Abstract
We revisit Stanley Garn’s theory related to sex differences in endocortical and periosteal apposition during adolescence using a 12-year mixed longitudinal study design. We used peripheral quantitative computed tomography to examine bone parameters in 230 participants (110 boys, 120 girls; 11.0 yrs at baseline). We assessed total (Tt.Ar, mm2), cortical (Ct.Ar, mm2), and medullary canal area (Me.Ar, mm2), Ct.Ar/Tt.Ar, cortical bone mineral density (Ct.BMD, mg/cm3) and polar strength-strain index (SSIp, mm3) at the tibial midshaft (50% site). We used annual measures of height and chronological age to identify age at peak height velocity (APHV) for each participant. We compared annual accrual rates of bone parameters between boys and girls, aligned on APHV using a linear mixed effects model. At APHV, boys demonstrated greater Tt.Ar (Ratio: 1.27; 95% CI: [1.21, 1.32]), Ct.Ar (1.24; [1.18, 1.30]), Me.Ar (1.31; [1.22, 1.40]) and SSIp (1.36; [1.28, 1.45]), and less Ct.Ar/Tt.Ar (0.98; [0.96, 1.00]) and Ct.BMD (0.97; [0.96, 0.97]) compared with girls. Boys and girls demonstrated periosteal bone formation and net bone loss at the endocortical surface. Compared with girls, boys demonstrated greater annual accrual rates pre-APHV for Tt.Ar (1.18; [1.02, 1.34]) and Me.Ar (1.34; [1.11, 1.57]), lower annual accrual rates pre-APHV for Ct.Ar/Tt.Ar (0.56; [0.29, 0.83]) and Ct.BMD (−0.07; [−0.17, 0.04]) and similar annual accrual rates pre-APHV for Ct.Ar (1.10; [0.94, 1.26]) and SSIp (1.14; [0.98, 1.30]). Post-APHV, boys demonstrated similar annual accrual rates for Ct.Ar/Tt.Ar (1.01; [0.71, 1.31]) and greater annual accrual rates for all other bone parameters compared with girls (Ratio: 1.23 – 2.63; 95% CI: 1.11 to 3.45). Our findings support those of Garn and others of accelerated periosteal apposition during adolescence, more evident in boys than girls. However, our findings challenge the notion of greater endocortical apposition in girls, suggesting instead that girls experience diminished endocortical resorption compared with boys.
Keywords: pQCT, bone accrual, bone strength, peak height velocity, growth
Introduction
An abundance of research now supports that childhood and adolescence are critical periods for bone mineral accrual.(1–4) However, the intricacies of how bone is gained (in childhood) and lost (in later life) are still not completely understood. In the 1960s and 1970s, Garn and colleagues examined the surface-specific changes that accompany bone growth and development.(5–7) They conducted cross-sectional radiographic studies of the second metacarpal and concluded that both boys and girls exhibit endocortical and periosteal apposition within the diaphysis of the second metacarpal during adolescent growth, but that girls experience more endocortical apposition compared with boys and that boys experience more periosteal apposition compared with girls.(5–7) It has been proposed that this sexual dimorphism contributes to increased bone fragility in women compared with men in older adulthood.(8,9)
The pioneering studies of Garn and colleagues advanced our understanding of sex differences in bone development. However, this early work was limited by the use of planar radiographs to evaluate bone surfaces and caution must be applied when generalizing Garn’s theory to all skeletal sites, as his radiographic studies focused on the non-weight bearing metacarpals. The advent of peripheral quantitative computed tomography (pQCT) permits scrutiny of the commonly held tenets regarding bone apposition and resorption on surfaces of growing bone. In a 20-month longitudinal study of boys and girls (10 to 13 years at baseline) using pQCT, we did not observe endocortical bone apposition or resorption in girls (assessed as the area of the medullary canal) at the tibial midshaft.(10) Similarly, boys demonstrated no endocortical apposition, but compared with girls, boys displayed significant endocortical resorption.(10) In contrast, a 2-year longitudinal study of girls (10–13 years at baseline) reported both periosteal apposition and endocortical apposition at the tibia shaft after menarche.(11) The contradictory findings of endocortical apposition in the studies by Garn et al.(5–7) and Wang et al.(11) compared with the absence of endocortical apposition in the study by Kontulainen et al.(10) have yet to be explored in a longitudinal study that spans a longer period of adolescent growth.
Garn and colleagues also did not control for maturational status in their cross-sectional studies. Failure to consider the tremendous variation in maturational status of children at the same chronological age can dramatically affect outcomes of cross-sectional and intervention studies.(12) Age at peak height velocity (APHV) is most commonly used as an indicator of somatic maturity in longitudinal studies of childhood and adolescent growth(12,13) and is highly correlated with sexual maturation.(13,14) APHV refers to the age when maximum linear growth in height occurs and generally occurs in boys and girls at a maturational time point when approximately 90% of adult stature has been achieved.(15) Serial measurements surrounding maximal height velocity are required to determine APHV.
Therefore, we aimed to advance the classic studies of Garn and colleagues by evaluating the sex- and surface-specific pattern of bone accrual on the periosteal and endocortical surfaces of the weight bearing mid-tibia in boys and girls, aligned by maturity. Our objectives were to: 1) compare rates of bone apposition and/or resorption at the periosteal and endocortical surfaces of the tibia and 2) compare rates of cortical bone density and bone strength accrual, between boys and girls pre and post-APHV. Our current study extends our previous 20-month pQCT study to 12 years to further evaluate these bone surface-specific events.
Methods
Study design
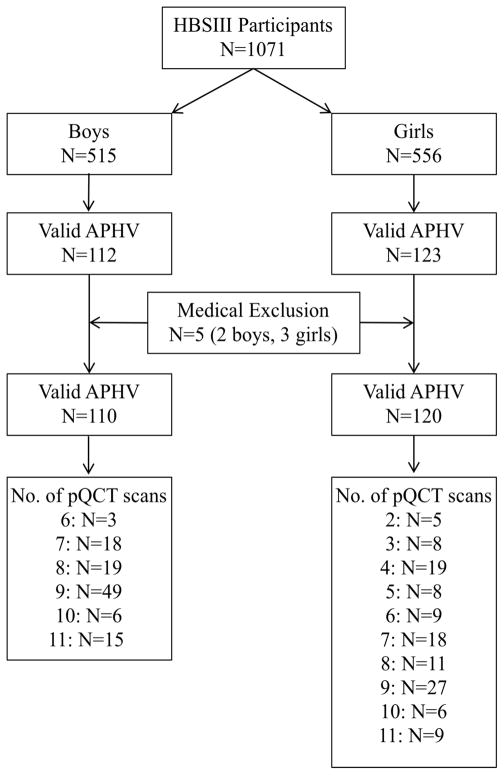
Participants were drawn from a cohort of healthy girls (n=556) and boys (n=515) aged 8 to 12 years at baseline who comprised the University of British Columbia Healthy Bones Study III (HBSIII; Figure 1). The HBSIII cohort includes participants from three school-based studies, described in detail elsewhere.(16–21) Briefly, we recruited participants from elementary schools in Vancouver and Richmond, British Columbia (BC), Canada between 1999 and 2009. We recruited the first cohort (n=436), for HBS II(16–18) and the Bounce at the Bell study,(21) in the fall of 1999 and 2000, respectively, from grades 4, 5 and 6 classes in 14 schools in Richmond, BC. In February 2003, we recruited the second cohort (n=515) for the Action Schools! BC study(19) from grade 4 and 5 classes at 10 elementary schools in Vancouver and Richmond, BC. Participants in both cohorts attended annual follow-up measurements each spring after cessation of the school-based interventions. We combined the cohorts in 2006 because they employed nearly identical protocols. In the fall of 2009, we recruited a third cohort (n=120) from grade 4 and 5 classes in 5 schools in Vancouver and Richmond, BC. We recruited this cohort to study bone microstructural changes from pre-puberty through young adulthood using more advanced imaging technology (high resolution pQCT) that was unavailable for our earlier studies.(20)
Figure 1.
Number of participants recruited and the number of valid peripheral quantitative computed tomography (pQCT) follow-up scans for boys and girls.
In the present analysis, we included bone data from annual measurements conducted between May 2001 (first year of pQCT measurements) and June 2012. Of the full sample (n=1071) there was a median of 3 annual measurements (interquartile range: 2 to 7). We excluded observations from children actively participating in an intervention (n=451, spring 2004)(19) as we previously demonstrated a positive effect of a physical activity intervention on bone accrual.(16,18) However, we included all additional follow-up measurements (2005–2011) regardless of group assignment, as participation in an exercise intervention was not associated with sustained benefits at the tibial shaft.(22) Specifically, we explored the influence of group assignment on bone parameters from 2005–2011; since group assignment was not a statistically significant predictor of bone parameters (data not shown), we pooled the data for the intervention and control groups. For the purpose of this analysis, we refer to data obtained at the first pQCT measurement as baseline.
We obtained written informed consent from the parents or legal guardians, written assent from participants younger than 18 years of age and informed consent from participants 18 years of age and older. The University of British Columbia’s Clinical Research Ethics Board approved the studies.
Anthropometry
We assessed stature during standing and sitting using standard stretch stature techniques.(23) Measurements were recorded to the nearest millimeter using a customized wall-mounted stadiometer (1999 – 2002) and thereafter using the Seca wall-mounted stadiometer (Model 242, Hanover, MD). Body mass was measured to the nearest 0.1 kg on a calibrated electronic scale (Seca Model 840, Hanover, MD), with participants dressed in light clothing. Tibia length was measured to the nearest millimeter using standard methods and a metal anthropometric tape. All measurements were taken in duplicate by trained research assistants, unless differences were > 0.4cm or 0.2kg and then a third measure was taken. We used the mean of two values or the median of three for all analyses. In our laboratory, reproducibility (CV%) is <0.3% for measures of stature and <3.5% for tibia length.
Age at Peak Height Velocity (APHV)
To control for well-known maturational differences between adolescent boys and girls of the same chronological age, we calculated age at peak height velocity (APHV, years) as an estimate of biological maturity. A detailed description of this process is provided in Supplementary Document 1. In brief, we fit an interpolating cubic spline(2) to each participant’s height velocity data. The magnitude of PHV was identified as growth per year (cm) that occurred at APHV. We used APHV to calculate a biological maturity offset (in years) by subtracting the APHV from chronological age at time of measurement. Thus, we generated a continuous measure of biological age (e.g., −1 year is equivalent to 1 year prior to attainment of APHV; +1 to one year after APHV). Due to missing and mistimed measurements surrounding APHV, we were able to identify APHV for 235 of the 1071 participants (112 boys, 123 girls).
Health history, ethnicity and lifestyle
Parents completed a health history questionnaire for their child at baseline and participants completed a shorter version of the questionnaire at subsequent annual visits. Based on questionnaire responses we identified five participants who had conditions that prevented their participation in regular physical activity and/or reported medical conditions known to influence bone metabolism (osteogenesis imperfecta, fetal alcohol syndrome, Type 1 diabetes, Leukemia, congenital heart defect). We excluded these participants from our analysis. Thus, we included 230 healthy participants (110 boys, 120 girls) in our analysis.
We determined each participant’s ethnicity based on their parents’ and/or grandparents’ place of birth as reported on the health history questionnaire at baseline. Parents were asked to classify their own, and their child’s ethnicity. We classified participants as “Asian” if both parents or three of four grandparents were born in Hong Kong, China, Japan, Taiwan, Philippines, Korea or India; “white” if both parents or three of four grandparents were born in North America or Europe; and “Other” if participant’s parents were of other or mixed ethnicities.
We assessed leisure-time physical activity using a modified version of the physical activity questionnaire for children (PAQ-C).(24) We calculated a general physical activity score as an average of the PAQ-C items in a continuous range between 1 (low activity) and 5 (high activity). Based on participants’ estimates of time spent in common sports and activities in Item 1 we also estimated time spent in moderate-to-vigorous physical activity (MVPA, min/day) and time spent in activities designated as loaded (impact > walking, load time; hrs/week). We used a validated food frequency questionnaire (FFQ)(25) to assess dietary calcium intake (mg/day).
Bone geometry, density and strength
We describe our methods for acquisition and analyses of pQCT scans in detail elsewhere.(10,26) Briefly, we acquired a 2.3 mm slice at the midshaft (50% site; proximal to the distal tibial endplate) of the left tibia using the XCT-2000 (Norland/Stratec Medizintechnic GmbH, Pforzheim, Germany) from 2003–2007 and the XCT-3000 (Norland/Stratec Medizintechnic GmbH, Pforzheim, Germany) from 2008 onward. We previously reported excellent agreement between the XCT-2000 and XCT-3000 (CVrms 0.6–1.5% for tibial midshaft bone parameters).(27) We used a scan speed of 30mm/sec and a resolution (voxel size) of 0.5mm in participants recruited prior to 2003 (n=78) and a resolution of 0.4mm thereafter (n=152). Previous work confirmed no significant differences in total area (Tt.Ar) or total bone mineral density (Tt.BMD) measures between 0.4mm and 0.5mm resolution scans at the distal radius using pQCT.(28) We acquired a 30 mm planar scout view over the joint line to define the anatomic reference line and used the same anatomical reference line to assess the same relative site each year.
Our outcome variables were total area (Tt.Ar, mm2) to assess change on the periosteal (outer) surface; cortical area (Ct.Ar, mm2) to evaluate change in cross-sectional area between the periosteal and endocortical surfaces; the ratio of Ct.Ar to Tt.Ar (Ct.Ar/Tt.Ar) as a surrogate to assess change in cortical thickness; medullary canal area (Me.Ar, mm2) to assess change on the endocortical (inner) surface; cortical density (Ct.BMD, mg/cm3) and the polar strength-strain index (SSIp, mm3), an estimate of torsional bone strength.
We analyzed all scans using Stratec software version 6.0 as per our standard protocol.(10,26,29) We used Peel mode 2 (540 mg/cm3), Cortmode/Separation mode 1 (default, 711 mg/cm3), and Contmode 1 (default, 711 mg/cm3) to calculate values for Tt.Ar, Ct.Ar and Ct.BMD. We calculated Me.Ar as Ct.Ar subtracted from Tt.Ar. We used Peel mode 2 (540 mg/cm3) and Cortmode/Separation mode 1 (480 mg/cm3) to determine SSIp. We determined in-vivo precision with repositioning at the 50% site using the XCT-2000 in 14 participants (12–27 years). The coefficient of variation (CV, %) was less than 2% for all bone variables.(30) Scans were acquired by one of 8 trained operators over the 12-year period. A pQCT anthropomorphic phantom was scanned daily to maintain quality assurance. We included all participants with at least one pQCT scan and for whom we were able to obtain APHV. We excluded all scans with motion artifacts (n = 9 across 12-years).
Data Cleaning
Prior to modeling our data, we first examined scatter plots generated for bone area, density and strength versus maturity offset for each participant. We specifically looked for negative changes in anthropometry and/or bone parameters – known to occur due to slight differences in positioning, limb length measures or other measurement error. We identified potential measurement errors using the following protocol. A negative change in height during childhood and adolescence would likely represent measurement error, as would a negative change in Tt.Ar at the tibia midshaft. Thus, we considered a negative change in Tt.Ar as an indicator of measurement error for bone measures. We allowed for a 0.7% buffer based on in vivo precision estimates from our lab.(10,30) When Tt.Ar was > 0.7% lower than the previous years recorded value, we flagged this measurement time point for further inspection. We first visually confirmed all ‘flagged’ Tt.Ar data points. We then applied a linear interpolation between the data point prior to and after the flagged data point for all bone parameters for that measurement time point (n=49/1756 total measures). For example, if Tt.Ar was flagged, linear interpolation was applied to measures of Tt.Ar, Ct.Ar, Me.Ar, Ct.BMD and SSIp at that measurement period. If a data point was flagged at the last measurement period (n=15), we used the previous year’s value. For density measures, we allowed a two-year buffer around APHV within which negative changes were accepted, as density measures may transiently decrease during maturation.(31) Lastly, in 2009 a scanner malfunction with the XCT-3000 required the use of a different XCT-3000 for all HBSIII scans. Phantom scans indicated a systematic underestimation of bone density measures by the replacement XCT-3000; thus, we interpolated all Ct.BMD measures for 2009 (n=158 scans). We then manually examined scatter plots of all bone measures against maturity offset for each participant to verify linear interpolation.
Statistical Analysis
We performed all analyses using STATA, Version 12.1 (StataCorp, College Station, TX). We used linear mixed effects models (also called random coefficients regression models or multilevel models) to compare the annual rate of change in the bone parameters between girls and boys pre-APHV and post-APHV as maturity offset = 0 is a common maturational landmark used in pediatric studies.(12) For each bone variable, we fit a piecewise linear model with a breakpoint at APHV, i.e. maturity offset = 0. Sex, ethnicity, a linear spline for maturity offset (MO1, MO2) and the interaction of sex and maturity offset were included as fixed effects in the model. A random intercept and random slopes were included, allowing each individual’s profile to vary about the average curve.
The regression model is:
where yij is the bone parameter on measurement occasion i in the jth individual, (b0j, b1j, b2j)~ N(0, Σ) is the vector of random effects for the jth individual and εij ~ N(0,σ2) is the within-subject error.
The average curves for an Asian participant, for example, are:
Thus, the intercepts β0 and (β0 + β3) represent the average value of the bone parameter when maturity offset is zero for girls and boys, respectively. Similarly, the slopes β1 and (β1+β4) represent the average annual rates of change pre-APHV and β2 and (β2+β5) represent the average annual rates of change post-APHV. Model adequacy was checked graphically using plots of transformed residuals.(32) Diagnostic checking of the fitted models revealed some serial correlation in the residuals; however, attempting to incorporate a serial correlation component into the model led to problems with model convergence, an issue that has been identified by others.(32) Models that included serial correlation and only a random intercept yielded similar results to the random coefficients only model. Between-sex differences were summarized as rate ratios and were estimated as nonlinear combinations of the model’s coefficients. P-values <0.05 were considered statistically significant.
Results
Descriptive Characteristics
We provide participant characteristics and baseline bone parameters in Table 1. The proportion of girls and boys included in the present analysis and ethnic diversity was similar to that in the larger HBSIII cohort (52% girls, 47% white). Baseline height was also similar between participants included in the current analyses (n=230) and those we excluded if we were unable to calculate APHV. Excluded participants were 0.1 years older and weighed 1.6 kg more at baseline, on average, compared with those participants included in the analyses.
Table 1.
Characteristics of boys and girls at first pQCT measurement. Data are reported as mean (standard deviation) unless otherwise indicated.
| Boys (n=110) | Girls (n=120) | |
|---|---|---|
| Age (yrs) | 11.0 (1.2) | 10.9 (1.0) |
| # 9/10/11/12/13/14 (yrs) | 20/42/25/20/0/3 | 22/58/15/25/0/0 |
| #Asian/ White/Other | 45/56/9 | 56/52/12 |
| APHV (yrs) | 13.1 (1.2) | 11.5 (0.8) |
| Height (cm) | 146.3 (10.1) | 145.5 (9.7) |
| Weight (kg) | 40.1 (10.3) | 39.1 (10.6) |
| Sitting height (cm) | 76.6 (4.9) | 76.5 (5.1) |
| Tibial length (mm) | 338.8 (28.2) | 337.0 (26.5) |
| Physical activity score | 3.1 (0.6) | 2.9 (0.5) |
| MVPA (min/day) | 110.5 (68.5) | 83.1 (55.7) |
| Load time (hrs/wk) | 7.4 (5.2) | 5.3 (4.4) |
| Dietary calcium (mg/day) | 956 (538) | 880 (426) |
| Tt.Ar (mm2) | 329.4 (68.9) | 311.2 (62.0) |
| Ct.Ar (mm2) | 211.3 (44.6) | 204.6 (42.2) |
| Ct.Ar/Tt.Ar | 0.64 (0.05) | 0.66 (0.05) |
| Me.Ar (mm2) | 118.1 (31.9) | 106.6 (29.2) |
| Ct.BMD (mg/cm3) | 1039.0 (33.9) | 1060.9 (33.9) |
| SSIp (mm3) | 1132.7 (361.2) | 1060.7 (312.0) |
MVPA=moderate-to-vigorous physical activity; APHV=age at peak height velocity; pQCT bone parameters: Tt.Ar = Total area; Ct.Ar = Cortical area; Me.Ar = Medullary canal area; Ct.BMD= Cortical bone mineral density; SSIp = strength-strain index.
Comparisons of Bone Parameters between Boys and Girls at APHV
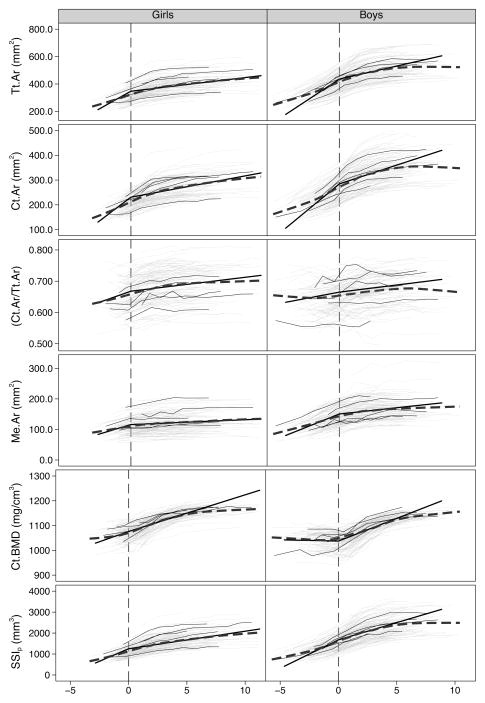
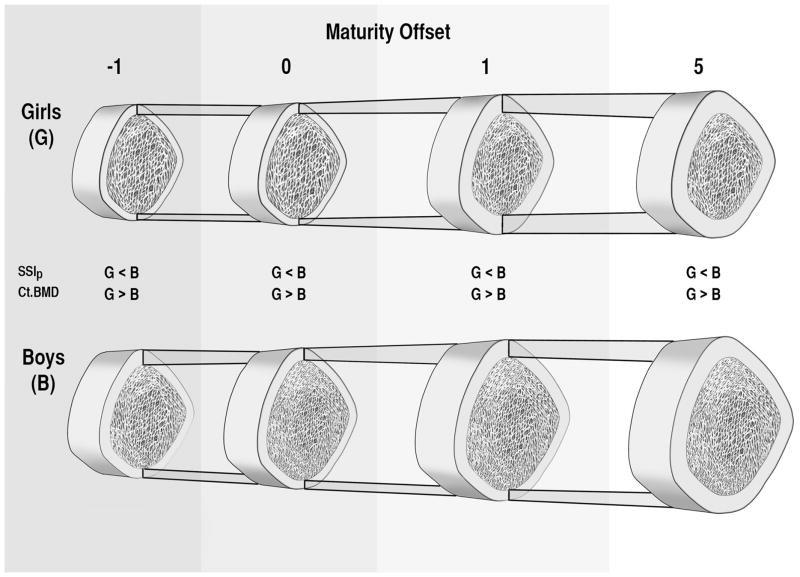
We provide mean values at APHV for all bone parameters for boys and girls in Table 2. For all bone variables (except Ct.Ar/Tt.Ar and Ct.BMD) boys’ mean values at APHV were 24–36% greater than girls’. Boys had 2 and 3% lower values for Ct.Ar/Tt.Ar and Ct.BMD, respectively, at APHV compared with girls. These differences are depicted in the individual growth curves and lowess curves aligned on maturity offset (Figure 2) and in a schematic representation of differences in bone parameters in relation to maturity offset (Figure 3). At APHV, white and Other participants had significantly greater Tt.Ar (Ratio: 1.11; 95% CI: [1.06, 1.16], 1.15; [1.07, 1.23]), Ct.Ar (1.13; [1.08, 1.18], 1.15; [1.07, 1.23]), Me.Ar (1.07; [1.00, 1.14], 1.15; [1.03, 1.27]) and SSIp (1.13; [1.06, 1.19], 1.17; [1.06, 1.23]), respectively, compared with Asian participants. White participants had significantly greater Ct.Ar/Tt.Ar (1.02; [1.00, 1.04]) and lower Ct.BMD (0.99, [0.98. 1.00]) compared with Asian participants at APHV (Supplemental Figure 1).
Table 2.
Estimates of model intercepts. Intercepts represent the average value of the bone parameter at APHV (maturity offset = 0). Numbers in brackets are the standard error of the parameter estimate or the 95% confidence interval for the ratio.
| Boys | Girls | Ratio | p-value | |
|---|---|---|---|---|
| Tt.Ar (mm2) | 413.4 (7.2) | 326.6 (6.8) | 1.27 (1.21 to 1.32) | <0.001 |
| Ct.Ar (mm2) | 267.9 (4.7) | 215.6 (4.5) | 1.24 (1.18 to 1.30) | <0.001 |
| Ct.Ar/Tt.Ar | 0.647 (0.006) | 0.662 (0.006) | 0.98 (0.96 to 1.00) | 0.027 |
| Me.Ar (mm2) | 145.5 (3.7) | 111.0 (3.5) | 1.31 (1.22 to 1.40) | <0.001 |
| Ct.BMD (mg/cm3) | 1042.9 (3.6) | 1080.7 (3.5) | 0.97 (0.96 to 0.97) | <0.001 |
| SSIp (mm3) | 1585.8 (36.6) | 1165.5 (35.0) | 1.36 (1.28 to 1.45) | <0.001 |
Tt.Ar, total area; Ct.Ar, cortical area; Me.Ar, medullary canal area; Ct.BMD, cortical bone mineral density; SSIp strength-strain index
For our purposes, intercepts presented refer to Asian participants
Figure 2.
Individual growth curves (thin, light gray lines), individual growth curves of five randomly selected girls and boys (thin, black lines), a lowess-smoothing curve (thick, dark gray dashed line) and the piecewise linear regression curves (thick, black line) of total area (Tt.Ar), cortical area (Ct.Ar), ratio of Ct.Ar to Tt.Ar (Ct.Ar/Tt.Ar), medullary canal area (Me.Ar), cortical bone mineral density (Ct.BMD) and polar strength-strain index (SSIp), plotted against maturity offset. The vertical line indicates maturity offset (years from age at peak height velocity) of 0.
Figure 3.
A schematic representation of differences in total area (Tt.Ar), cortical area (Ct.Ar), Ct.Ar/Tt.Ar, medullary canal area (Me.Ar), and cortical bone mineral density (Ct.BMD) in boys and girls in relation to maturity offset (years from age at peak height velocity). For our purposes we present maturity offset at −1, 0, 1 and 5 years. Significant differences between girls and boys are shown for strength-strain index (SSIp), where boys’ values exceed girls’ at all time points, and Ct.BMD, where girls’ values exceed boys’ at all time points. (Diagram not to exact scale).
Comparison of Annual Accrual Rates for Bone Parameters between Boys and Girls Pre- and Post-APHV
We aligned participants on maturity offset and provide between-sex comparisons for annual accrual rates pre- and post-APHV for each bone parameter (Table 3). Boys and girls demonstrated periosteal bone formation (represented by an increase in Tt.Ar) and net bone loss at the endocortical surface (represented by a net increase in Me.Ar) over the measurement period. Boys demonstrated significantly greater annual accrual rates compared with girls for Tt.Ar and Me.Ar pre-APHV, and significantly lower annual accrual rates for Ct.Ar/Tt.Ar and Ct.BMD pre-APHV compared with girls. There were no significant between-sex differences in annual accrual rates pre-APHV for Ct.Ar and SSIp. Post-APHV, there were no significant between-sex differences in annual accrual rates for Ct.Ar/Tt.Ar; however, boys demonstrated significantly greater annual accrual rates for all other bone parameters compared with girls (Figure 2).
Table 3.
Estimates of fixed effects slopes and comparison between boys and girls. Slopes represent annual rates of accrual pre- and post-age at peak height velocity (APHV). Numbers in brackets are the standard error of the parameter estimate or the 95% confidence interval for the ratio.
| Slope Pre-APHV | Slope Post-APHV | |||||||
|---|---|---|---|---|---|---|---|---|
| Boys | Girls | Ratio | p-value | Boys | Girls | Ratio | p-value | |
| Tt.Ar (mm2/yr) | 55.4 (2.0) | 47.0 (2.7) | 1.18 (1.02 to 1.34) | 0.011 | 19.2 (0.6) | 10.2 (0.6) | 1.89 (1.64 to 2.13) | <0.001 |
| Ct.Ar (mm2/yr) | 38.3 (1.6) | 35.0 (2.2) | 1.10 (0.94 to 1.26) | 0.211 | 15.2 (0.5) | 8.7 (0.5) | 1.75 (1.50 to 1.99) | <0.001 |
| Ct.Ar/Tt.Ar | 0.007 (0.001) | 0.013 (0.002) | 0.56 (0.29 to 0.83) | 0.021 | 0.005 (0.0005) | 0.005 (0.0005) | 1.01 (0.71 to 1.31) | 0.928 |
| Me.Ar (mm2/yr) | 15.2 (0.6) | 11.4 (0.9) | 1.34 (1.11 to 1.57) | <0.001 | 4.1 (0.2) | 1.6 (0.2) | 2.63 (1.80 to 3.45) | <0.001 |
| Ct.BMD (mg/cm3/yr) | −1.0 (0.9) | 16.4 (1.6) | −0.07 (−0.17 to 0.04) | <0.001 | 18.2 (0.5) | 14.8 (0.6) | 1.23 (1.11 to 1.35) | <0.001 |
| SSIp (mm3/yr) | 271.6 (10.0) | 237.4 (14.6) | 1.14 (0.98 to 1.30) | 0.054 | 164.0 (4.8) | 84.2 (5.0) | 1.95 (1.69 to 2.20) | <0.001 |
Tt.Ar, total area; Ct.Ar, cortical area; Me.Ar, medullary canal area; Ct.BMD, cortical bone mineral density; SSIp strength-strain index.
Discussion
We revisit Garn’s classic studies(5–7) and more recent reports(8,9) that suggest bone strength during adolescence is accrued primarily through periosteal expansion in boys as compared with endocortical apposition in girls. In this analysis, we extended Garn’s methods (cross-sectional study of planar radiographs of the second metacarpal) by aligning boys and girls on a common maturational landmark (APHV). We then modeled 3-dimensional aspects of tibial bone geometry, density and strength (acquired using pQCT) across 12-years. Should Garn’s general findings persist in our study, boys would accrue more bone on the periosteal surface (contributing to increased Tt.Ar) and girls would accrue more bone on the endocortical surface (contributing to a narrower medullary cavity (Me.Ar)).
Although both boys and girls accrued substantial amounts of bone on the periosteal surface (as estimated by changes in Tt.Ar), boys’ accrual rates were approximately 18% greater compared with girls’ pre-APHV (55.4 and 47.0 mm2/year for boys and girls, respectively) and 89% greater compared with girls’ post-APHV (19.2 and 10.2 mm2/year for boys and girls, respectively). These findings support those of Garn who reported boys had 48% greater periosteal apposition at the second metacarpal compared with girls from childhood to late adolescence (8–22 years of age).(5–7) Our current results also extend our earlier findings across 20-months where we reported 8% greater periosteal apposition in boys compared with girls during early- and peri-puberty and 14% greater periosteal apposition in boys post-puberty.(10) The distribution of bone away from the neutral axis confers a considerable strength advantage to boys at long bone shafts such as the mid-tibia.(33)
We used Me.Ar to estimate changes at bone’s endocortical surface. We observed expansion of the medullary canal for both boys and girls pre- and post-APHV. However, annual accrual rates were 34% greater pre-APHV and 163% greater post-APHV for boys compared with girls. Contrary to Garn’s findings that bone formation at the endocortical surface was enhanced in girls during puberty,(5–7) our 12-year longitudinal data demonstrated a small net loss of bone at the endocortical surface in girls. This was represented by a small net increase in Me.Ar (11 mm2/year pre-APHV and 2 mm2/year post-APHV, on average). Our findings also differ from Wang et al. who analyzed data from a 2-year longitudinal study and observed an increase in Me.Ar in girls until menarche and a slight decrease thereafter.(11)However, fewer than 10 participants were assessed at 24-months post menarche.(11) Our findings across a longer measurement period are consistent with our previous work, where we reported a 5–8% increase in Me.Ar across 20-months in early- to post-pubertal girls.(10) Thus, our data do not support that endocortical bone formation increases during adolescence as noted by Garn. On the contrary we noted that endocortical bone resorption predominates in both boys and girls; however, girls’ bone was preserved to a greater extent (less resorption) compared with boys.
There are several plausible explanations for the differences between Garn’s early findings and the present study. First, bone formation and resorption differ considerably by anatomical region.(34,35) In earlier studies we reported that cortical BMD also varied across sectors within the same bone cross-section.(36,37) As the metacarpal is non-weight bearing compared with the weight-bearing tibia, the substantially different forces experienced at each site may contribute to site-specific differences between the two studies. To enhance bone strength in response to greater loads associated with bending and torsion, the tibia preferentially adapts by distributing bone further away from the neutral axis (through an increase in Me.Ar in addition to increases in Tt.Ar). This could be achieved with either less endocortical apposition or more endocortical resorption compared with the non weight-bearing metacarpal.(33) Second, there are significant limitations associated with estimating bone cross-sectional geometry using 2-dimensional radiographs.(38) Third, despite relatively large sample sizes, Garn used cross-sectional data for his analyses. The limitations (e.g., selection/attrition, cohort differences) of age-heterogeneous cross-sectional samples for evaluating rates of change are reviewed in detail elsewhere.(39) Longitudinal data are better able to represent the tremendous growth-related variability among children and also permit separation of age-related mean trends from estimates of association between age-related variables. Finally, Garn et al. did not control for substantial maturational differences between boys and girls of the same chronological age, which may have influenced their findings.
We used the ratio of Ct.Ar to Tt.Ar (Ct.Ar/Tt.Ar) as a surrogate of cortical thickness to examine changes across time as we did in our previous 20-month study.(10) We observed relative thickening of the cortex (increase in Ct.Ar/Tt.Ar) and thus a reciprocal decrease in medullary canal area relative to total bone size in both boys and girls across the study period. The cortex contributed relatively less to overall bone size in boys compared with girls at APHV. Consistent with our previous study over a 20-month period,(10) boys’ greater overall growth rate (Tt.Ar and Ct.Ar) post-APHV resulted in no observed difference in Ct.Ar/Tt.Ar between boys and girls during this time.
We report significantly lower Ct.BMD for boys compared with girls in the current study as per previous reports at the tibial midshaft(10,30,36) and the proximal radius(40) assessed using pQCT, and at the distal tibia and radius assessed using HR-pQCT.(20) More dense cortices in girls might reflect lower rates of intracortical remodeling associated with a smaller magnitude of growth during adolescence in girls, compared with boys.(41) Although we note that sex differences in Ct.BMD appeared prior to APHV, we were unable to clearly discern if these differences were present at earlier maturational stages. Girls in our study were relatively close to APHV at baseline and mean curves appeared to converge (Figure 2). Importantly, given the greater contribution of geometry as compared with density to bone strength at long bone shafts,(33,42) girls’ more dense bones would only partially compensate for their smaller bone size, on average, compared with boys. In adults, larger bone size rather than differences in density or the amount of bone within the periosteal envelope is thought to account for men’s greater bone strength as compared with women.(8)
Although bone size, shape and density contribute to adult bone strength,(43) the relative contribution of these components to bone strength in children is largely unknown. On average, boys had significantly greater bone strength (represented by SSIp) at APHV and experienced significantly greater annual accrual rates post-APHV (95%) compared with girls. In boys, the surge of testosterone during puberty is largely responsible for the greater magnitude and prolonged duration of the pubertal growth spurt, and results in greater gains in periosteal and longitudinal bone formation across puberty in boys compared with girls.(44) As bone’s ability to resist bending forces is directly proportional to the distribution of mass about the neutral bending axis, an incremental increase in the external diameter of a long bone (increased bone size) improves bones’ resistance to bending and torsional loading and substantially increases bone’s strength.(42,45) Thus, in the current study, boys’ larger bones (greater Tt.Ar), enhanced rates of change in bone parameters and prolonged duration of longitudinal growth, compared with girls, contributed to boys’ greater bone strength (SSIp). Should this advantage persist similar to the continuity of bone area from young to middle adulthood as reported by Garn and colleagues,(46) boys and men would be conferred a bone strength advantage throughout life.
Although there are known ethnic differences in the timing and tempo of maturation(47) (Asian participants in our cohort attained APHV approximately 7 months prior to white participants), we attempted to account for this in our analysis by aligning boys and girls on APHV. White participants had 1% lower Ct.BMD and 2–13% greater values for all other bone parameters compared with their Asian peers at APHV. These findings are consistent with our previous reports demonstrating larger Ct.Ar (at the tibial midshaft by pQCT) in pre- and early-pubertal white boys and girls(30) and lower Ct.BMD (at the distal tibia by HR-pQCT) in mid- to late-pubertal white boys and girls compared with their Asian peers.(48) We cannot discount the possibility that ethnic differences in bone parameters may have been present prior to the onset of puberty and influenced by a shorter duration of growth (earlier onset of maturation) in Asian participants. We note that although our sample reflects the ethnic diversity of Metro Vancouver, where visible minority groups represent 47% of the population (compared with 16% in the rest of Canada), it may not be representative of populations outside this geographic area.
The purpose of our analysis was to compare rates of change pre-APHV and post-APHV. Thus, we fit a piecewise linear regression model; we did not attempt to model the bone parameter growth curves. We highlight several strengths of our study. Most importantly, longitudinal data are difficult, time consuming and costly to collect and are therefore relatively rare. The few previous studies that reported changes on bone surfaces during growth utilized cross-sectional data or longitudinal data across a short time frame(5–7,11) and were therefore unable to capture the tremendous variability that accompanies growth of tissues throughout adolescence. Our 12-year longitudinal data enabled us to identify APHV for participants and in turn, align boys and girls on the same maturational landmark. Additionally, we examined 3-dimensional changes in bone geometry and strength at a weight-bearing site, the tibial midshaft, using an advanced imaging technique (pQCT).
We acknowledge several limitations of our study. First, as in any repeated measures study of growing bone it is not possible to reassess the same bone cross-section over time. Long bone growth is both complex and disproportionate; at the tibia, 57% of longitudinal growth occurs at the proximal metaphysis and 43% occurs at the distal metaphysis.(49) Therefore, we used a standard anatomical landmark to identify the same relative site along the length of the tibia at each measurement in every child. Second, based on differences in maturational timing between boys and girls at the same chronological age, many of the girls in our study approached APHV at baseline. Thus, we were unable to compare boys and girls across several years prior to APHV and may have underestimated average annual rates of change pre-APHV for girls. Third, our results are specific to adaptations at the tibial shaft and do not necessarily represent other skeletal regions. Although we did not discern net endocortical apposition in girls at the tibia, it may occur at other skeletal sites or even at different sites along the length of the tibia. Fourth, APHV is a global measure and does not account for differences in appendicular and axial growth patterns;(50,51) however, we used APHV because it is a well-established measure of somatic maturity.(12) Fifth, the resolution of pQCT (0.4mm voxel size) may have limited our ability to detect small changes in cortical bone area and subsequently small changes on the endocortical surface. Partial volume effects may have limited the accurate separation of cortical and trabecular bone in regions with thin cortices. Finally, we acknowledge that our convenience sampling methods limit the external validity of our results.
In conclusion, the pioneering studies of Garn and colleagues established an important premise that prompted researchers to more closely examine sex differences on the surfaces of growing long bones. Boys’ larger bones confer a greater bone strength advantage during adolescence. However, it would be of interest to better understand whether benefits persist into adulthood and older age. Although evidence from animal studies(52) and retrospective studies of athletes(53–55) support that enhanced long bone strength accrued during the younger years persists, the implications of this on preventing osteoporosis and fragility fractures in later life, remains largely unknown.
Supplementary Material
Acknowledgments
Funding Source: Canadian Institutes of Health Research (MOP-84575), the Michael Smith Foundation for Health Research (MSFHR), British Columbia Health Research Foundation (2400–2 and 10898–2) and Legacies Now 2010.
We gratefully acknowledge the HBSIII participants, their families and the support from principals and teachers at participating schools in the Richmond and Vancouver School Districts. We would also like to acknowledge the HBSIII research teams and the supervision of imaging acquisition and processing from Dr. Danmei Liu (Centre for Hip Health and Mobility, Vancouver Coastal Health Research Institute). HBSIII was supported by the Canadian Institutes of Health Research (CIHR; MOP-84575), the Michael Smith Foundation for Health Research (MSFHR), the British Columbia Health Research Foundation (2400–2 and 10898–2) and 2010 Legacies Now. LG was supported by a CIHR Doctoral Research Award.
Footnotes
Authors’ roles: Study design: HAM and HMM. Data collection: LG, YA and HMM. Data analysis: LG, LN, PB. Data interpretation: LG, LN, PB, HMM. Drafting and revising manuscript: LG, LN, PB, SM, YA, PB, HMM, HAM. All authors approved the final version of this manuscript. LG takes responsibility for the integrity of the data analysis.
Conflicts of Interest:
All authors state that they have no conflicts of interest.
References
- 1.Bailey DA, McKay HA, Mirwald RL, Crocker PR, Faulkner RA. A six-year longitudinal study of the relationship of physical activity to bone mineral accrual in growing children: the University of Saskatchewan bone mineral accrual study. J Bone Miner Res. 1999;14(10):1672–1679. doi: 10.1359/jbmr.1999.14.10.1672. [DOI] [PubMed] [Google Scholar]
- 2.Baxter-Jones ADG, Faulkner RA, Forwood MR, Mirwald RL, Bailey DA. Bone mineral accrual from 8 to 30 years of age: an estimation of peak bone mass. J Bone Miner Res. 2011;26(8):1729–1739. doi: 10.1002/jbmr.412. [DOI] [PubMed] [Google Scholar]
- 3.Bailey D, McCulloch R. Osteoporosis: Are there childhood antecedents for an adult health problem? Can J Pediat. 1992;4:130–134. [Google Scholar]
- 4.Raisz LG. Local and systemic factors in the pathogenesis of osteoporosis. N Eng J Med. 1988;318(13):818–828. doi: 10.1056/NEJM198803313181305. [DOI] [PubMed] [Google Scholar]
- 5.Frisancho AR, Garn SM, Ascoli W. Subperiosteal and endosteal bone apposition during adolescence. Hum Biol. 1970;42(4):639–664. [PubMed] [Google Scholar]
- 6.Garn SM. The course of bone gain and the phases of bone loss. Orthop Clin North Am. 1972;3(3):503–520. [PubMed] [Google Scholar]
- 7.Garn SM. The earlier gain and later loss of cortical bone. Springfield, Il: Charles C Thomas Publisher; 1970. [Google Scholar]
- 8.Seeman E. The structural and biomechanical basis of the gain and loss of bone strength in women and men. Endocrinol Metab Clin North Am. 2003;32(1):25–38. doi: 10.1016/s0889-8529(02)00078-6. [DOI] [PubMed] [Google Scholar]
- 9.Seeman E. Periosteal bone formation--a neglected determinant of bone strength. N Eng J Med. 2003;349(4):320–323. doi: 10.1056/NEJMp038101. [DOI] [PubMed] [Google Scholar]
- 10.Kontulainen SA, Macdonald HM, Khan KM, McKay HA. Examining bone surfaces across puberty: a 20-month pQCT trial. J Bone Miner Res. 2005;20(7):1202–1207. doi: 10.1359/JBMR.050214. [DOI] [PubMed] [Google Scholar]
- 11.Wang Q, Alén M, Nicholson P, Lyytikäinen A, Suuriniemi M, Helkala E, Suominen H, Cheng S. Growth patterns at distal radius and tibial shaft in pubertal girls: a 2-year longitudinal study. J Bone Miner Res. 2005;20(6):954–961. doi: 10.1359/JBMR.050110. [DOI] [PubMed] [Google Scholar]
- 12.Baxter-Jones ADG, Eisenmann JC, Sherar LB. Controlling for maturation in pediatric exercise science. Ped Exerc Sci. 2005;17:18–30. [Google Scholar]
- 13.Malina RM, Bouchard C, Bar-Or O. Growth, maturation, and physical activity. 2. Champaign, IL: Human Kinetics; 2004. [Google Scholar]
- 14.Cole TJ, Ahmed ML, Preece MA, Hindmarsh P, Dunger DB. The relationship between Insulin-like Growth Factor 1, sex steroids and timing of the pubertal growth spurt. Clin Endocrinol. 2014 doi: 10.1111/cen.12682. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bailey DA. The Saskatchewan Pediatric Bone Mineral Accrual Study: bone mineral acquisition during the growing years. Int J Sports Med. 1997;18(Suppl 3):S191–4. doi: 10.1055/s-2007-972713. [DOI] [PubMed] [Google Scholar]
- 16.MacKelvie KJ, McKay HA, Khan KM, Crocker PR. A school-based exercise intervention augments bone mineral accrual in early pubertal girls. J Pediatr. 2001;139(4):501–508. doi: 10.1067/mpd.2001.118190. [DOI] [PubMed] [Google Scholar]
- 17.MacKelvie KJ, Petit MA, Khan KM, Beck TJ, McKay HA. Bone mass and structure are enhanced following a 2-year randomized controlled trial of exercise in prepubertal boys. Bone. 2004;34(4):755–764. doi: 10.1016/j.bone.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 18.MacKelvie KJ, McKay HA, Petit MA, Moran O, Khan KM. Bone mineral response to a 7-month randomized controlled, school-based jumping intervention in 121 prepubertal boys: associations with ethnicity and body mass index. J Bone Miner Res. 2002;17(5):834–844. doi: 10.1359/jbmr.2002.17.5.834. [DOI] [PubMed] [Google Scholar]
- 19.Macdonald HM, Kontulainen SA, Khan KM, McKay HA. Is a school-based physical activity intervention effective for increasing tibial bone strength in boys and girls? J Bone Miner Res. 2007;22(3):434–446. doi: 10.1359/jbmr.061205. [DOI] [PubMed] [Google Scholar]
- 20.Nishiyama KK, Macdonald HM, Moore SA, Fung T, Boyd SK, McKay HA. Cortical porosity is higher in boys compared with girls at the distal radius and distal tibia during pubertal growth: An HR-pQCT study. J Bone Miner Res. 2012;27(2):273–282. doi: 10.1002/jbmr.552. [DOI] [PubMed] [Google Scholar]
- 21.McKay HA, MacLean L, Petit M, MacKelvie-O’Brien K, Janssen P, Beck T, Khan KM. “Bounce at the Bell”: a novel program of short bouts of exercise improves proximal femur bone mass in early pubertal children. Br J Sports Med. 2005;39(8):521–526. doi: 10.1136/bjsm.2004.014266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Macdonald HM, MacKelvie KJ, MacLean LB, McKay HA. Does tibial bone structure differ between girls who completed a 20-month exercise intervention and controls? Med Sci Sports Exerc. 2003;35:S360. [Google Scholar]
- 23.Ross W, Marfell-Jones M. Kinanthropometry. In: Green H, editor. Physiological testing of the high performance athlete. Champaign, IL: Human Kinetics; 1991. [Google Scholar]
- 24.Crocker PR, Bailey DA, Faulkner RA, Kowalski KC, McGrath R. Measuring general levels of physical activity: preliminary evidence for the Physical Activity Questionnaire for Older Children. Med Sci Sports Exerc. 1997;29(10):1344–1349. doi: 10.1097/00005768-199710000-00011. [DOI] [PubMed] [Google Scholar]
- 25.Barr SI. Associations of social and demographic variables with calcium intakes of high school students. J Am Diet Assoc. 1994;94(3):260–6. doi: 10.1016/0002-8223(94)90366-2. [DOI] [PubMed] [Google Scholar]
- 26.Macdonald HM, Kontulainen SA, Mackelvie-O’Brien KJ, Petit MA, Janssen P, Khan KM, McKay HA. Maturity- and sex-related changes in tibial bone geometry, strength and bone-muscle strength indices during growth: a 20-month pQCT study. Bone. 2005;36(6):1003–1011. doi: 10.1016/j.bone.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 27.Burrows M, Cooper DML, Liu D, McKay HA. Bone and muscle parameters of the tibia: agreement between the XCT 2000 and XCT 3000 instruments. J Clin Densitom. 2009;12(2):186–194. doi: 10.1016/j.jocd.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 28.Ashe MC, Liu-Ambrose T, Khan KM, White N, McKay HA. Optimizing results from pQCT: reliability of operator-dependent pQCT variables in cadavers and humans with low bone mass. J Clin Densitom. 2005;8(3):335–340. doi: 10.1385/jcd:8:3:335. [DOI] [PubMed] [Google Scholar]
- 29.Stratec Medizintechnik GmbH. XCT 2000 Manual, Software Version 5.50. 2004. [Google Scholar]
- 30.Macdonald H, Kontulainen S, Petit M, Janssen P, McKay H. Bone strength and its determinants in pre- and early pubertal boys and girls. Bone. 2006;39(3):598–608. doi: 10.1016/j.bone.2006.02.057. [DOI] [PubMed] [Google Scholar]
- 31.Wang Q, Wang X-F, Iuliano-Burns S, Ghasem-Zadeh A, Zebaze R, Seeman E. Rapid growth produces transient cortical weakness: a risk factor for metaphyseal fractures during puberty. J Bone Miner Res. 2010;25(7):1521–1526. doi: 10.1002/jbmr.46. [DOI] [PubMed] [Google Scholar]
- 32.Verbeke G, Molenberghs G. Linear Mixed Models for Longitudinal Data. New York, NY: Springer-Verlag; 2000. [Google Scholar]
- 33.Seeman E. Clinical review 137: Sexual dimorphism in skeletal size, density, and strength. J Clin Endocrinol Metab. 2001;86(10):4576–4584. doi: 10.1210/jcem.86.10.7960. [DOI] [PubMed] [Google Scholar]
- 34.Lai YM, Qin L, Hung VW, Chan KM. Regional differences in cortical bone mineral density in the weight-bearing long bone shaft--a pQCT study. Bone. 2005;36(3):465–471. doi: 10.1016/j.bone.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 35.Nonaka K, Fukuda S, Aoki K, Yoshida T, Ohya K. Regional distinctions in cortical bone mineral density measured by pQCT can predict alterations in material property at the tibial diaphysis of the Cynomolgus monkey. Bone. 2006;38(2):265–272. doi: 10.1016/j.bone.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 36.Kontulainen SA, Macdonald HM, McKay HA. Change in cortical bone density and its distribution differs between boys and girls during puberty. J Clin Endocrinol Metab. 2006;91(7):2555–2561. doi: 10.1210/jc.2006-0136. [DOI] [PubMed] [Google Scholar]
- 37.Cooper DML, Ahamed Y, Macdonald HM, McKay HA. Characterising cortical density in the mid-tibia: intra-individual variation in adolescent girls and boys. Br J Sports Med. 2008;42(8):690–695. doi: 10.1136/bjsm.2008.049528. [DOI] [PubMed] [Google Scholar]
- 38.Van Gerven DP, Armelagos GJ, Bartley MH. Roentgenographic and direct measurement of femoral cortical involution in a prehistoric Mississippian population. Am J Phys Anthropol. 1969;31(1):23–38. doi: 10.1002/ajpa.1330310105. [DOI] [PubMed] [Google Scholar]
- 39.Hofer SM, Sliwinski MJ. Understanding Ageing. An evaluation of research designs for assessing the interdependence of ageing-related changes. Gerontology. 2001;47(6):341–352. doi: 10.1159/000052825. [DOI] [PubMed] [Google Scholar]
- 40.Schoenau E, Neu CM, Rauch F, Manz F. Gender-specific pubertal changes in volumetric cortical bone mineral density at the proximal radius. Bone. 2002;31(1):110–113. doi: 10.1016/s8756-3282(02)00802-5. [DOI] [PubMed] [Google Scholar]
- 41.Tanner JM, Whitehouse RH, Marubini E, Resele LF. The adolescent growth spurt of boys and girls of the Harpenden growth study. Ann Hum Biol. 1976;3(2):109–126. doi: 10.1080/03014467600001231. [DOI] [PubMed] [Google Scholar]
- 42.Bouxsein ML. Determinants of skeletal fragility. Best Pract Res Clin Rheumatol. 2005;19(6):897–911. doi: 10.1016/j.berh.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 43.Manske SL, Macdonald HM, Nishiyama KK, Boyd SK, McKay HA. Clinical tools to evaluate bone strength. Clinic Rev Bone Miner Metab. 2010;8(3):122–134. [Google Scholar]
- 44.Venken K, Callewaert F, Boonen S, Vanderschueren D. Sex hormones, their receptors and bone health. Osteoporos Int. 2008;19(11):1517–1525. doi: 10.1007/s00198-008-0609-z. [DOI] [PubMed] [Google Scholar]
- 45.Currey JD. Bone strength: what are we trying to measure? Calcif Tissue Int. 2001;68(4):205–210. doi: 10.1007/s002230020040. [DOI] [PubMed] [Google Scholar]
- 46.Garn SM, Hawthorne VM, Larkin FA, Sullivan TV, Decker SA. Long-term continuity of bone cortical area. N Eng J Med. 1991;324(12):850. doi: 10.1056/NEJM199103213241214. [DOI] [PubMed] [Google Scholar]
- 47.Cole TJ, Rousham EK, Hawley NL, Cameron N, Norris SA, Pettifor JM. Ethnic and sex differences in skeletal maturation among the Birth to Twenty cohort in South Africa. Arch Dis Child. 2015;100(2):138–143. doi: 10.1136/archdischild-2014-306399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim S, Macdonald HM, Nettlefold L, McKay HA. A comparison of bone quality at the distal radius between Asian and Caucasian adolescents and young adults: An HR-pQCT study. J Bone Miner Res. 2013;28(9):2035–42. doi: 10.1002/jbmr.1939. [DOI] [PubMed] [Google Scholar]
- 49.Anderson M, Green WT, Messner MB. Growth and predictions of growth in the lower extremities. J Bone Joint Surg Am. 1963;45-A:1–14. [PubMed] [Google Scholar]
- 50.Gasser T, Kneip A, Binding A, Prader A, Molinari L. The dynamics of linear growth in distance, velocity and acceleration. Ann Hum Biol. 1991 May;18(3):187–205. doi: 10.1080/03014469100001522. [DOI] [PubMed] [Google Scholar]
- 51.Iuliano-Burns S, Hopper J, Seeman E. The Age of Puberty Determines Sexual Dimorphism in Bone Structure: A Male/Female Co-Twin Control Study. J Clin Endocrinol Metab. 2009 May;94(5):1638–43. doi: 10.1210/jc.2008-1522. [DOI] [PubMed] [Google Scholar]
- 52.Warden SJ, Fuchs RK, Castillo AB, Nelson IR, Turner CH. Exercise when young provides lifelong benefits to bone structure and strength. J Bone Miner Res. 2007;22(2):251–259. doi: 10.1359/jbmr.061107. [DOI] [PubMed] [Google Scholar]
- 53.Warden SJ, Mantila Roosa SM, Kersh ME, Hurd AL, Fleisig GS, Pandy MG, Fuchs RK. Physical activity when young provides lifelong benefits to cortical bone size and strength in men. Proc Natl Acad Sci. 2014;111(14):5337–5342. doi: 10.1073/pnas.1321605111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Eser P, Hill B, Ducher G, Bass S. Skeletal benefits after long-term retirement in former elite female gymnasts. J Bone Miner Res. 2009;24(12):1981–1988. doi: 10.1359/jbmr.090521. [DOI] [PubMed] [Google Scholar]
- 55.Erlandson MC, Kontulainen SA, Chilibeck PD, Arnold CM, Faulkner RA, Baxter-Jones ADG. Former premenarcheal gymnasts exhibit site-specific skeletal benefits in adulthood after long-term retirement. J Bone Miner Res. 2012;27(11):2298–2305. doi: 10.1002/jbmr.1689. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.