Abstract
Gene therapy may be a promising approach for the treatment of Leber hereditary optic neuropathy. The aim of this study was to evaluate patients with this condition who were recruited into an upcoming gene therapy clinical trial and to assess any changes in the detection parameters to provide support for the clinical trial. Sixteen patients with Leber hereditary optic neuropathy were evaluated using visual function tests 12 months before the initiation of gene therapy. Then, the results of visual acuity (VA), visual field (VF), RNFL (retinal nerve fiber layer) thickness, and Pattern-reversal Visual evoked potential (PR-VEP) were compared and analyzed. A total of 32 eyes of 16 patients were evaluated. Based on the best-corrected visual acuity (BCVA), 24 eyes were relatively stable compared with the baseline evaluation, and 8 eyes had significant changes, including 5 eyes that showed improvement and 3 eyes that showed impairment. In all eyes, the changes in the best-corrected visual acuity were significantly correlated with the changes in the visual field index (VFI), mean defect (MD), and P100 of the visual evoked potential. In the eyes with relatively stable BCVA and those with an obvious improvement in the BCVA, only the visual mean defect showed a significant change; the other indicators were not significantly different. Aside from the patients showing a tendency of spontaneous improvement, the others were in accordance with the requirement. The effects of Leber hereditary optical neuropathy (LHON) gene therapy should be evaluated primarily based on visual acuity. Additionally, visual field, neural fiber thickness, and electrophysiology should be considered in the evaluation.
Keywords: clinical trial, gene therapy, Leber hereditary optical neuropathy
1. Introduction
Leber hereditary optic neuropathy (LHON) is a form of hereditary blindness caused by a mutation in a mitochondrial gene.[1,2] A mutation in the G11778A locus is present in 90% of cases of LHON in China and Taiwan.[3,4] The pathogenic mutation mainly affects young adult men, who suffer from rapid, painless loss of vision.[1,5,6] Currently, no effective treatment for LHON has been developed. Preliminary results suggest that gene therapy may be effective for the treatment of hereditary eye disease,[7–10] and gene therapy has gained interest in research on LHON.
We have investigated the use of gene therapy for LHON and have performed basic studies in animals.[11–14] In the current study, we present an initial clinical trial with 16 patients who were selected from volunteers. Twelve months prior to the initiation of the formal gene therapy clinical trials, visual function was evaluated in these patients. Through clinical observation, we aimed to address the following issues prior to initiating our clinical trial: (1) Because 4% to 33% of LHON patients with the G11778A mutation undergo spontaneous visual recovery,[15,16] we submitted these patients for clinical observation for 12 months before the operation. (2) We sought to examine various methods for evaluating visual function, including the best-corrected visual acuity (BCVA) and objective examination indicators (optical coherence tomography, OCT; visual evoked potential, VEP; electroretinography, ERG) to facilitate the rational evaluation of the effect of gene therapy in LHON patients.
As a subjective examination parameter, visual acuity is the most important aspect for patients. In this study, we used the BCVA as the key reference standard. A comprehensive analysis of the BCVA and objective parameters, such as the visual field, OCT, VEP, and ERG, was conducted.
2. Methods
2.1. Patients
Our study was registered with clinicaltrials.gov. Number NCT01267422 (December 2010) and approved by the ethics committee of the Ezhou Central Hospital (Ezhou, China), and informed consent was obtained from adult patients and guardians of minors. All experiments followed the provisions of the Declaration of Helsinki, and written informed consent was obtained from the subjects in the study.
Sixteen LHON patients (Table 1) with the G11778A mutation were identified using a polymerase chain reaction-based test. For careful consideration, we plan to carry out the formal gene therapy phase in 2 phases. Therefore, the clinical evaluation at 1 year prior to gene therapy has also been divided into 2 stages. The first group (5 patients) was completed between July 2010 and July 2011, the second group (11 patients) was completed between October 2011 and October 2012. There were 12 male and 4 female volunteers ranging in age from 9 to 46 years, and the average age was 18.52 ± 10.35 years. All patients underwent a complete ophthalmic examination, including determination of the BCVA, automated visual field testing, OCT, pattern electroretinography (PERG), and VEP evaluation. These examinations were conducted by the same technician in the Ophthalmology Department.
Table 1.
Clinical data of the 16 LHON patients.
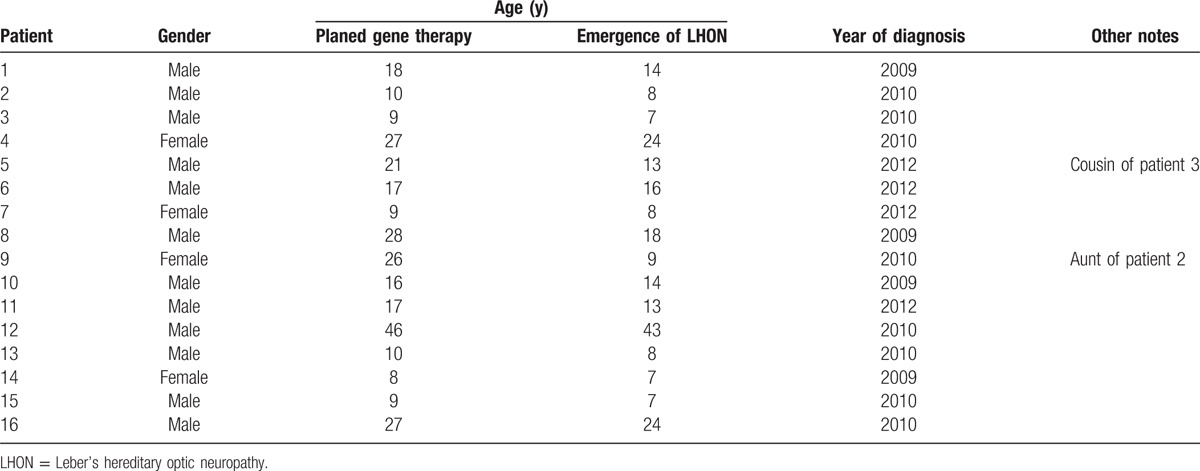
Patients needed to meet the following inclusion criteria: (a) All the enrollees had been diagnosed with LHON and were carriers of the mitochondrial point mutation at locus 11778. (b) All patients were between the ages of 8 and 60 years and provided signed written informed consent. (c) Each patient's physical examination results was normal, and a series of ophthalmic tests or treatments could be tolerated.
Patients were excluded if they had other ocular diseases or systemic diseases that may have affected their visual function. Patients who were included in the gene therapy clinical trial were instructed not to take drugs[17–21] that may have an impact on the accuracy of the measurements, including certain vitamins, antioxidants, idebenone, and so on, without supervision.
2.2. Best-corrected visual acuity
The BCVA was measured using the 2.5-m standard log MAR chart (Star Kang Medical Technology Co., Ltd. Wen Zhou China), and all measurements were obtained by the same ophthalmologist. The patients were examined repeatedly to confirm any change in their vision. Changes in the BCVA ≥0.3 log MAR were considered to indicate an improvement or decline, and a change in the BCVA of <0.3 log MAR was considered to indicate that the BCVA was stable.[22–24]
2.3. Visual field test
A Humphrey field analyzer (Carl Zeiss 740i, Carl Zeiss, Shanghai) was used for the visual field test. The testing procedure included the 30-2 central threshold test and the SITA Fast. Major parameters, including visual field index (VFI), mean defect (MD), and pattern standard deviation (PSD) were recorded.
2.4. Optical coherence tomography
The thickness of the retinal nerve fiber layer (RNFL) in 4 quadrants of the retina (the temporal, superior, nasal, and inferior quadrants in each group) was analyzed using a Spectralis HRA + OCT (Heidelberg Engineering, Heidelberg, Germany). All values obtained from OCT were automatically calculated using the existing software.
2.5. Pattern-reversal visual evoked potential (PR-VEP)
The function of the optic nerve was examined, and the value of the P100 wave was determined with a DV-100 (Shanghai Dikon Medical, Shanghai, China). The tested eyes were optically corrected, and the binocular viewing condition was adopted. Data acquisition and analysis were implemented with a connected computer. All steps were conducted according to the currently reported procedures.[25,26]
2.6. Pattern electroretinogram (PERG)
The pattern electroretinogram (PERG) was automatically analyzed with the RetiPort System (Roland Instruments, RETIport32 4.1.1, Germany) and with the original software of the system.
2.7. Statistical analysis
The correlations between the changes in the objective examination parameters (VF, OCT, ERG, and VEP) and the changes in the BCVA were analyzed using the Spearman correlation. In all eyes in which the BCVA remained relatively stable or in which the visual acuity improved, statistically significant differences in the objective parameters were detected by the signed rank-sum test. All values were expressed as the mean (SD). Statistical significance was defined as P < 0.05.
3. Results
3.1. Best-corrected visual acuity
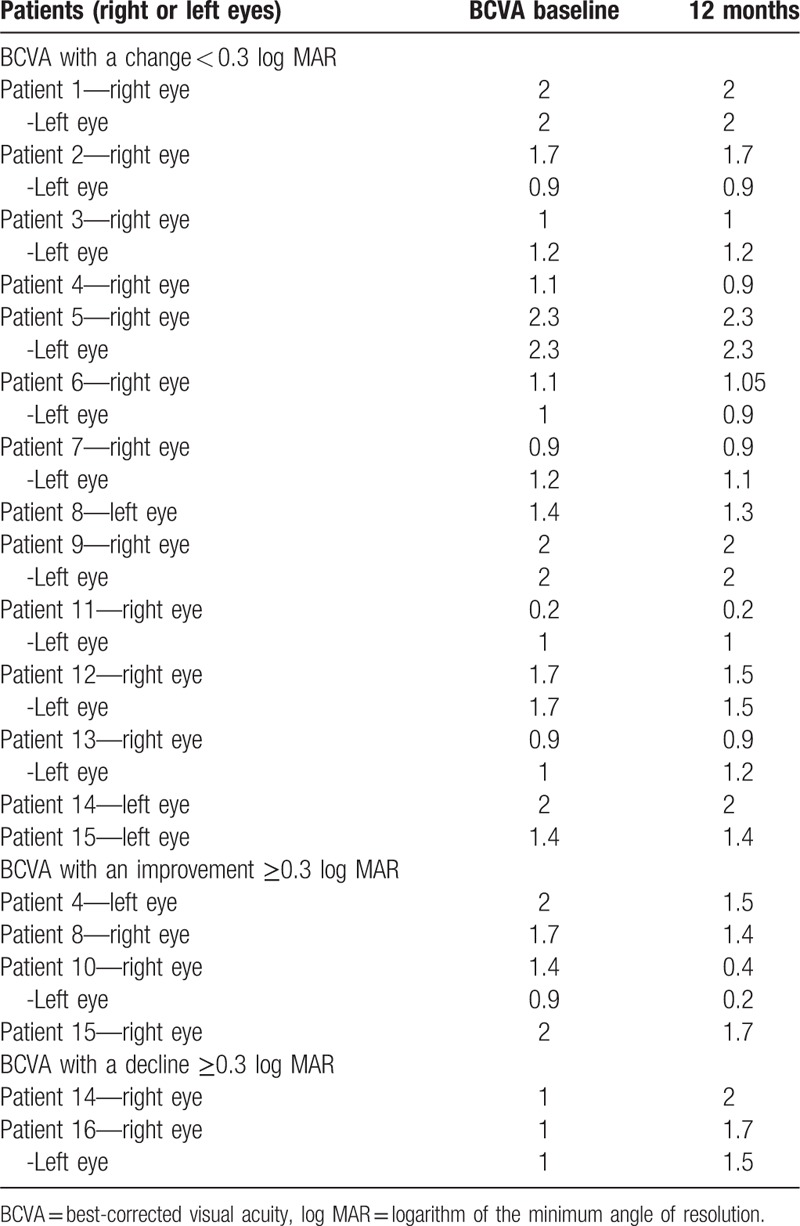
For the first evaluation, the mean BCVA was log MAR = 1.406 ± 0.092; for the second examination, the BCVA was log MAR = 1.364 ± 0.099. The BCVA was relatively stable in 24 eyes (Fig. 1A, Table 2), and the mean visual acuities were 1.417 ± 0.543 and 1.385 ± 0.545 at the first and second examinations, respectively. In addition, the visual acuity of 8 eyes significantly changed after 12 months (4 patients). In 5 eyes (4 patients), the visual acuity improved (Fig. 1B, Table 2), with mean values of 1.600 ± 0.464 and 1.040 ± 0.688 at the first and second examinations, respectively. The visual acuity of 3 eyes (3 patients) significantly decreased (Fig. 1C, Table 2), with mean values of 1.000 ± 0.000 and 1.733 ± 0.252 at the first and second examinations, respectively. Changes in the BCVA ≥0.3 log MAR were considered to indicate an improvement or decline, and a change in the BCVA of <0.3 log MAR was considered to indicate that the BCVA was stable. The correlation between the changes in the objective examination parameters and the change in the BCVA was evaluated twice.
Figure 1.
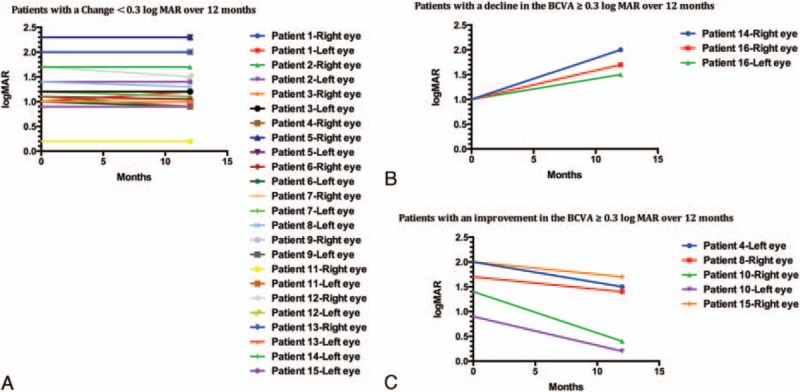
Best corrected visual acuity. (A) Eyes with changes of <0.3 log MAR over 12 months, (B) eyes showing an improvement of 0.3 or more log MAR over 12 months, (C) eyes showing a decline of 0.3 or more log MAR over 12 months. log MAR = logarithm of the minimum angle of resolution.
Table 2.
Log MAR vision acuity of the 16 patients.
3.2. Correlation with visual acuity
For the visual field analysis, changes in MD and VFI were significantly correlated with changes in the BCVA (r = −0.413, P = 0.019; r = −0.603, P < 0.001) (Fig. 2A). The changes in the PSD were not significantly correlated with the changes in the BCVA (r = 0.102, P = 0.577). Regarding the OCT results, changes in the superior, inferior, temporal, and nasal nerve fiber layer were not significantly correlated with changes in the BCVA (r = −0.084, P = 0.647; r = 0.150, P = 0.413; r = −0.040, P = 0.827; r = −0.050, P = 0.785; r = 0.015, P = 0.933). The changes in the P100 of the VEP were significantly correlated with the changes in the BCVA (r = 0.452, P = 0.009) (Fig. 2B). The changes in amplitude were not significantly correlated with the changes in the BCVA (r = −0.024, P = 0.896). Regarding the MfERG detection, complete data were available for only 10 eyes. The changes in the P50 and the amplitude were not significantly correlated with changes in the BCVA (r = 0.119, P = 0.743; r = −0.305, P = 0.392).
Figure 2.
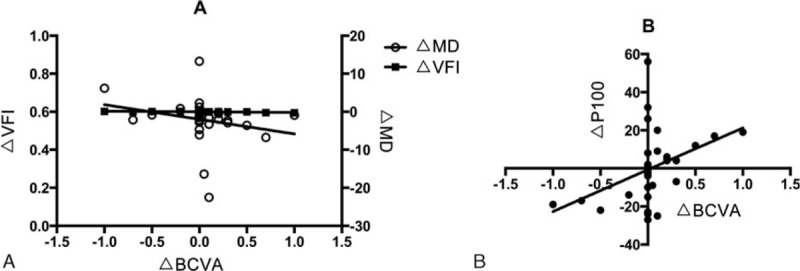
The correlation between changes in the objective examination parameters and the change in the BCVA during the 12 months prior to initiation of gene therapy. (A) The changes in the MD and VFI were significantly correlated with changes in the BCVA. (B) The changes in the P100 of the VEP were significantly correlated with the changes in the BCVA. BCVA = best corrected visual acuity, MD = mean defect, VEP = visual evoked potential, VFI = visual field index.
We evaluated the stability of the objective test parameters compared to the BCVA in eyes with a stable BCVA (a change in the BCVA of <0.3 log MAR).
There were significant differences in MD (P = 0.02), but not in the VFI or PDS (PSD: P = 0.331, VFI: P = 0.455). Regarding the results of OCT, the superior, inferior, temporal, nasal, and average nerve fiber thicknesses were not significantly different (P = 0.795, 0.248, 0.346, 0.768, 0.988, respectively). For the VEP test, there was no significant difference in the P100 or the amplitude at the 2 examinations (P = 0.932, P = 0.879). Although the BCVA was relatively stable in 24 eyes, complete data from the ERG detection were available for only 8 eyes in which the P50 and amplitude had no significant difference (P = 0.596, P = 0.674, respectively).
We evaluated the sensitivity of the objective test parameters compared to the BCVA in eyes that had significant improvement (the improvement of the BCVA was equal to or larger than 0.3 log MAR).
There were significant differences in the MD (P = 0.043) and VFI (P = 0.043) but not the PDS (P = 0.345) in the visual field test. In the OCT test, there were no significant differences in the superior, inferior, temporal, nasal, or average nerve fiber thickness (P = 0.891, 0.680, 0.588, 0.102, 0.223). In the VEP test, there was no significant difference in the P100 and the amplitude at the 2 examinations (P = 0.138, P = 0.345, respectively). The BCVA significantly improved in 5 eyes, but sufficient data for ERG was available for only 2 eyes, and so we did not perform a statistical analysis.
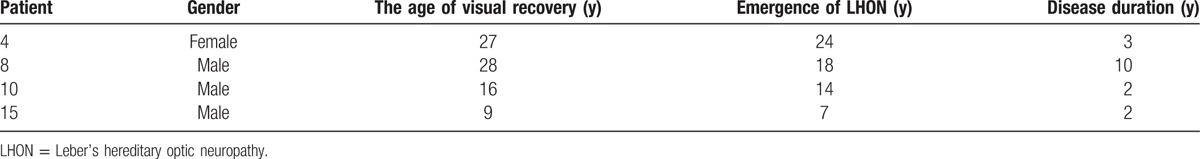
We also analyzed patients who showed spontaneous visual recovery. Four patients were aged between 7 and 24 years, and there were 3 patients with a duration of disease ≤ 3 years. Among them, 2 patients had the disease for <2 years, accounting for 29% (2/7) of all patients whose duration of disease was within 2 years, one patient had the disease for 1 year, and another patient had the disease for 10 years, accounting for 22% (2/9) of all patients whose duration of disease were >2 years (Table 3).
Table 3.
Clinical data of the 5 LHON patients undergo spontaneous visual recovery.
4. Discussion
To ensure the accuracy of our clinical studies, we have performed 12 months of clinical observation in 16 patients who were enrolled in an upcoming clinical trial of gene therapy. We aimed to exclude patients who underwent spontaneous recovery and compared the parameters of visual function synthetically. In this study, 5 eyes presented with obvious improvements in the BCVA (BCVA improved ≥ 0.3 log MAR). LHON patients with the G11778A mutation can undergo spontaneous visual recovery, and this has been reported to occur 4% to 33% of patients.[15,16,27] However, considering the sample size and the different detection methods, these data are one-sided to a certain degree. In our study, there were 4 patients (25%) who underwent spontaneous visual recovery, and this phenomenon was mainly observed in youth and children (disease duration ≤ 3 years). This conclusion may be an indication for subsequent formal clinical trials. In addition, Lam BL et al showed that visual recovery of LHON patients with the G11778A mutation is rare, and when it occurs, it is partial and limited.[28] Although we also acknowledge that the occurrence of spontaneous recovery may interfere with evaluation of the effect of LHON gene therapy in the late stage, clinical observation of patients in LHON gene therapy clinical trials with a large sample size may resolve this issue. By performing clinical observation 1 year before the surgery, we aimed to reduce the impact of this factor on the accuracy of the subsequent clinical trials. In view of these results, spontaneous visual recovery cannot be completely avoided. The therapeutic effect needs to be comparatively analyzed in subsequent gene therapy trials. For example, the time-node of visual acuity improvement occurred within 3 to 6 months after treatment.
In addition, this study aimed to determine the most appropriate methods for evaluating the effects of gene therapy in the planned clinical trial. BCVA is the most important and suitable primary outcome indicator to monitor the effect of treatment and can guide the interpretation of objective indicators (VF, OCT, VEP, ERG). It is important to understand the relationships between these indicators and the BCVA and the stability and sensitivity of the objective indicators compared with BCVA.
Our results show that changes in the nerve fiber layer thickness were not correlated with the change in BCVA. Previous studies found that thickness of the nerve fiber layer was not correlated with the BCVA in LHON patients,[27,29,30] but the thickness of the optic nerve fiber layer can be used as a measure of the damage to the optic nerve fiber layer to determine the safety of the treatment in clinical trials.
In LHON patients, there were correlations among the changes in the VFI, MD, and BCVA. Along with the change in the BCVA, these parameters are good indicators of the effectiveness of the treatment, and they can be used to evaluate the effect on the basis of the BCVA. Ran et al[31] showed that the visual field defects might provide a clinical basis for better diagnosis of LHON. In the VEP test, the changes in the P100 wave were also correlated with changes in the BCVA, and the VEP itself as a method for detecting subclinical damage to the optic nerve. The VEP be used to evaluate the efficacy and safety of gene therapy in future clinical trials.
ERG detection requires direct contact with the cornea, and some young patients had difficulty with this test. Of the 32 eyes (16 patients), complete ERG test data were obtained from 10 eyes (5 patients). Therefore, it may be difficult to collect enough data based on this measure in future clinical trials, and as a result the ERG will not be included as a main parameter.
In the eyes with a relatively stable BCVA, there was no statistically significant difference between the optic nerve fiber layer thicknesses. The results also showed that the RNFL thickness can remain relatively stable over a long period of time. The RNFL can be used to evaluate the degree of optic nerve fiber layer damage and will be more important than the evaluation of effectiveness in future clinical trials. There were no significant differences in the PDS, VFI, or the P100 of VEP, but the statistically significant difference in the MD demonstrated that the MD was relatively independent of the other parameters.
We also focused on the eyes with improved BCVA. In addition to the PSD, the MD and VFI were increased in the visual field, and the sensitivity of the VFI and MD response to changes in the BCVA was higher than the other parameters. The VFI and MD can be used together with the BCVA to evaluate the effect of gene therapy. In the OCT test, there was no obvious change in the optic nerve fiber layer thickness, and even in LHON patients with improved BCVA, the nerve fiber layer thickness remained relatively stable. For the VEP, the P100 wave was reduced in 5 eyes, and the amplitude was decreased in 4 eyes, but there was no significant difference. These results showed that the PSD, optic nerve fiber layer thickness, and VEP in patients with LHON are not associated with the BCVA. The individual VEP and ERG examinations showed great fluctuation in amplitude, which could be used as a reference to evaluate changes in visual function. However, the current study was limited to patients who are expected to take part in our formal LHON gene therapy trial, and the small sample size prevents further in-depth discussion. To examine the effect of LHON gene therapy, we plan to conduct additional studies and clinical trials in the future.
The primary endpoint of many clinical trials conducted in ophthalmology is the BCVA.[10,28,32] Detection of the optic nerve fiber layer thickness via OCT will be one of the main evaluation indicators of the safety of this therapy in LHON patients. VFI, MD, and P100 determined from VEP detection will serve as an indicator of the effectiveness of the gene therapy due to their association with the BCVA. ERG will serve as a secondary observation index due to poor acceptance among adolescents.
There are several limitations to our research. Due to the limited number of participants undergoing the gene therapy trial in the initial screening, there were no matched groups for patient age, gender, or other factors. Additionally, because the selected patients were from different regions of mainland China, and due to objective economic factors, the observation period spanned a long time interval. Another limitation is that some uncertainty is unavoidable in evaluation of vision because vision is subjective. We defined 0.3 log MAR as the criterion to identify changes in vision. The main purpose of this study was to clinically observe volunteers who were included in an upcoming gene therapy trial in order to exclude patients with spontaneous recovery, and we also evaluated and compared the detection indexes. Thus, a control group was not included in this study. The limitations described above may have a certain impact on the clinical utility and universality of our results. Addressing these problems will require a more in-depth exploration of the combination of the preliminary results of the gene therapy trial and those of the multicenter clinical trial.
5. Conclusions
In summary, patients showing a tendency towards spontaneous improvement were excluded, and the remaining patients fulfilled the requirements for the subsequent study. After conducting gene therapy in LHON patients, it will be important to comprehensively evaluate the improvement of visual function combined with multiple detection parameters.
Acknowledgments
The authors thank the patients and their family members for accepting gene therapy and for participating in this research.
Footnotes
Abbreviations: BCVA = best corrected visual acuity, ERG = electroretinogram, ERG = electroretinography, LHON = Leber's hereditary optic neuropathy, log MAR = logarithm of the minimum angle of resolution, MD = mean defect, OCT = optical coherence tomography, PR-VEP = pattern-reversal visual evoked potential, PSD = pattern standard deviation, RNFL = retinal nerve fiber layer, VA = visual acuity, VFI = visual field index.
SY and HY contributed equally to the study.
Authorship: SY designed and conducted the experiments, analyzed the data, and prepared the manuscript. BL, the guarantor of this work, designed and conducted the experiments, assisted with discussion, and reviewed the manuscript. QSM, SSW, JMZ, and HY conducted the experiments.
Funding: The research was supported by the National Nature Science Foundation of China (Grants No. 81271015).
The authors have no conflicts of interest to disclose.
References
- 1.Wallace DC, Singh G, Lott MT, et al. Mitochondrial DNA mutation associated with Leber's hereditary optic neuropathy. Science 1988; 242:1427–1430. [DOI] [PubMed] [Google Scholar]
- 2.Mackey DA, Oostra RJ, Rosenberg T, et al. Primary pathogenic mtDNA mutations in multigeneration pedigrees with Leber hereditary optic neuropathy. Am J Hum Genet 1996; 59:481–485. [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Y, Guo XM, Jia XY, et al. Clinical features and the mutation of Leber's hereditary optic neuropathy in Chinese patients. Chin J Med Genet 2005; 22:334–336. [PubMed] [Google Scholar]
- 4.Lin HZ, Pang CY, Chen SP, et al. Vision improvement in a Taiwanese (Han Chinese) family with Leber's hereditary optic neuropathy. Kaohsiung J Med Sci 2012; 28:679–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Man PYW, Turnbull DM, Chinnery PF. Leber hereditary optic neuropathy. J Med Genet 2002; 39:162–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sadun AA, La Morgia C, Carelli V. Leber's hereditary optic neuropathy. Curr Treat Options Neurol 2011; 13:109–117. [DOI] [PubMed] [Google Scholar]
- 7.Hauswirth WW, Aleman TS, Kaushal S, et al. Treatment of Leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: short-term results of a phase I trial. Hum Gene Ther 2008; 19:979–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maguire AM, High KA, Auricchio A, et al. Age-dependent effects of RPE65 gene therapy for Leber's congenital amaurosis: a phase 1 dose-escalation trial. Lancet 2009; 374:1597–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simonelli F, Maguire AM, Testa F, et al. Gene therapy for Leber's congenital amaurosis is safe and effective through 1.5 years after vector administration. Mol Ther 2010; 18:643–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Testa F, Maguire AM, Rossi S, et al. Three-year follow-up after unilateral subretinal delivery of adeno-associated virus in patients with Leber congenital Amaurosis type 2. Ophthalmology 2013; 120:1283–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi H, Gao J, Pei H, et al. Adeno-associated virus-mediated gene delivery of the human ND4 complex I subunit in rabbit eyes. Clin Experiment Ophthalmol 2012; 40:888–894. [DOI] [PubMed] [Google Scholar]
- 12.Pei H, Wan X, Hu W, et al. Construction and detection of a novel type of recombinant human rAAV2/2-ND4. Eye Sci 2013; 28:55–59. [PubMed] [Google Scholar]
- 13.Du H, Wang GP, Pei H, et al. Constructing and detecting of rAAV2/2-ND4 in gene therapy of Leber hereditary optic neuropathy. Recent Adv Ophthalmol 2012; 32:201–203. [Google Scholar]
- 14.Yang S, He H, Zhu Y, et al. Chemical and material communication between the optic nerves in rats. Clin Experiment Ophthalmol 2015; 43:742–748. [DOI] [PubMed] [Google Scholar]
- 15.Riordan-Eva P, Sanders MD, Govan GG, et al. The clinical features of Leber's hereditary optic neuropathy defined by the presence of a pathogenic mitochondrial DNA mutation. Brain 1995; 118:319–337. [DOI] [PubMed] [Google Scholar]
- 16.Stone EM, Newman NJ, Miller NR, et al. Visual recovery in patients with Leber's hereditary optic neuropathy and the 11778 mutation. J Clin Neuroophthalmol 1992; 12:10–14. [PubMed] [Google Scholar]
- 17.Carelli V, La Morgia C, Valentino ML, et al. Idebenone treatment in Leber's hereditary optic neuropathy. Brain 2011; 134:e188–e1188. [DOI] [PubMed] [Google Scholar]
- 18.Mashima Y, Kigasawa K, Wakakura M, et al. Do idebenone and vitamin therapy shorten the time to achieve visual recovery in Leber hereditary optic neuropathy? J Neuroophthalmol 2000; 20:166–170. [DOI] [PubMed] [Google Scholar]
- 19.Rudolph G, Dimitriadis K, Büchner B, et al. Effects of idebenone on color vision in patients with Leber hereditary optic neuropathy. J Neuroophthalmol 2013; 33:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barnils N, Mesa E, Muñoz S, et al. Response to idebenone and multivitamin therapy in Leber's hereditary optic neuropathy. Arch Soc Esp Oftalmol 2007; 82:377–380. [DOI] [PubMed] [Google Scholar]
- 21.Iyer S. Novel therapeutic approaches for Leber's hereditary optic neuropathy. Discov Med 2013; 15:141–149. [PMC free article] [PubMed] [Google Scholar]
- 22.Beck RW, Moke PS, Turpin AH, et al. A computerized method of visual acuity testing: adaptation of the early treatment of diabetic retinopathy study testing protocol. Am J Ophthalmol 2003; 135:194–205. [DOI] [PubMed] [Google Scholar]
- 23.Lin Z, Wu C, Chen X, et al. Repeatability of ETDRS visual acuity measurement in children. Eye Sci 2008; 24:48–52. [PubMed] [Google Scholar]
- 24.Manny RE, Hussein M, Gwiazda J, et al. Repeatability of ETDRS visual acuity in children. Invest Ophthalmol Vis Sci 2003; 44:3294–3300. [DOI] [PubMed] [Google Scholar]
- 25.Wan X, Pei H, Zhao MJ, et al. Efficacy and safety of rAAV2-ND4 treatment for Leber's hereditary optic neuropathy. Sci Rep 2016; 6:21587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang S, Ma S, Wan X, et al. Long-term outcomes of gene therapy for the treatment of Leber's hereditary optic neuropathy. EBioMedicine 2016; 10:258–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakamura M, Yamamoto M. Variable pattern of visual recovery of Leber's hereditary optic neuropathy. Br J Ophthalmol 2000; 84:534–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lam BL, Feuer WJ, Schiffman JC, et al. Trial end points and natural history in patients with G11778A Leber hereditary optic neuropathy: preparation for gene therapy clinical trial. JAMA Ophthalmol 2014; 132:428–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Z, Sun CB, Tong Y, et al. Measurement of retinal nerve fiber layer thickness in Leber hereditary optic neuropathy by optical coherence tomography. Chin J Ophthalmol 2012; 48:888–892. [PubMed] [Google Scholar]
- 30.Zhang Y, Huang H, Wei S, et al. Characterization of retinal nerve fiber layer thickness changes associated with Leber's hereditary optic neuropathy by optical coherence tomography. Exp Ther Med 2014; 7:483–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ran R, Yang S, He H, et al. A retrospective analysis of characteristics of visual field damage in patients with Leber's hereditary optic neuropathy. Springerplus 2016; 5:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Campochiaro PA, Heier JS, Feiner L, et al. Ranibizumab for macular edema following branch retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology 2010; 117:1102–1112. [DOI] [PubMed] [Google Scholar]


