Supplemental Digital Content is available in the text
Keywords: anti-neutrophil cytoplasmic antibody, anti-neutrophil cytoplasmic antibody-associated vasculitis, diagnosis
Abstract
Currently no validated diagnostic system for antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) is available. Therefore, diagnosing AAV is often challenging. We aimed to identify factors that lead to a clinical diagnosis AAV in ANCA positive patients in a teaching hospital in The Netherlands.
In this study, all patients that tested positive for ANCA proteinase 3 (PR3) and/or myeloperoxidase (MPO) between 2005 and 2015 were analysed. Patients with a clinical diagnosis of AAV were compared with patients without a clinical diagnosis of AAV. Clinical symptoms and laboratory variables at presentation, including the ANCA titre, were collected for both patients with and without AAV. Clinical and laboratory variables related with AAV were investigated, using multivariable logistic regression.
Two hundred thirty seven consecutive patients with a positive ANCA were included, of whom 119 were clinically diagnosed with AAV. Of the 118 ANCA positive patients without AAV, 87 patients had an alternative diagnosis, including inflammatory bowel disease (n = 24), other rheumatic diseases (n = 23), infection (n = 11), malignancy (n = 4), and other diagnoses (n = 25). In a multivariable regression model, a high ANCA titre (odds ratio [OR] 14.16, 95% confidence interval [CI] 6.93–28.94) and a high number of affected organ systems (OR 7.67, 95% CI 3.69–15.94) were associated with AAV.
MPO and PR3 ANCA can be positive in a variety of diseases that mimic AAV. A higher ANCA titre and multiple affected organ systems may help to discriminate between AAV and other systemic illnesses in anti-PR3 and anti-MPO positive patients. A diagnostic scoring system incorporating these factors should be considered.
1. Introduction
Antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) is a rare, necrotizing vasculitis that predominantly affects small vessels. AAV includes microscopic polyangiitis (MPA), granulomatosis with polyangiitis (GPA), and eosinophilic granulomatosis with polyangiitis (EGPA).[1,2] These clinical conditions are often associated with circulating ANCA directed against either proteinase 3 (PR3) or myeloperoxidase (MPO). Untreated and severe AAV can be fatal within months.[3] Fortunately, advances in therapies have led to an improved prognosis over the last decades.[4,5]
Adequate therapy requires an early diagnosis, but diagnosing AAV can be challenging.[6,7] Ideally the diagnosis AAV is confirmed with a biopsy.[8] However, in clinical practice and even in large trials, a biopsy is not always performed and a diagnosis is often made based on clinical features in combination with positive ANCA serology.[5,9,10] Importantly, positive ANCA serology can also be found in other conditions with systemic symptoms, or even in asymptomatic patients.[11–13] Classification systems, such as the American College of Rheumatology (ACR) criteria[14] and the Chapel Hill Consensus Conference (CHCC) guidelines[1] have several limitations.[15] The ACR criteria were developed in the 1980s, when ANCA was not routinely assessed and the classification of vasculitis did not yet include MPA. The CHCC classification was developed as a nomenclature system and does not provide clear diagnostic criteria. Hence, the lack of an established diagnostic system, the complex symptomatology of AAV and the limited specificity of ANCA may lead to a delayed and unstandardised diagnosis AAV in clinical practice.
The aim of the present study was to identify clinical and laboratory variables that lead to the diagnosis AAV in ANCA positive patients in clinical practice. Furthermore, the sensitivity and specificity of several ANCA cut-off values for a clinical diagnosis AAV were explored.
2. Methods
We performed a retrospective cohort study in the Northwest Clinics a teaching hospital in Alkmaar, The Netherlands. The institutional review board approved the study and the medical ethical committee waived requirements for informed consent, due to the retrospective nature of the study. A computerised search for the assessment of ANCA in the local laboratory between February 1, 2005 and February 1, 2015 was performed. ANCA serology was examined by indirect immunofluorescence (IIF) on neutrophil substrate (NOVA Lite ANCA, INOVA Diagnostics Inc, San Diego) and, if positive, followed by immunoassays for the detection of antibodies to PR3 and myeloperoxidase MPO (Autostat II Anti-PR-3 and Anti-MPO ELISAs, Hycor Biomedical Ltd, UK, from February 2005 until August 2012, and EliA PR3S and EliA MPOS run on a Phadia 250 analyzer, Thermo Fisher Scientific, Immunodiagnostics, Sweden from Augustus 2012 until the end of the study period). In patients with a positive IIF, all subsequent ANCA assessments were performed with anti-PR3 and anti-MPO specific immunoassays immediately, leaving out IIF. Upper limits of the normal range were provided by the manufacturer of the assays: MPO >5 IU/mL and PR3 >8 IU/mL before 2012 and MPO >5.0 IU/mL and PR3 >3.0 IU/mL after 2012.
Medical records of all patients with one or more positive MPO and/or PR3 ANCA test were reviewed for a clinical diagnosis of AAV (i.e., GPA, MPA, or EGPA). Demographic and clinical parameters were collected: age at presentation, sex, symptoms at presentation, number of affected organ systems, date and level of the first positive ANCA titre, laboratory parameters, and comorbidities. Furthermore, the clinical diagnosis (i.e., AAV or alternative diagnosis), date of diagnosis, and histological data were recorded. If a diagnosis was revised over time, this was recorded as well. Symptoms per organ system were recorded similar to symptoms as described in the Birmingham Vasculitis Activity Score (BVAS/WG).[16]
2.1. Statistical analysis
Patients with a clinical diagnosis AAV were compared with patients without a clinical diagnosis AAV. Chi-square tests were used for categorical data. Continuous data were analysed by the unpaired Student t test. The number of affected organ systems was analysed with the use of the Mann–Whitney U test. The results of the different ANCA assays were transformed into the multiplicity of their respective cut-off values. A receiver-operating characteristics (ROC)-curve was calculated for the sensitivity and specificity of several ANCA cut-off values for a clinical diagnosis. In order to identify indicators for AAV in ANCA positive patients a multivariable logistic regression model was developed. Fifty bootstrap samples were applied with backward elimination (P < 0.05) in order to establish the final predictors in the model. Hereafter the calculated shrinkage factor was used to adjust the original coefficients, in order to correct for optimism. A P value <0.05 was considered to be statistically significant. A sensitivity analysis was performed by repeating the analysis after the exclusion of patients with a clinical diagnosis AAV that was not biopsy proven. For data management and statistical analysis, Statistical Package for Social Sciences (SPSS) version 20.0 (IBM, Armonk, NY, USA) and RStudio 0.98.932 (Boston, MA, USA) were used.
3. Results
3.1. Enrolment
Between February 1, 2005 and February 1, 2015 a total of 8403 IIF for ANCA was performed of which 1238 tested positive (27% p-ANCA, 71% c-ANCA pattern, 1% aspecific pattern) in 279 patients. A total of 5370 immunoassays for PR3 and/or MPO ANCA was performed of which 1218 samples tested positive in 239 patients (Fig. 1). Two of the 239 anti-MPO or anti-PR3 positive patients were excluded due to a lack of data in the medical records.
Figure 1.
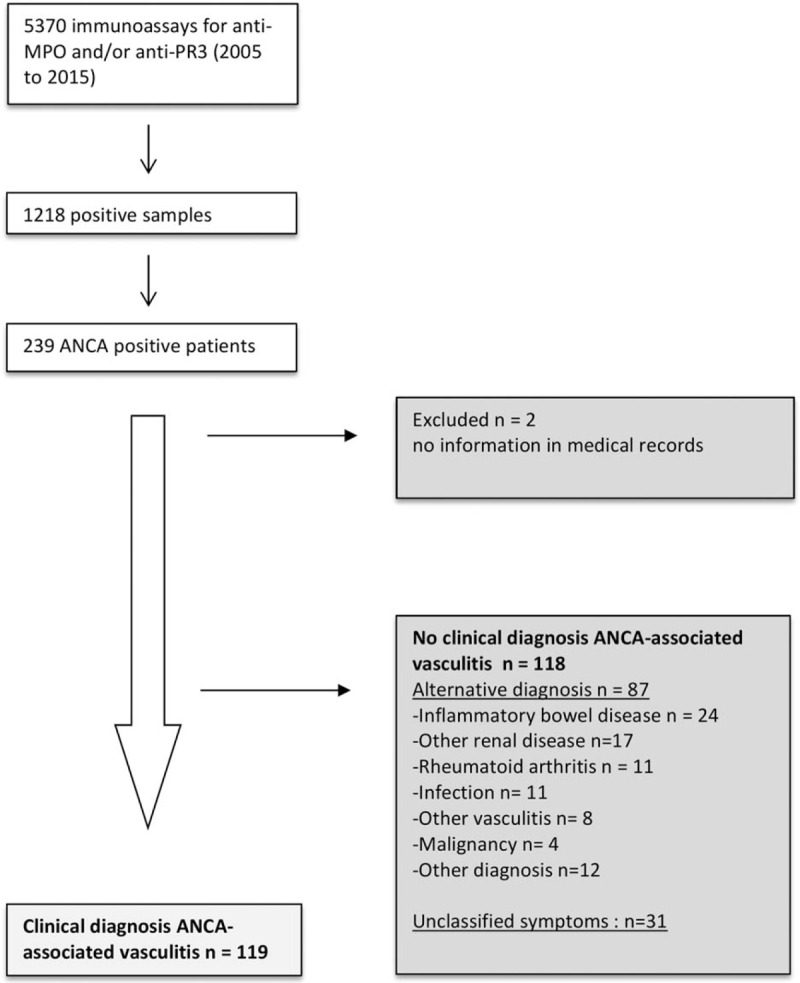
Flow chart of the inclusion of ANCA positive patients between February 2005 and February 2015. ANCA = antineutrophil cytoplasmic antibody, MPO = myeloperoxidase, PR3 = proteinase 3.
3.2. Patients
Of the 237 included MPO and/or PR3 ANCA positive patients, 57% was men with a mean age of 57 ± 19 years. ANCA was PR3 positive in 51% versus MPO positive in 49%. The median follow-up was 5.8 (percentiles 2.7–9.4) years and the median time between the request of the first positive ANCA titre and the diagnosis AAV was 15 days (9.0–36.0). Of the 237 patients, 119 patients (50%) were diagnosed with AAV between 1991 and 2015. In 9 patients the time until the diagnosis AAV was more than 4 months. None of the diagnoses were revised during follow-up. A total of 54 (45%) had a biopsy-proven vasculitis (34 renal biopsies, 12 [deep] skin biopsies, 4 nose biopsies, 3 lung biopsies) and in 28 patients (24%) a biopsy revealed aspecific, inflammatory findings.
Characteristics of patients with and without a clinical diagnosis AAV are summarised in Table 1. Patients with a clinical diagnosis of AAV had more often renal symptoms (66% vs. 36%, P < 0.001), pulmonary symptoms (45% vs. 25%, P = 0.01), ear nose throat (ENT) symptoms (45% vs. 13%, P < 0.001) and more affected organ systems (median 1 vs. 2, P < 0.001), as compared with patients without clinical diagnosis of AAV. Of the 118 patients without a diagnosis of AAV, 87 patients had an alternative diagnosis, including: inflammatory bowel disease (n = 24), renal insufficiency due to another cause (n = 17), rheumatoid arthritis (n = 11), infections (n = 11), other vasculitis (n = 8), malignancy (n = 4), or other (n = 12) (Table 2). Thirty-one patients had unclassified symptoms. Patients with an alternative diagnosis were more often anti-MPO positive than anti-PR3 positive (58% vs. 42%, [Table 2]). Patients with more affected organ systems were more likely to be diagnosed with AAV (Fig. 2).
Table 1.
Characteristics of ANCA positive patients with and without clinical diagnosis of ANCA-associated vasculitis.
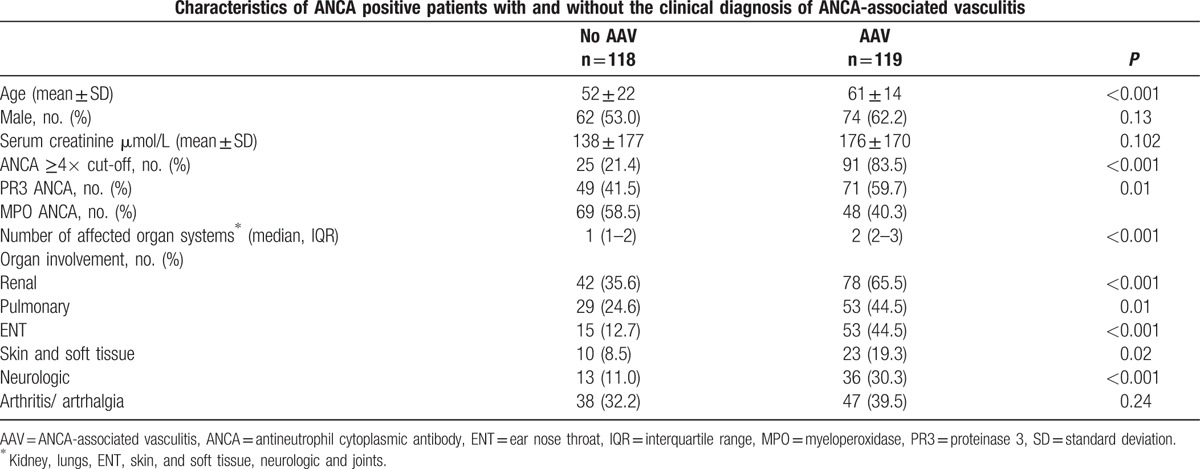
Table 2.
Characteristics of patients with alternative diagnoses in 115 ANCA positive patients without ANCA-associated vasculitis.
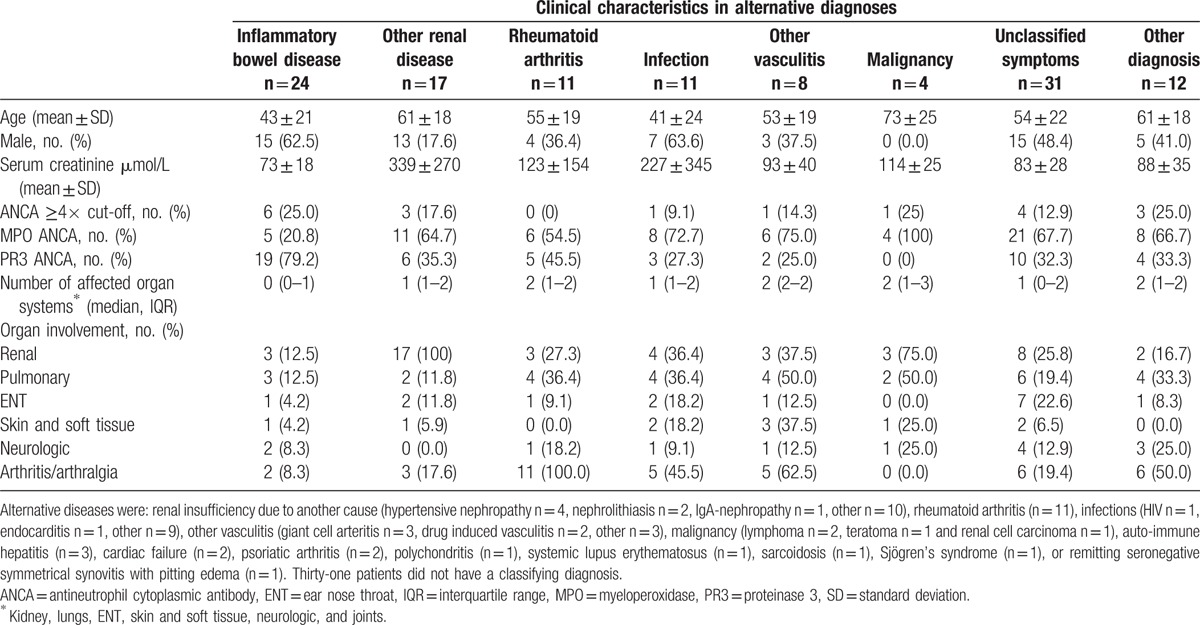
Figure 2.
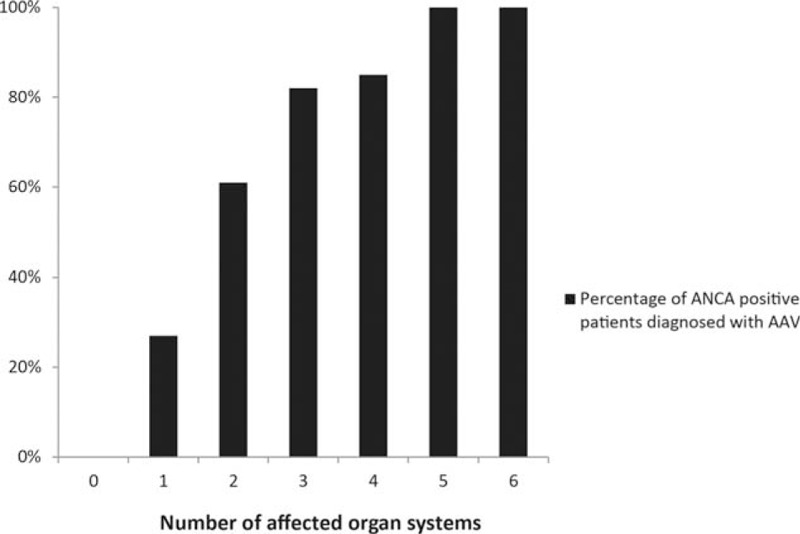
Percentage of patients with a clinical diagnosis of AAV subdivided by the number of affected organ systems. AAV = ANCA-associated vasculitis.
3.3. ANCA titres
First ANCA titres were available in 226 patients, since 5 patients were referred from another hospital and 6 patients were diagnosed before the use of immunoassays for the detection of antibodies (1993). These patients were excluded from the analysis. An ROC curve of ANCA cut-off values as a determinant for clinical diagnosis showed an area under the curve of 0.87 (95% CI 0.82–0.92), shown in Fig. 3. Coordinates of the sensitivity and specificity several cut-off levels are shown in Table 3. An ANCA titre of ≥4 times the usual cut-off resulted in a sensitivity of 83.5% and a specificity of 78.6% for a clinical diagnosis of AAV. In patients with an alternative diagnosis, the ANCA level was ≥4 times the cut-off in only 21%. The association between the ANCA titre and the clinical diagnosis AAV was comparable for the different tests that were used in the laboratory before and after 2012, shown in Fig. 4. Fig. 5 shows the proportion of patients diagnosed with AAV subdivided by the level of the ANCA titre.
Figure 3.

Receiver operating characteristic curve for distinguishing ANCA-associated vasculitis from other diagnoses using several cut-off values of ANCA titres. ANCA = antineutrophil cytoplasmic antibody.
Table 3.
Sensitivity and specificity of the number of times the cut-off value. Pooled analysis for different ANCA immunoassay techniques in the local laboratory.
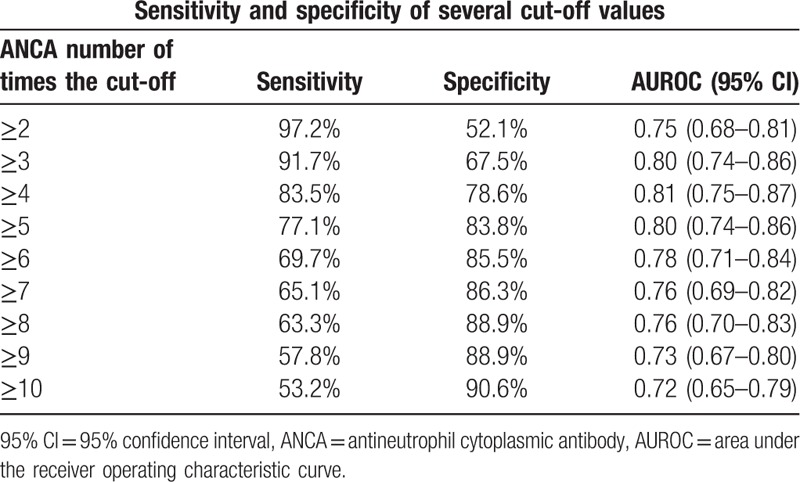
Figure 4.
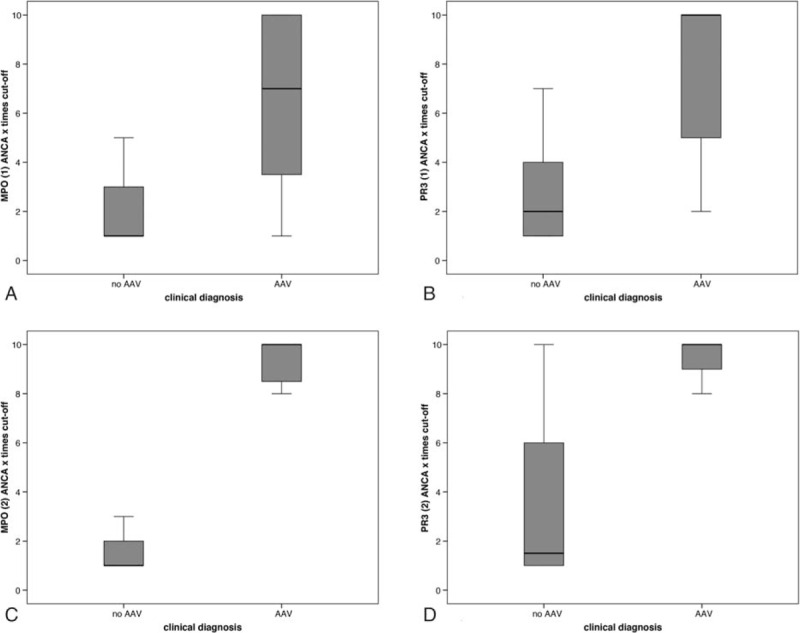
Number of times the ANCA cut-off in patients with and without a clinical diagnosis AAV in 226 ANCA positive patients. Results are shown for each laboratory test and all test pooled. (A) correlation of anti-MPO (1) with AAV in n = 93. (B) Correlation of anti-PR3 (1) with AAV in n = 91. (C) Correlation of anti-MPO (2) with AAV in n = 21. (D) Correlation of anti-PR3 (2) with AAV in n = 21. 1. AutostatTM II Anti-PR-3 and Anti-MPO ELISAs, Hycor Biomedical Ltd, UK. 2. EliA PR3S and EliA MPOS run on a Phadia 250 analyzer, Thermo Fisher Scientific, Immunodiagnostics, Sweden. AAV = ANCA-associated vasculitis, ANCA = antineutrophil cytoplasmic antibody, MPO = myeloperoxidase, PR3 = proteinase 3.
Figure 5.
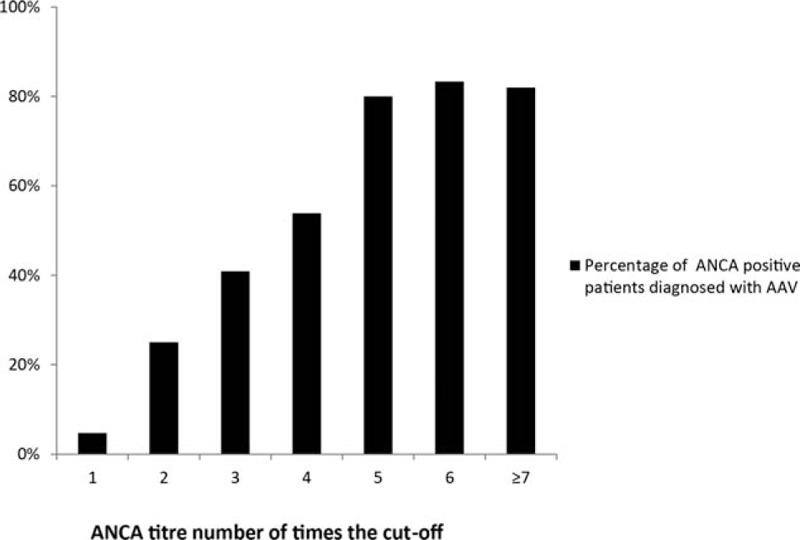
Percentage of patients with a clinical diagnosis of AAV subdivided by the ANCA titre in number of times the cut-off value. ANCA = antineutrophil cytoplasmic antibody. AAV = ANCA-associated vasculitis.
3.4. Multivariable analysis
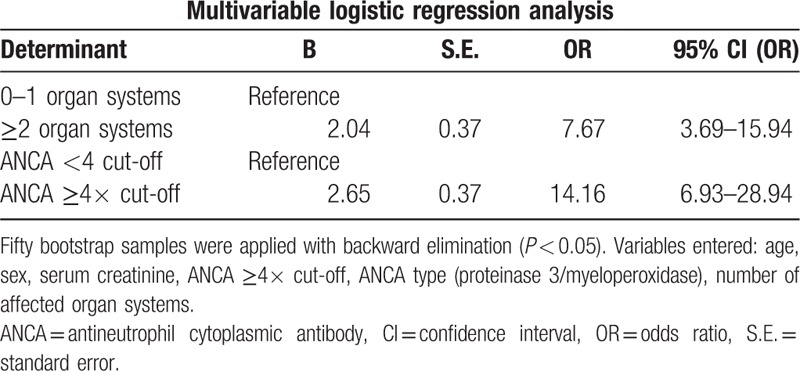
After the exclusion of patients with missing first ANCA titres (n = 11), 226 patients were included in the multivariable model. In the multivariable analysis a high ANCA titre (ANCA ≥4 times cut-off value, odds ratio [OR] 14.16, 95% confidence interval [CI] 6.93–28.94) and a high number of affected organ systems (≥2 organ systems, OR 7.67, 95% CI 3.69–15.94) were strongly associated with a clinical diagnosis AAV, with a c-statistic of 0.88 (Table 4). In a sensitivity analysis after the exclusion of patients with a clinical diagnosis AAV that was not biopsy proven, a high ANCA titre (OR 10.60, 95% CI 4.61–24.34) and more affected organ systems (OR 6.07, 95% CI 2.58–14.26) were still independently related with AAV (see Table 5–12 in the supplemental content for detailed information about these models and the sensitivity analysis).
Table 4.
Multivariable logistic regression analysis of factors related to a clinical diagnosis of ANCA-associated vasculitis in ANCA positive patients (c-statistic 0.88).
4. Discussion
Our findings confirm that both MPO and PR3 ANCA can be positive in a variety of clinical conditions. Higher ANCA levels, PR3 as well as MPO, and more affected organ systems were associated with a clinical diagnosis AAV in our cohort.
To the best of our knowledge, this is the first study that addresses the role of the level of both the MPO and PR3 ANCA titre in diagnosing AAV. In the 4 different immunoassays that were used for the ANCA test, ≥4 times the upper limit appeared a reasonable cut-off point to discriminate between AAV and alternative diagnoses. Recent studies have provided some evidence for an association between the ANCA titre and disease activity. A recent study found a moderate association between the ANCA level and relapses in patients with renal involvement.[17] Another study found an association between high PR3 ANCA levels and decreased patient survival.[18] The value of these data in clinical practice is still under debate.[19] Data on the role of ANCA titre as a diagnostic tool is scarce. In a study by Noel et al,[20] 19 patients diagnosed with GPA had higher PR3 ANCA titres as compared with patients without GPA. This observation so far, had not been described in patients with anti-MPO positivity. Our data suggest that the ANCA titre is potentially useful in clinical practice as a diagnostic tool. Additional studies in other cohorts with different tests would be required to establish the optimal cut-off of ANCA titres that could be incorporated in a diagnostic score system.
As shown by others and confirmed by our own data, ANCA can be detected following a variety of medical conditions apart from AAV. Some of the conditions that had been reported previously are: chronic inflammatory processes, malignancies, infections, and the use of drugs such as propylthiouracil.[21,22] The significance of the ANCA positivity in patients without AAV remains largely unexplained. In animal models it has been demonstrated that both MPO and PR3 ANCA are pathogenic. For instance, mice infused with ANCA auto-antibodies presented with clinical and histological features of glomerulonephritis.[23,24] Clinical evidence for ANCA pathogenicity in humans is scarce. One case report that is often referred to, describes a newborn child who develops MPA secondary to transplacental transfer of maternal MPO antibodies.[25] Indirect clinical evidence of pathogenicity is the efficacy of plasma exchange in severe AAV.[26] In patients with immunoglobulin A (IgA) nephropathy and seemingly accidental positive ANCA serology, ANCA positive patients showed more severe clinical and histological features when compared with ANCA-negative IgA nephropathy patients.[27] This implies ANCA pathogenicity, even in patients without AAV. On the other hand, the fact that in our cohort patients with apparently mild symptoms were ANCA positive, is an argument against ANCA pathogenicity. One possible explanation for these conflicting data is the recent finding that some ANCA are more pathogenic than others, depending on epitope specificity of the antibody. In MPO antibodies, certain epitopes were found to be specific for active disease, while other epitopes remained present during remission or were also present in healthy individuals.[28,29] Perhaps the association between the ANCA titre and a clinical diagnosis reflects an additional pathophysiologic mechanism, in which a certain antibody load is required for the development of AAV. This hypothesis is supported by an animal model of AAV in which the percentage of immunised rats developing crescentic glomerulonephritis, depended on the administered anti-MPO load and was 46%, 64%, and 100% in the groups receiving 400, 800, and 1600 μg/kg anti-MPO, respectively.[30]
As previously mentioned, in clinical practice the diagnosis AAV is often not biopsy proven nor standardised by a diagnostic scoring system.[5,9,10] In our AAV population, diagnosis was biopsy proven in 45%. A kidney biopsy was performed in 29%, which is comparable with 25% of patients in the Rituximab in Associated Vasculitis (RAVE) trial.[5] In patients without a biopsy proven AAV the diagnosis AAV was based on ANCA serology combined with clinical features and expert opinion. A current study by the ACR and the European League Against Rheumatism is developing and validating diagnostic and classification criteria for primary systemic vasculitis which could be helpful in clinical practice and in future trials.[7] Our results suggest that the ANCA titre and the number of affected organ systems could be considered in the development of future diagnostic classification systems. Future, prospective studies, should also consider novel tools in the diagnosis of vasculitis. For example, imaging: 18-F-Fluorodeoxyglucose Positron Emission Tomography with Computed Tomography has been shown to identify organ localizations of GPA at presentation.[31,32] Its value in the diagnosis of AAV needs to be further addressed. Biomarkers such as C3a, C5a, IL-18BP in blood and MCP-1 and C5a in urine samples have shown to be of value in discriminating between active and inactive disease.[33] Further investigations should confirm their reliability in predicting a clinical diagnosis AAV.
An important strength of this study was that our cohort was complete. The Northwest Clinics is connected to a large laboratory that is the only laboratory performing ANCA tests in the region. Besides that, the quality of the medical records was high and data from only two patients were missing. Therefore, it is likely that our cohort is representative of the entire ANCA positive population in the region with a population of approximately 470,000 patients. A limitation of our study was that our search strategy neglected ANCA negative AAV patients, that may account for approximately 10% of the MPA and GPA population and approximately up to 70% of EGPA patients.[34,35] However, data suggest that ANCA negative patients represent clinically different subtypes and should, therefore, be studied separately.[36,37] We used the clinical diagnosis of AAV as a gold standard, since there is no accurate diagnostic system available, which could potentially leave room for subjectivity. Nevertheless, with a median follow-up of 5.8 years we were able to record a reliable final diagnosis over time. Furthermore, the statistical model was repeated with the biopsy proven AAV patients, after which it remained largely unchanged. Finally, because of the retrospective nature of this study we were unable to record a classifying diagnosis of AAV: GPA, MPA or EGPA. Our population consisted of slightly more anti-PR3 positive patients, often with ENT symptoms, possibly indicating a trend for GPA.
In conclusion we demonstrated that ANCA can be positive in a variety of diseases that mimic AAV. In ANCA positive patients in a teaching hospital in The Netherlands, there was a strong association between the ANCA titre and a clinical diagnosis of AAV. The ANCA titre and the number of affected organ systems could be considered as diagnostic markers in AAV in clinical practice.
Supplementary Material
Acknowledgments
The authors wish to thank Dr T. Hoekstra and Dr C.L.M. de Roij van Zuijdewijn, VU medical center, Amsterdam, the Netherlands for statistical and epidemiological support.
Footnotes
Abbreviations: AAV = ANCA-associated vasculitis, ACR = American College of Rheumatology, ANCA = antineutrophil cytoplasmic antibody, CHCC = Chapel Hill Consensus Conference, EGPA = eosinophilic granulomatosis with polyangiitis, ENT = ear nose throat, EULAR = European League Against Rheumatism, FDG-PET/CT = 18-F-Fluorodeoxyglucose Positron Emission Tomography with Computed Tomography, GPA = granulomatosis with polyangiitis, IIF = indirect immunofluorescence, IQR = interquartile range, MPA = microscopic polyangiitis, MPO = myeloperoxidase, OR = odds ratio, PR3 = proteinase-3.
Research support was received from Roche Nederland B.V.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- 1.Jennette JC. Overview of the 2012 revised International Chapel Hill Consensus Conference nomenclature of vasculitides. Clin Exp Nephrol 2013; 17:603–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Watts RA, Lane SE, Bentham G, et al. Epidemiology of systemic vasculitis: a ten-year study in the United Kingdom. Arthritis Rheum 2000; 43:414–419. [DOI] [PubMed] [Google Scholar]
- 3.WALTON EW. Giant-cell granuloma of the respiratory tract (Wegener's granulomatosis). Br Med J 1958; 2:265–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sriskandarajah S, Aasarod K, Skrede S, et al. Improved prognosis in Norwegian patients with glomerulonephritis associated with anti-neutrophil cytoplasmic antibodies. Nephrol Dial Transplant 2015; 30 suppl 1:i67–i75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Specks U, Merkel PA, Seo P, et al. Efficacy of remission-induction regimens for ANCA-associated vasculitis. N Engl J Med 2013; 369:417–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Exley AR, Carruthers DM, Luqmani RA, et al. Damage occurs early in systemic vasculitis and is an index of outcome. QJM 1997; 90:391–409. [DOI] [PubMed] [Google Scholar]
- 7.Luqmani RA, Suppiah R, Grayson PC, et al. Nomenclature and classification of vasculitis—update on the ACR/EULAR diagnosis and classification of vasculitis study (DCVAS). Clin Exp Immunol 2011; 164 suppl 1:11–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaffo AL. Diagnostic approach to ANCA-associated vasculitides. Rheum Dis Clin North Am 2010; 36:491–506. [DOI] [PubMed] [Google Scholar]
- 9.Timlin H, Lee SM, Manno RL, et al. Rituximab for remission induction in elderly patients with ANCA-associated vasculitis. Semin Arthritis Rheum 2015; 45:67–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stone JH. Limited versus severe Wegener's granulomatosis: baseline data on patients in the Wegener's granulomatosis etanercept trial. Arthritis Rheum 2003; 48:2299–2309. [DOI] [PubMed] [Google Scholar]
- 11.Jennette JC, Falk RJ, Wilkman AS. Anti-neutrophil cytoplasmic autoantibodies—a serologic marker for vasculitides. Ann Acad Med Singapore 1995; 24:248–253. [PubMed] [Google Scholar]
- 12.McLaren JS, Stimson RH, McRorie ER, et al. The diagnostic value of anti-neutrophil cytoplasmic antibody testing in a routine clinical setting. QJM 2001; 94:615–621. [DOI] [PubMed] [Google Scholar]
- 13.de BM, Meyer O, Haim T, et al. Antineutrophil cytoplasmic antibodies in rheumatoid arthritis patients. Br J Rheumatol 1996; 35:38–43. [DOI] [PubMed] [Google Scholar]
- 14.Fries JF, Hunder GG, Bloch DA, et al. The American College of Rheumatology 1990 criteria for the classification of vasculitis. Summary. Arthritis Rheum 1990; 33:1135–1136. [DOI] [PubMed] [Google Scholar]
- 15.Watts RA, Mahr A, Mohammad AJ, et al. Classification, epidemiology and clinical subgrouping of antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis. Nephrol Dial Transplant 2015; 30 suppl 1:i14–i22. [DOI] [PubMed] [Google Scholar]
- 16.Stone JH, Hoffman GS, Merkel PA, et al. A disease-specific activity index for Wegener's granulomatosis. modification of the Birmingham Vasculitis Activity Score. International Network for the Study of the Systemic Vasculitides (INSSYS). Arthritis Rheum 2001; 44:912–920. [DOI] [PubMed] [Google Scholar]
- 17.Kemna MJ, Damoiseaux J, Austen J, et al. ANCA as a predictor of relapse: useful in patients with renal involvement but not in patients with nonrenal disease. J Am Soc Nephrol 2015; 26:537–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Westman KW, Selga D, Isberg PE, et al. High proteinase 3-anti-neutrophil cytoplasmic antibody (ANCA) level measured by the capture enzyme-linked immunosorbent assay method is associated with decreased patient survival in ANCA-associated vasculitis with renal involvement. J Am Soc Nephrol 2003; 14:2926–2933. [DOI] [PubMed] [Google Scholar]
- 19.Koh JH, Kemna MJ, Tervaert JW, et al. Editorial: Can a rise in ANCA titer predict relapses in ANCA-associated vasculitis? Arthritis Rheumatol 2016. [DOI] [PubMed] [Google Scholar]
- 20.Noel N, Andre C, Bengoufa D, et al. Performance evaluation of three assays for the detection of PR3-ANCA in granulomatosis with polyangiitis in daily practice. Autoimmun Rev 2013; 12:1118–1122. [DOI] [PubMed] [Google Scholar]
- 21.McAdoo SP, Hall A, Levy J, et al. Proteinase-3 antineutrophil cytoplasm antibody positivity in patients without primary systemic vasculitis. J Clin Rheumatol 2012; 18:336–340. [DOI] [PubMed] [Google Scholar]
- 22.Gao Y, Zhao MH. Review article: drug-induced anti-neutrophil cytoplasmic antibody-associated vasculitis. Nephrology (Carlton) 2009; 14:33–41. [DOI] [PubMed] [Google Scholar]
- 23.Xiao H, Heeringa P, Hu P, et al. Antineutrophil cytoplasmic autoantibodies specific for myeloperoxidase cause glomerulonephritis and vasculitis in mice. J Clin Invest 2002; 110:955–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Little MA, Al-Ani B, Ren S, et al. Anti-proteinase 3 anti-neutrophil cytoplasm autoantibodies recapitulate systemic vasculitis in mice with a humanized immune system. PLoS One 2012; 7:e28626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bansal PJ, Tobin MC. Neonatal microscopic polyangiitis secondary to transfer of maternal myeloperoxidase-antineutrophil cytoplasmic antibody resulting in neonatal pulmonary hemorrhage and renal involvement. Ann Allergy Asthma Immunol 2004; 93:398–401. [DOI] [PubMed] [Google Scholar]
- 26.Jayne DR, Gaskin G, Rasmussen N, et al. Randomized trial of plasma exchange or high-dosage methylprednisolone as adjunctive therapy for severe renal vasculitis. J Am Soc Nephrol 2007; 18:2180–2188. [DOI] [PubMed] [Google Scholar]
- 27.Yang YZ, Shi SF, Chen YQ, et al. Clinical features of IgA nephropathy with serum ANCA positivity: a retrospective case-control study. Clin Kidney J 2015; 8:482–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roth AJ, Ooi JD, Hess JJ, et al. Epitope specificity determines pathogenicity and detectability in ANCA-associated vasculitis. J Clin Invest 2013; 123:1773–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Land J, Rutgers A, Kallenberg CG. Anti-neutrophil cytoplasmic autoantibody pathogenicity revisited: pathogenic versus non-pathogenic anti-neutrophil cytoplasmic autoantibody. Nephrol Dial Transplant 2014; 29:739–745. [DOI] [PubMed] [Google Scholar]
- 30.Little MA, Smyth L, Salama AD, et al. Experimental autoimmune vasculitis: an animal model of anti-neutrophil cytoplasmic autoantibody-associated systemic vasculitis. Am J Pathol 2009; 174:1212–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soussan M, Abisror N, Abad S, et al. FDG-PET/CT in patients with ANCA-associated vasculitis: case-series and literature review. Autoimmun Rev 2014; 13:125–131. [DOI] [PubMed] [Google Scholar]
- 32.Kemna MJ, Vandergheynst F, Voo S, et al. Positron emission tomography scanning in anti-neutrophil cytoplasmic antibodies-associated vasculitis. Medicine (Baltimore) 2015; 94:e747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kronbichler A, Kerschbaum J, Grundlinger G, et al. Evaluation and validation of biomarkers in granulomatosis with polyangiitis and microscopic polyangiitis. Nephrol Dial Transplant 2016; 31:930–936. [DOI] [PubMed] [Google Scholar]
- 34.Westman KW, Bygren PG, Olsson H, et al. Relapse rate, renal survival, and cancer morbidity in patients with Wegener's granulomatosis or microscopic polyangiitis with renal involvement. J Am Soc Nephrol 1998; 9:842–852. [DOI] [PubMed] [Google Scholar]
- 35.Comarmond C, Pagnoux C, Khellaf M, et al. Eosinophilic granulomatosis with polyangiitis (Churg-Strauss): clinical characteristics and long-term followup of the 383 patients enrolled in the French Vasculitis Study Group cohort. Arthritis Rheum 2013; 65:270–281. [DOI] [PubMed] [Google Scholar]
- 36.Reinhold-Keller E, de GK, Holl-Ulrich K, et al. Severe CNS manifestations as the clinical hallmark in generalized Wegener's granulomatosis consistently negative for antineutrophil cytoplasmic antibodies (ANCA). A report of 3 cases and a review of the literature. Clin Exp Rheumatol 2001; 19:541–549. [PubMed] [Google Scholar]
- 37.Sinico RA, Di TL, Maggiore U, et al. Prevalence and clinical significance of antineutrophil cytoplasmic antibodies in Churg-Strauss syndrome. Arthritis Rheum 2005; 52:2926–2935. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


