostate cancer cells were treated with 10 nM dihydrotestosterone (DHT) for 24 hours.
2.13. MTS assay
Cells were suspended in RPMI-1640 medium containing 10% FCS and 2% streptomycin/penicillin. After counting cells, they were resuspended (0.125 × 105 cells/mL) and 100 μL of cells were added to the center of the wells of a 96-well flat bottom culture plate. The medium was carefully aspirated on days 1, 3, 5, or 7 and replaced with 100 μL RPMI-1640 medium containing 10% FCS and 2% streptomycin/penicillin containing 20 μL of MTS assay reagent (Promega). Proliferation was determined by the formation of a colored formazan product. Plates were read at 490 nm using a plate reader. The Olympus fluorescent microscope was used at each required time point to image cells. The MTS assay result was supported by cell count data in some experiments.
2.14. Transwell migration assays
Cell migration was assessed by an assay using transwells fitted with uncoated 8 μm pore size polycarbonate membranes. Inserts containing 100 μL of FCS-free growth medium were placed into wells of a 24 well plate possessing 10% FCS containing medium. The transwell devices were left to equilibrate at 37 °C for 1 hour. The FCS free growth medium was then replaced with new FCS-free growth media containing LNCaP cells at a density of 0.25 × 105 cells/mL and incubated at 37 °C for 1 and 3 days. Cells attached to the bottom surface of the membrane at 2 and 3 days post-seeding were fixed using methanol, followed by hematoxylin staining. The membrane was then washed 3 times and cells remaining on the upper surface of the membrane were removed using a cotton swab. The bottom of the membrane was then visualized using light microscopy at 200× magnification. Five random high powered fields of view were captured and used to analyze migration. Migrated cells were distinguished by their dark blue opaque appearance.
2.15. Western blotting for endogenous IGFBP-3 cleavage in DU145 cells
DU145 cells were starved in 0.1% serum-containing medium overnight. The following day, cells were cultured in 0.1% serum containing medium in the absence or presence of 1 μM KB-R7785 for 30 minutes before adding IGF-I (100 ng/mL). Cells were then cultured for 24 hours. Serum-free conditioned cell culture supernatants were concentrated using the Biomax 5k Nmwl membrane (Millipore). The western blot for IGFBP-3 cleavage was conducted on concentrated cell culture medium as previously described.[23] The rabbit anti-human IGFBP-3 antibody was a kind gift from Rob Baxter (Kolling Institute).
2.16. Statistical data analysis
Statistical analysis was carried out with the assistance of Professor Max Bulsara (The University of Notre Dame) using IBM SPSS statistics software. Nonparametric t tests (Mann–Whitney) were performed to compare mediums of sample medium values when “n” was greater than 3. Statistical significance was determined if the probability of the null hypothesis was less than 0.05 (P ≤ 0.05). IBM SPSS statistics was used to plot all graphs.
3. RESULTS
3.1. Human prostate carcinoma tissue displays elevated ADAM28 expression
Human prostate tumor biopsies and paired adjacent benign prostate tissue samples were immunostained for ADAM28 expression. The prostate tumor biopsies possessed Gleason grades ranging from 7 to 8. First, prostate tumor samples demonstrated negligible background immunoreactivity staining when they were stained with non-immune mouse IgG1 (Fig. 1A). However, we found that human prostate carcinoma samples have increased ADAM28 expression (Fig. 1C, D) indicated by brown staining when compared to normal human prostate tissue (Fig. 1B). These data provided an incentive to explore the effects of androgen on ADAM28 expression, which we were able to evaluate in prostate cancer cells.
Figure 1.
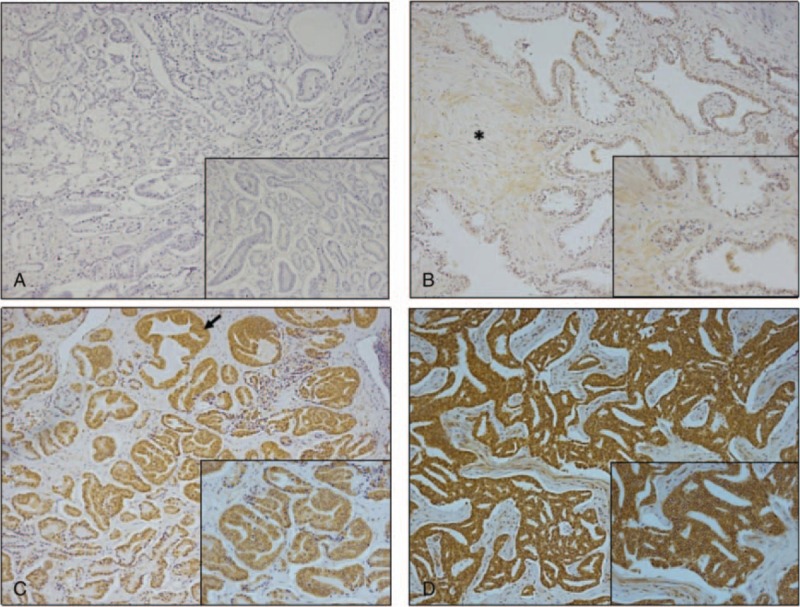
Immunostaining of a disintegrin and metalloproteinase (ADAM)28 in human prostate cancer. (A) non-immune mouse IgG immunostaining of human prostate tumor (Gleason grade 3 + 4 = 7), (B) ADAM28 immunostaining (brown) of benign prostate, (C) prostate tumor (Gleason grade 3 + 4 = 7), and (D) prostate tumor (Gleason grade 4 + 4 = 8). All main photomicrographs are 100× magnification while insets are 300× magnification. Asterisk (panel B) indicates stroma and arrow (panel C) indicates glandular hyperplasia.
We also obtained normal and prostate tumor (Gleason score 9) tissue for the assessment of ADAM28 protein expression by western blotting. The active membrane-bound form of ADAM28 (55 kDa) is elevated 2-fold in tumor tissue compared to normal prostate tissue (Supplementary Fig. 1).
3.2. ADAM28 expression is increased in human prostate cancer cells after dihydrotestosterone treatment
As androgen is a major driver in prostate cancer, we examined whether ADAM28 expression is enhanced by exposure to androgens, such as DHT. When androgen sensitive human LNCaP prostate cancer cells were treated with and without DHT, ADAM28 protein expression was increased greater than 3-fold in the presence of DHT (Fig. 2). This was mirrored by an expected increase in the expression of prostate specific antigen, the positive control, in the DHT treated samples compared to the controls. Taken together, these data suggest that not only is ADAM28 expression associated with more poorly differentiated tumors, it may be regulated by androgen in a feed-forward manner.
Figure 2.
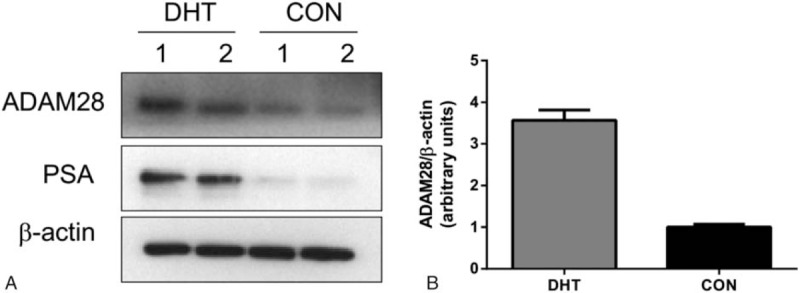
ADAM28 protein expression is increased in the presence of DHT in androgen responsive human LNCaP prostate cancer cells. LNCaP cells were either treated with DHT (10 nM) or untreated (CON) for 24 hours, in duplicate. (A) Western blot indicates that the presence of DHT increases the expression of both ADAM28 and PSA when compared with the untreated controls. (B) Quantitation of ADAM28 expression. ADAM = a disintegrin and metalloproteinase, DHT = dihydrotestosterone, PSA = prostate specific antigen.
3.3. ADAM28 overexpression studies
We next evaluated the functional effects of transient overexpression of ADAM28 in human prostate carcinoma cells (DU145 and LNCaP). A plasmid encoding human ADAM28 cDNA was transiently transfected into the cells, resulting in ADAM28 overexpression in both cell lines after 48 hours. Immunohistochemistry was performed on the cells using anti-ADAM28 antibody (297-2F3). ADAM28 expression was identified by strong brown cytoplasmic staining (Supplementary Fig. 2). The over-expressed ADAM28 was detected as the active (55 kDa) membrane bound form (Supplementary Fig. 3).
We next explored the effect of ADAM28 overexpression on proliferation of DU145 and LNCaP human prostate cancer cell lines compared to normal RWPE1 prostate epithelial cells. We show by MTS cell proliferation assay that overexpression of ADAM28 promotes proliferation of DU145 (Fig. 3A), LNCaP (Fig. 3B), and RWPE1 cells (Fig. 3C). This result also confirmed that overexpression of ADAM28 stimulates proliferation of human normal prostate epithelial and cancerous prostate cells.
Figure 3.
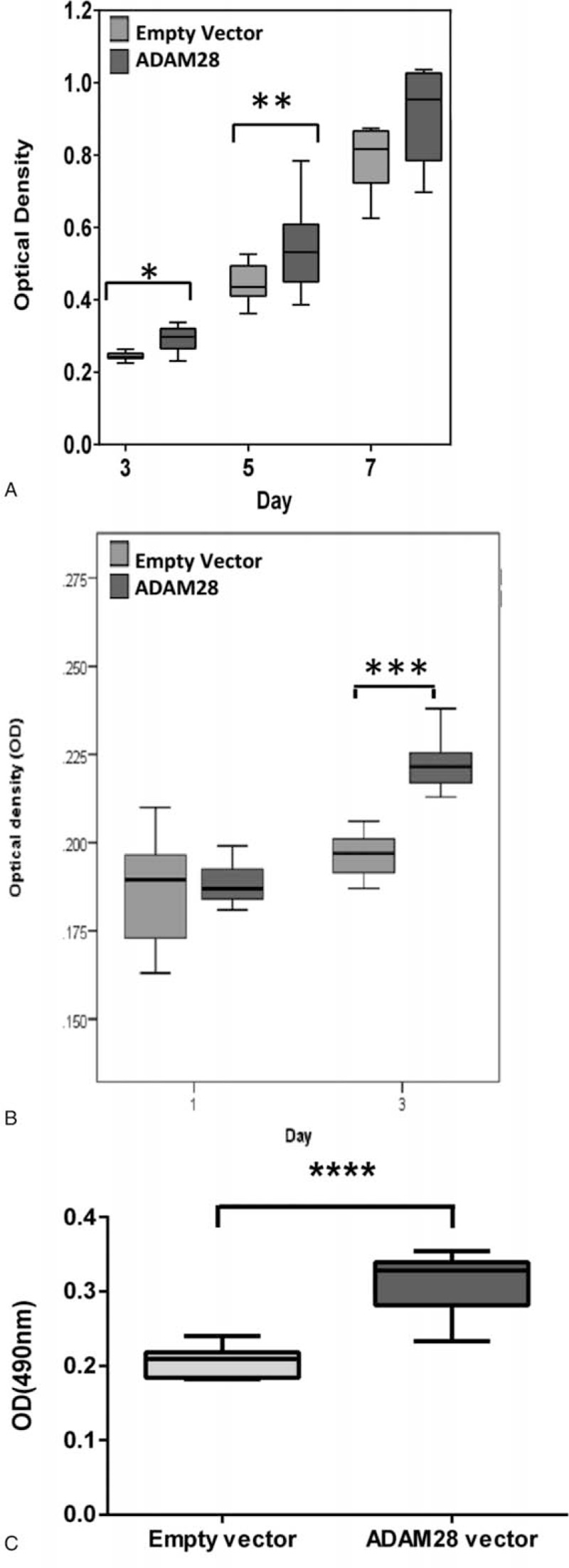
Overexpression of ADAM28 in human prostate cancer cells and normal prostate epithelial cells promotes proliferation. DU145 (A), LNCaP (B), and RWPE1 (C) cells were either transiently transfected with empty vector (pCMV-Tag4a) or an ADAM28 expressing vector (pCMV-Tag4a ADAM28). Proliferation of cells was measured utilizing MTS reagent and read at 490 nm (∗P < 0.02; ∗∗P < 0.012; ∗∗∗P < 0.001; ∗∗∗∗P = 0.0001, Mann–Whitney) n = 6–16. Data are presented as a box and whisker plot. ADAM = a disintegrin and metalloproteinase.
3.4. Inhibition of ADAM28 reduces IGFBP-3 cleavage, while overexpression of ADAM28 promotes IGF-I, but not IL-6 release from human prostate carcinoma cells
Our previous studies highlight that the ADAM inhibitor KB-R7785 is highly capable of inhibiting ADAM28-mediated cleavage.[20] We now show that KB-R7785 may reduce IGFBP-3 cleavage (22 kDa product) in DU145 prostate cancer cells (Fig. 4A).
Figure 4.
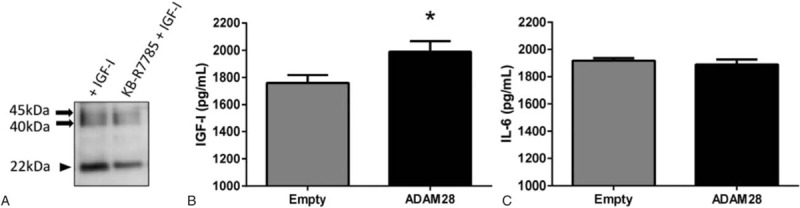
Inhibition of ADAM28 reduces IGFBP-3 cleavage, while overexpression of ADAM28 in DU145 human prostate cancer cells elevates IGF-1, but not IL-6 expression. (A) IGFBP-3 cleavage is inhibited by the ADAM inhibitor KB-R7785. (B) IGF-1 is significantly increased when ADAM28 is overexpressed. (C) There is no change in the expression of IL-6 when ADAM28 is over expressed; n = 6–9 samples per treatment. Data are presented as mean ± SEM. ∗P = 0.03. ADAM = a disintegrin and metalloproteinase, IGF = insulin-like growth factor, IGFBP-3 = IGF binding protein-3, IL-6 = interleukin 6, SEM = standard error of mean.
Previous studies have shown that IGF-I and IL-6 influence the invasiveness of prostate cancer cells, supporting the concept that IGF-I and IL-6 are identifiable predictors of prostate cancer prognosis. This is particularly relevant as ADAM28 is already known to be a potential upstream mediator of IGF-I release from cells.[23] An IGF-I ELISA performed on supernatant from DU145 cells that were transiently transfected with empty vector or ADAM28 vector revealed that overexpression of ADAM28 significantly increased IGF-I release (Fig. 4B). However, IL-6 ELISA data showed that ADAM28 overexpression does not affect IL-6 release (Fig. 4C). Therefore, in a human prostate cancer setting, the liberation of IGF-I appears to be regulated by ADAM28.
3.5. Pharmacological inhibition of ADAM28 activity by KB-R7785 attenuates proliferation of human prostate carcinoma cells
Given that overexpression of ADAM28 can drive the proliferation of human prostate cancer carcinoma cells, we next investigated the effect of inhibition of ADAM28 activity by KB-R7785. Pharmacological inhibition of ADAM28 by KB-R7785 significantly reduced the proliferation of DU145 (Fig. 5A) and LNCaP (Fig. 5B) human prostate cancer cells. These results provide further confirmation that ADAM28 plays a role in promoting human prostate carcinoma cell proliferation.
Figure 5.
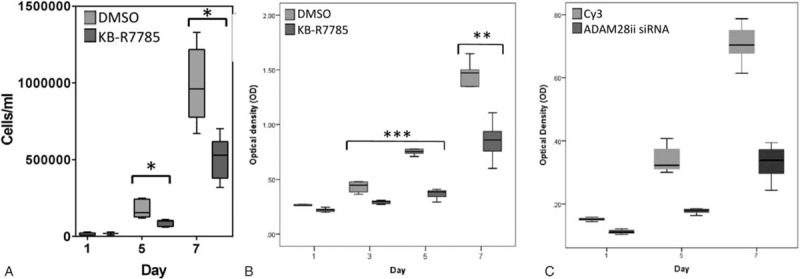
Proliferation of human prostate cancer cells is reduced when ADAM28 is pharmacologically inhibited by KB-R7785 or silenced by siRNA transfection. (A) Proliferation (cells/mL) of DU145 cells treated with KB-R7785 (5 μM) or DMSO (vehicle control) over a time course of 7 days, and (B) proliferation (optical density at 490 nm) of LNCaP cells treated with KB-R7785 (1 μM) or DMSO (vehicle control) over 7 days. ∗P < 0.006, ∗∗P < 0.007, (∗∗∗P < 0.002; DMSO vs KB-R7785 for day 3 and 5); Mann–Whitney. Data are presented as a box and whisker plot (A and B), n = 3–8. (C) Graph depicts proliferation (optical density) of LNCaP cells when transiently transfected with ADAM28 siRNA or Cy3 scrambled control over 7 days. The proliferation of ADAM28ii siRNA transfected LNCaP prostate cancer cells (dark gray) is significantly reduced when compared to the Cy3 scrambled control (light gray) for all time-points. All P values < 0.000004; n = 7–8. Data are presented as a box and whisker plot. ADAM = a disintegrin and metalloproteinase, DMSO = dimethyl sulfoxide, siRNA = short-interfering RNA, SEM = standard error of mean.
3.6. siRNA knockdown of ADAM28 in human prostate cancer cells
We also assessed the effect of knocking down ADAM28 using siRNA. Fluorescent microscopy of LNCaP prostate carcinoma cells transfected with Cy3-labeled (red) scrambled siRNA confirmed high transfection efficiency (Supplementary Fig 4A and B). Real-time PCR for ADAM28 was performed to confirm ADAM28 siRNA-mediated knock down of ADAM28 mRNA (Supplementary Fig 4C). Importantly, we observed a significant decrease in the proliferation of LNCaP cells treated with ADAM28 siRNA relative to Cy3-labeled scrambled siRNA (Fig. 5C). This result was also repeated with a 2nd ADAM28 siRNA (data not shown).
3.7. Human prostate cancer cell migration is reduced by ADAM28 inhibition
Finally, we examined the role of ADAM28 in prostate carcinoma cell migration using Transwell chambers. We performed a migration assay using LNCaP cells treated with KB-R7785 or RWPE1 normal epithelial cells transfected with ADAM28 vector. When ADAM28 activity was inhibited by KB-R7785 for 3 days, migration of the LNCaP cells was significantly reduced compared to DMSO treated cells (Fig. 6A). Conversely, we found that RWPE1 cells transfected with ADAM28 vector exhibited significantly increased migration compared to cells transfected with empty vector after 2 days (Fig. 6B). Therefore, in addition to regulating prostate cancer cell proliferation, ADAM28 may mediate some aspects of prostate cancer cell migration.
Figure 6.
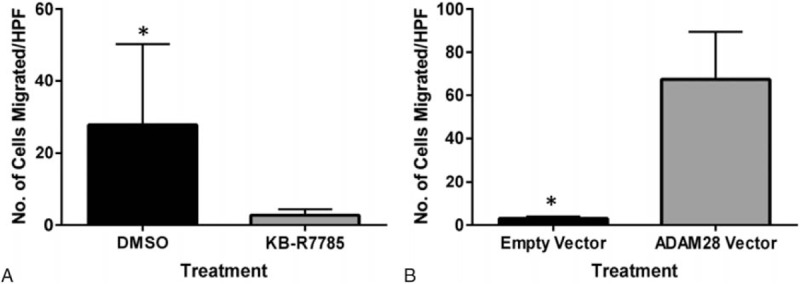
Human prostate cancer cell migration is reduced with ADAM28 inhibition and increased with ADAM28 overexpression. (A) Cell count for LNCaP cells showing that human prostate cancer cell migration is significantly reduced with ADAM28 inhibition by KB-R7785 (10 μM). (B) Normal epithelial prostate RWPE1 cells exhibit significantly heightened migration when transfected with an ADAM28 expression vector when compared to cells transfected with empty vector. All cell counts were averaged from 5 high power fields of view (HPF). Data are represented as mean ± SEM, ∗P < 0.05; n = 5/treatment for (A) and n = 15/treatment for (B). ADAM = a disintegrin and metalloproteinase, HPF = high powered field, SEM = standard error of mean.
4. DISCUSSION
This study highlights that ADAM28 is overexpressed in human prostate cancer tissue biopsies. Furthermore, we demonstrated that ADAM28 promotes proliferation and migration of human prostate carcinoma cells. Interestingly, overexpression of ADAM28 in human prostate carcinoma or normal prostate epithelial cells enhanced cellular proliferation and migration, while inhibition with an ADAM28 inhibitor or siRNA silencing of ADAM28 reduced cellular proliferation and migration of human prostate carcinoma cells. Collectively, these findings suggest that ADAM28 may promote the pathogenesis of prostate cancer via proliferation and migration. Thus, successful therapeutic targeting of ADAM28 to inhibit its activity could be a beneficial approach in prostate cancer.
Our team previously elucidated that overexpression of ADAM28 mediated breast carcinoma cell proliferation through the liberation of IGF-I after proteolysis of IGFBP3.[23] In a prostate cancer setting, IGF-I is a powerful mitogenic factor and may promote the proliferation of prostate carcinoma cells.[34] Based on these findings, the hypothesis that ADAM28 may play a key role in cell proliferation in human prostate carcinomas was formulated. In our current study, we found that IGF-I liberation is increased when ADAM28 is overexpressed. We propose that this may be via ADAM28-mediated proteolysis of IGFBP3 on prostate carcinoma cells as indicated in Fig. 4A.
The results in the present study indicated that inhibition of ADAM28 by KB-R7785 significantly reduced proliferation and migration of human prostate carcinoma cells compared to control cells. Although KB-R7785 has been shown to completely inhibit ADAM28 activity,[18] it does have off-target effects on ADAM12.[9,35] Interestingly, ADAM12 has been shown to be essential for tumor development and progression in a mouse model of prostate cancer.[36] Thus, in the studies using KB-R7785, some of the functional effects observed could be due to combined blockade of ADAM28 and ADAM12. Future studies will delineate these effects by knocking down ADAM12 expression in prostate cancer or alternatively through the use of an ADAM12 neutralizing antibody that would specifically block the active site of ADAM12 in functional studies. Although an important line of enquiry regards ADAM12, our data using siRNA against ADAM28 and ADAM28 overexpression, validates ADAM28's pathogenic role in prostate cancer.
Several studies have implicated the proinflammatory cytokine, IL-6, in prostate cancer cell proliferation and migration.[37–40] In our study, ADAM28 overexpression did not influence the level of IL-6 secretion from human prostate cancer cells. Therefore, IL-6 is not a fundamental driver of ADAM28-mediated proliferation and migration of human prostate cancer cells.
This initial study of ADAM28 and prostate cancer raises several questions, including how ADAM28 is regulated in prostate cancer and its mechanism of action. A recent study by our team has verified that the oncogene Src induces ADAM28 expression.[41] The oncogene Src is involved in regulating cellular proliferation, survival, migration, invasion, metastasis, and angiogenesis.[41–45] In addition, we have shown that ADAM28 cleaves IGFBP3 to liberate IGF-I, which in part contributes to carcinoma cell proliferation in breast cancer.[23] ADAM28 also promotes VEGF165 bioavailability by digestion of the VEGF165/CTGF complex.[24] Furthermore, anti-apoptosis of carcinoma cells within blood vessels is due in part to ADAM28 degrading VWF, enabling them to bypass VWF-induced apoptosis.[25] Thus, these mechanisms could be operative in prostate cancer.
Numerous studies have highlighted a strong correlation between the metabolic syndrome and prostate cancer stage.[46,47] Interestingly, our group has demonstrated that ADAM28 is significantly elevated with the metabolic syndrome.[20] There is mounting evidence that prostate cancers are more aggressive in men with the metabolic syndrome than in men without.[46–51] Interestingly, in our current clinical study, ADAM28 expression in prostate biopsies appeared greater in patients with prostate cancer (Fig. 1). It is possible, therefore, that expression of ADAM28 may be greater in men with the metabolic syndrome and prostate cancer.
In unpublished microarray data from a PhD dissertation,[52] it was shown that DHT treatment of LNCaP cells resulted in a 20-fold increase in ADAM28 mRNA expression. We now show that DHT treatment of LNCaP cells increases ADAM28 protein expression 3-fold compared to control cells (Fig. 2). Combined, these results suggest that DHT promotes elevated ADAM28 protein expression in LNCaP cells at the transcriptional level.
In conclusion, we have shown for the first time that ADAM28 is overexpressed in human prostate cancer and is involved in driving the proliferation and migration of human prostate carcinoma cells. Importantly, its activity can be inhibited readily by a small molecule inhibitor, suggesting that it may be possible to consider reduction of ADAM28 expression as a new therapeutic strategy in prostate cancer. Further work with validation in preclinical models will provide additional insight into this exciting possibility.
Supplementary Material
Acknowledgment
The authors thank Cancer Council of Western Australia for the support to CML.
Footnotes
Abbreviations: ADAM = a disintegrin and metalloproteinase, CTGF = connective tissue growth factor, DHT = dihydrotestosterone, IGF = insulin-like growth factor, IGFBP-3 = IGF binding protein-3, IL-6 = interleukin 6, RPMI = Roswell Park Memorial Institute, VEGF = vascular endothelial growth factor, VWF = von Willebrand factor.
Funding/support: This study was funded by the Cancer Council of Western Australia (to CML).
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136:E359–E386. [DOI] [PubMed] [Google Scholar]
- 2.Attard G, Parker C, Eeles RA, et al. Prostate Cancer. Lancet 2016; 387:70–82. [DOI] [PubMed] [Google Scholar]
- 3.Damber J, Aus G. Prostate cancer. Lancet 2008; 371:1710–1721. [DOI] [PubMed] [Google Scholar]
- 4.Higano CS. Side effects of androgen deprivation therapy: monitoring and minimizing toxicity. Urology 2003; 61:32–38. [DOI] [PubMed] [Google Scholar]
- 5.Miyamoto H, Messing EM, Chang C. Androgen deprivation therapy for prostate cancer: current studies and future prospects. Prostate 2004; 61:332–353. [DOI] [PubMed] [Google Scholar]
- 6.Blobel CP. ADAMs: key components in EGFR signalling and development. Nat Rev Mol Cell Biol 2005; 6:32–43. [DOI] [PubMed] [Google Scholar]
- 7.Edwards DR, Handsley MM, Pennington CJ. The ADAM metalloproteinases. Mol Aspects Med 2008; 29:258–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huovila APJ, Turner AJ, Pelto-Huikko M, et al. Shedding light on ADAM metalloproteinases. Trends Biochem Sci 2005; 30:413–422. [DOI] [PubMed] [Google Scholar]
- 9.Mochizuki S, Okada Y. ADAMs in cancer cell proliferation and progression. Cancer Sci 2007; 95:621–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fritzsche F, Jung M, Xu C, et al. ADAM8 Expression in prostate cancer is associated with parameters of unfavorable prognosis. Virchows Arch 2006; 449:628–636. [DOI] [PubMed] [Google Scholar]
- 11.Fritzsche F, Jung M, Tölle A, et al. ADAM9 expression is a significant and independent prognostic marker of PSA relapse in prostate cancer. Eur Urol 2008; 54:1097–1108. [DOI] [PubMed] [Google Scholar]
- 12.Josson S, Anderson CS, Sung SY, et al. Inhibition of ADAM9 expression induces epithelial phenotypic alterations and sensitizes human prostate cancer cells to radiation and chemotherapy. Prostate 2010; 71:232–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arima T, Enokida H, Kubo H, et al. Nuclear translocation of ADAM-10 contributes to the pathogenesis and progression of human prostate cancer. Cancer Sci 2007; 98:1720–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCulloch D, Harvey M, Herington A. The expression of the ADAMs proteases in prostate cancer cell lines and their regulation by dihydrotestosterone. Mol Cell Endocrinol 2000; 167:11–21. [DOI] [PubMed] [Google Scholar]
- 15.Kuefer R, Day K, Kleer C, et al. ADAM15 disintegrin is associated with aggressive prostate and breast cancer disease. Neoplasia 2006; 8:319–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiao J, Lin P, Lin F, et al. ADAM17 targets MMP-2 and MMP-9 via EGFR-MEK-ERK pathway activation to promote prostate cancer cell invasion. Int J Oncol 2012; 40:1714. [DOI] [PubMed] [Google Scholar]
- 17.Hoyne G, Rudnicka C, Sang QXA, et al. Genetic and cellular studies highlight that a disintegrin and metalloproteinase 19 is a protective biomarker in human prostate cancer. BMC Cancer 2016; 16:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mochizuki S, Shimoda M, Shiomi T, et al. ADAM28 is activated by MMP-7(matrilysin-1) and cleaves insulin-like growth factor binding protein-3. Biochem Biophys Res Commun 2004; 315:79–84. [DOI] [PubMed] [Google Scholar]
- 19.Howard L, Maciewicz RA, Blobel CP. Cloning and characterisation of ADAM28: Evidence for autocatalytic pro-domain removal and for cell surface localisation of mature ADAM28. Biochem J 2000; 348:21–27. [PMC free article] [PubMed] [Google Scholar]
- 20.Jowett J, Okada Y, Leedman P, et al. ADAM28 is elevated in humans with the metabolic syndrome and is a novel sheddase of human tumour necrosis factor-α. Immunol Cell Biol 2012; 90:966–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bridges L, Tani P, Hanson K, et al. The lymphocyte metalloprotease MDC-L (ADAM 28) is a ligand for the integrin alpha4beta1. J Biol Chem 2002; 277:3784–3792. [DOI] [PubMed] [Google Scholar]
- 22.Bridges L, Sheppard D, Bowditch R. ADAM disintegrin-like domain recognition by the lymphocyte integrins alpha4beta1 and alpha4beta7. Biochem J 2005; 387:101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mitsui Y, Mochizuki S, Kodama T, et al. ADAM28 is overexpressed in human breast carcinomas: Implications for carcinoma cell proliferation through cleavage of insulin-like growth factor binding protein-3. Cancer Res 2006; 66:9913–9920. [DOI] [PubMed] [Google Scholar]
- 24.Mochizuki S, Tanaka R, Shimoda M, et al. Connective tissue growth factor is a substrate of ADAM28. Biochem Biophys Res Commun 2012; 402:651–657. [DOI] [PubMed] [Google Scholar]
- 25.Mochizuki S, Soejima K, Shimoda M, et al. Effect of ADAM28 on carcinoma cell metastasis by cleavage of von willebrand factor. J Nat Cancer Inst 2012; 104:906–922. [DOI] [PubMed] [Google Scholar]
- 26.Giovannucci E. Insulin-like growth factor-I and binding protein-3 and risk of cancer. Hormone Res 1999; 51:34–41. [DOI] [PubMed] [Google Scholar]
- 27.Grzmil M, Hemmerlein B, Thelen P, et al. Blockade of the type I IGF receptor expression in human prostate cancer cells inhibits proliferation and invasion, up-regulates IGF binding protein-3, and suppresses MMP-2 expression. J Path 2004; 202:50–59. [DOI] [PubMed] [Google Scholar]
- 28.Saikali Z, Setya H, Singh G, et al. Role of IGF-I/IGF-IR in regulation of invasion in DU145 prostate cancer cells. Cancer Cell Int 2008; 8:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohtsuka T, Shiomi T, Shimoda M, et al. ADAM28 is overexpressed in human non-small cell lung carcinomas and correlates with cell proliferation and lymph node metastasis. Int J Cancer 2006; 118:263–273. [DOI] [PubMed] [Google Scholar]
- 30.Tyan YC, Yang MH, Chen SCJ, et al. Urinary protein profiling by liquid chromatography/tandem mass spectrometry: ADAM28 is overexpressed in bladder translational cell carcinoma. Rapid Commun Mass Spectrom 2011; 25:2851–2862. [DOI] [PubMed] [Google Scholar]
- 31.Stokes A, Joutsa J, Ala-Aho R, et al. Expression profiles and clinical correlations of degradome components in the tumor microenvironment of head and neck squamous cell carcinoma. Clin Cancer Res 2010; 16:2022–2035. [DOI] [PubMed] [Google Scholar]
- 32.Zhang XH, Wang CC, Jiang Q, et al. ADAM28 overexpression regulated via the PI3K/Akt pathway is associated with relapse in de novo adult B-cell acute lymphoblastic leukemia. Leuk Res 2015; 39:1229–1238. [DOI] [PubMed] [Google Scholar]
- 33.Shimoda M, Hashimoto G, Mochizuki S, et al. Binding of ADAM28 to P-selectin glycoprotein ligand-1 enhances P-selectin-mediated leukocyte adhesion to endothelial cells. J Biol Chem 2007; 282:25864–25874. [DOI] [PubMed] [Google Scholar]
- 34.Massoner P, Collesell D, Matscheski A, et al. Novel mechanism of IGF-binding protein-3 action on prostate cancer cells: inhibition of proliferation, adhesion, and motility. Endocrin Relat Cancer 2009; 16:795–808. [DOI] [PubMed] [Google Scholar]
- 35.Asakura M, Kitakaze M, Takashima S, et al. Cardiac hypertrophy is inhibited by antagonism of ADAM12 processing of HB-EGF: metalloproteinase inhibitors as a new therapy. Nat Med 2002; 8:35–40. [DOI] [PubMed] [Google Scholar]
- 36.Peduto L, Reuter V, Sehara-Fujisawa A, et al. ADAM12 is highly expressed in carcinoma-associated stroma and is required for mouse prostate tumor progression. Oncogene 2006; 25:5462–5466. [DOI] [PubMed] [Google Scholar]
- 37.Culig Z, Steiner H, Bartsch G, et al. Interleukin-6 regulation of prostate cancer cell growth. J Cell Biochem 2005; 95:497–505. [DOI] [PubMed] [Google Scholar]
- 38.Giri D, Ozen M, Ittmann M. Interleukin-6 is an autocrine growth factor in human prostate cancer. Am J Pathol 2001; 159:2159–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith P, Hobisch A, Lin D, et al. Interleukin-6 and prostate cancer progression. Cytokine Growth Factor Rev 2001; 12:33–40. [DOI] [PubMed] [Google Scholar]
- 40.Smith D, Kiba A, Zong Y, et al. Interleukin-6 and oncostatin-M synergize with the PI3K/AKT pathway to promote aggressive prostate malignancy in mouse and human tissues. Mol Cancer Res 2013; 11:1159–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abe H, Mochizuki S, Ohara K, et al. Src plays a key role in ADAM28 expression in v-src-transformed epithelial cells and human carcinoma cells. Am J Pathol 2013; 183:1667–1678. [DOI] [PubMed] [Google Scholar]
- 42.Aleshin A, Finn R. Src: A century of science brought to the clinic. Neoplasia 2010; 12:599–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mayer EL, Krop IE. Advances in targeting src in the treatment of breast cancer and other solid malignancies. Clin Cancer Res 2010; 16:3526–3532. [DOI] [PubMed] [Google Scholar]
- 44.Summy J, Gallick G. Src family kinases in tumour progression and metastasis. Cancer Metastasis Rev 2003; 22:337–358. [DOI] [PubMed] [Google Scholar]
- 45.Wheeler D, Dunn E. The role of Src in solid tumors. Oncologist 2009; 14:667–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Denunzio C, Freeland F, Miano R, et al. Metabolic syndrome is associated with high grade Gleason score when prostate cancer is diagnosed on biopsy. Prostate 2011; 71:1492–1498. [DOI] [PubMed] [Google Scholar]
- 47.Morote J, Ropero J, Planas J, et al. Metabolic syndrome increases the risk of aggressive prostate cancer detection. BJU Int 2013; 111:1031–1036. [DOI] [PubMed] [Google Scholar]
- 48.Bhindi B, Locke J, Alibhai S, et al. Dissecting the association between metabolic syndrome and prostate cancer risk: analysis of a large clinical cohort. Eur Urol 2015; 67:64–70. [DOI] [PubMed] [Google Scholar]
- 49.Kheterpal E, Sammon J, Diaz M, et al. Effect of metabolic syndrome on pathologic features of prostate cancer. Urol Oncol 2013; 31:1054–1059. [DOI] [PubMed] [Google Scholar]
- 50.Ozbek E, Otunctemur A, Dursun M, et al. The metabolic syndrome is associated with more aggressive prostate cancer. Asian Pac J Cancer Prev 2012; 15:4029–4032. [DOI] [PubMed] [Google Scholar]
- 51.Sanchis-Bonet A, Ortiz-Vico F, Morales-Palcios N, et al. Asociacion entre sindrome metabolico y cancer de prostata: efecto sobre su agresividad y progresion. Actas Urol Esp 2015; 39:154–160. [DOI] [PubMed] [Google Scholar]
- 52.Romanuik TL. Gene Expression in Prostate Cancer [Dissertation]. Vancouver: University of British Columbia; 2008. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


