Abstract
The Tat-responsive region (TAR) sequence is present at the 5' end of human immunodeficiency virus 1 mRNAs and as a cytoplasmic form of 58-66 nucleotides. TAR RNA blocks the activation and autophosphorylation of the double-stranded RNA-activated protein kinase in vitro. We show here that TAR RNA also prevents the double-stranded RNA-mediated inhibition of translation in a cell-free system. Mutagenic and structural analyses of TAR RNA indicate that a stem of at least 14 base pairs is required for this activity, whereas the loop and bulge required for transactivation by Tat are dispensable. Truncation of the RNA to 68 nucleotides results in the loss of translational rescue ability, suggesting that the short cytoplasmic TAR RNA produced by viral transcription in vivo may not have the capability to suppress activation of the kinase. However, because longer TAR transcripts stimulate expression in a transient assay in vivo, the TAR structure at the 5' end of viral mRNAs could still exert this function in cis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akusjärvi G., Svensson C., Nygård O. A mechanism by which adenovirus virus-associated RNAI controls translation in a transient expression assay. Mol Cell Biol. 1987 Jan;7(1):549–551. doi: 10.1128/mcb.7.1.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braddock M., Thorburn A. M., Chambers A., Elliott G. D., Anderson G. J., Kingsman A. J., Kingsman S. M. A nuclear translational block imposed by the HIV-1 U3 region is relieved by the Tat-TAR interaction. Cell. 1990 Sep 21;62(6):1123–1133. doi: 10.1016/0092-8674(90)90389-v. [DOI] [PubMed] [Google Scholar]
- Colvin R. A., Garcia-Blanco M. A. Unusual structure of the human immunodeficiency virus type 1 trans-activation response element. J Virol. 1992 Feb;66(2):930–935. doi: 10.1128/jvi.66.2.930-935.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen B. R. Regulation of HIV-1 gene expression. FASEB J. 1991 Jul;5(10):2361–2368. doi: 10.1096/fasebj.5.10.1712325. [DOI] [PubMed] [Google Scholar]
- Davies M. V., Furtado M., Hershey J. W., Thimmappaya B., Kaufman R. J. Complementation of adenovirus virus-associated RNA I gene deletion by expression of a mutant eukaryotic translation initiation factor. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9163–9167. doi: 10.1073/pnas.86.23.9163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Benedetti A., Baglioni C. Inhibition of mRNA binding to ribosomes by localized activation of dsRNA-dependent protein kinase. Nature. 1984 Sep 6;311(5981):79–81. doi: 10.1038/311079a0. [DOI] [PubMed] [Google Scholar]
- Edery I., Petryshyn R., Sonenberg N. Activation of double-stranded RNA-dependent kinase (dsl) by the TAR region of HIV-1 mRNA: a novel translational control mechanism. Cell. 1989 Jan 27;56(2):303–312. doi: 10.1016/0092-8674(89)90904-5. [DOI] [PubMed] [Google Scholar]
- Feinberg M. B., Baltimore D., Frankel A. D. The role of Tat in the human immunodeficiency virus life cycle indicates a primary effect on transcriptional elongation. Proc Natl Acad Sci U S A. 1991 May 1;88(9):4045–4049. doi: 10.1073/pnas.88.9.4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., Smiley J., Russell W. C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977 Jul;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Gunnery S., Rice A. P., Robertson H. D., Mathews M. B. Tat-responsive region RNA of human immunodeficiency virus 1 can prevent activation of the double-stranded-RNA-activated protein kinase. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8687–8691. doi: 10.1073/pnas.87.22.8687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann C. H., Dery C. V., Mathews M. B. Transactivation of host and viral genes by the adenovirus E1B 19K tumor antigen. Oncogene. 1987;2(1):25–35. [PubMed] [Google Scholar]
- Herrmann C. H., Mathews M. B. The adenovirus E1B 19-kilodalton protein stimulates gene expression by increasing DNA levels. Mol Cell Biol. 1989 Dec;9(12):5412–5423. doi: 10.1128/mcb.9.12.5412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao S. Y., Calman A. F., Luciw P. A., Peterlin B. M. Anti-termination of transcription within the long terminal repeat of HIV-1 by tat gene product. Nature. 1987 Dec 3;330(6147):489–493. doi: 10.1038/330489a0. [DOI] [PubMed] [Google Scholar]
- Kaufman R. J., Murtha P. Translational control mediated by eucaryotic initiation factor-2 is restricted to specific mRNAs in transfected cells. Mol Cell Biol. 1987 Apr;7(4):1568–1571. doi: 10.1128/mcb.7.4.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler M., Mathews M. B. Premature termination and processing of human immunodeficiency virus type 1-promoted transcripts. J Virol. 1992 Jul;66(7):4488–4496. doi: 10.1128/jvi.66.7.4488-4496.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler M., Mathews M. B. Tat transactivation of the human immunodeficiency virus type 1 promoter is influenced by basal promoter activity and the simian virus 40 origin of DNA replication. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10018–10022. doi: 10.1073/pnas.88.22.10018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laspia M. F., Rice A. P., Mathews M. B. HIV-1 Tat protein increases transcriptional initiation and stabilizes elongation. Cell. 1989 Oct 20;59(2):283–292. doi: 10.1016/0092-8674(89)90290-0. [DOI] [PubMed] [Google Scholar]
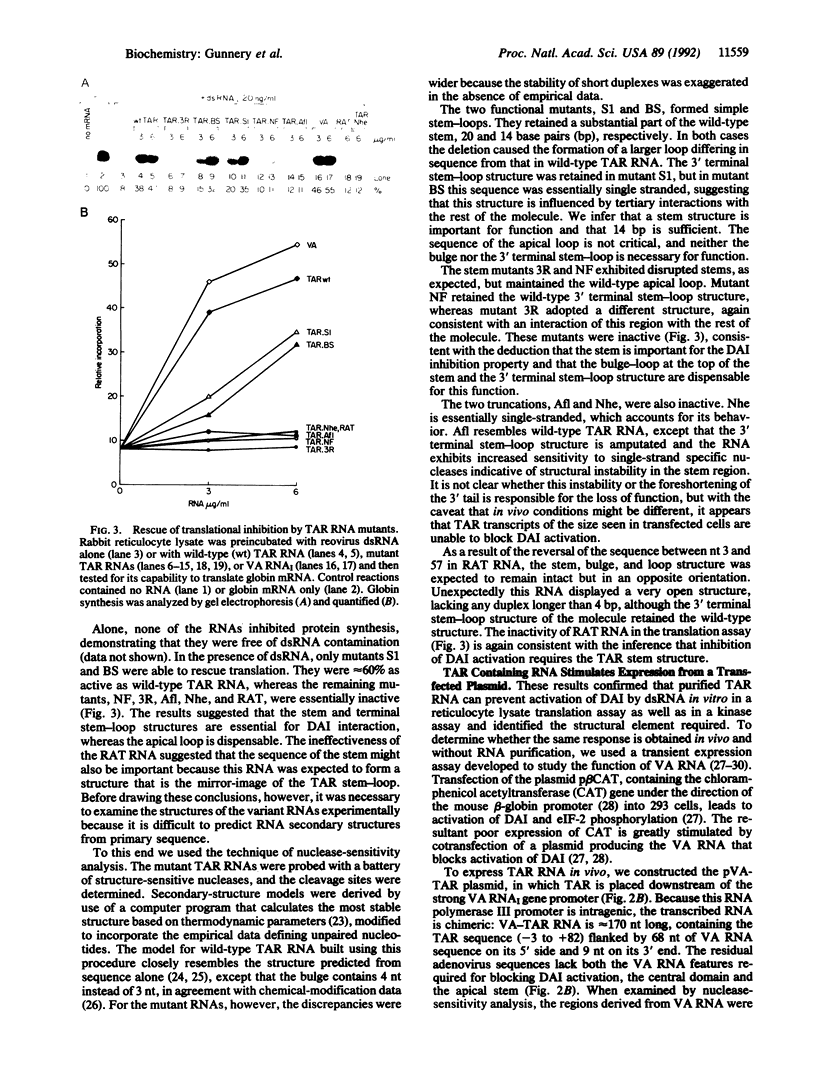
- Manche L., Green S. R., Schmedt C., Mathews M. B. Interactions between double-stranded RNA regulators and the protein kinase DAI. Mol Cell Biol. 1992 Nov;12(11):5238–5248. doi: 10.1128/mcb.12.11.5238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews M. B., Shenk T. Adenovirus virus-associated RNA and translation control. J Virol. 1991 Nov;65(11):5657–5662. doi: 10.1128/jvi.65.11.5657-5662.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellits K. H., Mathews M. B. Effects of mutations in stem and loop regions on the structure and function of adenovirus VA RNAI. EMBO J. 1988 Sep;7(9):2849–2859. doi: 10.1002/j.1460-2075.1988.tb03141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellits K. H., Pe'ery T., Manche L., Robertson H. D., Mathews M. B. Removal of double-stranded contaminants from RNA transcripts: synthesis of adenovirus VA RNAI from a T7 vector. Nucleic Acids Res. 1990 Sep 25;18(18):5401–5406. doi: 10.1093/nar/18.18.5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minks M. A., West D. K., Benvin S., Baglioni C. Structural requirements of double-stranded RNA for the activation of 2',5'-oligo(A) polymerase and protein kinase of interferon-treated HeLa cells. J Biol Chem. 1979 Oct 25;254(20):10180–10183. [PubMed] [Google Scholar]
- Muesing M. A., Smith D. H., Capon D. J. Regulation of mRNA accumulation by a human immunodeficiency virus trans-activator protein. Cell. 1987 Feb 27;48(4):691–701. doi: 10.1016/0092-8674(87)90247-9. [DOI] [PubMed] [Google Scholar]
- O'Malley R. P., Duncan R. F., Hershey J. W., Mathews M. B. Modification of protein synthesis initiation factors and the shut-off of host protein synthesis in adenovirus-infected cells. Virology. 1989 Jan;168(1):112–118. doi: 10.1016/0042-6822(89)90409-1. [DOI] [PubMed] [Google Scholar]
- Okamoto T., Wong-Staal F. Demonstration of virus-specific transcriptional activator(s) in cells infected with HTLV-III by an in vitro cell-free system. Cell. 1986 Oct 10;47(1):29–35. doi: 10.1016/0092-8674(86)90363-6. [DOI] [PubMed] [Google Scholar]
- Parkin N. T., Cohen E. A., Darveau A., Rosen C., Haseltine W., Sonenberg N. Mutational analysis of the 5' non-coding region of human immunodeficiency virus type 1: effects of secondary structure on translation. EMBO J. 1988 Sep;7(9):2831–2837. doi: 10.1002/j.1460-2075.1988.tb03139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlakis G. N., Felber B. K. Regulation of expression of human immunodeficiency virus. New Biol. 1990 Jan;2(1):20–31. [PubMed] [Google Scholar]
- Ratnasabapathy R., Sheldon M., Johal L., Hernandez N. The HIV-1 long terminal repeat contains an unusual element that induces the synthesis of short RNAs from various mRNA and snRNA promoters. Genes Dev. 1990 Dec;4(12A):2061–2074. doi: 10.1101/gad.4.12a.2061. [DOI] [PubMed] [Google Scholar]
- Roy S., Agy M., Hovanessian A. G., Sonenberg N., Katze M. G. The integrity of the stem structure of human immunodeficiency virus type 1 Tat-responsive sequence of RNA is required for interaction with the interferon-induced 68,000-Mr protein kinase. J Virol. 1991 Feb;65(2):632–640. doi: 10.1128/jvi.65.2.632-640.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
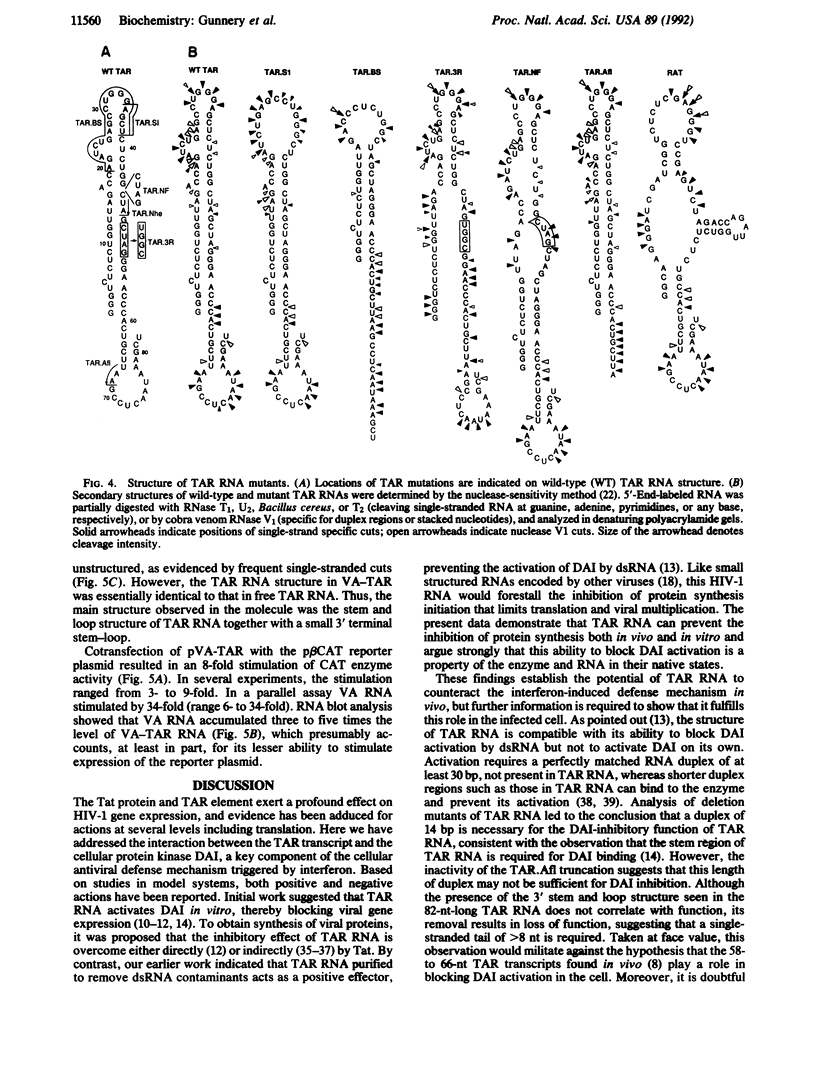
- Roy S., Katze M. G., Parkin N. T., Edery I., Hovanessian A. G., Sonenberg N. Control of the interferon-induced 68-kilodalton protein kinase by the HIV-1 tat gene product. Science. 1990 Mar 9;247(4947):1216–1219. doi: 10.1126/science.2180064. [DOI] [PubMed] [Google Scholar]
- Samuel C. E. Antiviral actions of interferon. Interferon-regulated cellular proteins and their surprisingly selective antiviral activities. Virology. 1991 Jul;183(1):1–11. doi: 10.1016/0042-6822(91)90112-o. [DOI] [PubMed] [Google Scholar]
- SenGupta D. N., Berkhout B., Gatignol A., Zhou A. M., Silverman R. H. Direct evidence for translational regulation by leader RNA and Tat protein of human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7492–7496. doi: 10.1073/pnas.87.19.7492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SenGupta D. N., Silverman R. H. Activation of interferon-regulated, dsRNA-dependent enzymes by human immunodeficiency virus-1 leader RNA. Nucleic Acids Res. 1989 Feb 11;17(3):969–978. doi: 10.1093/nar/17.3.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharmeen L., Bass B., Sonenberg N., Weintraub H., Groudine M. Tat-dependent adenosine-to-inosine modification of wild-type transactivation response RNA. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):8096–8100. doi: 10.1073/pnas.88.18.8096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenberg N. Measures and countermeasures in the modulation of initiation factor activities by viruses. New Biol. 1990 May;2(5):402–409. [PubMed] [Google Scholar]
- Svensson C., Akusjärvi G. A novel effect of adenovirus VA RNA1 on cytoplasmic mRNA abundance. Virology. 1990 Feb;174(2):613–617. doi: 10.1016/0042-6822(90)90116-9. [DOI] [PubMed] [Google Scholar]
- Svensson C., Akusjärvi G. Adenovirus VA RNAI mediates a translational stimulation which is not restricted to the viral mRNAs. EMBO J. 1985 Apr;4(4):957–964. doi: 10.1002/j.1460-2075.1985.tb03724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuker M., Stiegler P. Optimal computer folding of large RNA sequences using thermodynamics and auxiliary information. Nucleic Acids Res. 1981 Jan 10;9(1):133–148. doi: 10.1093/nar/9.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]