Abstract
Objective
To explore the “healthy user” and “healthy adherer” effects—hypothetical sources of bias thought to arise when patients who initiate and adhere to preventive therapies are more likely to engage in healthy behaviors than are other subjects.
Methods
The authors examined the association between statin initiation and adherence, and the subsequent use of preventive health services and incidence of clinical outcomes unlikely to be associated with the need for, or use of, a statin among older enrollees in two state-sponsored drug benefit programs.
Results
After adjustment for demographic and clinical covariates, patients who initiated statin use were more likely to receive recommended preventive services than noninitiators matched on age, sex, and state (hazard ratio [HR]: 1.10, 1.06–1.14 for males, HR: 1.09, 1.07–1.11 for females) and appeared to have a lower risk of a range of adverse outcomes (HR: 0.87, 0.85–0.89) thought to be unrelated to statin use. Adherence to a statin regimen was also associated with increased rates of preventive service use and a decreased rate of adverse clinical outcomes (HR: 0.93, 0.88–0.99).
Conclusions
These results suggest that patients initiating and adhering to chronic preventive drug therapies are more likely to engage in other health-promoting behaviors. Failure to account for this relationship may introduce bias in any epidemiologic study evaluating the effect of a preventive therapy on clinical outcomes.
Keywords: bias, confounding factors, epidemiologic methods, health behavior, pharmacoepidemiology
Introduction
The healthy user and healthy adherer effects have gained increasing attention as potential sources of bias in observational studies. Widely cited as a likely contributor to the divergence between observational and randomized controlled trial (RCT) evidence concerning the relationship between estrogen replacement therapy and cardiovascular mortality [1–4], the healthy user effect has also been raised as an explanation for the finding that elderly subjects receiving flu shots have lower mortality rates in the pre-flu season [5]. A close relative of the healthy user effect, the healthy adherer effect, has been implicated in the observation that patients adherent to placebo in RCTs have lower mortality rates than patients less adherent to placebo, which was documented in a recent meta-analysis of 21 RCTs [6]. These effects should be of interest and concern both to researchers conducting observational studies and to readers of such studies.
The healthy user effect, also known as “healthy user bias,” arises when healthier patients are more likely to initiate a preventive therapy, either through selective prescribing of preventive medications to healthier patients or through health-seeking patients being more likely to request and fill prescriptions for such medications [7–12]. The healthy adherer effect [6,13,14], also known as “adherence bias” [11], or “compliance bias” [1,3], occurs when such patients are more likely to remain adherent to a preventive therapy. These phenomena can lead to exaggerated or spurious protective effects of preventive therapies on adverse outcomes when general health status is not well measured and is associated with both initiation and/or adherence to the treatment of interest and the study outcome. Unfortunately, general health status and related constructs—frailty, cognitive function, and health-seeking tendencies—are often difficult to measure in many data sets used in observational research, resulting in the possibility of residual confounding.
Despite a growing awareness of the healthy user and healthy adherer effects, few studies to date have attempted to quantify these effects directly and assess their potential impact on observed rates of clinical events. In a recent study on the healthy adherer effect, we examined the association between adherence among new users of statins and the use of recommended preventive tests and services [15]. We found that patients who were adherent to statins received flu shots, pneumonia vaccinations, prostate specific antigen testing, mammograms, and fecal occult blood tests at significantly higher rates than nonadherent patients, suggesting that the former may be more health seeking. A second study documented an apparent protective effect of statin adherence on clinical outcomes that should be biologically unrelated to the clinical need for or use of a statin [16].
In the present study, we add to our existing work by exploring the healthy user effect. We hypothesize that initiation of a statin will be a marker for a healthier lifestyle, so that patients who initiate statins will have greater rates of preventive service use than age, sex-matched noninitiators, and lower rates of clinical outcomes that we expect to be associated with unhealthy lifestyle. Lastly, we assess the possibility of using receipt of preventive services in the year prior to statin initiation as a measure of health-seeking tendency. We anticipate that adjustment for receipt of these services will attenuate the association between statin use and the use of preventive health services and occurrence of adverse clinical outcomes during follow-up.
Methods
Data sources and study population
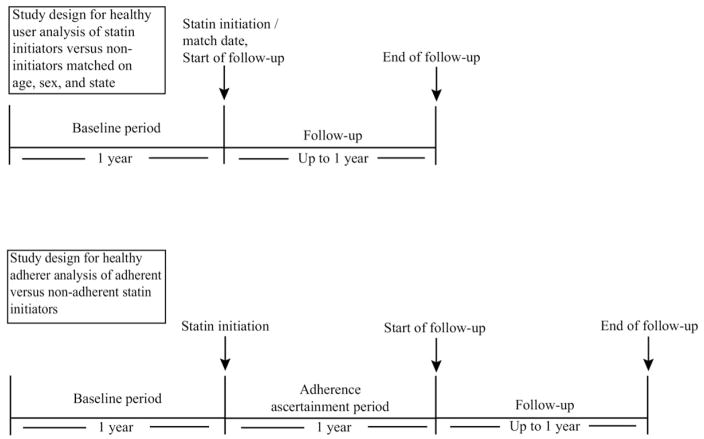
Our study cohort was drawn from a population of patients ≥65 years old, who were dually enrolled in Medicare and in the Pennsylvania Pharmaceutical Assistance Contract for the Elderly (PACE) or the New Jersey Pharmaceutical Assistance to the Aged and Disabled program (PAAD). PACE is a state-run pharmaceutical benefit program for households with incomes less than $17,200 USD, and covers all out-patient drugs with low patient co-payments of $6 to $9; PAAD provides similar benefits to New Jersey seniors with incomes up to $27,676. Our cohort of statin initiators consisted of PACE/Medicare and PAAD/Medicare enrollees who filled a statin prescription between 1997 and 2002 without having filled one in the prior 12 months. We excluded patients who started cerivastatin because the market withdrawal of cerivastatin in 2001 is likely to have influenced their statin adherence [17]. We limited our study to a primary prevention population by excluding all patients who had evidence of myocardial infarction, ischemic heart disease, coronary artery by-pass graft surgery, or angioplasty. We also excluded patients with diabetes and peripheral vascular disease, considered coronary artery disease equivalents. The primary prevention restriction was imposed in order to make the population more homogenous in its clinical need for a statin. For each statin initiator, a noninitiator matched on age, sex and state was selected from the pool of subjects who met study eligibility requirements and were not using a statin on that initiator’s start date.
Healthy adherer analysis
For our analysis of the association between statin adherence and the study outcomes, we further restricted the population to statin initiators who survived a 1-year adherence ascertainment period following their initiation date, and began follow-up at the end of this 1-year period. A 1-year adherence ascertainment period was selected in order to capture variability in adherence that might not be observed during a shorter period.
A schematic of the designs for the healthy user and healthy adherer analyses is given in Figure 1.
Fig. 1.
Schematic of study design for healthy user and healthy adherer analyses.
Exposure assessment
Healthy user analysis
In our analysis of the association between statin initiation and the study outcomes, we carried forward initial exposure status (initiator vs. noninitiator), with follow-up beginning on the initiation date for statin users and matched index date for nonusers.
Healthy adherer analysis
For our analysis of the association between adherence and the study outcomes, adherence was defined as refilling a statin prescription at least once during the adherence ascertainment period beginning on the index date for each subject and ending 1 year later (Fig. 1). Almost all prescriptions filled through the PACE and PAAD program are for a 30-day supply, so fully adherent subjects would be expected to fill 12 prescriptions. Subjects without a full 1-year adherence ascertainment period due to early death, loss of PACE or PAAD eligibility, or nursing home admission were omitted from the analysis. The adherence ascertainment period was shortened to 90 days in a sensitivity analysis. In a secondary analysis, we quantified adherence using the continuous measure proportion of days covered (PDC). The estimates from this analysis can be interpreted as the effect of full adherence versus complete nonadherence.
Covariates
We obtained baseline demographics, health services use, and health status information from Medicare and PACE/PAAD enrollment files and health care utilization during the year prior to the initiation of the statin prescription or to the index date for matched noninitiators. Covariates included age, sex, race, number of days spent in hospital, number of physician visits, and presence of specific diagnoses ascertained from inpatient and outpatient diagnosis codes.
Study outcomes
Outcomes studied were time to the occurrence of clinical outcomes and time to receipt of recommended preventive medical tests and services covered by Medicare. Outcomes were assessed in the year following the index date for matched initiators and noninitiators, and in the year following the adherence ascertainment period for the analyses of adherent versus non-adherent subjects. In consultation with clinicians, we selected clinical outcomes a priori to include those which are not known to be affected by the use of or clinical need for a statin, but which may be associated with an unhealthy lifestyle. These included outcomes sometimes associated with poor adherence to dietary recommendations (gout, diverticulitis, and gall stones), dehydration (kidney stones), alcohol consumption and smoking (peptic ulcers and gastrointestinal bleeds), poor personal hygiene (skin infections and dental problems such as gingivitis, periodontal diseases and diseases of the dental pulp), poor management of existing medical conditions (inpatient admissions for asthma or chronic obstructive pulmonary disease [COPD] among subjects with a previous asthma/COPD diagnosis), sun exposure (malignant melanoma), and careless or risky behavior (sexually transmitted diseases, fractures, open wounds, burns, poisoning, motor vehicle accidents, and falls). Outcomes defined by tests and preventive services received were also pre-specified and included bone mineral density (BMD) testing and screening mammography for women, prostate specific antigen (PSA) testing for men, and fecal occult blood tests, influenza vaccinations, and pneumococcal vaccinations for both sexes. PSA testing, fecal occult blood tests, influenza vaccinations, and pneumococcal vaccinations are provided at no cost to Medicare beneficiaries with traditional Part B coverage, while beneficiaries pay a 20% copayment for screening mammograms and BMD tests. Lastly, we studied myocardial infarction (MI) as an outcome likely to be related to statin use, and nursing home admission and death as outcomes related to frailty. Time to the first occurrence of each outcome was determined, with subjects censored by death, nursing home admission, loss of PACE or PAAD eligibility, or by the administrative end of follow-up (365 days after the start of follow-up).
We have previously reported results from an analysis of the association between adherence and preventive service use in PACE/Medicare enrollees [15]. Because our new analyses were conducted in a larger population including PAAD/Medicare enrollees, we repeated the original analyses on this population using the same adherence definition and methodology to provide comparable estimates.
Statistical analysis
The relationships between each outcome and statin initiation and adherence were examined using both an unadjusted and a multivariable adjusted Cox proportional hazards model. The first Cox model was stratified on 5-year age category, sex, and state. The second model was stratified on age, sex, and state and included the following covariates: Charlson comorbidity score [18], number of medications used, physician visits, days in hospital, days in nursing home during the baseline period, history during the baseline period of chronic obstructive pulmonary disease, obesity, stroke or transient ischemic attack, hypertension, congestive heart failure, atrial fibrillation, dementia, depression, liver disease, rheumatoid arthritis, osteoarthritis, hip fracture, and cancer. In a third analysis, we attempted to adjust for health-seeking tendency to see whether this attenuated our effect estimates. We treated preventive service use in the year prior to the index date as a proxy for health seeking, including indicators for use of each of the six preventive services in the model. Due to the anticipated small number of clinical outcomes and in the interest of obtaining a summary measure, we conducted an additional composite analysis in which we analyzed the total number of different clinical and service use event types for each patient during follow-up, using a Poisson regression model with follow-up time as an offset and controlling for the same covariates listed above. We estimated the over dispersion parameter using the Pearson method. In a secondary analysis, we used propensity score methods to adjust for differences between statin initiators and noninitiators and adherent and nonadherent subjects. These analyses are described in detail in the appendix. Data analyses were performed in SAS V9.0 (SAS Institute Inc., Cary, NC).
This study was approved by the Partners Healthcare Institutional Review Board and is covered under Data Use Agreements in place with the Centers for Medicare & Medicaid Services, PACE, and PAAD. All personal identifiers were removed from the analytic datasets.
Results
Study population
Healthy user analysis sample
A total of 98,400 patients initiated a statin and met system use criteria between 1996 and 2002. Excluding 54,819 patients with evidence of existing coronary artery disease, diabetes, or peripheral vascular disease and 138 lacking continuous Medicare Part B enrollment left a population of 43,443 patients, 38,585 of whom were matched by age, sex, and state to eligible nonusers. This population of statin initiators, described in column 1 of Table 1, was predominantly female (84%), had a mean age of 76, and had an average of nine physician visits during the 1-year baseline period. Osteoarthritis (18.3%), COPD (19.6%), and atrial fibrillation (8.1%) were common comorbid conditions. The matched statin noninitiators were more likely to have been hospitalized or in a nursing home in past year, were more likely to have asthma/COPD, a prior hip fracture, cancer, or rheumatoid arthritis, but were less likely to be obese. Noninitiators also had lower rates of all preventive services and physician visits, and took fewer different medications.
Table 1.
Characteristics of study subjects.
| Characteristic assessed during 1-year baseline period | Statin initiators and matched noninitiators included in healthy user analysis
|
Statin initiators surviving 1 year from initiation date included in healthy adherer analysis
|
||||
|---|---|---|---|---|---|---|
| Statin initiators | Noninitiators matched on age, sex, state | P value | Statin initiators— adherent | Statin initiators— nonadherent | P value | |
| Frequency (%)/mean | ||||||
| N | 38,585 | 38,585 | 26,165 | 3,510 | ||
| Pennsylvania residents | 46.61 | 46.61 | 1 | 48.05 | 39.37 | <0.0001 |
| Age (mean) | 76.05 | 76.06 | 0.862 | 75.74 | 76.24 | <0.0001 |
| Female | 84.23 | 84.23 | 1 | 84.88 | 85.36 | 0.46 |
| Race | <0.0001 | <0.0001 | ||||
| Black | 8.56 | 8.05 | 8.15 | 10.11 | ||
| White | 88.18 | 89.35 | 88.74 | 85.13 | ||
| Other | 3.26 | 2.60 | 3.11 | 4.76 | ||
| Number of physician visits (mean) | 8.55 | 8.04 | <0.0001 | 8.35 | 8.84 | <0.0001 |
| Number of medications used (mean) | 7.12 | 7.30 | <0.0001 | 7.14 | 7.38 | <0.0001 |
| Charlson comorbidity score (mean) | 1.12 | 1.14 | 0.1505 | 1.06 | 1.09 | 0.2036 |
| Acute care hospitalization | 11.80 | 13.53 | <0.0001 | 10.51 | 11.34 | 0.1362 |
| Nursing home stay | 1.35 | 2.04 | <0.0001 | 0.91 | 0.91 | 0.9724 |
| History of chronic obstructive pulmonary disease | 19.63 | 21.35 | <0.0001 | 18.73 | 20.74 | 0.0043 |
| History of stroke | 2.65 | 1.22 | <0.0001 | 2.20 | 1.79 | 0.1192 |
| History of dementia | 3.50 | 4.80 | <0.0001 | 2.68 | 2.45 | 0.4205 |
| History of depression | 7.97 | 8.54 | 0.0038 | 7.21 | 7.44 | 0.6306 |
| History of congestive heart failure | 5.91 | 5.70 | 0.2126 | 5.50 | 4.62 | 0.0294 |
| History of hip fracture | 0.85 | 1.37 | <0.0001 | 0.83 | 0.68 | 0.3786 |
| History of obesity | 3.07 | 2.65 | 0.0005 | 3.06 | 3.62 | 0.0751 |
| History of cancer | 1.13 | 2.35 | <0.0001 | 0.93 | 1.34 | 0.0203 |
| History of liver disease | 0.11 | 0.18 | 0.0046 | 0.10 | 0.20 | 0.0783 |
| History of osteoarthritis | 18.36 | 17.58 | 0.0052 | 18.28 | 18.80 | 0.4486 |
| History of rheumatoid arthritis | 1.73 | 2.52 | <0.0001 | 1.59 | 1.88 | 0.1949 |
| History of atrial fibrillation | 8.15 | 8.42 | 0.1785 | 7.43 | 6.78 | 0.1665 |
| History of hypertension | 69.63 | 58.38 | <0.0001 | 70.34 | 64.33 | <0.0001 |
| Preventive service use | ||||||
| Bone mineral density test* | 6.45 | 5.61 | <0.0001 | 5.73 | 5.56 | 0.67 |
| Fecal occult blood test | 12.95 | 10.77 | <0.0001 | 13.87 | 12.56 | 0.0353 |
| Influenza vaccination | 44.09 | 40.56 | <0.0001 | 44.29 | 36.75 | <0.0001 |
| Mammography* | 19.30 | 16.07 | <0.0001 | 18.98 | 15.30 | <0.0001 |
| Prostate specific antigen test* | 6.62 | 5.01 | <0.0001 | 6.33 | 6.84 | 0.24 |
| Pneumonia vaccination | 7.31 | 6.14 | <0.0001 | 7.40 | 6.61 | 0.093 |
Bone mineral density testing percent is among women ≥65 years old, mammography percent is among women, and prostate specific antigen percent is among men.
Healthy adherer analysis sample
For our analysis of the association between statin adherence and the study outcomes, we restricted our population of statin initiators to the 29,675 subjects who survived the 1-year adherence ascertainment period without leaving the PACE/PAAD program or entering a nursing home so that 1-year adherence patterns could be assessed. When adherence was defined as filling two or more statin prescriptions in the year following initiation, 88% of statin initiators were classified as adherent. The mean number of days covered during the year following initiation was 234 (standard deviation: 120; range: 7 to 365), yielding a mean proportion of days covered (PDC) of 64%. Compared to the 3,510 subjects who filled only one prescription, adherent subjects were less likely to have been hospitalized in the past year, had lower rates of COPD, had fewer physician visits and different medications in the prior year, and were more likely to have received all preventive services except for BMD testing in the prior year.
Preventive service use outcomes
Healthy user analysis
Of the 38,585 statin initiators, 39.1% received a flu shot, 9.4% received a fecal occult blood test, and 5.9% received a pneumonia vaccination in the 1-year follow-up period (Table 2). Among women, 19.2% received a mammogram, and 7.2% received a bone mineral density test. Among men, 23.6% received a PSA test. In an analysis adjusted for clinical and demographic covariates (model 2), statin initiators as compared to matched noninitiators had significantly higher rates of use for all of the tests studied excluding PSA testing which was borderline significant, resulting in a 9% higher overall rate of receiving preventive services (95% confidence interval [CI]: 7%–11%) among women and a 10% higher rate of preventive service use among men (95% CI: 6%–15%). Further adjustment for indicators of past preventive service use (model 3) reduced the overall preventive service use rate ratios to 1.05 (1.03–1.07) for women and 1.04 (0.99 –1.08) for men.
Table 2.
Analysis of healthy user effect: event counts, rates, and rate ratios in statin initiators vs. matched noninitiators.
| Statin initiators (N = 38,585)
|
Noninitiators matched on age, sex, and state (N = 38,585)
|
Rate ratio, statin initiators vs. matched noninitiators (95% CI)† | |||
|---|---|---|---|---|---|
| N events | Rate† | N events | Rate* | ||
| Myocardial infarction | 370 | 1.09 | 355 | 1.08 | 0.98 (0.84, 1.13) |
| Death | 601 | 1.77 | 1110 | 3.36 | 0.58 (0.52, 0.64) |
| Nursing home admission | 1236 | 3.63 | 1794 | 5.43 | 0.69 (0.64, 0.75) |
| Preventive service use outcomes | |||||
| Composite–males | 5072 | 96.49 | 4,300 | 85.52 | 1.10 (1.06, 1.14) |
| Composite–females | 26,582 | 92.44 | 23,402 | 83.55 | 1.09 (1.07, 1.11) |
| Bone mineral density test‡ | 2351 | 8.59 | 1236 | 3.63 | 1.17 (1.11, 1.25) |
| Prostate specific antigen test§ | 1437 | 36.42 | 1228 | 32.42 | 1.08 (1, 1.16) |
| Fecal occult blood test‡ | 3639 | 11.49 | 3291 | 10.72 | 1.06 (1.01, 1.11) |
| Mammography‡ | 6245 | 25.48 | 5199 | 21.75 | 1.15 (1.11, 1.19) |
| Influenza vaccination | 15,099 | 56.58 | 13,444 | 50.82 | 1.09 (1.06, 1.12) |
| Pneumonia vaccination | 2265 | 6.89 | 1915 | 5.96 | 1.15 (1.08, 1.23) |
| Clinical outcomes not known to be related to statin use | |||||
| Composite | 15,772 | 46.37 | 17,462 | 52.85 | 0.87 (0.85, 0.89) |
| Asthma/COPD hospitalization among subjects with asthma/COPD | 736 | 13.2 | 1141 | 19.96 | 0.70 (0.64, 0.77) |
| Burns | 139 | 0.41 | 117 | 0.35 | 1.16 (0.90, 1.49) |
| Dental problems | 92 | 0.27 | 107 | 0.32 | 0.86 (0.65, 1.14) |
| Diverticulitis | 2793 | 8.56 | 2757 | 8.71 | 0.99 (0.94, 1.05) |
| Falls | 1227 | 3.66 | 1567 | 4.83 | 0.78 (0.72, 0.84) |
| Food-borne bacterial illness | 359 | 1.06 | 389 | 1.18 | 0.90 (0.78, 1.05) |
| Fractures | 1774 | 5.34 | 2216 | 6.91 | 0.81 (0.76, 0.86) |
| Gall stones | 693 | 2.06 | 697 | 2.13 | 0.97 (0.87, 1.08) |
| Gout | 848 | 2.53 | 732 | 2.24 | 1.03 (0.94, 1.14) |
| Kidney stones | 424 | 1.25 | 409 | 1.25 | 0.99 (0.86, 1.14) |
| Malignant melanoma | 110 | 0.32 | 113 | 0.34 | 0.99 (0.76, 1.3) |
| Migraine | 311 | 0.92 | 302 | 0.92 | 1.00 (0.85, 1.17) |
| Motor vehicle accident | 103 | 0.3 | 73 | 0.22 | 1.41 (1.04, 1.91) |
| Open wound | 977 | 2.91 | 1186 | 3.65 | 0.81 (0.74, 0.88) |
| Peptic ulcer/gastrointestinal bleed | 2061 | 6.24 | 2201 | 6.87 | 0.91 (0.86, 0.97) |
| Poisoning | 272 | 0.8 | 281 | 0.85 | 0.97 (0.82, 1.15) |
| Sexually transmitted disease | 103 | 0.3 | 85 | 0.26 | 1.15 (0.86, 1.54) |
| Skin infections | 2358 | 7.19 | 2558 | 8.05 | 0.89 (0.85, 0.95) |
CI, confidence interval; COPD, chronic obstructive pulmonary disease.
Rate is per 100 person-years.
Rate ratios for individual outcomes were estimated from a Cox proportional hazards regression stratified on age, sex, and state of residence, and adjusted for race, Charlson comorbidity score, number of medications used, physician visits, days in hospital, and days in nursing home during the baseline period, and history during the baseline period of chronic obstructive pulmonary disease, obesity, stroke or transient ischemic attack, hypertension, congestive heart failure, atrial fibrillation, dementia, depression, liver disease, rheumatoid arthritis, osteoarthritis, hip fracture, and cancer (model 2). Rate ratios for composite outcomes were estimated using a Poisson regression adjusted for the same covariates.
Prostate specific antigen test, fecal occult blood test, and mammography analyses were restricted to subjects without a history of the cancer detected by the screening test. Bone mineral density testing analysis was restricted to women without a history of hip fracture.
Healthy adherer analysis
Consistent with our previously reported results [15], the 26,165 adherent subjects had higher rates of all tests than did the 3510 nonadherent subjects adjusting for clinical and demographic covariates (model 2, Table 3), yielding pooled rate ratios for preventive service use of 1.23 (95% CI: 1.17–1.28) among women and 1.15 (1.04–1.27) among men. Further adjustment for indicators of past preventive service use (model 3) attenuated these estimates to 1.14 (95% CI: 1.10–1.20) and 1.11 (1.01–1.23). Modeling the effect of adherence using PDC as a continuous outcome yielded more extreme estimates representing the effect of full adherence compared to complete nonadherence. The rate ratios for the composite preventive service use outcomes estimated from model 2 were 1.36 (95% CI: 1.30–1.42) among women and 1.25 (1.14–1.38) among men (Appendix table 1 at: doi:10.1016/j.jval.2010.10.033). These decreased to 1.22 (95% CI: 1.17–1.27) and 1.18 (1.07–1.29) after further adjustment for past preventive service use (model 3). Results from a sensitivity analysis in which the adherence ascertainment period was shortened to 90 days were attenuated slightly compared to those obtained using a 365-day adherence ascertainment period. (hazard ratio [HR] = 1.19 versus 1.23 among women, HR = 1.11 versus 1.15 among men, see appendix at: doi:10.1016/j.jval.2010.10.033).
Table 3.
Analysis of healthy adherer effect: event counts, rates, and rate ratios in adherent vs. nonadherent statin initiators.
| Adherent subjects (N =26,165)
|
Nonadherent subjects (N =3,510)
|
Rate ratio, adherent vs. nonadherent patients (95% CI)† | |||
|---|---|---|---|---|---|
| N events | Rate* | N events | Rate* | ||
| Myocardial Infarction | 221 | 0.96 | 39 | 1.27 | 0.74 (0.52, 1.04) |
| Death | 442 | 1.91 | 81 | 2.63 | 0.72 (0.57, 0.91) |
| Nursing home admission | 871 | 3.76 | 122 | 3.96 | 0.98 (0.81, 1.19) |
| Preventive service use outcomes | |||||
| Composite–males | 3269 | 96.04 | 371 | 84.66 | 1.15 (1.04, 1.27) |
| Composite–females | 19,216 | 97.24 | 2092 | 79.28 | 1.23 (1.17, 1.28) |
| Bone mineral density test‡ | 1646 | 8.75 | 177 | 6.96 | 1.35 (1.16, 1.58) |
| Prostate specific antigen test‡ | 962 | 37.54 | 115 | 35.2 | 1.13 (0.93, 1.37) |
| Fecal occult blood test‡ | 2521 | 11.7 | 283 | 9.81 | 1.18 (1.04, 1.33) |
| Mammography‡ | 4415 | 26.26 | 459 | 19.94 | 1.30 (1.18, 1.44) |
| Influenza vaccination | 10,339 | 57.29 | 1153 | 45.77 | 1.27 (1.19, 1.35) |
| Pneumonia vaccination | 1566 | 6.98 | 162 | 5.41 | 1.28 (1.09, 1.51) |
| Clinical outcomes not known to be related to statin use | |||||
| Composite | 11,139 | 48.08 | 1644 | 53.43 | 0.93 (0.87, 0.99) |
| Asthma/COPD hospitalization among subjects with asthma/COPD | 496 | 13.91 | 87 | 16.78 | 0.83 (0.66, 1.04) |
| Burns | 95 | 0.41 | 13 | 0.42 | 1.04 (0.58, 1.87) |
| Dental problems | 48 | 0.21 | 7 | 0.23 | 1.00 (0.45, 2.21) |
| Diverticulitis | 1974 | 8.91 | 291 | 9.95 | 0.95 (0.84, 1.08) |
| Falls | 944 | 4.14 | 129 | 4.27 | 0.97 (0.81, 1.17) |
| Food-borne bacterial illness | 266 | 1.15 | 53 | 1.74 | 0.69 (0.51, 0.92) |
| Fractures | 1242 | 5.49 | 182 | 6.09 | 0.93 (0.79, 1.09) |
| Gall stones | 466 | 2.03 | 81 | 2.66 | 0.78 (0.62, 0.99) |
| Gout | 603 | 2.64 | 67 | 2.21 | 1.16 (0.90, 1.49) |
| Kidney stones | 299 | 1.3 | 47 | 1.54 | 0.86 (0.63, 1.17) |
| Malignant melanoma | 66 | 0.29 | 4 | 0.13 | 2.22 (0.81, 6.11) |
| Migraine | 192 | 0.83 | 32 | 1.05 | 0.85 (0.59, 1.25) |
| Motor vehicle accident | 58 | 0.25 | 8 | 0.26 | 1.06 (0.50, 2.22) |
| Open wound | 695 | 3.04 | 96 | 3.16 | 0.97 (0.78, 1.2) |
| Peptic ulcer/gastrointestinal bleed | 1423 | 6.33 | 221 | 7.44 | 0.88 (0.77, 1.02) |
| Poisoning | 186 | 0.81 | 25 | 0.82 | 1.00 (0.66, 1.52) |
| Sexually transmitted disease | 65 | 0.28 | 14 | 0.46 | 0.73 (0.41, 1.31) |
| Skin infections | 1689 | 7.58 | 229 | 7.73 | 1.03 (0.89, 1.18) |
CI, confidence interval; COPD, chronic obstructive pulmonary disease.
Rate is per 100 person-years
Rate ratios for individual outcomes were estimated from a Cox proportional hazards regression stratified on age, sex, and state of residence, and adjusted for race, Charlson comorbidity score, number of medications used, physician visits, days in hospital, and days in nursing home during the baseline period, and history during the baseline period of chronic obstructive pulmonary disease, obesity, stroke or transient ischemic attack, hypertension, congestive heart failure, atrial fibrillation, dementia, depression, liver disease, rheumatoid arthritis, osteoarthritis, hip fracture, and cancer (model 2). Rate ratios for composite outcomes were estimated using a Poisson regression adjusted for the same covariates.
Prostate specific antigen test, fecal occult blood test, and mammography analyses were restricted to subjects without a history of the cancer detected by the screening test. Bone mineral density testing analysis was restricted to women without a history of hip fracture.
Clinical outcomes
Healthy user analysis
In the follow-up period, of the clinical outcomes studied, diverticulitis (7.2%), skin infections (6.1%), fractures (4.6%), and peptic ulcers and gastrointestinal bleeds (5.3%) were the most common clinical outcomes among statin initiators, as shown in Table 2. Compared to noninitiators, statin initiators had significantly lower rates of falls, fractures, open wounds, skin infections, peptic ulcer disease/gastrointestinal bleeds, and asthma/COPD hospitalizations among patients with a prior diagnosis of asthma or COPD. The rate ratio for clinical outcomes among statin initiators relative to noninitiators estimated from model 2 was 0.89 (95% CI: 0.86–0.91). Compared to noninitiators, statin initiators appeared to be protected against both death (HR: 0.58, 95% CI: 0.52– 0.64) and nursing home admission (HR: 0.69, 95% CI: 0.64–0.75). The rate of MI was not significantly different between these groups.
Healthy adherer analysis
As shown in Table 3, among statin initiators, adherent patients had significantly lower rates of food-borne bacterial illness and gall stones. Adherent patients had adverse clinical outcomes at a rate of 0.93 (95% CI: 0.87– 0.99) times that of nonadherent subjects as estimated from model 2. Modeling adherence as continuous PDC yielded a rate ratio of 0.89 (95% CI: 0.83– 0.95) for the composite clinical end point (see Appendix table 1 at: doi: 10.1016/j.jval.2010.10.033). Adjustment for past use of preventive services (model 3) did not attenuate either estimate. Shortening the adherence ascertainment period from 365 days to 90 days yielded results similar to those from the primary analysis (HR = 0.94, 0.89–0.98, see appendix at: doi:10.1016/j.jval.2010.10.033). The rate of MI in adherent subjects was 0.74 times that in nonadherent subjects (95% CI: 0.52–1.04). While adherence was associated with lower mortality rates (HR: 0.72, 95% CI: 0.57– 0.91), it had no apparent effect on nursing home admission (HR: 0.98, 0.81–1.19).
There were no meaningful differences between the results obtained within the individual states, New Jersey and Pennsylvania. Results adjusted for propensity score decile (appendix tables 2 and 3 at: doi:10.1016/j.jval.2010.10.033) were essentially identical to those presented in Tables 2 and 3.
Discussion
In patients without evidence of coronary artery disease, diabetes, or peripheral vascular disease, statin initiation and statin adherence were both associated with increased use of preventive services and an apparent protective effect against a range of clinical outcomes not known to be related to the need for or use of a statin, but possibly associated with an unhealthy lifestyle. Because statin initiation and adherence do not directly cause preventive service use or protect subjects against the clinical outcomes evaluated, the observed associations must be attributable to other factors. We think it likely that initiating and adhering to preventive treatment for an asymptomatic condition are markers of health-seeking tendencies and better general health status, such that these patients are more likely to engage in preventive service use and less likely to suffer outcomes potentially associated with an unhealthy lifestyle.
Adherent versus nonadherent subjects differed more in their preventive service use than did statin initiators versus noninitiators. One possible reason for this is that while patients play a role in statin initiation by deciding to fill an initial prescription, statin initiation is driven in large part by a physician’s decision to prescribe a statin based on cholesterol levels and cardiovascular risk factors. For this reason, statin initiation is a much weaker indicator of health-seeking behavior than is the subsequent decision to adhere to a statin. While statin initiators are likely to have greater cardiovascular disease risk, the higher rates of prior hip fracture, hospitalization, and nursing home admission among noninitiators suggest that noninitiators may be a physically frailer population, less likely to be prescribed a preventive therapy. Our observation that statin initiation was associated with a 42% reduction in the risk of mortality and a 31% reduction in the risk of nursing home admission is also consistent with this hypothesis. These findings are also consistent with past work which found a negative association between statin initiation and frailty markers, and a strong apparent protective effect of statin initiation on mortality [7,8].
Adjusting for prior use of preventive tests and services attenuated the association between statin use and the use of these services during follow-up, suggesting that past preventive services use may be a marker for health-seeking behavior. Thus, adjusting for past use of preventive services may be one way of reducing the association between preventive drug use and health-seeking behavior. However, even after adjustment for past service use, statin users still had higher rates of preventive service use during follow-up. Furthermore, adjusting for past service use did not attenuate the protective effects of statins on the clinical outcomes we studied. One possible explanation, supported by the residual association between statin use and preventive service use, is that prior use of BMD tests, flu shots, mammograms, PSA testing, and fecal occult blood tests are an incomplete measure of a patient’s overall health-seeking tendency. Furthermore, the tendency to seek formal medical care may be only one component of a healthy lifestyle, which in turn, may be only one possible contributor to a patient’s general health status.
The association between statin initiation and adherence and the majority of the clinical outcomes we studied tended toward a protective effect. However, this was not the case for malignant melanoma which occurred with a higher frequency in subjects who initiated and remained adherent to a statin. Malignant melanoma is likely subject to screening bias, such that patients who are more engaged in their own health are more likely to have cancerous lesions detected.
These results support the notion of healthy user and healthy adherer effects, but several alternative explanations should be considered. While our outcomes were selected by consensus among two clinicians, it is possible that future research will show that one or more of these outcomes is biologically affected by either statin use or by conditions such as cardiovascular disease or hypercholesterolemia related to the clinical need for a statin. For example, despite results of secondary analyses of RCT data [19], some clinicians believe statins do have a protective effect on hip fracture.
Our study suffers from several limitations. We defined adherence as refilling an initial statin prescription in order to be consistent with the definition used in our previous study [15], because refilling versus not refilling provides an extreme contrast, and because the effect of refilling versus not refilling is easily interpreted. While refilling is correlated with both the medication possession ratio (MPR), which is the adherence measure recommended by the International Society of Pharmaceutical Outcomes Research (ISPOR) Medication and Compliance Special Interest Group and with persistence, refilling is not a pure measure of either adherence, which has been defined by the ISPOR SIG as “the extent to which a patient acts in accordance with the prescribed interval, and dose of a dosing regimen” or persistence, which has been defined as “the duration of time from initiation to discontinuation of therapy” [20]. Our study is also limited in generalizability. We studied a predominantly female, frail, elderly population from two states and a single income bracket. Variability in health-seeking behavior and health status may be substantially different in younger, healthier, more affluent populations; a recent study among statin initiators in British Columbia found stronger apparent protective effects of adherence on outcomes such as accidents than those reported here [16]. In addition, the relationship between initiation, adherence, and health-seeking tendencies may vary by drug class and outcome; this association is likely stronger for medications used to treat asymptomatic conditions than symptomatic ones, and for outcomes with a potential behavioral component than those without.
It is possible that our sample selection process could have contributed to the observed associations between statin adherence and the outcomes of interest. In order for statin initiators to be selected into the primary analysis sample, they must have survived without a nursing home admission during the 1-year adherence ascertainment period. If meeting these selection criteria depends on variables such as health status, that are associated with increased use of preventive services and decreased risk of adverse clinical outcomes, and if adherence to statins also contributes to selection (e.g., by preventing mortality during the ascertainment period), then the study design itself may cause a spurious association known as “collider bias” between adherence and health status [21–23]. We tested the contribution of selection bias to our findings by shortening the adherence ascertainment period to 90 days in a sensitivity analysis. The results from this analysis were very similar to those from the primary analysis. Assuming that filling a statin two or more times versus only once is unlikely to affect 90-day survival, the results of our sensitivity analysis can be taken as evidence that our results were not completely attributable to conditioning on a collider.
Further research is needed to investigate other potential causes of the observed effects and ways of guarding against them in observational studies. Assuming adequate measures of health-seeking tendency and health status can be developed, statistical adjustment for such measures can reduce or ideally eliminate the healthy user and healthy adherer effects. For example, the apparent protective effect of flu shots on mortality prior to the flu season reported in a recent article was attenuated when the analysis was adjusted for measures of functional status [24]. Study design can also reduce the potential for healthy user and healthy adherer effects. Adherence bias can be reduced through the use of a new user design where exposure is assumed to continue through follow-up in an “intention-to-treat” approach as has been used in many recent studies of medications [25–29]. This design reduces the degree to which the treated group becomes enriched with adherent patients who may be healthier and more health seeking than patients in the comparator group. Healthy user bias can be minimized through the use of an active comparator group of subjects initiating a different therapy. Through this design, the exposed and comparator groups become similar in having initiated a drug and therefore in the behavioral and health status characteristics that may be associated with initiating a preventive therapy. While the choice of an appropriate comparator can be challenging, this design has been used recently in several studies [30,31]. One possible tool for detecting the presence of healthy user and healthy adherer bias is the use of control outcomes. For example, differences in rates of preventive service use or outcomes that should not be associated with exposure but might be influenced by health-seeking tendency can be assessed between treatment and comparator groups.
These findings suggest that differences in health-seeking behavior, as measured by differences in preventive service use, exist between initiators and noninitiators of statin treatment, as well as between adherent and nonadherent patients. The presence of these differences raises the possibility of healthy user and healthy adherer biases in studies of the association between initiation or adherence and adverse clinical outcomes. In fact, we find that both statin initiation and adherence are associated with reduced rates of clinical outcomes unlikely to be biologically related to either the need for or use of a statin. These findings—and the use of methods to diagnose and reduce the risk of confounding by healthy user and healthy adherer effects—should be considered by researchers conducting non-randomized studies of preventive therapies and by clinicians and policy-makers evaluating the findings from such studies.
Supplementary Material
Acknowledgments
Source of financial support: This study was funded by a grant from the National Institute on Aging (R01-AG018833-05). W.H.S. is supported by a career development award from the National Heart, Lung and Blood Institute (K23HL090505-01). S.M.C. is supported by a Canadian Institutes of Health Research Fellowship. M.A.B. is supported by a career development grant from the National Institute of Aging (AG-027400).
Footnotes
Supplemental material accompanying this article can be found in the online version as a hyperlink at doi:10.1016/j.jval.2010.10.033, or if hard copy of article, at www.valueinhealthjournal.com/issues (select volume, issue, and article).
The authors have no conflicts of interest to declare.
References
- 1.Petitti DB. Coronary heart disease and estrogen replacement therapy. Can compliance bias explain the results of observational studies? Ann Epidemiol. 1994;4:115–8. doi: 10.1016/1047-2797(94)90056-6. [DOI] [PubMed] [Google Scholar]
- 2.Barrett-Connor E, Grady D. Hormone replacement therapy, heart disease, and other considerations. Annu Rev Public Health. 1998;19:55–72. doi: 10.1146/annurev.publhealth.19.1.55. [DOI] [PubMed] [Google Scholar]
- 3.Rossouw JE. Debate: the potential role of estrogen in the prevention of heart disease in women after menopause. Curr Control Trials Cardiovasc Med. 2000;1:135–8. doi: 10.1186/cvm-1-3-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garbe E, Suissa S. Hormone replacement therapy and acute coronary outcomes: methodological issues between randomized and observational studies. Hum Reprod. 2004;19:8–13. doi: 10.1093/humrep/deh022. [DOI] [PubMed] [Google Scholar]
- 5.White HD. Adherence and outcomes: it’s more than taking the pills. Lancet. 2005;366:1989–91. doi: 10.1016/S0140-6736(05)67761-6. [DOI] [PubMed] [Google Scholar]
- 6.Simpson SH, Eurich DT, Majumdar SR, et al. A meta-analysis of the association between adherence to drug therapy and mortality. BMJ. 2006;333:15. doi: 10.1136/bmj.38875.675486.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glynn RJ, Knight EL, Levin R, et al. Paradoxical relations of drug treatment with mortality in older persons. Epidemiology. 2001;12:682–9. doi: 10.1097/00001648-200111000-00017. [DOI] [PubMed] [Google Scholar]
- 8.Glynn RJ, Schneeweiss S, Wang PS, et al. Selective prescribing led to overestimation of the benefits of lipid-lowering drugs. J Clin Epidemiol. 2006;59:819–28. doi: 10.1016/j.jclinepi.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 9.Haley RW, Dietschy JM. Is there a connection between the concentration of cholesterol circulating in plasma and the rate of neuritic plaque formation in Alzheimer disease? Arch Neurol. 2000;57:1410–2. doi: 10.1001/archneur.57.10.1410. [DOI] [PubMed] [Google Scholar]
- 10.Humphrey LL, Chan BK, Sox HC. Postmenopausal hormone replacement therapy and the primary prevention of cardiovascular disease. Ann Intern Med. 2002;137:273–84. doi: 10.7326/0003-4819-137-4-200208200-00012. [DOI] [PubMed] [Google Scholar]
- 11.Ray WA, Daugherty JR, Griffin MR. Lipid-lowering agents and the risk of hip fracture in a Medicaid population. Inj Prev. 2002;8:276–9. doi: 10.1136/ip.8.4.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Majumdar SR, McAlister FA, Eurich DT, et al. Statins and outcomes in patients admitted to hospital with community acquired pneumonia: population based prospective cohort study. BMJ. 2006;333:999. doi: 10.1136/bmj.38992.565972.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chewning B. The healthy adherer and the placebo effect. BMJ. 2006;333:18–9. doi: 10.1136/bmj.333.7557.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Avorn J. Review: good adherence (compared with poor adherence) to drug therapy is associated with a reduction in mortality. ACP J Club. 2006;145:80. [PubMed] [Google Scholar]
- 15.Brookhart MA, Patrick AR, Dormuth C, et al. Adherence to lipid-lowering therapy and the use of preventive health services: an investigation of the healthy user effect. Am J Epidemiol. 2007;166:348–54. doi: 10.1093/aje/kwm070. [DOI] [PubMed] [Google Scholar]
- 16.Dormuth CR, Patrick AR, Shrank WH, et al. Statin adherence and risk of accidents: a cautionary tale. Circulation. 2009;119:2051–7. doi: 10.1161/CIRCULATIONAHA.108.824151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mantel-Teeuwisse AK, Klungel OH, Egberts TC, et al. Failure to continue lipid-lowering drug use following the withdrawal of cerivastatin. Drug Saf. 2004;27:63–70. doi: 10.2165/00002018-200427010-00004. [DOI] [PubMed] [Google Scholar]
- 18.Charlson ME, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 19.Toh S, Hernandez-Diaz S. Statins and fracture risk. A systematic review. Pharmacoepidemiol Drug Saf. 2007;16:627–40. doi: 10.1002/pds.1363. [DOI] [PubMed] [Google Scholar]
- 20.Cramer JA, Roy A, Burrell A, et al. Medication compliance and persistence: terminology and definitions. Value Health. 2008;11:44–7. doi: 10.1111/j.1524-4733.2007.00213.x. [DOI] [PubMed] [Google Scholar]
- 21.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology. 1999;10:37–48. [PubMed] [Google Scholar]
- 22.Greenland S. Quantifying biases in causal models: classical confounding vs collider-stratification bias. Epidemiology. 2003;14:300–6. [PubMed] [Google Scholar]
- 23.Hernan MA, Hernandez-Diaz S, Robins JM. A structural approach to selection bias. Epidemiology. 2004;15:615–25. doi: 10.1097/01.ede.0000135174.63482.43. [DOI] [PubMed] [Google Scholar]
- 24.Jackson LA, Nelson JC, Benson P, et al. Functional status is a confounder of the association of influenza vaccine and risk of all cause mortality in seniors. Int J Epidemiol. 2006;35:345–52. doi: 10.1093/ije/dyi275. [DOI] [PubMed] [Google Scholar]
- 25.Wang PS, Schneeweiss S, Avorn J, et al. Risk of death in elderly users of conventional vs. atypical antipsychotic medications. N Engl J Med. 2005;353:2335–41. doi: 10.1056/NEJMoa052827. [DOI] [PubMed] [Google Scholar]
- 26.Brookhart MA, et al. Evaluating short-term drug effects using a physician-specific prescribing preference as an instrumental variable. Epidemiology. 2006;17:268–75. doi: 10.1097/01.ede.0000193606.58671.c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Setoguchi S, et al. Potential causes of higher mortality in elderly users of conventional and atypical antipsychotic medications. J Am Geriatr Soc. 2008;56:1644–50. doi: 10.1111/j.1532-5415.2008.01839.x. [DOI] [PubMed] [Google Scholar]
- 28.Cadarette SM, et al. Relative effectiveness of osteoporosis drugs for preventing nonvertebral fracture. Ann Intern Med. 2008;148:637–46. doi: 10.7326/0003-4819-148-9-200805060-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schneeweiss S, Setoguchi S, Brookhart A, et al. Risk of death associated with the use of conventional versus atypical antipsychotic drugs among elderly patients. CMAJ. 2007;176:627–32. doi: 10.1503/cmaj.061250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schneeweiss S, et al. Increasing levels of restriction in pharmacoepidemiologic database studies of elderly and comparison with randomized trial results. Med Care. 2007;45(10 Suppl 2):S131–42. doi: 10.1097/MLR.0b013e318070c08e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Solomon DH, Avorn J, Sturmer T, et al. Cardiovascular outcomes in new users of coxibs and nonsteroidal antiinflammatory drugs: high-risk subgroups and time course of risk. Arthritis Rheum. 2006;54:1378–89. doi: 10.1002/art.21887. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.