SUMMARY
Incubation of bovine sperm with ouabain, an endogenous cardiac glycoside that inhibits both the ubiquitous (ATP1A1) and testis-specific α4 (ATP1A4) isoforms of Na+/K+ATPase, induces tyrosine phosphorylation and capacitation. The objectives of this study were to investigate: (1) fertilizing ability of bovine sperm capacitated by incubating with ouabain; (2) involvement of ATP1A4 in this process; and (3) signaling mechanisms involved in the regulation of sperm capacitation induced by inhibition of Na+/K+ATPase activity. Fresh sperm capacitated by incubating with ouabain (inhibits both ATP1A1 and ATP1A4) or with anti-ATP1A4 immunoserum fertilized bovine oocytes in vitro. Capacitation was associated with relocalization of ATP1A4 from the entire sperm head to the post-acrosomal region. To investigate signaling mechanisms involved in oubain-induced regulation of sperm capacitation, sperm preparations were pre-incubated with inhibitors of specific signaling molecules, followed by incubation with ouabain. The phosphotyrosine content of sperm preparations was determined by immunoblotting, and capacitation status of these sperm preparations were evaluated through an acrosome reaction assay. We inferred that Na+/K+ATPase was involved in the regulation of tyrosine phosphorylation in sperm proteins through receptor tyrosine kinase, nonreceptor type protein kinase, and protein kinases A and C. In conclusion, inhibition of Na+/K+ATPase induced tyrosine phosphorylation and capacitation through multiple signal transduction pathways, imparting fertilizing ability in bovine sperm. To our knowledge, this is the first report documenting both the involvement of ATP1A4 in the regulation of bovine sperm capacitation and that fresh bovine sperm capacitated by the inhibition of Na+/K+ATPase can fertilize oocytes in vitro.
INTRODUCTION
Ejaculated sperm must reside in the female reproductive tract for a species-dependent interval to acquire fertilizing ability (Yanagimachi, 1994). During this interval, sperm undergo a series of structural and functional modifications, collectively known as capacitation, which enables them to achieve hyperactivated motility and to undergo the acrosome reaction following binding to the zona pellucida of oocytes (Yanagimachi, 1994; de Lamirande et al., 1997). Key molecular events leading to sperm capacitation include removal of decapacitation factors, removal of cholesterol from the sperm plasma membrane, increased bicarbonate uptake, activation of adenylyl cyclase and synthesis of cAMP, and tyrosine phosphorylation in a cohort of sperm proteins (Yanagimachi, 1994; Visconti et al., 1995; Galantino-Homer et al., 1997; Olds-Clarke, 2003; De Jonge, 2005; O’Flaherty et al., 2006; de Lamirande and O’Flaherty, 2008). Activation of several signaling pathways involving reactive oxygen species (ROS), protein kinase A (PKA), calcium, extracellular signal regulated kinase family of mitogen activated protein kinase pathway (MAPK), receptor tyrosine kinases (RTK), and protein kinase C (PKC), resulted in tyrosine phosphorylation and capacitation in sperm (Breitbart et al., 1992; Pawson, 1995; Galantino-Homer et al., 1997; Leclerc et al., 1997; Luconi et al., 2001; Thundathil et al., 2002; de Lamirande and Gagnon, 2002; Olds-Clarke, 2003; Urner and Sakkas, 2003; Naz and Rajesh, 2004; De Jonge, 2005; O’Flaherty et al., 2005, 2006; de Lamirande and O’Flaherty, 2008). However, the role of specific sperm membrane proteins involved in the regulation of sperm capacitation, is still under investigation.
It is well established that Na+/K+ATPase is a functionally active integral membrane protein composed of two subunits, α and β. There are four isoforms of the α subunit (α1, α2, α3, and α4), and three isoforms of the β subunit (β1, β2, and β3). Testicular tissue and germ cells contain α1, α4, β1, and β3 subunits (Blanco and Mercer, 1998). The α4 isoform (ATP1A4) is testis-specific and very sensitive to inhibition with ouabain (an endogenous cardiac glycoside; Blanco and Mercer, 1998). In somatic cells, ouabain treatment inhibited the activity of Na+/K+ATPase and induced signaling mechanisms, leading to cellular responses (namely an increase in intracellular calcium concentrations and generation of reactive oxygen species) similar to those associated with mammalian sperm capacitation (Kometiani et al., 1998; Woo et al., 2002; Xie and Askari, 2002; Liu et al., 2003). Therefore, Na+/K+ATPase may be involved in the regulation of sperm capacitation. Consistent with this hypothesis, inhibition of Na+/K+ATPase with ouabain induced tyrosine phosphorylation and capacitation in bovine sperm (Thundathil et al., 2006) and ATP1A4 was present in bovine sperm (Newton et al., 2009). However, the fertilizing ability of sperm capacitated by incubating with ouabain and involvement of ATP1A4 in this process, capacitation-associated changes in the distribution of this protein, and signaling mechanisms involved in the regulation of sperm capacitation induced by the inhibition of Na+/K+ATPase remain unknown; these were the objectives of the present study.
RESULTS
Incubation of Sperm With Ouabain or Anti-ATP1A4 Immunoserum Capacitated Fresh Bovine Sperm
The objective was to determine the ability of sperm incubated with ouabain or anti-ATP1A4 to bind with and fertilize oocytes, as these characteristics are hallmarks of sperm capacitation. Total motility of sperm was not significantly affected by incubation with ouabain, anti-ATP1A4 immunoserum, anti-ATP1A4 immunoserum pre-absorbed to its blocking peptide, or preimmune serum. In a sperm–oocyte binding assay, the mean number of sperm bound to the zona pellucida was higher for sperm incubated with ouabain or anti-ATP1A4 (26.95 μg/ml) and heparin (10 μg/ml) compared to the control groups (P <0.05; Table 1, Fig. 1A–C). Percentage of fertilized oocytes (demonstrating 2 pronuclei) was higher for sperm incubated with ouabain, anti-ATP1A4 immunoserum, or heparin compared to negative controls (P <0.05; Table 2, Fig. 1D,E). Consistent with these results, the percentage of oocytes that underwent cleavage was higher for sperm incubated with ouabain, anti-ATP1A4 immunoserum, or heparin, compared to negative controls (P <0.05; Table 3). However, rates of fertilization and cleavage for oocytes co-incubated with sperm capacitated in anti-ATP1A4 immunoserum were lower (P <0.05) than those with ouabain or heparin.
TABLE 1.
Fresh Bovine Sperm Capacitated by Incubating With Ouabain or Anti-ATP1A4 Bound to the Zona Pellucida of Bovine Oocytes In Vitro
| Group | No. of oocytes | Mean no. of sperm bound to the zona pellucida |
|---|---|---|
| Negative | 50 | 21.1 ± 8.6a |
| Heparin | 50 | 70.9 ± 16.7b |
| Ouabain | 53 | 71.5 ± 14.4b |
| Anti-ATP1A4 | 52 | 61.7.5 ± 14.9b |
| Pre-absorbed | 52 | 19.3.0 ± 9.1a |
| Pre-immune | 51 | 16.1 ± 9.4a |
Sperm preparations were incubated with either Sp-TALP alone (negative), heparin (positive control for capacitation), ouabain, anti-ATP1A4, anti-ATP1A4 pre-absorbed to its blocking peptide or pre-immune serum for 4 hr. After incubation, sperm were washed in SP-TALP, resuspended in final fertilization medium, and used for inseminating in vitro-matured bovine oocytes.
Values without a common superscript (a–b) differed (P <0.05).
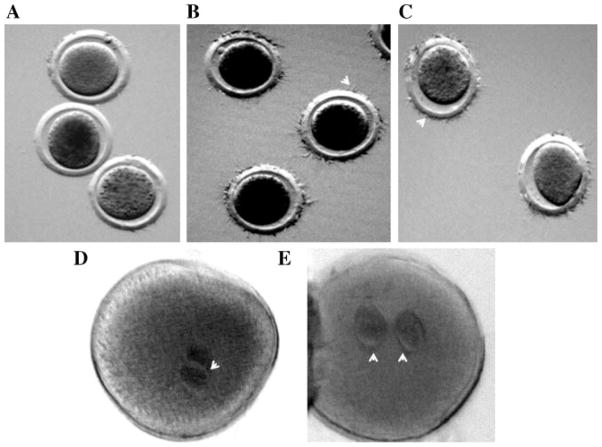
Figure 1.
Incubation of sperm with ouabain or anti-ATP1A4 immunoserum-capacitated fresh bovine sperm. In vitro matured cumulus dissociated bovine oocytes were coincubated with fresh bovine sperm capacitated by incubating under various conditions (A: Sp-TALP alone; B: ouabain; C: anti-ATP1A4) for 6 hr. The oocytes were removed from the fertilization medium, washed 7 times by pipetting to remove loosely attached spermatozoa, and then mounted on a poly-L-lysine coated microscopic glass slide. The oocytes were fixed in a solution of glacial acetic acid and absolute ethyl alcohol (1:3 ratio) for 24–36 hr and stained by placing in small drops of aceto-orcein. The number of sperm bound to the zona pellucida (B,C: arrow heads indicate sperm bound to the zona pellucida) was counted by phase-contrast microspcopy. This experiment was done in three replicates, using ejaculates from three bulls. To determine the ability of sperm capacitated under above conditions to penetrate oocytes and development into pronuclei (D,E: arrow heads indicate pronuclei), in vitro matured COC were fertilized with sperm incubated in different capacitating conditions (D: anti-ATP1A4; E: ouabain). After 6 hr of sperm–oocyte coincubation, COC were cumulus-dissociated and cultured for an additional 12 hr in SOF medium. Presumptive zygotes were fixed, stained and examined microscopically for pronuclei formation at 400× magnification. This experiment was done in three replicates, using semen from different bulls.
TABLE 2.
Fresh Bovine Sperm Capacitated by Incubating With Ouabain or Anti-ATP1A4 Developed Into Pronuclei Following Penetration of the Zona Pellucida of Bovine Oocytes In Vitro
| Group | No. of oocytes | Percentage of oocytes with two pronuclei (fertilization rate) |
|---|---|---|
| Negative | 51 | 23.5.0a |
| Heparin | 58 | 70.7.6b |
| Ouabain | 57 | 71.9b |
| Anti-ATP1A4 | 58 | 58.6c |
| Pre-absorbed | 51 | 21.5a |
| Pre-immune | 51 | 19.6a |
Sperm preparations were incubated with either Sp-TALP alone (negative), heparin (positive control for capacitation), ouabain, anti-ATP1A4, anti-ATP1A4 pre-absorbed to its blocking peptide or pre-immune serum for 4 hr. After incubation, sperm were washed in Sp-TALPH and resuspended in final fertilization medium, and used for inseminating in vitro-matured bovine oocytes. After 6 hr of sperm–oocyte coincubation, presumptive zygotes were cumulus-dissociated and cultured in SOF for an additional 12 hr for pronuclei development. These oocytes were fixed in acetic acid alcohol (1:3) for 24 hr and stained with 1% Orcein in glacial acetic acid and examined microscopically (400×; phase 2) for the demonstration of pronuclei.
Values without a common superscript (a–c) differed (P <0.05).
TABLE 3.
Fresh Bovine Sperm Capacitated by Incubating With Ouabain or Anti-ATP1A4 Initiated Cleavage of Bovine Oocytes In Vitro
| Group | No. of oocytes | Cleaved (%) |
|---|---|---|
| Negative | 153 | 13.0 ± 1.1a |
| Heparin | 132 | 79.5 ± 4.0b |
| Ouabain | 141 | 74.4 ± 3.3b |
| Anti-ATP1A4 | 167 | 53.8 ± 3.8c |
| Pre-absorbed | 157 | 19.1 ± 1.2a |
| Pre-immune | 149 | 12.0 ± 0.9a |
Sperm preparations were incubated with either Sp-TALP alone (negative), heparin (positive control for capacitation), ouabain, anti-ATP1A4, anti-ATP1A4 pre-absorbed to its blocking peptide or pre-immune serum for 4 hr. After incubation, sperm were washed in Sp-TALP and resuspended in final fertilization medium and used for inseminating in vitro-matured bovine oocytes.
Values without a common superscript (a–c) differed (P <0.05).
Effect of Anti-ATP1A4 Immunoserum on Sperm Capacitation
Tyrosine phosphoprotein content of proteins (50, 80, 110, 150, 200, and 260 kDa) in sperm preparations incubated with anti-ATP1A4 were similar to that of ouabain and heparin, but higher than that of the controls (Fig. 2A). The proportion of sperm undergoing an acrosome reaction in response to lysophosphatidylcholine (Fig. 2C) was consistent with the results of immunoblotting. Since incubation of bovine sperm with anti-ATP1A4 induced sperm capacitation as described above, an immunocytochemistry-based approach was used to confirm binding of anti-ATP1A4 to sperm during incubation. In these studies, incubation of sperm with anti-ATP1A4 caused binding of this antibody to sperm, as evidenced by fluorescence from sperm after 4 hr of incubation (Fig. 2D–G).
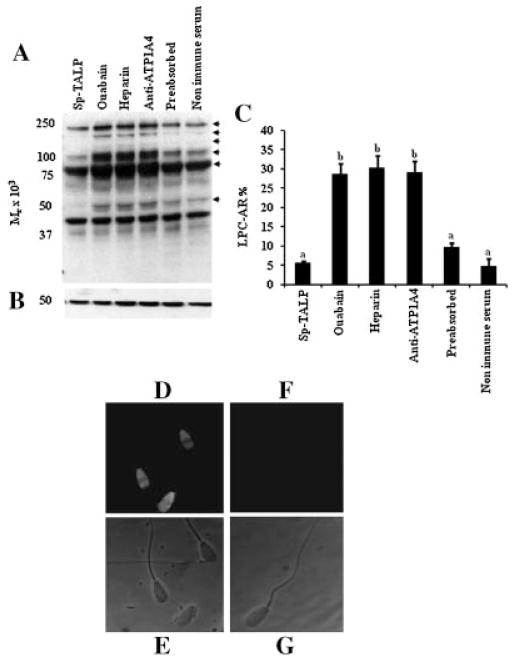
Figure 2.
Incubation of bovine sperm with anti-ATP1A4 immunoserum induced tyrosine phosphorylation and capacitation. Sperm preparations were incubated in Sp-TALP alone or with ouabain, heparin, anti-ATP1A4, pre-immune serum, or ATP1A4 preabsorbed to its blocking peptide. A: After 4 hr of incubation, these preparations were evaluated for the induction of tyrosine phosphorylation in sperm proteins by immunoblotting. Arrows at the right of the immunoblot identify proteins exhibiting a change in the content of tyrosine phosphorylation; the results are representative of three experiments, using sperm preparations from three bulls. B: Membranes were stripped and reprobed with anti-β tubulin to ensure that amount of proteins loaded was equal among wells. C: Parallel sperm preparations were evaluated for the induction of acrosome reaction in response to LPC (LPC-AR). The values are presented as the mean ± SD (n = 3; a,bP <0.05). To confirm the ability of anti-ATP1A4 to bind with sperm, suspensions of live sperm incubated with anti-ATP1A4 immunoserum or preimmune serum for 4 hr were processed for immunocytochemistry, as described in the Materials and Methods Section, except that these sperm preparations were not incubated with primary antibody (anti-ATP1A4) during the immunocytochemstry procedure. Fluorescent and phase contrast view of sperm incubated with anti-ATP1A4 (D and E, respectively) and preimmune serum (F and G, respectively) were shown.
Capacitation-Associated Changes in the Distribution of ATP1A4 in Bovine Sperm
Since the above studies provided clear evidence that ATP1A4 was involved in the regulation of sperm capacitation, we further investigated the possible involvement of this protein during capacitation induced by a physiological capacitating agent (heparin) by evaluating capacitation-associated redistribution of this protein. Immunolocalization of ATP1A4 in sperm during capacitation with heparin demonstrated gradual loss of immunofluorescence from the acrosomal region, with retention of fluorescence at the postacrosomal region, suggesting that this protein was relocated to the postacrosomal region during capacitation (Fig. 3A). This result could not be explained by a loss of the acrosome, since fluorescein isothiocyanate-conjugated Pisum sativum agglutinin (FITC-PSA), which is specific for the acrosome, similarly stained sperm at all times. Sperm with a complete loss of ATP1A4 from the acrosomal region had intact acrosomes (Fig. 3B). Furthermore, ≤8% of sperm underwent spontaneous acrosomal exocytosis at the end of 4 hr of capacitation (results not shown). Similarly, incubation of sperm with ouabain for 4 hr resulted in a complete loss of fluorescence from the acrosomal region (Fig. 3C), whereas these sperm retained their acrosome, as evidenced by the intense fluorescence of FITC-PSA from the acrosomal region (Fig. 3D). However, immunolocalization of ATP1A4 in sperm incubated with Sp-TALP alone (negative control) demonstrated fluorescence throughout the sperm head, as observed at 0 hr of incubation with ouabain or heparin. Sperm preparations incubated with pre-immune serum (Fig. 3E,F) or secondary antibody alone (Fig. 3G,H), did not elicit fluorescence.
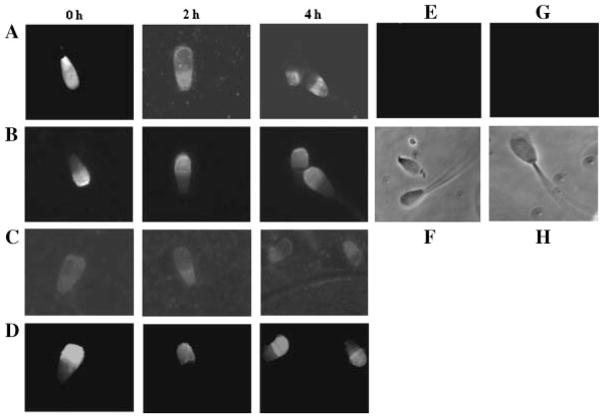
Figure 3.
Evaluation of capacitation-associated relocalization of ATP1A4 in bovine sperm. Bovine sperm preparations were incubated at 39°C in 5% CO2 and under high humidity with heparin (10 μg/ml) or ouabain (100 μM) for 4 hr. A: Sperm preparations incubated with heparin at 0, 2, and 4 hr of incubation were processed for immunolocalization of ATP1A4, using a custom anti-ATP1A4. B: The acrosomal integrity of these sperm was concurrently evaluated using a fluorescent probe (FITC-PSA). Similarly, distribution patterns of ATP1A4 following incubation of sperm with ouabain at 0, 2, and 4 hr and the acrosomal integrity of these sperm were shown in Panels C and D, respectively. Fluorescence and phase contrast views of sperm incubated with pre-immune serum (E and F, respectively) or secondary antibody alone (G and H, respectively) were shown as negative controls.
Effects of Various Kinase Inhibitors on Sperm Protein Tyrosine Phosphorylation and Capacitation Induced by Ouabain
Incubation of sperm in the presence of ouabain induced tyrosine phosphorylation in a cohort of sperm proteins (50, 80, 110, 150, 200, and 260 kDa) similar to those reported previously. This effect was inhibited in sperm pretreated with inhibitors for RTK (AG213; Fig. 4), PKC (chelerythrine, Fig. 4) and PKA (H89; Fig. 5) in a dose-dependent manner. However, inhibition of MEK (PD98059) did not have a consistent inhibitory effect on tyrosine phosphorylation, even at a higher (1 mM) concentration. Furthermore, 100 nM of PP2, an inhibitor of nonreceptor type PTK, consistently (replicated in several bulls) resulted in partial inhibition of tyrosine phosphorylation in sperm proteins (results not shown). Subjective examination of sperm preparations incubated with ouabain and various inhibitors did not detect any change in total motility compared to sperm incubated in Sp-TALP alone.
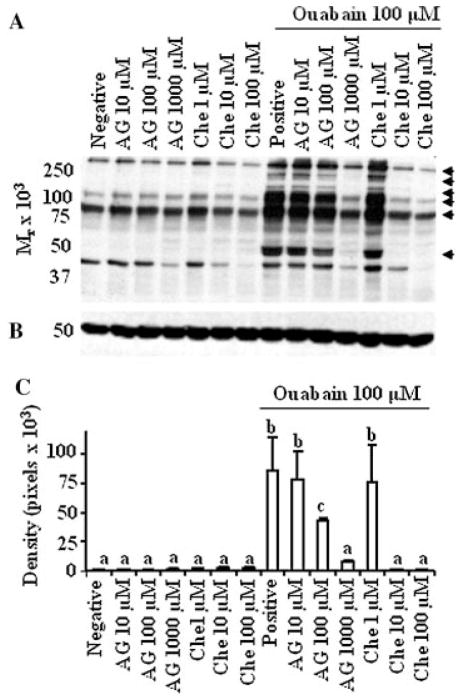
Figure 4.
Ouabain-induced tyrosine phosphorylation is regulated by RTK and PKC in a dose-dependent manner. A: Bovine sperm preparations were preincubated in Sp-TALP alone (negative) or with inhibitors for RTK (AG) and PKC (Che: chelerythrine) for 30 min. These sperm preparations were subsequently incubated for an additional 4 hr, without or with ouabain. A positive control group was maintained where sperm preparation was incubated in Sp-TALP containing only 100 μM ouabain. These sperm preparations were subjected to electrophoresis, electrotransferred and immunoblotted using antiphosphotyrosine antibody. Arrows at the right of the immunoblot identify proteins exhibiting a change in the content of tyrosine phosphorylation; the results are representative of three experiments, using sperm preparations from three bulls. B: Membranes were stripped and reprobed with anti-β tubulin to ensure that amount of proteins loaded was equal among wells. C: Densitometry analysis on the effect of inhibitors on the content of phosphotyrosine in sperm treated with ouabain. A representative band (50 kDa) from blot A was quantified. In the absence of differences, densitometry data were compared between treatment groups. Values presented are mean ± SD (n = 3). a–cValues without a common superscript differed (P <0.05).
Figure 5.
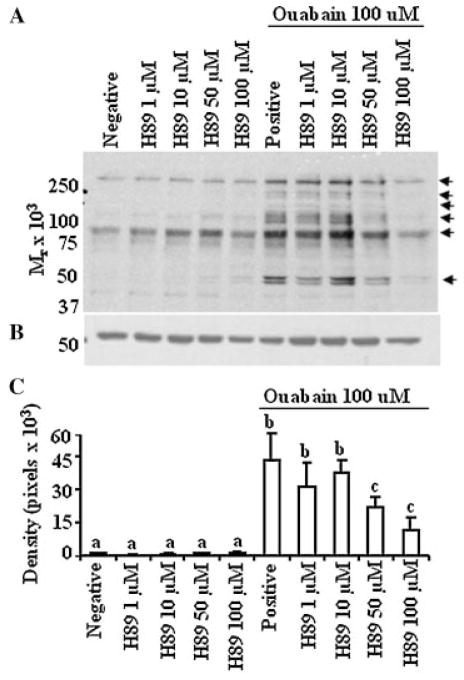
Tyrosine phosphorylation induced by ouabain was regulated by PKA in a dose-dependent manner. A: Bovine sperm preparations were processed and incubated as described in Figure 4, except that various concentrations of H89 (inhibitor for PKA) were used as the inhibitors. Arrows at the right of the immunoblot identify proteins exhibiting a change in the content of tyrosine phosphorylation; the results are representative of three experiments, using sperm preparations from three bulls. B: immunoblot was stripped and reprobed with anti β-tubulin to ensure equal loading between wells. C: Densitometry analysis on the effect of H89 on the content of phosphotyrosine in sperm treated with ouabain. A representative band (50 kDa) from blot A was quantified. In the absence of differences, densitometry data were directly compared between treatment groups. The results are representative of three experiments using sperm preparations from different bulls. Values presented are the mean ± SD. a–cValues without a common superscript differed (P <0.05).
Effects of Combinations of Kinase Inhibitors at 50% of Minimum Effective Concentrations on Protein Tyrosine Phosphorylation
Since the results described above indicated the involvement of various signaling molecules in pathways leading to tyrosine phosphorylation and the minimum concentration of inhibitors required for inhibiting protein tyrosine phosphorylation of ouabain-treated sperm, we subsequently evaluated additive effects between signaling molecules by blocking them in various combinations. Pre-incubation of sperm preparations with inhibitors for RTK and PKA, RTK and Src, RTK and PKC or RTK, Src and PKC in combination (at 50% of their minimum inhibitory concentrations) inhibited tyrosine phosphorylation in sperm proteins 50, 80, 110, 150, 200, and 260 kDa (Fig. 6A,C; P <0.05) compared to the group received ouabain alone (positive control). Although this inhibition of tyrosine phosphorylation was not significantly different from the individual effect of each inhibitor, these combinations of inhibitors significantly reduced the proportion of sperm undergoing capacitation (based on an acrosome reaction; Fig. 6D) in response to ouabain, compared to the individual effects of these inhibitors. That total motility of sperm (routine microscopic examination) in any experimental group was not affected either by ouabain or inhibitors (data not shown) confirmed sperm viability.
Figure 6.
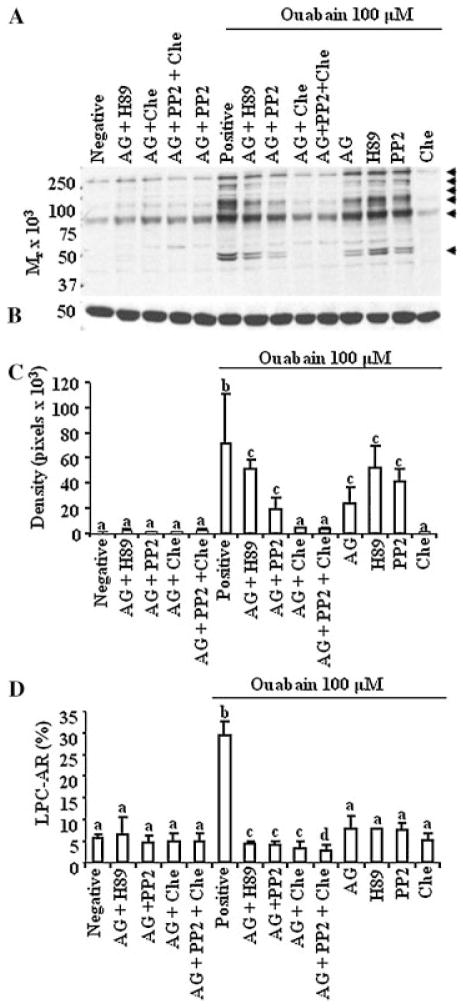
Cross-talk between RTK, Src, and PKC regulated capacitation induced by the inhibition of Na+/K+ATPase. A: Sperm preparations were processed and incubated as described previously, except that AG (RTK inhibitor), H89 (PKA inhibitor), chelerythrine (Che; PKC inhibitor) or PP2 (Src inhibitor) were used as inhibitors individually, or in various combinations. Arrows at the right of the immunoblot identify proteins exhibiting a change in the content of tyrosine phosphorylation; the results are representative of three experiments, using sperm preparations from three bulls. B: Membranes were stripped and re-probed with anti-beta tubulin to ensure equal loading of wells. C: Densitometry analysis of the effects of treatments on level of tyrosine phosphorylation in 50 kDa protein in blot A. In the absence of differences, densitometry data were directly compared among treatment groups. Values presented are the mean ± SD (n = 3; a–cP <0.05). D: Parallel sperm preparations resulting from Experiment A were incubated with or without lysophosphatidyl choline (LPC) for 30 min to induce an acrosome reaction (AR). Percentage of sperm undergoing the AR in response to LPC (LPC-AR) was considered as the percentage of capacitated spermatozoa. These values were corrected by subtracting the percentage of cells undergoing spontaneous AR from the respective groups. The values are presented as the mean ± SD (n = 3; a–dP <0.05).
DISCUSSION
The objectives of this study were to determine the fertilizing ability of sperm capacitated by incubating with ouabain and involvement of ATP1A4 in this process, and signaling mechanisms involved in the regulation of sperm capacitation induced by inhibition of Na+/K+ATPase. Sperm incubated with ouabain (presumably blocking ATP1A1 and ATP1A4) bound with oocytes, underwent pronuclear formation and the resulting zygotes normally progressed to the 2-cell stage (cleavage) in vitro. We also concluded that ATP1A4 was present in the sperm head and involved in sperm physiology, since incubation with an anti-ATP1A4 antiserum induced capacitation and facilitated in vitro fertilization. Furthermore, whereas ATP1A4 was uniformly distributed over the entire sperm head of uncapacitated sperm, it was confined to the post-acrosomal region in capacitated sperm. Therefore, we inferred that there was a capacitation-associated redistribution and a potential role for this protein in gamete interactions leading to fertilization. In this study, H89, chelerythrine, AG and PP2 decreased the level of tyrosine phosphorylation in bovine sperm induced by ouabain. In addition, use of these inhibitors in various combinations inhibited the proportion of sperm undergoing an acrosome reaction, compared to the individual effects of these inhibitors. We inferred that PKA, PKC, Src, and RTK may be involved in the signaling cascade leading to tyrosine phosphorylation and capacitation in sperm induced by inhibition of Na+/K+ATPase.
Effect of Incubation of Sperm With Ouabain or Anti-ATP1A4 Immunoserum on Capacitation and In Vitro Fertilization
We previously reported that incubation of sperm with ouabain induced tyrosine phosphorylation and capacitation in bovine sperm (Thundathil et al., 2006). Since acquiring the ability to interact with and fertilize oocytes are the ultimate result of successful capacitation, we performed a sperm–oocyte binding assay and fertilization assay (evaluation of pronuclei formation and cleavage) and confirmed that incubation of Na+/K+ATPase with ouabain or anti-ATP1A4 induced capacitation. Based on differences in signaling mechanisms in somatic cells versus sperm, and the presence of ATP1A4 in bovine sperm (Newton et al., 2009), further studies were conducted to determine the role of ATP1A4 in the regulation of sperm function. In our preliminary studies, we determined the specificity of anti-ATP1A4; this antibody specifically recognized a protein band at 110 kDa (Newton et al., 2009). Furthermore, LC-MS/MS studies on this protein band demonstrated that one of the proteins at 110 kDa was ATP1A4. In addition, since ATP1A4 and ATP1A1 are present in bovine sperm, we further tested the ability of anit-ATP1A4 to specifically recognize ATP1A4. In that regard, protein extracts prepared from bovine liver and kidney (both tissues express ATP1A1) were immunoblotted with anti-ATP1A4. Since the antibody did not recognize a band from these samples consistent with ATP1A1, we inferred that the antiserum used in this study was specific to ATP1A4.
Incubation of sperm with immunosera developed against specific sperm proteins has been used to study the role of individual sperm proteins in gamete interaction (Primakoff et al., 1987; Matwee et al., 2001; Ellerman et al., 2006). We used this approach to investigate the effect of incubation of sperm with ouabain or anti-ATP1A4 immunoserum on capacitation and in vitro fertilization. Pre-incubation of sperm with ATP1A4 immunoserum increased the proportion of sperm undergoing capacitation. Since those sperm were capable of binding with and fertilizing (as evidenced by pronuclear formation and cleavage) oocytes in vitro, we inferred that ATP1A4 was involved in the regulation of sperm capacitation. It was noteworthy that although sperm incubated with anti-ATP1A4 immunoserum underwent capacitation (based on the content of tyrosine phosphoproteins and LPC-induced acrosome reaction), their ability to fertilize oocytes in vitro was significantly lower, compared to that of ouabain (blocking both isoforms) and heparin. The underlying cause of these differences in capacitation and fertilization remains unknown. Perhaps both ubiquitous and testis-specific isoforms of this enzyme are involved in achieving the completion of capacitation process. Alternatively, the ATP1A4 isoform may be exclusively involved in these processes. However, incubation of sperm with ouabain presumably inhibited the activity of this protein more efficiently than incubation with anti-ATP1A4 immunoserum, inducing capacitation.
An immunocytochemical study demonstrated that the anti-ATP1A4 immunoserum developed against a peptide (MGLGGKKGAVTPHERNPNPGPT; XP_618149, position 1–22; Newton et al., 2009) appeared to bind live sperm. However, the exact mechanism by which the immunoserum accessed this N-terminus sequence (predicted to be intracellular) used for raising the antiserum, warrants further study. It has been proposed that ouabain binding to specific regions of the α1 subunit of ATPase inhibited cation transport by immobilizing transmembrane domains of Na+//K+ATPase (Palasis et al., 1996). This arrest of cation transport may increase intracellular calcium concentrations, decrease intracellular pH, and cause generation of reactive oxygen species, as observed in somatic cells (Kometiani et al., 1998; Woo et al., 2002; Xie and Askari, 2002; Liu et al., 2003), which promote tyrosine phosphorylation and capacitation. Similarly, interaction of anti-ATP1A4 immunoserum with ATP1A4 may arrest cation transport by immobilizing transmembrane domains, leading to the induction of sperm capacitation.
Capacitation-Associated Redistribution of ATP1A4 in Bovine Sperm
Based on immunocytochemical studies, there was a change in the localization of the ATP1A4 during sperm capacitation; uncapacitated sperm had a uniform distribution of this protein throughout the sperm head, whereas fluorescence was limited to the postacrosomal region in capacitated sperm. We used various approaches to confirm acrosome integrity of sperm in this study. Staining with FITC-PSA was concurrently used with immunolocalization of ATP1A4; there was a capacitation-associated change in the distribution of ATP1A4 in sperm that retained their acrosome. In addition, aliquots from capacitated sperm preparations used for the above study were evaluated by fluorescent microscopy, and no more than 8% of sperm were acrosome-reacted after 4 hr of incubation with ouabain. Therefore, we inferred that loss of the ATP1A4 from the acrosomal region during capacitation was not due to acrosomal exocytosis, but may have been at least partially due to the reorganization of this protein in the sperm plasma membrane during capacitation. Consistent with this observation, capacitation-associated redistribution of heat shock protein 70 (Kamaruddin et al., 2004) and rat epididymal glycoprotein DE (Rochwerger and Cuasnicu, 1992) have been reported. Redistribution of ATP1A4 at the postacrosomal region of capacitated sperm suggests additional roles for this protein in the regulation of sperm–oocyte interaction, which require further investigation.
Role of Kinases in the Regulation of Tyrosine Phosphorylation and Capacitation Induced by Ouabain
During somatic cell signaling, inhibition of Na+/K+ ATPase by ouabain initially activated receptor type kinase (RTK; for example, epidermal growth factor receptor) and nonreceptor type protein tyrosine kinase (Src), which lead to downstream activation of signaling cascades, e.g., ERK 1/2 (Xie and Askari, 2002). In addition, ouabain inhibition of Na+/K+ATPase activated PKC, shown to be necessary for ERK 1/2 activation (Mohammadi et al., 2001). Although the involvement of cAMP/PKA pathway in signaling mechanisms leading to tyrosine phosphorylation due to the inhibition of Na+/K+ATPase has not been demonstrated in somatic cells, this pathway was involved in tyrosine phosphorylation and capacitation in bovine sperm (Galantino-Homer et al., 1997). Therefore, the roles of RTK, Src, and PKC and MEK (an upstream activator of ERK 1/2) and PKA in the regulation of ouabain-induced sperm capacitation, were investigated in this study.
Activation of Src leading to protein tyrosine phosphorylation is the earliest event identified in somatic cells during oubain-induced signal transduction (Xie and Askari, 2002). In somatic cells, activation of RTK resulted in binding of Srchomology-2 (SH2) domains to specific receptor targets, stimulating their activity. Phosphorylation and activation of specific proteins within these domains (eg. Src), lead to the activation of downstream cascades in the signal transduction process (Pawson, 1995). Additionally, adaptor proteins with no intrinsic enzymatic activity can interact with activated receptors, linking RTK activation to downstream signal transduction pathways (Zwick et al., 2001; Thundathil et al., 2002). Consistent with these reports, since AG, an inhibitor of RTK, inhibited tyrosine phosphorylation and sperm capacitation, we inferred that RTK was involved in signal transduction, leading to these events in bovine sperm.
The role of PKA in somatic cell signaling, as a result of Na+/K+ATPase inhibition, is unknown. An increase in adenyl cyclase activity increases cAMP content; most actions of cAMP are mediated through activation of PKA, which leads to tyrosine phosphorylation of specific proteins (Leclerc et al., 1996), through the effects of PKA on tyrosine kinase or phosphatases (Visconti et al., 2002; O’Flaherty et al., 2004). Inhibition of PKA in sperm from mice (Visconti et al., 1995), humans (Leclerc et al., 1996), and bulls (Galantino-Homer et al., 1997) blocked tyrosine phosphorylation and capacitation. Therefore, we investigated the role of PKA in protein tyrosine phosphorylation and capacitation induced by incubation of sperm with ouabain (which presumably inhibits Na+/K+ATPase). As expected, inhibition of PKA inhibited tyrosine phosphorylation and capacitation in bovine sperm treated with ouabain, suggesting that PKA was involved in events induced by the inhibition of Na+/K+ATPase. The minimum dose of H89 (50 μM) required for the complete inhibition of PKA in bovine sperm was similar to that reported previously (Galantino-Homer et al., 1997).
In cardiac myocytes, ouabain inhibition of Na+/K+ ATPase activates PKC, which is required for downstream activation of ERK1 and 2, and protein tyrosine phosphorylation (Mohammadi et al., 2001). Although PKC is present in bovine sperm (Breitbart et al., 1992), its exact role in sperm function is still under investigation. In capacitated human sperm, calcium-dependent phospholipase C was activated, leading to generation of inositol-triphosphate (IP3) and dia-cylglycerol (Roldan, 1998; Baldi et al., 2002). The latter molecule is a physiological activator of PKC, and is activated during capacitation of human sperm (Naor and Breitbart, 1997) and the acrosome reaction (Bonaccorsi et al., 1998), suggesting the activation of PKC during sperm capacitation. In support of this hypothesis, inhibition of PKC by chelerythrine inhibited tyrosine phosphorylation of several proteins associated with human sperm capacitation (O’Flaherty et al., 2005). Based on the observation that inhibition of PKC inhibited tyrosine phosphorylation and capacitation in oubain-treated sperm, we inferred that PKC was an essential component of the signal transduction pathway leading to these events induced by the incubation of sperm with ouabain.
Inhibition of signaling molecules (RTK and PKA, RTK and Src, RTK and PKC or RTK, Src, and PKC), using inhibitors at 50% of their minimum inhibitory concentrations, blocked tyrosine phosphorylation induced by ouabain, but these inhibitory effects were not significantly different from the individual effect of inhibitors. However, combinations of inhibitors significantly reduced the proportion of sperm undergoing capacitation induced by ouabain. These differences in the effects of the signaling molecules on tyrosine phosphorylation and capacitation remain unknown. It is noteworthy that combination of inhibitors decreased the intensity of phosphotyrosine content in several proteins (50, 80, 110, 150, 200, and 260 kDa; Fig. 6A) compared to the effects of treating with an individual inhibitor. These partial inhibitions of tyrosine phosphorylation in a cohort of sperm proteins may be sufficient to inhibit capacitation in a significantly higher proportion of sperm. In that regard, capacitation status of sperm may be a better indicator of cross-talk between signaling molecules. Based on these studies, we infer that there were two independent signaling pathways involved in ouabain-induced tyrosine phosphorylation and capacitation, namely the cAMP/PKA pathway and a pathway involving RTK, Src, and PKC.
Although these studies demonstrated that Na+/K+ ATPase is involved in the regulation of sperm capacitation, the mechanisms by which this protein induce capacitation appear to be different from classical capacitating agents. For example, increased intracellular pH is an essential component of heparin-mediated bovine sperm capacitation (Parrish et al., 1989; Vredenburgh-Wilberg and Parrish, 1995). Conversely intracellular acidification inhibits capacitation (Galantino-Homer et al., 2004) or simply raising intracellular pH (in the absence of heparin) induce capacitation. Although intracellular pH in sperm incubated with ouabain was not measured in our study, it appears that inhibition of the activity of Na+/K+ATPase caused intracellular acidification, based on studies from somatic cells (Souza et al., 2000). Inhibition of Na+/K+ATPase by ouabain involving both ATP1A-1 and ATP1A-4 leads to depolarization of the sperm cell (Thundathil et al., 2006), whereas the process of capacitation has been shown to lead to sperm cell hyperpolarization (Arnoult et al., 1999). In addition, increases in intracellular calcium concentrations is an important feature of heparin-induced capacitation. However, there was no apparent increase in calcium concentrations in sperm when capacitation was induced by ouabain (Thundathil et al., 2006). Therefore, the signaling mechanisms leading to capacitation induced by the interaction of Na+/K+ATPase with its antibody or ouabain appeared to be very distinct from that induced by classical capacitation-inducing agents. Since activity of Na+/K+ATPase following incubation of sperm with ouabain was not tested in this study, we cannot rule out the possibility that the observed effects of this protein on sperm capacitation may have been mediated through its enzymatic activity. Alternatively, perhaps signaling mechanisms leading to the regulation of capacitation may be exerted through a classical ligand (ouabain)-receptor (Na+/K+ ATPase) mechanism, that is completely distinct from the enzymatic activity of this protein. These differences in mechanisms leading to capacitation induced by ouabain may have contributed to the above described differences between the capacitation process induced by ouabain and other capacitation agents.
In summary, signaling mechanisms leading to tyrosine phosphorylation and capacitation in bovine sperm induced by ouabain involved PKC, RTK, Src, and PKA. We concluded that ATP1A4 was involved in the regulation of sperm capacitation and a capacitation-associated relocalization of ATP1A4 to the post-acrosomal region was observed. The mechanisms leading to capacitation induced by the interaction of Na+/K+ATPase with its antibody or ouabain appeared to be distinct from those of other classical capacitating agents. To our knowledge, this is the first report describing the role of a testis-specific isoform of Na+/K+ATPase in the regulation of bovine sperm capacitation and in vitro fertilization of bovine oocytes using fresh bovine sperm capacitated with ouabain.
MATERIALS AND METHODS
Materials
All chemicals purchased were reagent grade or better. The following were purchased from Sigma–Aldrich Canada Ltd. (Oakville, ON, Canada): P. sativum agglutinin conjugated to fluorescein isothiocyanate (FITC-PSA), lysophosphatidylcholine (LPC), heparin sodium salt (from porcine intestinal mucosa, 180 -USP U/mg), BSA (fraction V), luminol (97%, HPLC grade), p-coumaric acid, dimethyl sulfoxide (DMSO), and ouabain. Furthermore, H89, chelerythrine, PP2, AG 213, and PD98059 were purchased from Calbiochem (Gibbstown, NJ), whereas Tris, Ponceau S, sodium vanadate, sodium azide and glycine were purchased from Fisher Scientific (Edmonton, AB, Canada). Nitrocellulose membrane, Precision Plus Protein Kaleidoscope molecular mass standards, were purchased from Bio-Rad Laboratories (Mississauga, ON, Canada).
Percoll was obtained from GE Health Care Biosciences (Baie D’Urfe, Quebec, Canada). Anti-phosphotyrosine monoclonal antibody (clone 4G10) developed in mouse, anti-β tubulin developed in rabbit and goat anti-mouse IgG conjugated with horseradish peroxidase were purchased from Upstate Technologies (Temecula, CA). Kodak Scientific Imaging X-OMAT LS film was obtained from Mandel Scientific (Guelph, ON, Canada). Fetal calf serum was purchased from Hyclone (Logan, UT). Anti-ATP1A4 was custom made at the Antibody Services of the University of Calgary. Bovine ovaries were purchased from XL Beef (Calgary, AB, Canada).
Effect of Incubation of Sperm With Ouabain or Anti-ATP1A4 Immunoserum on Capacitation and In Vitro Fertilization
Our previous studies demonstrated that incubation of sperm with ouabain induced tyrosine phosphorylation and capacitation in bovine sperm. However, the fertilizing ability of sperm capacitated by this method remains unknown. Moreover, since ouabain is inhibitory to both ATP1A1 and ATP1A4, the specific role of ATP1A4 (testis-specific) in this process remains unknown. To address these questions, a series of homologous sperm–oocyte binding and fertilization (based on pronuclei formation and cleavage) assays were conducted to evaluate the ability of sperm capacitated with ouabain or anti-ATP1A4 to bind with and fertilize oocytes (pronuclei formation and cleavage), as described below.
In vitro maturation of cumulus oocyte complexes (COC)
In brief, bovine ovaries were recovered at an abattoir and transported to the laboratory in physiological saline (maintained at 35°C). Cumulus oocyte complexes were aspirated from antral follicles and assessed under a stereo microscope. The COCs were selected for in vitro maturation (IVM). These oocytes were washed (3×) in flushing medium (Tissue Culture Medium 199 (TCM; with HEPES), 1 μg/ml gentamicin, and 3 mg/ml BSA. Maturation media (TCM-199 containing 25 μM sodium pyruvate, 1 μg/ml gentamicin, 0.5 μg/ml FSH, 5 μg/ml LH, 2 μg/ml estradiol-17β, and 10% fetal calf serum) was transferred to a multiwell dish (500 μl/well), covered with embryo-tested mineral oil, and equilibrated for 4 hr (5% CO2 in air, 95% humidity, 39°C). Twenty COC were transferred to each well and incubated for 24 hr.
Preparation of sperm for capacitation
Fresh ejaculates collected from mature Holstein bulls were obtained from a local artificial insemination centre (Alta Genetics, Calgary, AB, Canada) on the day following oocyte recovery. Animal maintenance and handling, as well as all experimental procedures, were performed in accordance with the guidelines of the Canadian Council on Animal Care and the policies of the Animal Care Committee of the University of Calgary. Semen with at least 70% progressively motile sperm (based on subjective evaluation at 400×) were used. Semen samples were immediately diluted 1:3 with Sp-TALPH (Galantino-Homer et al., 1997) and transported to the laboratory, within 30 min after collection, in a vacuum bottle maintained at 35°C. Semen samples were subjected to a Percoll wash on two-layer Percoll gradients (45–90%) by centrifugation (700g for 30 -min). The resulting sperm pellet was re-suspended in Sp-TALPH and washed to remove Percoll (700g, 5 min).
Capacitation of fresh bovine sperm
Fresh bovine sperm as prepared above were incubated in Sp-TALP medium (Galantino-Homer et al., 1997; Thundathil et al., 2006) alone, Sp-TALP supplemented with either anti-ATP1A4 immunoserum (26.95 μg/ml; Newton et al., 2009), heparin (10 μg/ml; positive control for capacitation), ouabain (100 μM; inhibitory to both ubiquitous and ATP1A4), pre-immune serum obtained from the rabbits used for the production of anti-ATP1A4 immunoserum, or the immunoserum pre-absorbed to its blocking peptide (at fivefold higher concentration) for 4 hr (39°C, 5% CO2). Following the completion of capacitation treatments, these sperm samples were washed in Sp-TALPH (5 min, 500g) and re-suspended in equilibrated final fertilization medium (stock medium of FERT-TALP (Parrish et al., 1988), supplemented with 6 mg/ml fatty acid-free BSA, 25 μM sodium pyruvate, 20 μM penicillin/streptomycin, and 0.3 mg/ml penicillamine, 0.1 mg/ml hypotaurine and 45.8 μg/ml epinephrine) and used for the following experiments.
Sperm–oocyte binding assay
The objective of this experiment was to compare the ability of sperm capacitated as described above to bind with in vitro matured bovine oocytes. In vitro matured COC were treated with 1% hyaluronidase in PBS for 2 min and vortexed for 1 min to dissociate cumulus cells. Cumulus dissociated oocytes were washed 3 times in TALP-HEPES and once in FERT-TALP, randomly allotted to pre-equilibrated (incubated at 39°C, in 5% CO2 for 4 hr) fertilization droplets (40 μl each; 10 oocytes per droplet) prepared using the final fertilization medium. Motility of sperm capacitated as described above was evaluated subjectively (400× magnification), in increments of 10%. Concentration of sperm preparations were determined with a hemocytometer and diluted to a final concentration of 1 × 106/ml motile sperm in final fertilization medium. An aliquot (5 μl) of sperm suspension was used to fertilize oocytes in 40-μl fertilization drops. The final volume of each fertilization droplet was adjusted to 50 μl by adding pre-warmed final fertilization medium.
After 6 hr of co-incubation with sperm, the oocytes were removed from the fertilization medium, washed 7 times by pipetting to remove loosely attached spermatozoa, and then placed in the refrigerator until fixation. For fixation, five oocytes were placed between two polylysine-coated glass slides with a small drop of TALP-HEPES and gently flattened. After flattening, the oocytes were fixed in a solution of glacial acetic acid and absolute ethyl alcohol in a 1:3 ratio for 24–36 hr and stained by placing small drops of aceto-orcein (1% orcein in glacial acetic acid) at the corners of the cover glass. The number of spermatozoa bound to the zona pellucida was counted by phase-contrast microspcopy. This experiment was done in three replicates, using ejaculates from three bulls.
Evaluation of pronuclei formation
The objective of this experiment was to determine the ability of sperm capacitated under above conditions to penetrate oocytes and development into pronuclei. In vitro matured COC were washed 3 times through TALP-HEPES and once through FERT-TALP and fertilized with sperm incubated in various capacitating conditions, as described above. After 6 hr of sperm–oocyte coincubation, COC were removed from the fertilization medium, cumulus dissociated and transferred to synthetic oviduct fluid (SOF) medium and cultured for an additional 12 hr for the development of pronuclei. Presumptive zygotes were fixed, stained and examined microscopically for pronuclei formation at 400× magnification. This experiment was done in three replicates using semen from different bulls.
Evaluation of cleavage
The objective of this experiment was to compare the ability of oocytes fertilized by sperm capacitated as described above to initiate first cleavage division. After 6 hr of sperm–oocyte coincubation, presumptive zygotes were cultured (20 in each drop) in SOF medium (Thundathil et al., 2007) and cleavage was determined after 2 days (Day 0 = Day of fertilization). This experiment was done in five replicates. Parthenogenic development of embryos was evaluated in each replicate by exposing in vitro matured oocytes to final fertilization medium for 6 hr, followed by cumulus cell dissociation and culturing as described above. Percentage of oocytes developed by parthenogenic activation during each replicate was subtracted from the number of zygotes cleaved from each experimental group.
Involvement of ATP1A4 in the Regulation of Sperm Capacitation
Since sperm incubated with anti-ATP1A4 immunoserum fertilized oocytes, we investigated the role of this protein in the regulation of sperm capacitation. In that regard, sperm preparations were incubated with either ouabain (100 μM), heparin (10 μg/ml) or a custom-made anti-ATP1A4 immunoserum (Newton et al., 2009). Sperm were also incubated with pre-immune serum obtained from the rabbits used for the production of anti-ATP1A4 immunoserum, or this immunoserum pre-absorbed to its blocking peptide (at fivefold higher concentration) for 4 hr (39°C, 5% CO2). At the end of the capacitation period, parallel sperm preparations, incubated in conditions as described above, were evaluated for viability by examining sperm motility. For this, an aliquot (10 μl) was placed onto a warm (37°C) microscope slide, covered with a cover slip, and the proportion of progressively motile sperm was subjectively estimated (in increments of 10%). Capacitation status of sperm preparations were evaluated based on the ability of sperm to undergo tyrosine phosphorylation (using immunoblotting) and an LPC-induced acrosome reaction, as described below (Thundathil et al., 2006).
SDS–PAGE, Electrotransfer, and Immunoblotting
Sperm proteins were separated (reducing conditions) on 10% polyacrylamide electrophoresis gels and electrotransferred to nitrocellulose membranes. After blocking with skim milk (5%, w/v) in Tris (20 mM, pH 7.8)-buffered saline containing Tween 20 (TTBS; 0.1% v/v) for 45 min, membranes were incubated with anti-phosphotyrosine antibody (clone 4G10; 1:5,000) overnight at 4°C, washed with TTBS, and incubated with goat anti-mouse IgG conjugated with horseradish peroxidase for 1 hr. Following washing, positive immunoreactive bands were detected using chemiluminescence. The specificity of the anti-phosphotyrosine antibody was verified by incubation with phosphotyrosine (5 mM). To ensure equal protein loading among wells, membranes were stripped and re-probed with a polyclonal anti β-tubulin antibody.
Evaluation of Capacitation Status of Sperm by Induction of the Acrosome Reaction
Parallel sperm preparations from the above experiment (involvement of ATP1A4 in the regulation of sperm capacitation) were used for the evaluation of the ability of sperm to undergo an acrosome reaction in response to LPC (n = 3). At the end of the capacitation period, sperm preparations were split into two and incubated with either LPC (100 μg/ml) or Sp-TALPH at 39°C in 5% CO2 and high humidity for 30 min. Then, smears were prepared and stained with FITC-conjugated P. sativum agglutinin (100 μg/ml; Galantino-Homer et al., 1997; Thundathil et al., 2006), and at least 200 sperm were counted. The proportion of sperm undergoing the LPC-induced acrosome reaction was corrected for a spontaneous acrosome reaction (samples in Sp-TALPH alone).
Evaluation of the Ability of Anti-ATP1A4 to Bind With Live Sperm
Since the above studies indicated that incubation of fresh sperm with anti-ATP1A4 induced capacitation, we further conducted an experiment to confirm the binding of anti-ATP1A4 during incubation of this antibody with a suspension of live sperm. In that regard, sperm incubated with the immunoserum (26.95 μg/ml of Sp-TALP) or preimmune serum for 4 hr were processed for immunocytochemistry, as described below, except that these sperm preparations were not incubated with primary antibody (anti-ATP1A4) during the immunocytochemistry procedure. This experiment was based on the assumption that if incubation of live sperm with anti-ATP1A4 causes binding of anti-ATP1A4 with sperm, a fluorescence signal will be obtained during immunocytochemistry of the sperm with a fluorescence tagged secondary antibody (anti-rabbit cy3) alone without any fluorescent signal from sperm incubated with preimmune serum.
Capacitation-Associated Changes in the Distribution of ATP1A4
The objective was to investigate the effects of capacitation on the distribution of ATP1A4 to determine the involvement of this protein during capacitation induced by a physiological capacitating agent (heparin) and ouabain. Sperm preparations were incubated with heparin (10 μg/ml) or ouabain for 0, 2, or 4 hr, placed on polylysine coated coverslips for 1–2 hr, and fixed in 2.5% paraformaldehyde (10 min at 20°C). Cells on the coverslips were blocked with 5% goat serum (in PBS containing 0.1% BSA) and incubated for 2.5 hr at 20°C with: (a) protein A-purified anti-ATP1A4 immunoserum (26.95 μg/ml); (b) pre-immune serum from the rabbits used for antibody production; or (c) PBS containing 0.1% BSA (secondary antibody control). Coverslips were washed in PBS containing 0.1% BSA, and cells were briefly permeabilized with glacial ethanol. Coverslips were then incubated with anti-rabbit cy3 (secondary antibody to label anti-ATP1A4) at a dilution of 1:1,000 and FITC-conjugated P. sativum agglutinin (100 μg/ml) for 1 hr at room temp, and washed extensively. Mounted coverslips were viewed and images were captured with a Carl Zeiss Axiovert 200M deconvolution microscope and camera (Carl Zeiss Microimaging, GmbH 37081, Gottingen, Germany). This procedure facilitated concurrent evaluation of the same sperm for the distribution of ATP1A4 and acrosome integrity.
Effects of Various Kinase Inhibitors on Sperm Protein Tyrosine Phosphorylation and Capacitation Induced by Ouabain
Since the above studies demonstrated the involvement of Na+/K+ATPase in the regulation of tyrosine phosphorylation and capacitation, additional experiments were conducted to understand the signaling mechanisms leading to sperm capacitation induced by the inhibition of Na+/K+ATPase. Sperm preparations were preincubated in Sp-TALP (100 × 106/ml) containing BSA (6 mg/ml) with inhibitors for RTK (AG 213; 10 μM, 100 μM, or 1 mM), PKC (chelerythrine; 1, 10, or 100 μM), PKA (H89; 1, 10, 50, or 100 μM), nonreceptor TK (PP2; 1, 10, or 100 nM), and MEK (PD 98059; 10 μM, 100 μM, or 1 mM) for 30 min at 39°C in 5% CO2 under high humidity, to determine the lowest concentration of these inhibitors required to inhibit tyrosine phosphorylation and capacitation induced by ouabain (n = 3). Subsequently, 100 μM ouabain (Thundathil et al., 2006) was added to induce capacitation, and sperm were incubated for 4 hr (39°C, 5% CO2, high humidity) and the inhibitors for signaling molecules remained in the incubation medium during capacitation. Each trial also contained both a negative (incubation medium; Sp-TALP containing 6 mg/ml BSA) and a positive (ouabain) control (we previously observed that tyrosine phosphorylation and the time course of capacitation in bovine sperm induced by ouabain was similar to that of heparin; Thundathil et al., 2006). For some inhibitors initial stock solutions were prepared in dimethyl sulfoxide (DMSO), and working solutions for these inhibitors were prepared by diluting this stock solution in complete Sp-TALP medium on the day of use. The concentration of DMSO used for initial dilutions of inhibitors did not exceed 1% in the sperm incubation medium; at this concentration, sperm viability was not affected (de Lamirande and Gagnon, 2002).
In another series of experiments, the same inhibitors were used at 50% of the minimum concentration required to prevent ouabain-induced tyrosine phosphorylation and used in combinations (see Results Section for specific combinations tested). Sperm were pretreated with the combinations of inhibitors and incubated, with or without ouabain, for 4 hr. Samples were then washed in 1 ml Sp-TALPH containing 0.2 mM Na2VO3 (centrifuged at 15,000g for 5 min). The pellet was re-suspended in sample buffer for SDS–PAGE containing 0.2 mM Na2VO3, mixed well, boiled for 5 min, centrifuged (15,000g, 5 min) and used for immunoblotting, as described below. At the end of the capacitation period, parallel sperm preparations incubated with inhibitors were evaluated for viability by examining sperm motility. For this, an aliquot (10 ml) was placed onto a warm (37°C) microscope slide, covered with a cover slip, and the proportion of progressively motile sperm was subjectively estimated (in increments of 10%). Evaluation of sperm motility was used as a method for testing the effect of vehicles on sperm viability.
Statistical Analysis
Data analysis was done using the program STATA (STATA IC 10.0, College Station, TX). Three replicates were performed simultaneously for binding and fertilization assay (evaluation of pronuclei formation). Sperm binding was compared between treatment groups by one-way ANOVA, followed by Scheffe’s posthoc analysis. Fertilization (three replicates) and cleavage rates (based on five replicates) were compared using a Chi square test. For all analyses, a difference was considered significant at P <0.05. Immunoblots from each of the experiments were stripped and re-probed with anti-β-tubulin antibody to ensure equal loading of protein among wells. Since there was no significant difference in the density of β-tubulin among wells within an experiment, densitometry values were directly compared among treatment groups. Protein density of the 50 kDa protein from immunoblots from the signaling experiments was determined using Kodak Digital Science 1D imaging software (Kodak Scientific Imaging Systems, Version 3.0.1, New Haven, CT). Although a large cohort of sperm proteins undergo tyrosine phosphorylation during capacitation, we selected a representative protein (50 kDa) for densitometric analysis, as this band consistently appeared as a result of capacitation. Results for the acrosome reaction and protein densitometry (three replicates) were analyzed using ANOVA to compare means among treatment groups. For all analyses, a difference was considered significant at P <0.05.
Acknowledgments
We thank Alta Genetics, Calgary, AB for providing semen samples for this study. We also thank Dr. Eve de Lamirande, Urology Laboratory, Royal Victoria Hospital, McGill University for helpful discussions and critical reading of this manuscript. L.D. Newton was supported through a studentship from the CIHR Training Program in Genetics, Child Development and Health. S. Krishnakumar received a studentship from the Faculty of Veterinary Medicine, University of Calgary. This study received funding support from the University of Calgary (Grant # RT 750114), and Agricultural & Food Council, and the Alberta Livestock Industry Development Fund (Grant # FC 2007F048R).
References
- Arnoult C, Kazam IG, Visconti PE, Kopf GS, Villaz M, Florman HM. Control of the low voltage-activated calcium channel of mouse sperm by egg ZP3 and by membrane hyperpolarization during capacitation. Proc Natl Acad Sci USA. 1999;96:6757–6762. doi: 10.1073/pnas.96.12.6757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldi E, Luconi M, Bonaccorsi L, Forti G. Signal transduction pathways in human spermatozoa. J Reprod Immunol. 2002;53:121–131. doi: 10.1016/s0165-0378(01)00089-4. [DOI] [PubMed] [Google Scholar]
- Blanco G, Mercer RW. Isozymes of the Na-K-ATPase: Heterogeneity in structure, diversity in function. Am J Physiol. 1998;275:F633–F650. doi: 10.1152/ajprenal.1998.275.5.F633. [DOI] [PubMed] [Google Scholar]
- Bonaccorsi L, Krausz C, Pecchioli P, Forti G, Baldi E. Progesterone-stimulated intracellular calcium increase in human spermatozoa is protein kinase C-independent. Mol Hum Reprod. 1998;4:259–268. doi: 10.1093/molehr/4.3.259. [DOI] [PubMed] [Google Scholar]
- Breitbart H, Lax J, Rotem R, Naor Z. Role of protein kinase C in the acrosome reaction of mammalian spermatozoa. Biochemistry. 1992;J15:473–476. doi: 10.1042/bj2810473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jonge C. Biological basis for human capacitation. Hum Reprod. 2005;11:205–214. doi: 10.1093/humupd/dmi010. [DOI] [PubMed] [Google Scholar]
- de Lamirande E, Gagnon C. The extracellular signal-regulated kinase (ERK) pathway is involved in human sperm function and modulated by the superoxide anion. Mol Hum Reprod. 2002;8:124–135. doi: 10.1093/molehr/8.2.124. [DOI] [PubMed] [Google Scholar]
- de Lamirande E, O’Flaherty C. Sperm activation: Role of reactive oxygen species and kinases. Biochim Biophys Acta Proteins Proteomics. 2008;1784:106–115. doi: 10.1016/j.bbapap.2007.08.024. [DOI] [PubMed] [Google Scholar]
- de Lamirande E, Leclerc P, Gagnon C. Capacitation as a regulatory event that primes spermatozoa for the acrosome reaction and fertilization. Mol Hum Reprod. 1997;3:175–194. doi: 10.1093/molehr/3.3.175. [DOI] [PubMed] [Google Scholar]
- Ellerman DA, Cohen DJ, Da Ros VG, Morgenfeld MM, Busso D, Cuasnicú PS. Sperm protein “DE” mediates gamete fusion through an evolutionarily conserved site of the CRISP family. Dev Biol. 2006;297:228–237. doi: 10.1016/j.ydbio.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Galantino-Homer HL, Visconti PE, Kopf GS. Regulation of protein tyrosine phosphorylation during bovine sperm capacitation by a cyclic adenosine 3′,5′-monophosphate dependent pathway. Biol Reprod. 1997;56:707–719. doi: 10.1095/biolreprod56.3.707. [DOI] [PubMed] [Google Scholar]
- Galantino-Homer HL, Florman HM, Storey BT, Dobrinski I, Kopf GS. Bovine sperm capacitation: Assessment of phospho-diesterase activity and intracellular alkalinization on capacitation-associated protein tyrosine phosphorylation. Mol Reprod Dev. 2004;67:487–500. doi: 10.1002/mrd.20034. [DOI] [PubMed] [Google Scholar]
- Kamaruddin M, Kroetsch T, Basrur PK, Hansen PJ, King WA. Immunolocalization of heat shock protein 70 in bovine spermatozoa. Andrologia. 2004;36:327–334. doi: 10.1111/j.1439-0272.2004.00629.x. [DOI] [PubMed] [Google Scholar]
- Kometiani P, Luigi JL, Gnudi L, Kahn BB, Askari A, Xie Z. Multiple signal transduction pathways link Na+/K+-ATPase to growth-related genes in cardiac myocytes. J Biol Chem. 1998;273:15249–15256. doi: 10.1074/jbc.273.24.15249. [DOI] [PubMed] [Google Scholar]
- Leclerc P, de Lamirande E, Gagnon C. Cyclic adenosine 3′,5′monophosphate-dependent regulation of protein tyrosine phosphorylation in relation to human sperm capacitation and motility. Biol Reprod. 1996;55:684–692. doi: 10.1095/biolreprod55.3.684. [DOI] [PubMed] [Google Scholar]
- Leclerc P, de Lamirande E, Gagnon C. Regulation of protein-tyrosine phosphorylation and human sperm capacitation by reactive oxygen derivatives. Free Radic Biol Med. 1997;22:643–656. doi: 10.1016/s0891-5849(96)00379-6. [DOI] [PubMed] [Google Scholar]
- Liu L, Mohammadi K, Aynafshar B, Wang H, Li D, Liu J, Ivanov AV, Xie Z, Askari A. Role of calveolae in signal-transducing function of cardiac Na+/K+ ATPase. Am J Physiol. 2003;284:C1550–C1560. doi: 10.1152/ajpcell.00555.2002. [DOI] [PubMed] [Google Scholar]
- Luconi M, Barni T, Vanelli GB, Crausz C, Marra F, Benedetti PA, Evangelista V, Matwee C, Kamaruddin M, Betts DH, Basrur PK, King WA. The effects of antibodies to heat shock protein 70 in fertilization and embryo development. Mol Hum Reprod. 2001;7:829–833. doi: 10.1093/molehr/7.9.829. [DOI] [PubMed] [Google Scholar]
- Matwee C, Kamaruddin M, Betts DH, Basrur PK, King WA. The effects of antibodies to heat shock protein 70 in fertilization and embryo development. Mol Hum Reprod. 2001;7:829–837. doi: 10.1093/molehr/7.9.829. [DOI] [PubMed] [Google Scholar]
- Mohammadi K, Kometiani P, Xie Z, Askari A. Role of protein kinase C in the signal pathways that link Na+/K+-ATPase to ERK1/2. J Biol Chem. 2001;9:42050–42056. doi: 10.1074/jbc.M107892200. [DOI] [PubMed] [Google Scholar]
- Naor Z, Breitbart H. Protein kinase C and mammalian spermatozoa acrosome reaction. Trends Endocrinol Metab. 1997;8:337–342. doi: 10.1016/s1043-2760(97)00134-3. [DOI] [PubMed] [Google Scholar]
- Naz RK, Rajesh PB. Role of tyrosine phosphorylation in sperm capacitation/acrosome reaction. Reprod Biol Endocrinol. 2004;2:1–12. doi: 10.1186/1477-7827-2-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton L, Kastelic JP, Wong B, van der Hoorn V, Thundathil J. Elevated testicular temperature modulates expression patterns of sperm proteins in Holstein bulls. Mol Reprod Dev. 2009;76:109–118. doi: 10.1002/mrd.20934. [DOI] [PubMed] [Google Scholar]
- O’Flaherty C, de Lamirande E, Gagnon C. Phosphorylation of the Arginine-X-X-(Serine/Threonine) motif in human sperm proteins during capacitation: Modulation and protein kinase A dependency. Mol Hum Reprod. 2004;10:355–363. doi: 10.1093/molehr/gah046. [DOI] [PubMed] [Google Scholar]
- O’Flaherty C, de Lamirande E, Gagnon C. Reactive oxygen species and protein kinases modulate the level of phospho-MEK-like proteins during human sperm capacitation. Biol Reprod. 2005;73:94–105. doi: 10.1095/biolreprod.104.038794. [DOI] [PubMed] [Google Scholar]
- O’Flaherty C, de Lamirande E, Gagnon C. Reactive oxygen species modulate independent protein phosphorylation pathways during human sperm capacitation. Free Rad Biol Med. 2006;40:1045–1055. doi: 10.1016/j.freeradbiomed.2005.10.055. [DOI] [PubMed] [Google Scholar]
- Olds-Clarke P. Unresolved issues in mammalian fertilization. Int Rev Cytol. 2003;232:129–184. doi: 10.1016/s0074-7696(03)32004-2. [DOI] [PubMed] [Google Scholar]
- Palasis M, Kuntzweiler TA, Argüello JM, Lingrel JB. Ouabain interactions with the H5–H6 hairpin of the Na,K-ATPase reveal a possible inhibition mechanism via the cation binding domain. J Biol Chem. 1996;271:14176–14182. doi: 10.1074/jbc.271.24.14176. [DOI] [PubMed] [Google Scholar]
- Parrish JJ, Susko-Parrish J, Winer MA, First NL. Capacitation of bovine sperm by heparin. Biol Reprod. 1988;38:1171–1180. doi: 10.1095/biolreprod38.5.1171. [DOI] [PubMed] [Google Scholar]
- Parrish JJ, Susko-Parrish JL, First NL. Capacitation of bovine sperm by heparin: Inhibitory effect of glucose and role of intracellular pH. Biol Reprod. 1989;41:683–699. doi: 10.1095/biolreprod41.4.683. [DOI] [PubMed] [Google Scholar]
- Pawson T. Protein modules and signaling networks. Nature. 1995;16:373, 573–580. doi: 10.1038/373573a0. [DOI] [PubMed] [Google Scholar]
- Primakoff P, Hyatt H, Tredick-Kline J. Identification and purification of a sperm surface protein with a potential role in sperm-egg membrane fusion. J Cell Biol. 1987;104:141–149. doi: 10.1083/jcb.104.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochwerger L, Cuasnicu PS. Redistribution of a rat sperm epididymal glycoprotein after in vitro and in vivo capacitation. Mol Reprod Dev. 1992;3:34–41. doi: 10.1002/mrd.1080310107. [DOI] [PubMed] [Google Scholar]
- Roldan ER. Role of phospholipases during sperm acrosomal exocytosis. Front Biosci. 1998;3:D1109–D1119. doi: 10.2741/a348. [DOI] [PubMed] [Google Scholar]
- Souza MM, Gross S, Boyle RT, Lieberman M. Na+/K+-ATPase inhibition during cardiac myocyte swelling: Involvement of intracellular pH and Ca2+ Mol Cell Biochem. 2000;210:173–183. doi: 10.1023/a:1007154412805. [DOI] [PubMed] [Google Scholar]
- Thundathil J, de Lamirande E, Gagnon C. Different signal transduction pathways are involved during human sperm capacitation induced by biological and pharmacological agents. Mol Hum Reprod. 2002;8:811–816. doi: 10.1093/molehr/8.9.811. [DOI] [PubMed] [Google Scholar]
- Thundathil J, Anzar M, Buhr MM. Sodium potassium ATPase as a signaling molecule during bovine sperm capacitation. Biol Reprod. 2006;75:308–317. doi: 10.1095/biolreprod.105.047852. [DOI] [PubMed] [Google Scholar]
- Thundathil J, Whiteside D, Shea B, Ludbrook D, Elkin B, Nishi J. Preliminary assessment of reproductive technologies in wood bison (Bison bison athabascae): Implications for preserving genetic diversity. Theriogenology. 2007;68:93–99. doi: 10.1016/j.theriogenology.2007.04.020. [DOI] [PubMed] [Google Scholar]
- Urner F, Sakkas D. Protein phosphorylation in mammalian spermatozoa. Reproduction. 2003;125:17–26. doi: 10.1530/rep.0.1250017. [DOI] [PubMed] [Google Scholar]
- Visconti PE, Bailey JL, Moore GD, Pan D, Olds-Clarke P, Kopf GS. Capacitation of mouse spermatozoa. I. Correlation between the capacitation state and protein tyrosine phosphorylation. Development. 1995;121:1139–1150. doi: 10.1242/dev.121.4.1129. [DOI] [PubMed] [Google Scholar]
- Visconti PE, Westbrook VA, Chertihin O, Demarco I, Sleight S, Diekman AB. Novel signaling pathways involved in sperm acquisition of fertilizing capacity. J Reprod Immunol. 2002;53:133–150. doi: 10.1016/s0165-0378(01)00103-6. [DOI] [PubMed] [Google Scholar]
- Vredenburgh-Wilberg WL, Parrish JJ. Intracellular pH of bovine sperm increases during capacitation. Mol Reprod Dev. 1995;40:490–502. doi: 10.1002/mrd.1080400413. [DOI] [PubMed] [Google Scholar]
- Woo AL, James PF, Lingrel JB. Roles of the Na,K-ATPase alpha4 isoform and the Na+/H+ exchanger in sperm motility. Mol Reprod Dev. 2002;62:348–356. doi: 10.1002/mrd.90002. [DOI] [PubMed] [Google Scholar]
- Xie Z, Askari A. Na+/K+-ATPase as a signal tranducer. Eur J Biochem. 2002;269:2434–2439. doi: 10.1046/j.1432-1033.2002.02910.x. [DOI] [PubMed] [Google Scholar]
- Yanagimachi R. Mammalian fertilization. In: Knobil E, Neill JD, editors. The physiology of reproduction. New York: Raven Press LTD; 1994. pp. 189–317. [Google Scholar]
- Zwick E, Bange J, Ullrich A. Receptor tyrosine kinase signaling as a target for cancer intervention strategies. Endocr Relat Cancer. 2001;8:161–173. doi: 10.1677/erc.0.0080161. [DOI] [PubMed] [Google Scholar]