Abstract
Background
Falls are common among community-dwelling stroke survivors. The aim of this study was to (1) compare motor and cognitive outcomes between individuals who fell in the six months post-discharge from in-patient stroke rehabilitation and those who did not fall, and (2) explore potential mechanisms underlying the relationship between falls and recovery of motor and cognitive function.
Methods
Secondary analysis of a prospective cohort study of individuals discharged home from in-patient rehabilitation was conducted. Participants were recruited at discharge and completed a six-month falls monitoring period using postcards with follow-up. Non-fallers and fallers were compared at the six-month follow-up assessment on the Berg Balance Scale (BBS), Chedoke-McMaster Stroke Assessment (CMSA), gait speed, and Montreal Cognitive Assessment (MoCA). Measures of balance confidence and physical activity were also assessed.
Results
23 fallers were matched to 23 non-fallers on age and functional balance scores at discharge. A total of 43 falls were reported during the study period (8 participants fell more than once). At follow-up, BBS scores (p=0.0066) and CMSA foot scores (p=0.0033) were significantly lower for fallers than non-fallers. The two groups did not differ on CMSA leg scores (p=0.049), gait speed (p=0.47) or MoCA (p=0.23). There was no significant association between change in balance confidence scores and change in physical activity levels among all participants from the first and third questionnaire (r=0.27, p=0.08).
Conclusions
Performance in balance and motor recovery of the foot were compromised in fallers when compared to non-fallers at six months post-discharge from in-patient stroke rehabilitation.
INTRODUCTION
Compared to individuals with severe stroke, those with moderate stroke tend to benefit more from in-patient stroke rehabilitation,1–3 where patients receive specialized care from an interdisciplinary team. Patients typically attend in-patient rehabilitation in the sub-acute phase after stroke (i.e. less than three months post-stroke), and most recovery takes place in the first three to six months after stroke.4 The majority of stroke survivors attending in-patient rehabilitation (64–83%) are discharged to community living.3, 5, 6 Thus, functional recovery, demonstrated by the improved ability to perform activities (e.g. activities of daily living (ADL), and motor tasks),7 continues after discharge. In addition, there is evidence that mobility can continue to improve with ongoing physical activity in the chronic phase of stroke recovery (i.e. more than six months post-stroke).8
Individuals with stroke are at a high risk of falls,9 and the highest rates (37–73%) seem to occur within the first six months after discharge from hospital.10–12 Several studies have investigated risk factors for falls among community-dwelling stroke survivors;10, 11, 13–15 however, only a few have documented the consequences of falling beyond injury. Falls may have psychological sequelae, such as fear of falling, which was reported in 88% of stroke survivors who fell in the community,16 and impaired balance self-efficacy, which has been shown to predict physical function and perceived health status after stroke.17 Falls and fear of falling among individuals with stroke can result in activity restriction,18 and reduced social activity and depression.10 These consequences can put an individual at further risk for falls by accelerating deconditioning, and lead to a loss of independence,9 which may limit cognitive recovery through reduced participation and engagement in everyday activities. It is not yet known what the implications of falls and their consequences are on the functional level of individuals returning home from rehabilitation hospital after stroke. Thus, because of the potential for fear, decreased physical and social activity, it is possible that even falls that do not result in a physical injury may adversely affect continued motor and cognitive recovery after stroke.
The primary objective of this study was to compare motor and cognitive outcomes between individuals who fell in the six months post-discharge from in-patient stroke rehabilitation and those who did not fall. We hypothesized that individuals with stroke who fell in the community would have worse motor and cognitive outcomes (i.e. functional balance, motor recovery of the lower extremities, gait speed, and cognitive status) than those who did not fall when assessed six months after discharge from hospital. The secondary objective was to explore potential mechanisms underlying the relationship between falls and recovery of motor and cognitive function. It was hypothesized that poor motor and cognitive outcomes would be associated with decreased balance confidence and reduced physical activity levels.
METHODS
Study design
This study involved secondary analysis of a prospective cohort study,19 which aimed to determine if measures of reactive balance control, as assessed at discharge from in-patient rehabilitation, predicted falls in the six months post-discharge among individuals with stroke. Recruitment took place on the stroke rehabilitation unit at the Toronto Rehabilitation Institute – University Health Network between October 20, 2010 and March 21, 2013. This study was approved by the Toronto Rehabilitation Institute Research Ethics Board, and all participants provided written informed consent.
Participants
Participants were recruited at discharge following a course of in-patient stroke rehabilitation if they ambulated independently, completed a balance assessment in a specialized clinic, and returned home after discharge (n=95). For the purpose of this study, participants were eligible to be included in the analysis if they returned to the hospital for a follow-up assessment at the end of the six-month falls monitoring period (n=65).
Falls monitoring
Falls monitoring took place for six months post-discharge; participants were asked to report any falls or near falls using postcards mailed back to the investigators every two weeks. This prospective method of data collection is considered the “gold standard” for falls reporting.20 All participants were mailed monthly newsletters to remind them to return their completed postcards. Additionally, a research assistant contacted participants by telephone if they did not return a postcard to ask if they had experienced any falls. A fall was defined as any time an individual came to rest unintentionally on the ground, floor, or other lower level.6 Participants who fell were contacted by phone to complete a structured falls questionnaire, modified from one used by Maki and colleagues,21 to gather more details about the circumstances surrounding the fall (e.g. what the participant was doing at the time, where, when and how the fall occurred, and the consequences of the fall, if any). Falls and near falls were reclassified by study investigators according to the participant’s description of the event, if necessary (e.g. one participant reported a near fall when they lost their footing and lowered themselves into a chair; however, a chair is considered a lower level, and therefore this event was re-classified as a fall).
Data collection
Information to describe the study cohort was collected from the patient chart: age, sex, date of stroke, and stroke type. In addition, the National Institutes for Health Stroke Scale22 was administered by study staff at enrolment. To address the primary objective of falls on stroke recovery, measures of motor and cognitive function were administered at discharge from in-patient stroke rehabilitation and again six months later, and are described below.
Functional balance was assessed using the Berg Balance Scale (BBS),23 which is a 14-item observational rating scale. Participants were asked to perform each of the 14 tasks and their ability to perform the task was rated on a scale from 0 to 4, up to a maximum score of 56. The maximum score indicates good balance, and a score below 45 indicates increased fall risk in the elderly population.24 The BBS is a reliable and valid measure of functional balance in the stroke population,25 with excellent inter-rater reliability (intraclass correlation coefficient (ICC)=0.98).23 The Chedoke-McMaster Stroke Assessment (CMSA)26 leg and foot sub-scales of the impairment inventory were used to determine participants’ motor recovery. Each sub-scale was rated between stages 1 to 7, where lower scores indicate more impairment and 7 indicates full or almost full recovery of function. These assessments were completed as part of a routine battery of tests by the treating physiotherapist at discharge, and repeated by a research physiotherapist at the follow-up assessment. Excellent inter-rater reliability of the CMSA has been reported among stroke patients on a rehabilitation unit (ICC=0.97).26
Gait speed is an objective measure that is sensitive to change over time,27 and has been used as a key indicator of community ambulation among stroke survivors.28, 29 Assessment of gait was performed using the GAITRite walkway system (CIR Systems Inc., Clifton, New Jersey, USA), which is a four-metre long pressure-sensitive mat that records the placement and timing of each footfall. Participants started walking at least one metre away from the mat and walked across the mat until they reached at least one metre from the end of the mat to account for acceleration and deceleration. Each participant walked at their preferred pace and completed enough passes to allow for at least 18 footfalls to be captured by the pressure-sensitive mat (approximately two to four passes). Gait speed was subsequently calculated using the GAITRite software, where all passes of self-selected pace were grouped into one trial. The GAITRite system has been shown to have good concurrent validity in a rehabilitation population,30 good test-retest reliability (ICC=0.72–0.98),31, 32 and good inter- (ICC>0.81) and intra-reliability (ICC>0.77)33 at comfortable walking speeds among individuals with stroke.
Cognitive deficits are common after stroke, and can influence quality of life and independence.34 The Montreal Cognitive Assessment (MoCA)35 is a screening tool for mild cognitive impairment that examines visuospatial skills, executive function, memory, attention, and orientation. The maximum score for the test is 30 points, and a total score less than 26 is considered an indicator of potential cognitive deficit. The MoCA was administered by a research assistant at discharge and follow-up. Excellent test-retest reliability (ICC=0.92) and excellent concurrent validity with the Mini-Mental State Examination (r=0.87) was demonstrated for the MoCA among patients with and without mild cognitive impairment.35
In addition to the primary outcomes, secondary measures of balance confidence and physical activity were assessed to understand the potential mechanisms underlying the hypothesized relationship between falls and functional recovery. The Activities-specific Balance Confidence (ABC) scale36 is a 16-item questionnaire that determines confidence in maintaining balance while performing 16 everyday tasks. Participants were asked to rate each item on a scale from 0–100%, where 0% indicates no confidence, and 100% indicates the highest confidence. The items were averaged to obtain an overall balance confidence score ranging from 0% to 100%. The ABC scale was administered by a research assistant at both the discharge and follow-up assessments to detect changes in balance confidence. In geriatric populations, ABC scale scores have been shown to correlate with performance on balance measures and risk for falls.37, 38 The construct validity of the ABC scale with measures of balance and physical function has been established in the stroke population,39 and the test-retest reliability of the scale is excellent (ICC=0.85).40 The Physical Activity Scale for Individuals with Physical Disabilities (PASIPD)41 is a 13-item questionnaire that assesses physical activity by reporting the number of days per week and hours per day an individual participates in recreational, household, and occupational activities over the last seven days. Each question was scored by multiplying the average hours per day by a metabolic equivalent value associated with the intensity of the activity.41 The total score was calculated by taking the sum of items 2 to 13. In this study, the PASIPD was conducted over the phone at three time points during the six-month falls monitoring period (i.e. approximately every two months). The PASIPD has good test-retest reliability (r=0.77) and the criterion validity against accelerometers has been shown to be comparable to other self-report questionnaires in a rehabilitation population.42
Statistical analysis
The sample was divided into non-fallers (i.e. participants who did not fall in the community; n=39) and fallers (i.e. participants who fell at least once in the community; n=26). A matched sample of non-fallers to fallers was achieved through stratified random sampling on categories of BBS scores at discharge (i.e. 0–35, 36–45, 46–55, 56 points) and age (i.e. <40, 40–59, 60–79, >80 years) in order to create two comparable groups. Characteristics of non-fallers and fallers were compared at discharge from in-patient rehabilitation using the Mann-Whitney U test for continuous or ordinal data, and the chi-square or Fisher’s exact test for nominal data. The alpha level for comparing baseline characteristics of the two groups was set at 0.05. To test the outcome measures of interest, non-fallers and fallers were compared using the Mann-Whitney U test on all measures, except for gait speed which was compared using a Student’s t-test. Non-parametric testing was necessary after assumptions of normality were violated. To examine the primary hypothesis, a one-tailed test was used, and alpha was set at 0.01 (i.e., Bonferonni-corrected for multiple comparisons; 0.05 divided by five primary outcome measures). Associations between the change in balance confidence, physical activity levels, and each of the primary outcomes were analysed using Spearman’s rank correlation coefficients. For these correlations, an alpha level of 0.0167 determined statistical significance (i.e. Bonferonni-corrected for multiple comparisons; 0.05 divided by three comparisons). In the case of missing data at discharge or follow-up, the participant was removed from the analysis of that particular outcome. Statistical analyses were conducted using SAS version 9.2.
RESULTS
A sample of 46 participants (23 non-fallers and 23 fallers) matched on BBS categories and age was formed. Nineteen participants were not included (non-fallers=16, fallers=3; age: 62.7 years, average time post-stroke: 46.9 days); three fallers were lost as a result of not having a non-faller match (10 falls; one faller experienced 8 falls). There were no significant differences on demographic and stroke characteristics between non-fallers and fallers at discharge from rehabilitation (Table 1).
Table 1.
Participant characteristics at discharge from rehabilitation.
| Characteristic | Non-fallers (n=23) | Fallers (n=23) | p value |
|---|---|---|---|
| Age (years) | 64.8 (12.6) | 65.4 (10.4) | 0.96 |
| Number of women | 10 (43) | 9 (39) | 0.76 |
| Time post-stroke (days) | 47.4 (19.2) | 53.4 (19.6) | 0.14 |
| Length of in-patient rehabilitation stay (days) | 30.2 (11.1) | 35.9 (13.7) | 0.17 |
| Type of stroke | |||
| Ischemic | 20 (87) | 18 (78) | 0.74 |
| Hemorrhagic | 2 (9) | 3 (13) | |
| Transforming to hemorrhagic | 1 (4) | 2 (9) | |
| National Institutes of Health Stroke Scale (score) | 2.7 (2.0) | 3.0 (2.9) | 0.96 |
| Berg Balance Scale (0–56 points) | 51.2 (3.4) | 49.1 (4.1) | 0.079 |
NOTE: Values are means (standard deviation) for continuous or ordinal variables, and counts (% rounded to the nearest integer) for categorical variables. The p value is for the Mann-Whitney U test, chi-square or Fisher’s exact test comparing non-fallers to fallers at discharge.
A total of 43 falls were reported during the six-month monitoring period, and eight fallers (8/23, 35%) fell more than once. Eleven fallers (11/23, 48%) experienced their first fall within one month of discharge, and 15/43 of all falls (35%) took place in the first two months (Figure 1).
Figure 1.
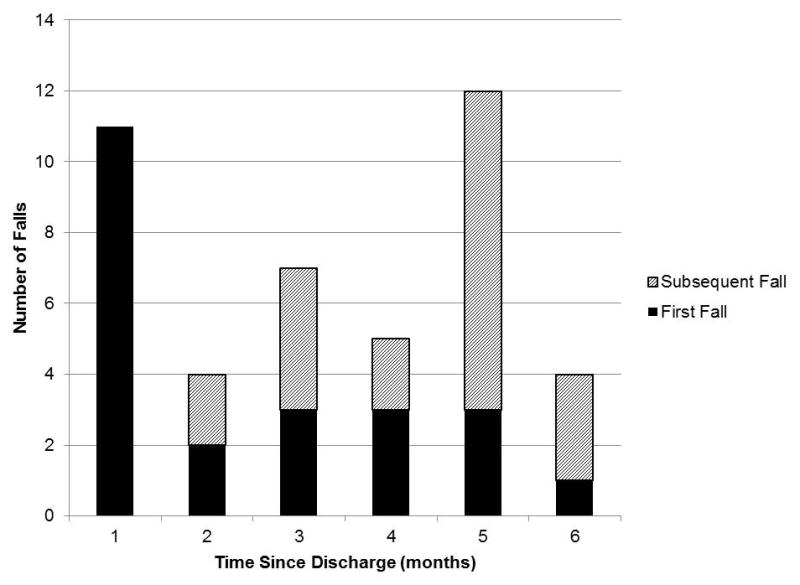
Timing of falls in the community (n=43 falls). Each fall was included with reference to the discharge date among 23 fallers.
The follow-up assessment was conducted at an average time post-stroke of 7.7 months. The Mann-Whitney U test revealed that non-fallers and fallers differed on BBS (W(1)=657.5, Z=2.58, p=0.0066) and CMSA foot scores (W(1)=573, Z=2.85, p=0.0033) at the six-month follow-up assessment. While CMSA leg scores, walking speed, and MOCA scores were generally lower for fallers than non-fallers, there were no statistically significant differences between groups on these measures (p>0.049; Table 2).
Table 2.
Differences in outcome measures at discharge from in-patient stroke rehabilitation and six months post-discharge.
| n | Non-fallers | n | Fallers | p value | |
|---|---|---|---|---|---|
| Berg Balance Scale (points) | |||||
| Discharge | 23 | 51.2 (3.4) | 23 | 49.1 (4.1) | 0.079 |
| Follow-up | 23 | 53.2 (2.8) | 23 | 50.2 (4.0) | 0.0066* |
| Chedoke-McMaster – leg (stage) | |||||
| Discharge | 21 | 5.2 (0.9) | 22 | 4.9 (1.1) | 0.17 |
| Follow-up | 21 | 5.7 (0.6) | 22 | 5.2 (0.9) | 0.049 |
| Chedoke-McMaster – foot (stage) | |||||
| Discharge | 21 | 5.0 (1.2) | 22 | 4.7 (1.4) | 0.23 |
| Follow-up | 21 | 5.7 (0.7) | 22 | 4.8 (1.0) | 0.0033* |
| Gait speed (metres/second) | |||||
| Discharge | 23 | 0.86 (0.3) | 23 | 0.80 (0.4) | 0.57 |
| Follow-up | 23 | 0.97 (0.3) | 23 | 0.90 (0.4) | 0.47 |
| Montreal Cognitive Assessment (points) | |||||
| Discharge | 9 | 22.1 (3.7) | 11 | 22.3 (3.6) | 0.94 |
| Follow-up | 9 | 24.4 (2.7) | 11 | 22.8 (4.2) | 0.23 |
NOTE: Values are means (standard deviation) for continuous or ordinal variables. The p value is for the Mann-Whitney U test comparing both groups for all measures except for gait speed, which was calculated using a Student’s t-test.
p value is significant, where p<0.01 (Bonferroni-corrected for multiple comparisons).
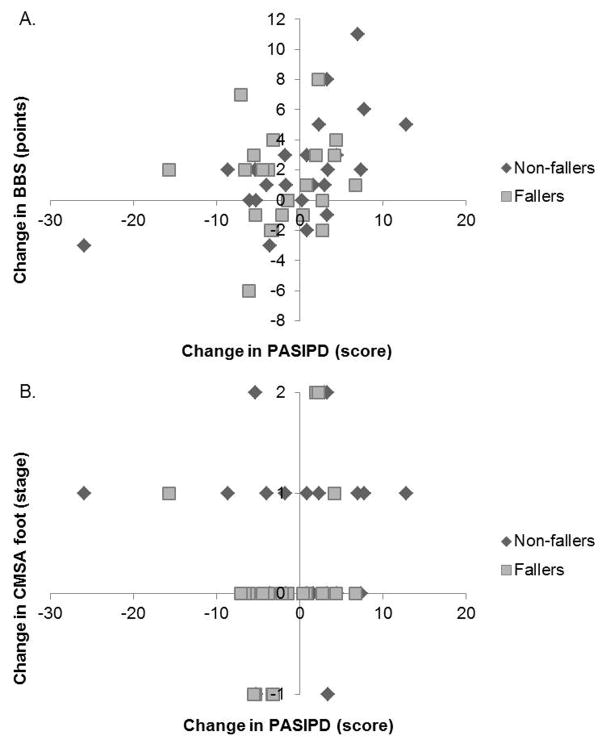
At follow-up, non-fallers reported mean (standard deviation) ABC scores of 79% (19.6%), whereas fallers reported 77% (15.9%). Fallers averaged PASIPD scores of 8.4 (7.7) points, compared to 9.9 (8.2) points for non-fallers at the third time point, which occurred at an average time post-discharge of 5.3 months. There was no statistically significant correlation between change in ABC scores and change in PASIPD scores among all participants (r=0.27, p=0.08). Correlations of physical activity with functional balance and motor recovery of the foot for all participants are presented in Table 3. There was a significant positive correlation between change in PASIPD score and change in functional balance as measured by the BBS (Figure 2 A). However, there was no significant correlation between change in PASIPD score and change CMSA foot score (Figure 2 B).
Table 3.
Spearman correlation coefficients between physical activity and outcomes at six-month follow-up for all participants.
| Change in ABC | Change in BBS | Change in CMSA foot | |
|---|---|---|---|
| Change in PASIPD | r=0.27 | r=0.35 | r=0.19 |
| p=0.08 | p=0.0163* | p=0.22 |
NOTE: ABC=Activities-specific Balance Confidence scale; BBS=Berg Balance Scale; CMSA=Chedoke-McMaster Stroke Assessment; PASIPD=Physical Activity Scale for Individuals with Physical Disabilities.
Statistically significant correlation at alpha<0.0167 (Bonferroni-corrected for multiple comparisons).
Figure 2. Correlations of change in physical activity scores with functional balance and motor recovery of the foot for all participants.
A significant relationship was found in the change in Physical Activity Scale for Individuals with Physical Disabilities (PASIPD) scores and Berg Balance Scale (BBS) scores (A) but not Chedoke-McMaster Stroke Assessment (CMSA) foot scores (B).
DISCUSSION
In partial support of our hypothesis, we found that individuals with stroke who fell in the six months post-discharge from in-patient rehabilitation performed worse on some motor outcomes (i.e. BBS and CMSA foot) than individuals who did not fall. Conversely, CMSA leg scores, gait speed, and cognitive function (MoCA scores) were not significantly different between non-fallers and fallers. These findings agree with those of Blennerhassett and colleagues who completed a follow-up observational study post-discharge from in-patient stroke rehabilitation, and found that fallers had lower scores on balance and mobility tests at follow-up.43 However, in this previous study, the groups differed on the balance and mobility outcome measures at baseline, and the follow-up assessment occurred at a median time of 14.5 months post-discharge. By matching fallers to non-fallers on important measures retrospectively, we minimized the differences between groups at baseline and provide stronger support for the hypothesis that the occurrence of falls influenced motor and cognitive recovery from stroke.
Almost half of all fallers (11/23, 48%) experienced their first fall within the first month after discharge home from in-patient rehabilitation. We hypothesized that falls occurring early post-discharge would lead participants to develop fear of falling and reduced physical activity over time, and that reduced physical and social activity would result in lower performance scores at the six-month follow-up. However, this hypothesis was not fully supported. The results suggest that balance confidence was not reduced among those individuals who experienced a fall, and reduced balance confidence (ABC scores) did not translate to reduced physical activity (PASIPD scores). Nevertheless, individuals who decreased physical activity over the course of the follow-up period demonstrated smaller changes in BBS scores.
On average, individuals may have declined in balance confidence post-discharge as a result of attempting to execute certain activities since returning home to the community that they may not have been exposed to in hospital; thus reducing their confidence based on experience and increased insight into post-stroke limitations. Simpson and colleagues suggested that balance confidence may mediate an effect on falls through the interaction with other variables;44 however, the current study did not find similar results. Levels of physical activity may play a role in stroke recovery, and the reason for inactivity can include post-stroke fatigue, availability of social support, and the residual deficits that impair mobility.45 The low level of physical activity for both groups in this study was not surprising, and the correlation of the changes with BBS scores does not aid in the directionality of the relationship between falls and activity. It is possible that falls led to restricted physical activity, as reported in previous work,18, 43 or that deconditioning as a result of low activity increased the risk of falls. With increased physical activity, greater change in mobility measures may have followed.8 Therefore, a potential area for intervention to influence stroke recovery is increasing balance confidence and physical activity, both of which are modifiable factors at the individual level.
Both non-fallers and fallers were high-functioning at discharge from in-patient rehabilitation, leaving little room for individuals to improve at follow-up. For example, the mean BBS scores were less than six points from the maximum score of 56, suggesting a possible ceiling effect for many individuals; therefore, the BBS may be a tool more suitable for individuals in the acute and sub-acute stages of stroke. This problem may be addressed by incorporating a balance measure that is less susceptible to ceiling effects, such as the Community Balance and Mobility Scale;46 however, this measure was not conducted as part of the primary study, and was therefore not available for secondary analysis. Similarly, the average gait speed for both non-fallers and fallers was considered to be in the community ambulation range (greater than 0.8 m/s),28 and participants enrolled in this study were independently walking at the time of discharge. Achieving levels of community ambulation is important for recovery due to the relationship between improved gait speed and improved participation and quality of life.28, 29, 47 Gait speed has been termed the sixth vital sign,48 and therefore, should be monitored among fallers to ensure continued recovery after stroke.
While it was hypothesized that falls may lead to fear of falling and reduced physical activity, and therefore, less capacity for ongoing recovery, it is possible that fallers were simply on a slower recovery trajectory than non-fallers. This may be partially supported by the longer inpatient rehabilitation stay of the faller group (although not significantly different). This difference could have also been due to factors affecting stroke severity, such as genetics, type, size, and location of stroke, which are predictive of post-stroke outcomes.49 Thus, while CMSA scores were not different between the two groups at discharge, it is possible that fallers had lower foot scores than their non-faller matches at the time of the fall, and the fall was potentially due to a trip that resulted from poor foot clearance when walking as many falls (14/43, 33%) resulted from tripping.
Previous studies of cognitive deficits after stroke suggest that improvement in cognitive function is possible over time, especially within six months post-stroke;50–52 however, another study suggested that cognitive deficits remain stable in the sub-acute phase of stroke.53 In this study, the proportion of participants who scored less than 26 points on the MoCA at discharge dropped slightly by 10% at follow-up. Nevertheless, recovery of cognitive deficits after stroke remains important, as difficulties with attention and perception can influence falls risk.
As individuals return home after stroke, they may be adjusting to a new environment with reduced supervision and assistance compared to the hospital setting.18 Falls that occur early after discharge are likely due to individuals dealing with persistent limitations that may affect balance and mobility, which could in turn increase the risk of falling. On the contrary, Yates and colleagues found that individuals with accumulated impairments may be less mobile, and therefore at a reduced risk of falls.15 Hyndman and colleagues studied individuals with chronic stroke (average time post-stroke: 50 months), and found that falls in the community were associated with reduced rehabilitation potential and functional recovery;14 however, the current study aimed to determine the impact of falls on recovery earlier after stroke in what may be a critical time for regaining function.
Study limitations
This study may have limited ability to detect differences between groups due to small sample size. Post-hoc power analysis was conducted for all non-significant results using meaningful differences in means from the literature26, 54–56 and standard deviations from the current study (i.e. low statistical power ranged from 12% (MoCA) to 63% (CMSA leg score)). Across both groups, data were missing frequently for the MoCA as many participants spoke English as a second language, could not complete the assessment due to scheduling difficulties, or declined. Therefore, additional studies may be required to further investigate the effect of falls on these important motor and cognitive outcomes. This study only included participants from the original study who returned for the six-month follow-up assessment. There may be differences between those who were included and the participants who were not assessed. Poor recovery in function may be one reason why participants did not return for the follow-up assessment, among other reasons (e.g. passed away, lived a far distance from hospital). However, of those who completed the full falls monitoring period, it does not appear as though fallers were less likely to return (8/35, 23%) than non-fallers (22/60, 37%).
Conclusions
The current study found that performance in balance and motor recovery of the foot were compromised in fallers compared to non-fallers at six months post-discharge from in-patient stroke rehabilitation. These differences may be mediated by reduced activity following a fall, resulting in either deconditioning or decreased capacity for ongoing motor recovery. Stroke survivors may benefit from supported discharge including post-falls management strategies.
Acknowledgments
This project has been generously funded by a grant from the Ontario Ministry of Health and Long-Term Care, administered and supported by the Ontario Stroke Network (OSN1101-000117). The authors also acknowledge the support of the Toronto Rehabilitation Institute. Equipment and space have been funded with grants from the Canada Foundation for Innovation, Ontario Innovation Trust and the Ministry of Research and Innovation. The views expressed do not necessarily reflect those of the funders. JSW received funding from the Toronto Rehabilitation Institute Student Scholarship (Ontario Student Opportunity Trust Funds). DB holds a Canada Research Chair. At the time of this study, EI was supported by the Canadian Institutes of Health Research Fellowship (Health Professions). AM holds a New Investigator Award from the Canadian Institutes of Health Research (MSH-141983). Preliminary results were presented at the 2015 International Society for Posture & Gait Research World Congress in Seville, Spain.
Footnotes
Conflict of interest: None.
References
- 1.Pereira S, Graham JR, Shahabaz A, et al. Rehabilitation of individuals with severe stroke: synthesis of best evidence and challenges in implementation. Topics in stroke rehabilitation. 2012;19:122–31. doi: 10.1310/tsr1902-122. [DOI] [PubMed] [Google Scholar]
- 2.Ancheta J, Husband M, Law D, et al. Initial functional independence measure score and interval post stroke help assess outcome, length of hospitalization, and quality of care. Neurorehabilitation and neural repair. 2000;14:127–34. doi: 10.1177/154596830001400205. [DOI] [PubMed] [Google Scholar]
- 3.Gagnon D, Nadeau S, Tam V. Clinical and administrative outcomes during publicly-funded inpatient stroke rehabilitation based on a case-mix group classification model. Journal of rehabilitation medicine: official journal of the UEMS European Board of Physical and Rehabilitation Medicine. 2005;37:45–52. doi: 10.1080/16501970410015055. [DOI] [PubMed] [Google Scholar]
- 4.Kwakkel G, Kollen B, Lindeman E. Understanding the pattern of functional recovery after stroke: facts and theories. Restorative neurology and neuroscience. 2004;22:281–99. [PubMed] [Google Scholar]
- 5.Jørgensen HS, Nakayama H, Raaschou HO, et al. Outcome and time course of recovery in stroke. Part I: Outcome. The Copenhagen stroke study. Archives of Physical Medicine and Rehabilitation. 1995;76:399–405. doi: 10.1016/s0003-9993(95)80567-2. [DOI] [PubMed] [Google Scholar]
- 6.Reistetter TA, Graham JE, Deutsch A, et al. Utility of functional status for classifying community versus institutional discharges after inpatient rehabilitation for stroke. Arch Phys Med Rehabil. 2010;91:345–50. doi: 10.1016/j.apmr.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teasell R, Hussein N. Evidence-Based Review of Stroke Rehabilitation. Background Concepts in Stroke Rehabilitation 2013;Chapter 3 [Google Scholar]
- 8.Ferrarello F, Baccini M, Rinaldi LA, et al. Efficacy of physiotherapy interventions late after stroke: a meta-analysis. Journal of neurology, neurosurgery, and psychiatry. 2011;82:136–43. doi: 10.1136/jnnp.2009.196428. [DOI] [PubMed] [Google Scholar]
- 9.Weerdesteyn V, de Niet M, van Duijnhoven HJ, et al. Falls in individuals with stroke. J Rehabil Res Dev. 2008;45:1195–213. [PubMed] [Google Scholar]
- 10.Forster A, Young J. Incidence and consequences of falls due to stroke: a systematic inquiry. Bmj. 1995;311:83–6. doi: 10.1136/bmj.311.6997.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mackintosh SF, Hill KD, Dodd KJ, et al. Balance score and a history of falls in hospital predict recurrent falls in the 6 months following stroke rehabilitation. Arch Phys Med Rehabil. 2006;87:1583–9. doi: 10.1016/j.apmr.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 12.Kerse N, Parag V, Feigin VL, et al. Falls after stroke: results from the Auckland Regional Community Stroke (ARCOS) Study, 2002 to 2003. Stroke; a journal of cerebral circulation. 2008;39:1890–3. doi: 10.1161/STROKEAHA.107.509885. [DOI] [PubMed] [Google Scholar]
- 13.Harris JE, Eng JJ, Marigold DS, et al. Relationship of balance and mobility to fall incidence in people with chronic stroke. Physical therapy. 2005;85:150–8. [PubMed] [Google Scholar]
- 14.Hyndman D, Ashburn A, Stack E. Fall events among people with stroke living in the community: circumstances of falls and characteristics of fallers. Arch Phys Med Rehabil. 2002;83:165–70. doi: 10.1053/apmr.2002.28030. [DOI] [PubMed] [Google Scholar]
- 15.Yates JS, Lai SM, Duncan PW, et al. Falls in community-dwelling stroke survivors: an accumulated impairments model. J Rehabil Res Dev. 2002;39:385–94. [PubMed] [Google Scholar]
- 16.Watanabe Y. Fear of falling among stroke survivors after discharge from inpatient rehabilitation. International journal of rehabilitation research Internationale Zeitschrift fur Rehabilitationsforschung Revue internationale de recherches de readaptation. 2005;28:149–52. doi: 10.1097/00004356-200506000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Salbach NM, Mayo NE, Robichaud-Ekstrand S, et al. Balance Self-Efficacy and Its Relevance to Physical Function and Perceived Health Status After Stroke. Archives of Physical Medicine and Rehabilitation. 2006;87:364–70. doi: 10.1016/j.apmr.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 18.Mackintosh SF, Hill K, Dodd KJ, et al. Falls and injury prevention should be part of every stroke rehabilitation plan. Clin Rehabil. 2005;19:441–51. doi: 10.1191/0269215505cr796oa. [DOI] [PubMed] [Google Scholar]
- 19.Mansfield A, Wong JS, McIlroy WE, et al. Do measures of reactive balance control predict falls in people with stroke returning to the community? Physiotherapy. 2015 doi: 10.1016/j.physio.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 20.Lamb SE, Jørstad-Stein EC, Hauer K, et al. Development of a Common Outcome Data Set for Fall Injury Prevention Trials: The Prevention of Falls Network Europe Consensus. Journal of the American Geriatrics Society. 2005;53:1618–22. doi: 10.1111/j.1532-5415.2005.53455.x. [DOI] [PubMed] [Google Scholar]
- 21.Maki BE, Holliday PJ, Topper AK. A prospective study of postural balance and risk of falling in an ambulatory and independent elderly population. Journal of gerontology. 1994;49:M72–84. doi: 10.1093/geronj/49.2.m72. [DOI] [PubMed] [Google Scholar]
- 22.Brott T, Adams HP, Jr, Olinger CP, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke; a journal of cerebral circulation. 1989;20:864–70. doi: 10.1161/01.str.20.7.864. [DOI] [PubMed] [Google Scholar]
- 23.Berg K, Wood-Dauphinee S, Williams JI. The Balance Scale: reliability assessment with elderly residents and patients with an acute stroke. Scandinavian journal of rehabilitation medicine. 1995;27:27–36. [PubMed] [Google Scholar]
- 24.Berg KO, Wood-Dauphinee SL, Williams JI, et al. Measuring balance in the elderly: validation of an instrument. Canadian journal of public health = Revue canadienne de sante publique. 1992;83(Suppl 2):S7–11. [PubMed] [Google Scholar]
- 25.Blum L, Korner-Bitensky N. Usefulness of the Berg Balance Scale in stroke rehabilitation: a systematic review. Physical therapy. 2008;88:559–66. doi: 10.2522/ptj.20070205. [DOI] [PubMed] [Google Scholar]
- 26.Gowland C, Stratford P, Ward M, et al. Measuring physical impairment and disability with the Chedoke-McMaster Stroke Assessment. Stroke; a journal of cerebral circulation. 1993;24:58–63. doi: 10.1161/01.str.24.1.58. [DOI] [PubMed] [Google Scholar]
- 27.Salbach NM, Mayo NE, Higgins J, et al. Responsiveness and predictability of gait speed and other disability measures in acute stroke. Arch Phys Med Rehabil. 2001;82:1204–12. doi: 10.1053/apmr.2001.24907. [DOI] [PubMed] [Google Scholar]
- 28.Perry J, Garrett M, Gronley JK, et al. Classification of walking handicap in the stroke population. Stroke; a journal of cerebral circulation. 1995;26:982–9. doi: 10.1161/01.str.26.6.982. [DOI] [PubMed] [Google Scholar]
- 29.Schmid A, Duncan PW, Studenski S, et al. Improvements in speed-based gait classifications are meaningful. Stroke; a journal of cerebral circulation. 2007;38:2096–100. doi: 10.1161/STROKEAHA.106.475921. [DOI] [PubMed] [Google Scholar]
- 30.Webster KE, Wittwer JE, Feller JA. Validity of the GAITRite walkway system for the measurement of averaged and individual step parameters of gait. Gait & posture. 2005;22:317–21. doi: 10.1016/j.gaitpost.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 31.Kuys SS, Brauer SG, Ada L. Test-retest reliability of the GAITRite system in people with stroke undergoing rehabilitation. Disability and rehabilitation. 2011;33:1848–53. doi: 10.3109/09638288.2010.549895. [DOI] [PubMed] [Google Scholar]
- 32.Lewek MD, Randall EP. Reliability of spatiotemporal asymmetry during overground walking for individuals following chronic stroke. Journal of neurologic physical therapy: JNPT. 2011;35:116–21. doi: 10.1097/NPT.0b013e318227fe70. [DOI] [PubMed] [Google Scholar]
- 33.Wong JS, Jasani H, Poon V, et al. Inter- and intra-rater reliability of the GAITRite system among individuals with sub-acute stroke. Gait & posture. 2014;40:259–61. doi: 10.1016/j.gaitpost.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 34.Tatemichi TK, Desmond DW, Stern Y, et al. Cognitive impairment after stroke: frequency, patterns, and relationship to functional abilities. Journal of neurology, neurosurgery, and psychiatry. 1994;57:202–7. doi: 10.1136/jnnp.57.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society. 2005;53:695–9. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 36.Powell LE, Myers AM. The Activities-specific Balance Confidence (ABC) Scale. The journals of gerontology Series A, Biological sciences and medical sciences. 1995;50A:M28–34. doi: 10.1093/gerona/50a.1.m28. [DOI] [PubMed] [Google Scholar]
- 37.Lajoie Y, Gallagher SP. Predicting falls within the elderly community: comparison of postural sway, reaction time, the Berg balance scale and the Activities-specific Balance Confidence (ABC) scale for comparing fallers and non-fallers. Archives of gerontology and geriatrics. 2004;38:11–26. doi: 10.1016/s0167-4943(03)00082-7. [DOI] [PubMed] [Google Scholar]
- 38.Myers AM, Powell LE, Maki BE, et al. Psychological indicators of balance confidence: relationship to actual and perceived abilities. The journals of gerontology Series A, Biological sciences and medical sciences. 1996;51:M37–43. doi: 10.1093/gerona/51a.1.m37. [DOI] [PubMed] [Google Scholar]
- 39.Salbach NM, Mayo NE, Hanley JA, et al. Psychometric evaluation of the original and Canadian French version of the activities-specific balance confidence scale among people with stroke. Arch Phys Med Rehabil. 2006;87:1597–604. doi: 10.1016/j.apmr.2006.08.336. [DOI] [PubMed] [Google Scholar]
- 40.Botner EM, Miller WC, Eng JJ. Measurement properties of the Activities-specific Balance Confidence Scale among individuals with stroke. Disability and rehabilitation. 2005;27:156–63. doi: 10.1080/09638280400008982. [DOI] [PubMed] [Google Scholar]
- 41.Washburn RA, Zhu W, McAuley E, et al. The physical activity scale for individuals with physical disabilities: development and evaluation. Arch Phys Med Rehabil. 2002;83:193–200. doi: 10.1053/apmr.2002.27467. [DOI] [PubMed] [Google Scholar]
- 42.van der Ploeg HP, Streppel KR, van der Beek AJ, et al. The Physical Activity Scale for Individuals with Physical Disabilities: test-retest reliability and comparison with an accelerometer. Journal of physical activity & health. 2007;4:96–100. doi: 10.1123/jpah.4.1.96. [DOI] [PubMed] [Google Scholar]
- 43.Blennerhassett JM, Dite W, Ramage ER, et al. Changes in balance and walking from stroke rehabilitation to the community: a follow-up observational study. Arch Phys Med Rehabil. 2012;93:1782–7. doi: 10.1016/j.apmr.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 44.Simpson LA, Miller WC, Eng JJ. Effect of Stroke on Fall Rate, Location and Predictors: A Prospective Comparison of Older Adults with and without Stroke. PLoS ONE. 2011;6:e19431. doi: 10.1371/journal.pone.0019431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gordon NF, Gulanick M, Costa F, et al. Physical Activity and Exercise Recommendations for Stroke Survivors: An American Heart Association Scientific Statement From the Council on Clinical Cardiology, Subcommittee on Exercise, Cardiac Rehabilitation, and Prevention; the Council on Cardiovascular Nursing; the Council on Nutrition, Physical Activity, and Metabolism; and the Stroke Council. Stroke; a journal of cerebral circulation. 2004;35:1230–40. doi: 10.1161/01.STR.0000127303.19261.19. [DOI] [PubMed] [Google Scholar]
- 46.Inness EL, Howe JA, Niechwiej-Szwedo E, et al. Measuring Balance and Mobility after Traumatic Brain Injury: Validation of the Community Balance and Mobility Scale (CB&M) Physiotherapy Canada Physiotherapie Canada. 2011;63:199–208. doi: 10.3138/ptc.2009-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lord SE, McPherson K, McNaughton HK, et al. Community ambulation after stroke: how important and obtainable is it and what measures appear predictive? Arch Phys Med Rehabil. 2004;85:234–9. doi: 10.1016/j.apmr.2003.05.002. [DOI] [PubMed] [Google Scholar]
- 48.Fritz S, Lusardi M. White paper: “walking speed: the sixth vital sign”. Journal of geriatric physical therapy (2001) 2009;32:46–9. [PubMed] [Google Scholar]
- 49.Kwakkel G, Kollen BJ. Predicting activities after stroke: what is clinically relevant? International Journal of Stroke. 2013;8:25–32. doi: 10.1111/j.1747-4949.2012.00967.x. [DOI] [PubMed] [Google Scholar]
- 50.Rasquin SM, Lodder J, Ponds RW, et al. Cognitive functioning after stroke: a one-year follow-up study. Dementia and geriatric cognitive disorders. 2004;18:138–44. doi: 10.1159/000079193. [DOI] [PubMed] [Google Scholar]
- 51.Nys GM, Van Zandvoort MJ, De Kort PL, et al. Domain-specific cognitive recovery after first-ever stroke: a follow-up study of 111 cases. Journal of the International Neuropsychological Society: JINS. 2005;11:795–806. doi: 10.1017/s1355617705050952. [DOI] [PubMed] [Google Scholar]
- 52.Hochstenbach JB, den Otter R, Mulder TW. Cognitive recovery after stroke: a 2-year follow-up 1. Archives of Physical Medicine and Rehabilitation. 2003;84:1499–504. doi: 10.1016/s0003-9993(03)00370-8. [DOI] [PubMed] [Google Scholar]
- 53.Rasquin SM, Welter J, van Heugten CM. Course of cognitive functioning during stroke rehabilitation. Neuropsychological rehabilitation. 2013;23:811–23. doi: 10.1080/09602011.2013.821950. [DOI] [PubMed] [Google Scholar]
- 54.Fulk GD, Ludwig M, Dunning K, et al. Estimating clinically important change in gait speed in people with stroke undergoing outpatient rehabilitation. Journal of neurologic physical therapy: JNPT. 2011;35:82–9. doi: 10.1097/NPT.0b013e318218e2f2. [DOI] [PubMed] [Google Scholar]
- 55.Boss HM, Van Schaik SM, Deijle IA, et al. A randomised controlled trial of aerobic exercise after transient ischaemic attack or minor stroke to prevent cognitive decline: the MoveIT study protocol. BMJ Open. 2014:4. doi: 10.1136/bmjopen-2014-007065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marzolini S, Oh P, McIlroy W, et al. The effects of an aerobic and resistance exercise training program on cognition following stroke. Neurorehabilitation and neural repair. 2013;27:392–402. doi: 10.1177/1545968312465192. [DOI] [PubMed] [Google Scholar]