SUMMARY
Exposure to nickel has been shown to cause damage to the testis in several animal models. It is not known if the testis expresses protein(s) that can bind nickel. To test this, we used a nickel-binding assay to isolate testicular nickel-binding proteins. We identified glutamate–ammonia ligase (GLUL) as a prominent nickel-binding protein by mass spectrometry. Protein analysis and reverse transcriptase polymerase chain reaction showed that GLUL is expressed in the testis, predominantly in interstitial cells. We determined that GLUL has a higher affinity for nickel than for its regular co-factor manganese. We produced an enzymatically active, recombinant GLUL protein. Upon binding, nickel interferes with the manganese-catalyzed enzymatic activity of recombinant GLUL protein. We also determined that GLUL activity in testes of animals exposed to nickel sulfate is reduced. Our results identify testicular GLUL as the first testicular protein shown to be affected by nickel exposure.
INTRODUCTION
Nickel is important in lipid metabolism, iron absorption and the action of biotin, folate, and vitamin B12 (Stangl and Kirchgessner, 1996; for review, see Nielsen, 1984). In animals, nickel deficiency has been linked to retarded growth, reduced reproduction rates, and alterations in serum lipids and glucose (Nielsen et al., 1974; Barceloux, 1999). Nickel deficiency may also diminish sperm quantity and movement in rats (Yokoi et al., 2003). In humans, the deficiency-related pathology has not been clearly defined. In contrast, studies of overexposure to nickel compounds have indicated that nickel may constitute a major challenge to human health. Workers exposed to nickel were found to have an elevated incidence of lung and nasal cancers (Doll, 1958; Grimsrud et al., 2003). In addition, nickel exposure has been linked to skin allergies (Hostynek, 2006). Nickel is widely used in many industrial and consumer products, including stainless steel, magnets, coinage, plating, and special alloys, and hence may constitute an important source of environmental pollution (Bruske-Hohlfeld, 2009). Nickel pollution takes four different forms: water soluble, sulfidic, oxidic, and metallic. The water-soluble form may pose the highest risk to human health as this form is believed to be responsible for cancer development reported in a group of Norwegian nickel refinery workers (Grimsrud et al., 2002). Animal studies of nickel toxicity have shown various pathogenic effects of nickel. In adult male Sprague–Dawley rats treated for 13 days with low dosage of nickel sulfate in drinking water, no or minor effects were found, but when animals were treated with a high dosage, body-weight loss and a significant decrease in aspects of metabolism and cellular activities were noted (Obone et al., 1999; Das and Dasgupta, 2000). Biochemical analysis of bronchoalveolar lavage fluid and lung tissue showed lung damage at the high dosage (Obone et al., 1999). Noticeably, nickel compounds were found to accumulate in many organs, with testis as one of the major sites of accumulation (Whanger, 1973; Severa et al., 1995). Obone et al. (1999) reported that testis is the second accumulation site among a number of organs tested, preceded by kidney, and followed by lung and liver.
When accumulated, nickel may affect human reproduction. Incubation of ejaculated spermatozoa with nickel in vitro has shown a concentration-dependent, biphasic effect of nickel on expression of mannose receptors (Benoff et al., 2000a): at a low concentration, nickel stimulated mannose receptor expression, but at high concentrations, nickel exposure resulted in decreased mannose receptor expression. Mannose receptors are involved in the binding of human sperm to carbohydrate residues on the zona pellucida of oocytes, initiating the acrosome reaction. For other transition and heavy metals, such as cadmium (Cd2+) and lead (Pb2+), occupational exposure is associated with decreased semen quality and male subfertility (Benoff et al., 2000b and reference therein).
In rodents, experimental data have shown that accumulation of nickel in the testis can cause damage to the reproductive system. After exposure to the metal, male rats exhibited reductions in sperm motility, sperm count, and fertility, and their offspring displayed male-mediated, dominant lethal types of mutations (Xie et al., 1995; Das and Dasgupta, 2000; Doreswamy et al., 2004). Examination of the testis from nickel-treated animals revealed that nickel induced diverse pathological manifestations, depending on dosage and route of nickel administration, which include changes in the ratio of organ-to-body weight (testis and epididymis vs. body weight), degeneration, shrinkage and edema of seminiferous tubules, changes in sperm morphology, and reduction in the number of basal spermatogonia (Hoey, 1966; Mathur et al., 1977; Xie et al., 1995; Pandey et al., 1999; Das and Dasgupta, 2000; Doreswamy et al., 2004).
Although nickel effects on mammalian testis are well documented, there is little information regarding testicular proteins that can bind nickel and on how exposure to this metal can modify the function of these proteins in terms of fertility and/or hormone production. To identify proteins that bind nickel in testis, we used a nickel-binding assay and mass spectrometry for identification. We found that glutamate–ammonia ligase (GLUL) is a prominent and novel nickel binding protein, and that nickel binding to GLUL significantly inhibits its enzymatic activity. In vivo, nickel treatment causes a reduction in testicular GLUL enzyme activity in testis of treated rats.
RESULTS
Identification of Glutamate–Ammonia Ligase as a Major Nickel Binding Protein in Rat Testis
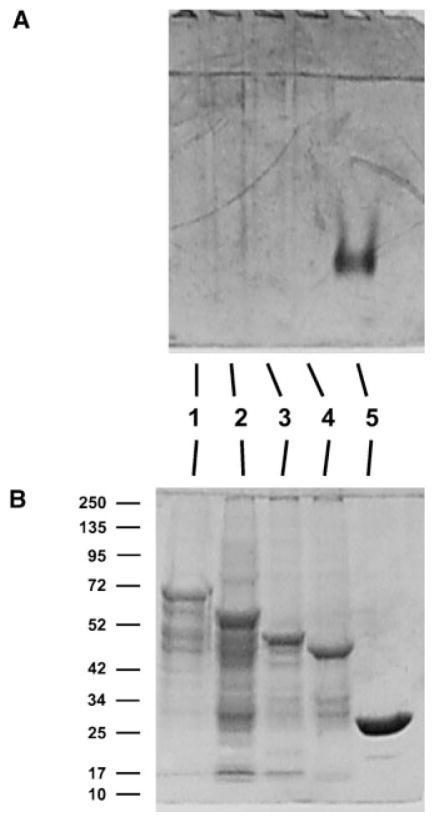
Studies in rats have shown that the testis is one of the organs that accumulate nickel following exposure (e.g., Obone et al., 1999). Experimental data have shown that accumulation of nickel in rat testis can cause damage to the reproductive system. To identify which proteins in the testis can bind nickel, we used a modified metal-chelate affinity chromatography protocol followed by protein separation by SDS–PAGE. Pre-cleared rat testis extracts were incubated with nickel agarose beads. Proteins bound to nickel beads were eluted, fractionated by SDS–PAGE, and stained by Coomassie blue G-250. The results shown in Figure 1A demonstrate that rat testis expresses several proteins that bind to nickel, the most prominent of which has an apparent molecular mass of 42 kDa (Fig. 1A, arrow). Gel slices containing this protein were excised and analyzed for identification by mass spectrometry. A MASCOT search of the Matrixscience database by peptide mass fingerprinting demonstrated that peptides derived from the 42 kD protein match GLUL, which has a nominal mass (Mr) of 42,259 and a calculated pI value of 6.83 (Fig. 1B).
Figure 1.
Identification of testicular nickel-binding proteins. A: Rat testis homogenates were prepared. The protein extracts were pre-cleared with 10% agarose to remove non-specific binding proteins and analyzed directly (lane 2) or were incubated with Ni-NTA agarose after which bound protein(s) were analyzed (lane 3). The arrow indicates a prominent 42 kDa band that was subsequently identified by mass spectrometry as containing glutamate–ammonia ligase. Protein markers are shown in lane 1. B: MASCOT Search identification of glutamate–ammonia ligase. Proteins in the 42 kDa gel slice were analyzed by mass spectrometry. Matched peptides are shown in bold. The match to GLUL was highly significant with a score of 149, expect 8.6E–11 and number of peptides matched 12 among 31 searched. Glutamate–ammonia ligase has a nominal mass of 42,259 and a calculated pI value of 6.83. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com]
Rat Testis GLUL is a Nickel-Binding Protein
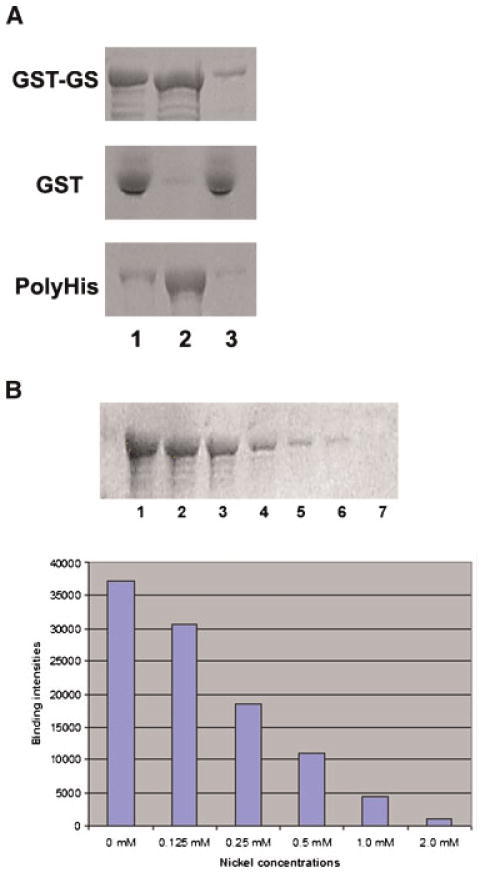
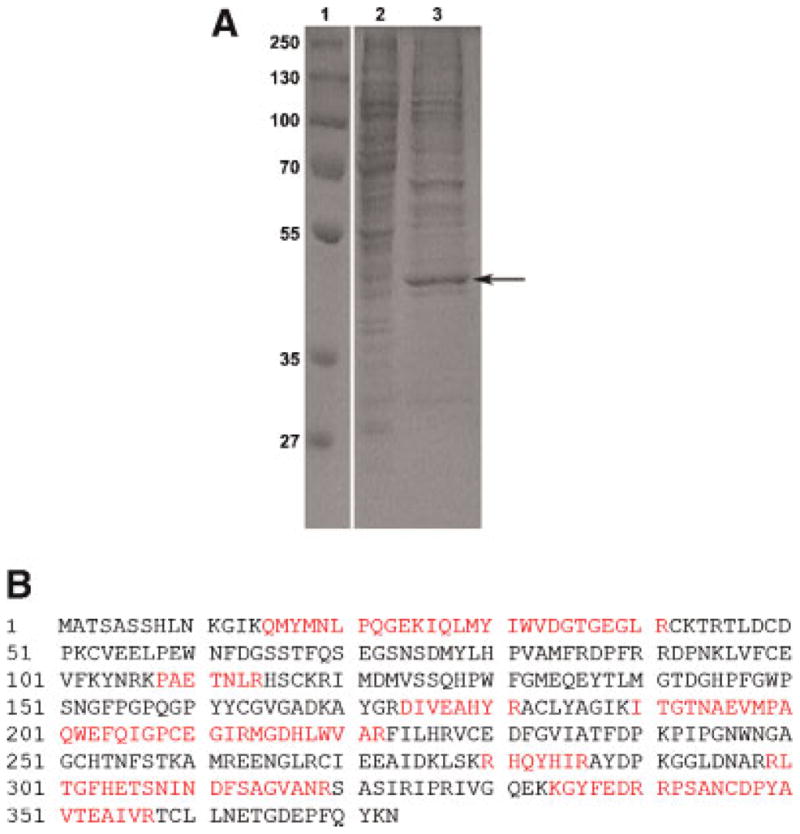
It is possible that more than one protein was present in the 42 kD band taken from SDS–PAGE gels for mass spectrometry identification, and that a protein different from GLUL binds nickel. Therefore, to exclude this possibility and to demonstrate that rat testis GLUL is able to bind nickel, we cloned rat testis GLUL and produced a recombinant glutathione S-transferase (GST)–GLUL protein for analysis of its nickel-binding capability. The GST–GLUL fusion protein was extracted from bacteria using a urea-solubilization and dialysis process. The GST–GLUL fusion protein was incubated with nickel agarose beads, and bound proteins were eluted and examined by SDS–PAGE. Figure 2 shows that GST–GLUL binds to nickel beads (Fig. 2A, panel GST–GS, lane 2). A poly(His)-tagged sperm tail protein was used as positive control for this assay and GST protein was used as negative control. As expected, the positive control binds to nickel beads (Fig. 2A, panel PolyHis, lane 2) while GST does not (Fig. 2A, panel GST, lane 2).
Figure 2.
Glutamate–ammonia ligase is a Ni2+ binding protein. A: Recombinant 68 kDa GST–GLUL protein was prepared in bacteria and analyzed for its capability to bind nickel by incubation with Ni-NTA agarose beads. GST–GS, recombinant 68 kDa GST–GLUL protein. GST, GST protein as a negative control. PolyHis, a poly(His)-tagged sperm tail protein used as a positive control. Lane 1, input protein; lane 2, proteins bound to Ni-NTA agarose beads; lane 3, unbound material (flow-through). B: Ni2+ competes with glutamate–ammonia ligase binding to Ni-NTA agarose beads. GST–GLUL recombinant protein was incubated with different amounts of nickel sulfate before incubation with Ni-NTA agarose beads. Then, GST–GLUL and nickel were incubated with Ni-NTA agarose beads and the bound protein was analyzed by SDS–PAGE. Top panel: Lane 1, 0 mM Ni2+; lanes 2–6, 0.125, 0.25, 0.50, 1.0, and 2.0 mM Ni2+, respectively. Bottom panel: Quantitation of the results is shown as a graph. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com]
To further demonstrate that GLUL is a bona fide nickel-binding protein, the GST–GLUL fusion protein was pre-incubated with various concentration of NiSO4 before incubation with nickel beads. The results show that, as a result of competition by NiSO4, the binding of GST–GLUL to the nickel beads decreased (Fig. 2B). These results confirm the original characterization and demonstrate for the first time that testicular GLUL has the capability to bind nickel.
Expression of GLUL Protein in Rat Testis
In many tissues the expression of GLUL is not homogeneous, but highly cell-type specific. In rat liver, for instance, the expression of GLUL is restricted to cells adjacent to central veins (Gebhardt and Mecke, 1983; van Straaten et al., 2006). In mouse testis and epididymis, GLUL protein was reported to be expressed in Leydig cells and in epithelial cells of caput epididymis (van Straaten et al., 2006).
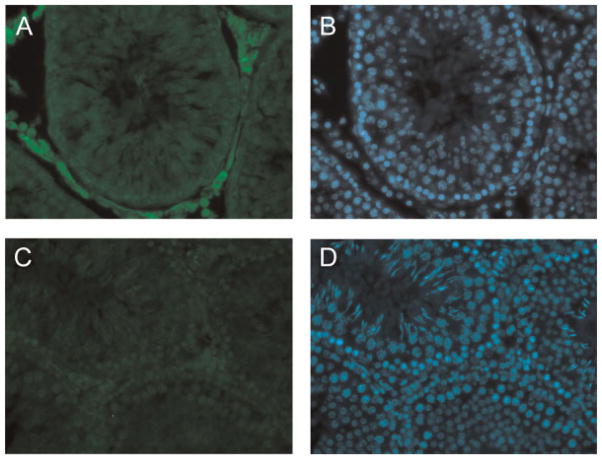
To examine the site(s) of GLUL protein expression in rat testis, we first performed Western blot analysis of total testis and interstitial tissue fractions using an anti-GLUL antibody to confirm its specificity. Our result shows that the 42 kDa GLUL protein is present in both whole rat testis extracts and interstitial tissue fractions (Fig. 3). Next, we used the anti-GLUL antibody in immunofluorescence to examine the distribution of GLUL protein within rat testis. The results from immunohistochemistry shown in Figure 4 indicate that GLUL is predominantly expressed in interstitial cells (Fig. 4, panels A and B) in agreement with previous publications. Negative controls showed that anti-GLUL antibody pre-incubated with recombinant GST–GLUL protein did not produce a signal (Fig. 4, panels C and D). The pre-immune serum also did not produce signal (not shown).
Figure 3.
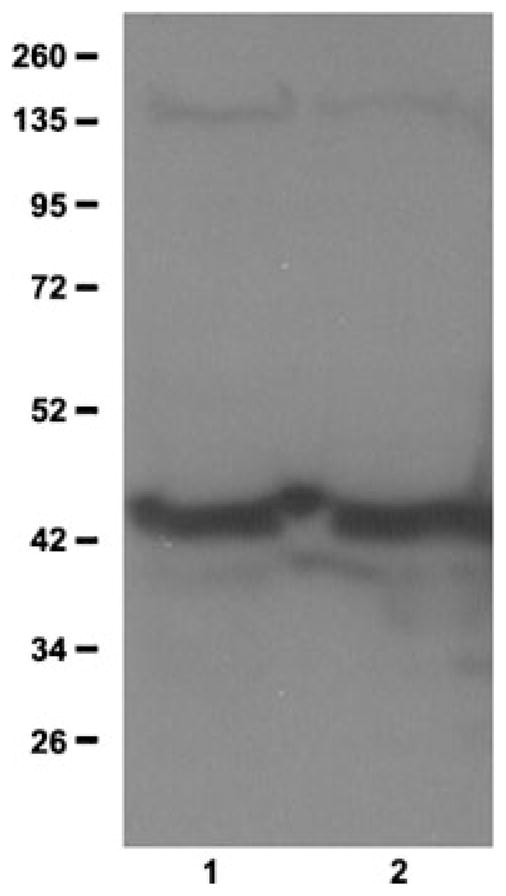
Glutamate–ammonia ligase protein expression in testis and interstitial cells. Western blot analysis was done using anti-GLUL antibodies. Lane 1, total testis; lane 2, interstitial cell fraction. Molecular sizes of marker proteins are indicated on the left.
Figure 4.
Expression of glutamate–ammonia ligase in testis. Detection of GLUL protein expression in testis by immunofluorescence microscopy. Rat testes were frozen in OCT and sectioned. Testis sections were stained with an anti-GLUL antibody followed by a secondary antibody conjugated with Cy3. Panel A: Sections were stained using anti-GLUL antibody. Panel B: The same section was stained with DAPI. Panel C: Sections were stained using anti-GLUL antibody that was first blocked with GST–GLUL recombinant protein (negative control). Panel D: The same section was stained with DAPI.
Nickel Inhibits GST–GLUL Recombinant Protein Enzymatic Activity
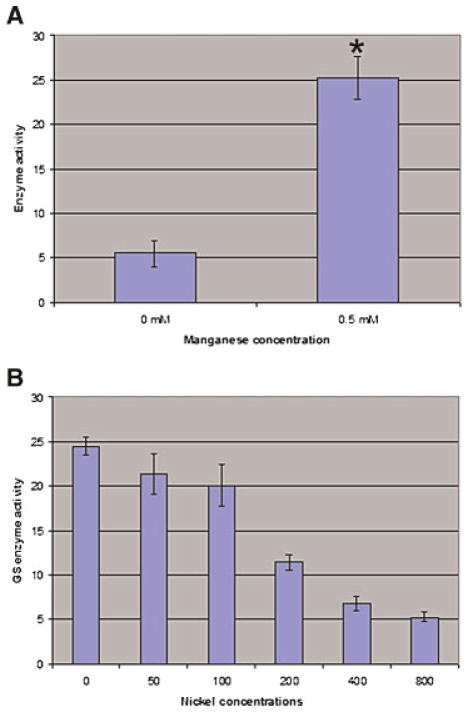
Next, we tested if the GST–GLUL recombinant protein displays GLUL-specific enzymatic activity. GLUL catalyses the formation of L-glutamine from glutamate and ammonia. As well, this enzyme also has a glutamyl-transferase activity that produces gamma-glutamyl-hydroxamate from glutamine and hydroxylamine (Meister, 1985). The gamma-glutamyl-transfer reaction is commonly used to determine GLUL enzymatic activity by a simple colorimetric assay (Minet et al., 1997). In its native state, active mammalian GLUL holoenzyme requires two divalent metal ions (magnesium, manganese or cobalt) per subunit for activity, and among these, manganese has the higher affinity for GLUL (Eisenberg et al., 2000). We therefore analyzed the activity of GST–GLUL in the presence or absence of its required co-factor Mn2+. Our results shown in Figure 5A indicate that in the absence of Mn2+ GST–GLUL recombinant protein only displays low basal glutamyl-transferase activity. Addition of Mn2+ at 0.5 mM, a concentration commonly used for measuring GLUL enzymatic activity (Monder, 1965), results in a significant increase in glutamyl-transferase activity of the recombinant protein. This indicates that the recombinant GST–GLUL protein is enzymatically active.
Figure 5.
Recombinant GST–GLUL protein is enzymatically active. A: Recombinant GST–GLUL protein was incubated without Mn2+ or with 0.5 mM Mn2+ for 1 hr at room temperature. Next, GLUL activity was detected by a spectrometry assay described in Materials and Methods. Values are the mean ±SE from six independent analyses. The asterisk indicates a significant difference between the two groups, P <0.01. B: Recombinant GST–GLUL protein was incubated in the presence of 0.5 mM manganese and different concentrations of NiSO4 (0, 50, 100, 200, 400, and 800 μM) for 1 hr at room temperature. Next, GLUL activity was measured using the spectrometry assay. Values are the mean ± SE from 6 independent assays. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com]
We next asked if nickel can compete with manganese for binding to GLUL and can affect the enzymatic activity of GLUL. We incubated GST–GLUL protein with an optimal amount of Mn2+ and increasing amounts of nickel sulfate, and then determined enzymatic activity. Figure 5B shows that GLUL enzymatic activity is significantly inhibited by nickel in a dose-dependent fashion (P <0.01). Our results also show that the 50% inhibitory concentration (IC50) of NiSO4 for GLUL activity is ~200 μM, significantly below the optimal manganese concentration (500 μM). Thus, our results indicate that nickel inhibits GLUL enzymatic activity in the micromolar range.
The GLUL Nickel-Binding Domain Overlaps With the Catalytic Domain
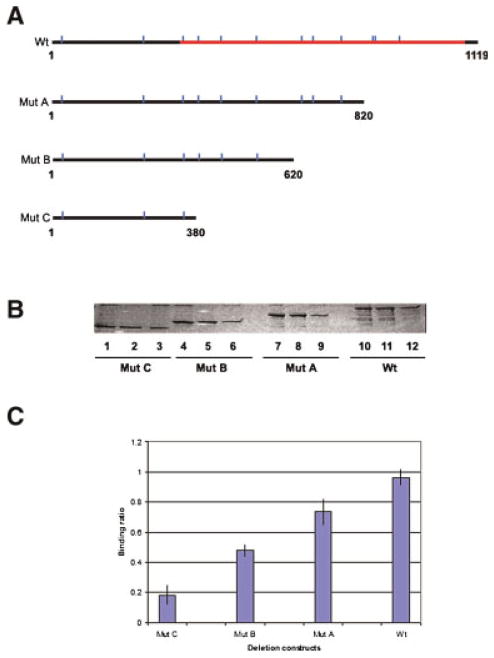
Nickel has been found to bind to a number of enzymes, including urease, hydrogenase, CO-dehydrogenase, methyl-coenzyme M reductase, Ni-superoxide dismutase, glyoxalase I, and cis–trans isomerase (for review, see Wattt and Ludden, 1999). For these metalloenzymes, nickel binding is essential for their activity and the nickel binding site, which contains the amino acids histidine and cysteine, spans the active site of these enzymes. To determine the nickel binding domain of GLUL, we first performed a bioinformatics analysis of rat testis GLUL protein: this indicated that (a) GLUL has 12 histidine residues distributed throughout the entire protein and (b) the catalytic domain is predicted to be very large, and located in a region from amino acid residue 110 to residue 361 (Fig. 6A, Wt). This domain contains 10 of the 12 His residues.
Figure 6.
Determination of the Ni2+-binding domain of glutamate–ammonia ligase protein. A: Diagram showing the positions of histidine residues in GLUL. Indicated are the sizes of recombinant deletion mutant proteins produced. Vertical lines, histidines. The GLUL catalytic domain is indicated. B: Nickel affinity binding and SDS–PAGE. Lanes 1–3, GST–GLUL protein mutant C (41 kDa, spanning nucleotides 1–380). Lanes 4–6, GST–GLUL protein mutant B (50 kDa, spanning nucleotides 1–620). Lanes 7–9, GST–GLUL protein mutant A (57 kDa, spanning nucleotides 1–820). Lanes 10–12, GST–GLUL full length (68 kDa, spanning nucleotides 1–1,119). Lanes 1, 4, 7, and 10, input protein samples. Lanes 2, 5, 8, and 11, protein bound to Ni-NTA agarose beads. Lanes 3, 6, 9, and 12, flow through proteins. C: Quantification of nickel-binding activity of deletion mutants and full length GLUL protein. After nickel binding and SDS–PAGE, the intensity of each fractionated protein band was quantified. The diagram shows the average ratio of the intensity of each eluted protein band vs. the input protein band from three independent experiments. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com]
To identify the nickel binding domain, we prepared a series of deletions in GLUL protein (shown in Fig. 6A). Wild type (Wt) and deletion mutants were incubated with nickel agarose beads. After elution and fractionation, the intensity of each band was quantified. The results of the binding assays are shown in Figure 6B and quantitation represented in Figure 6C. This demonstrates that compared to full length GLUL protein, deletion mutants A, B, and C show a gradual decrease of 74%, 48%, and 18% of Wt binding activity, respectively. Importantly, the region deleted in mutant C corresponds to the hypothetical catalytic domain of GLUL protein. This result suggests that the nickel-binding domain is spread out over the central and C-terminal regions of GLUL, which overlaps with the catalytic domain of GLUL, rather than being defined by a small motif.
It is known that in its native state as holoenzyme, mammalian GLUL protein forms an eight-subunit oligomer (Eisenberg et al., 2000 and references therein). Thus, Wt GST–GLUL holoenzyme should have a molecular weight of at least 400 kDa (for Wt GST–GLUL protein, the molecular weight of the holoenzyme is 69 × 8 kDa). The observed reduction in nickel-binding capability of mutant GLUL proteins may thus result from an inability to form oligomers. To explore this, we set out to analyze the size of non-denatured Wt and mutant protein GLUL proteins using native polyacrylamide gel electrophoresis. Proteins with a native molecular weight larger than 300 kDa are unable to enter into the gel under our conditions. Bacterially expressed Wt and mutant GLUL proteins were extracted and renatured as for the above nickel-binding assay. Samples were analyzed on a 5% native polyacrylamide gel. The results are shown in Figure 7A and indicate that neither Wt nor any of the mutant GST–GLUL proteins entered the gel while the control (GST protein only) migrates as expected. To exclude the possibility that Wt and mutant proteins were degraded during preparation, the same samples were denatured and analyzed on a 12% denaturing polyacrylamide gel (Fig. 7B). The results demonstrate that all proteins were intact. Thus, the decrease in nickel-binding activity of mutant GLUL proteins is not the result of an inability to form oligomers.
Figure 7.
Wt and deletion mutant glutamate–ammonia ligase proteins form oligomers. Non-denaturing (native) polyacrylamide gel electrophoresis analysis of Wt and mutant GST–GLUL protein oligomers was carried out. All samples were analyzed by native and denaturing polyacrylamide gel electrophoresis. Panel A: native PAGE. Panel B: denaturing SDS–PAGE. Lane 1, Wt GST–GLUL protein. Lanes 2–4, mutants A–C, respectively. Lane 5, GST protein as the control. Molecular sizes of marker proteins are indicated for panel B.
Determination of the Dosage of Nickel That Causes Pathological Changes in Rat Testis
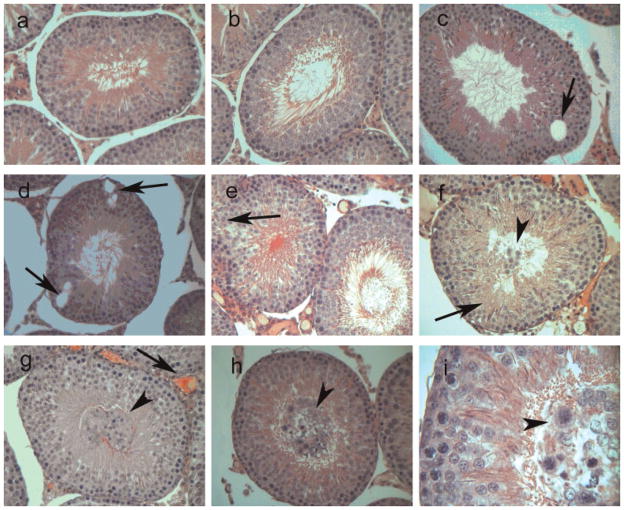
The GLUL analyses so far have been done in vitro. We next wanted to compare these in vitro results with data obtained in vivo using a rat animal model. We first determined the dose of nickel required to produce pathological effects in the testis of rats treated with nickel administered intraperitoneally. Throughout the treatment, food and water consumption did not differ between treated and control groups, and weight loss was not observed in treated animals. After euthanization, measurement of organ weight indicated that there was no significant change in either average weight of testes or the ratio of testis and whole body weight. Histological examination of testes of treated and control animals showed changes in animals treated with the medium (2.5 mg/kg) and high dose (5.0 mg/kg) (Fig. 8): vacuoles were found inside seminiferous tubules close to the basement membrane at the medium dosage (Fig. 8, panels C and D, arrows). The germ cell layer appeared in several instances to be interrupted (Fig. 8, panels E–H, arrows). Also, at medium and high dosages, detached germ cells were observed inside the lumen of seminiferous tubules (Fig. 8, panels F, G, and H, arrowheads): these cells appeared to have abnormal chromatin staining (Fig. 8, panel I, arrowhead). We used the high dosage for subsequent animal GLUL activity experiments.
Figure 8.
Pathology of nickel-treated rat testis. Rats were treated with nickel intraperitoneally at high (5 mg/kg) dosage, and their testes were analyzed for nickel-induced effects. A,B: Control animal that received saline. C–I: Animals treated with 5 mg/kg nickel sulfate. C,D: Arrows indicate vacuoles. E: Arrow indicates occasionally observed interruption in the germ cell layer. F: Arrow denotes disarrangement of spermatocytes. G: the arrow denotes blood vessel expansion in the interstitium. F–H: Arrowheads indicate detached germ cells into the lumen of the seminiferous tubules. I: The arrowhead points to detached germ cells present in the lumen of seminiferous tubules displaying abnormal staining of chromatin. A–H: 40× magnification. I: 100× magnification.
Nickel Inhibits the Activity of Rat Testicular GLUL Protein
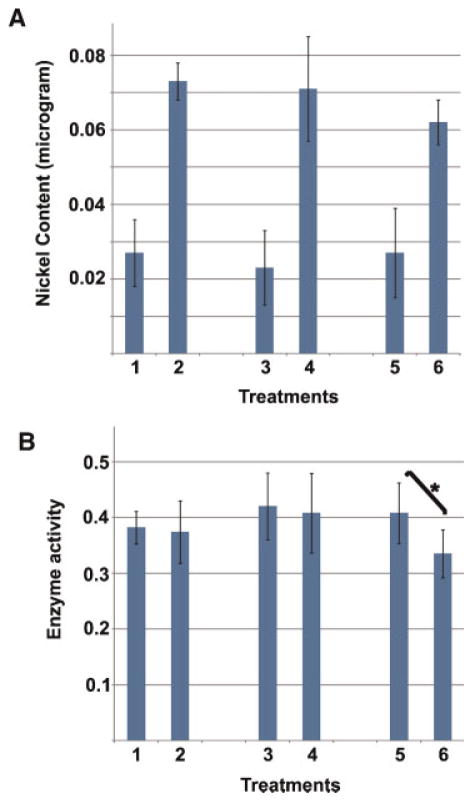
We next measured the effect of nickel on GLUL activity in testes of nickel-treated rats. We treated animals at the high dosage with nickel for 10, 20, and 30 days. We then determined the nickel concentration in testes of treated animals in comparison with controls receiving saline, and measured GLUL activity in testes extracts of these animals. The results (Fig. 9A) indicate that, on average, testes of treated animals contained approximately three times more nickel than controls at all time points. Nickel concentration did not increase with prolonged time of treatment. Determination of GLUL enzyme activity showed that enzyme activity was significantly reduced in testes of animals treated with nickel for 30 days in comparison to mock controls (P <0.05) (Fig. 9B). No significant decrease was observed at earlier time points (see Discussion Section). Together with our in vitro analysis of GLUL, these data indicated that testicular GLUL is capable of binding nickel, and that its activity is inhibited by nickel.
Figure 9.
Treatment with nickel inhibits testicular glutamate–ammonia ligase activity. Nickel concentration and enzyme activity were measured in testes of rats subjected to nickel treatment. Treatments indicated in panels A and B were as follows: Lane 1, 10 days of saline treatment (control); lane 2, 10 days of nickel treatment; lane 3, 20 days of saline treatment (control); lane 4, 20 days of nickel treatment; lane 5, 30 days of saline treatment (control); lane 6, 30 days of nickel treatment. Panel A: Nickel concentration per gram of testes after treatment with saline or nickel. Panel B: GLUL enzymatic activity (mM/mg protein/min) in testes of animals treated with saline or nickel. Bars represent standard deviation. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com]
DISCUSSION
While there is evidence showing that humans exposed to nickel develop skin allergies and lung cancer, there are no reports on the effects of nickel exposure on the human male reproductive system. Animal studies, however, have shown that nickel deficiency can diminish sperm quantity and sperm tail movement in rats (Yokoi et al., 2003) whereas over-exposure can cause a number of pathologic changes to testicular structure (Pandey et al., 1999; Das and Dasgupta, 2000; Doreswamy et al., 2004). This suggests that nickel can accumulate in testis and affect proteins in various but poorly understood ways, for example, through changes in protein interactions, protein post-translational modifications, and enzymatic activity. Here we identify, for the first time, a testicular protein capable of binding nickel, viz. GLUL. In mammals, GLUL is expressed in every tissue, including testis (Lie-Venema et al., 1997 and references therein). In rat testis, GLUL is expressed predominantly in interstitial cells with possibly a very low level of expression in germ cells. GLUL requires manganese for optimal activity, and we show that it binds more efficiently to nickel, which inhibits its activity both in vitro using a recombinant, enzymatically active GLUL and in vivo for testicular GLUL.
Although there has been no report documenting nickel toxicity for the human male reproductive system, rodents have been used as models to analyze such toxicity (Hoey, 1966; Mathur et al., 1977; Pandey et al., 1999; Das and Dasgupta, 2000; Doreswamy et al., 2004). Perhaps as a result of the different routes and dosage of administration, tested animals displayed different manifestations. There appeared, however, to be a correlation between higher dosage and a longer duration with more severe damage (Mathur et al., 1977). In this study, we observed pathological changes to rat testis at high dosage, including disturbance of the germ cell layer close to the basement membrane of seminiferous tubules and the presence of detached germ cells inside the lumen of seminiferous tubules. These latter cells may be related to the observation of necrotic material in the ductuli of nickel-treated rats reported previously (Hoey, 1966). It remains unknown how nickel causes these changes in testis, but several possibilities present themselves. First, nickel accumulation can result in production of reactive oxygen species, which could mediate oxidative damage to macromolecules. In somatic tissues, nickel has been found to enhance lipid peroxidation, which could trigger a free radical oxidation process and produce highly reactive lipid hydrogenperoxides, H2O2, hydroxyl radicals, and malondialdehyde (e.g., see Athar et al., 1987; Kasprzak and Hernandez, 1989; Chen et al., 1998). Lipid peroxidation activity has been found in the testis of rats treated with nickel (Doreswamy et al., 2004). A second possibility is that nickel accumulation in testis interferes with the activities of enzymes. Pandey et al. (1999) reported that there are significant alterations in the activities of marker testicular enzymes, such as sorbitol dehydrogenase (decrease), lactate dehydrogenase (increase), and gamma-glutamyl transpeptidase (increase). Iscan et al. (2002) demonstrated that, when given a single dose of nickel chloride, reductions were seen in the activity of the testicular enzymes 7-ethoxyresorufin O-deethylase and GST. A third mechanism is represented by proteins that depend on the association of a protein with a metal for activity: one group of these is metalloenzymes that use a different metal as a cofactor, but can bind nickel resulting in either an increase or decrease in their activity. Mouse aminoacylase 3 is an example of this, and when expressed in E. coli contains approximately 0.35 zinc atoms per monomer. Incubation with nickel increased activity of aminoacylase 3 several times (Tsirulnikov et al., 2009). It remains to be discovered how nickel toxicity relates to reproduction. The effects could be multiple, including effects in interstitial cells that affect spermatogenesis, as well as effects in caput epididymis where high levels of GLUL activity had been reported (Kvidera and Carey, 1994).
Using a nickel-binding affinity protocol and mass spectrometry we discovered that rat testis GLUL is a protein that is capable of binding nickel. GLUL holoenzyme is a multimer and catalyzes a variety of reactions. It requires two divalent metal ions (manganese preferred) per subunit for optimal activity (Ginsburg and Stadtman, 1970; Tholey et al., 1987; Eisenberg et al., 2000). We prepared an enzymatically active recombinant GLUL protein in bacteria and used it to show that (1) GLUL appears to have a higher affinity for nickel than for manganese, (2) upon nickel binding manganese-catalyzed enzymatic activity of GLUL is significantly reduced, and (3) nickel binding is not limited to one small motif within GLUL protein, but instead is conferred across the larger catalytic domain, a finding that mirrors that of mouse aminoacylase 3 whose metal binding domain also overlaps with its enzymatically active site. To relate these in vitro findings with effects in whole testis, we next investigated the effect of nickel on the endogenous GLUL enzyme in testis of nickel-treated and control rats. The results from the in vivo experiments (carried out under conditions that cause pathological abnormalities in rat testis) corroborate the in vitro data: the testis accumulates nickel and GLUL activity is significantly reduced. Although the testicular nickel concentration is high even at early time points, we did not observe a significant inhibition of GLUL enzyme until 30 days of treatment. Possible explanations include the likely presence of several nickel-binding proteins in cells that also express GLUL, thus effectively lowering the availability of nickel to GLUL, and unknown factors that may prevent a reduction in GLUL activity in vivo, including detoxification, de novo synthesis of GLUL, etc. Be that as it may, to our knowledge, this is the first report that demonstrates that testicular GLUL enzyme activity is inhibited by nickel.
We do not know if and how the inhibition of GLUL may be correlated to the observed nickel-induced testicular abnormalities, but we show that in rat testis, GLUL is present at high levels in interstitial cells and in elongating spermatids. GLUL has several key roles. GLUL plays an important role in the removal of glutamate and/or ammonia, hence contributing to the detoxification of testis. This is supported by evidence from mice where it was demonstrated that in organs with a high cellular concentration of GLUL, it functions primarily in the detoxification of glutamate and/or ammonia, whereas in organs with a low cellular GLUL concentration it is predominately involved in synthesis of glutamine (van Straaten et al., 2006). GLUL was also shown to be important for cell proliferation: inhibition of GLUL activity resulted in decreased cell proliferation (DeMarco et al., 1999). Rotoli et al. (2005) found that inhibition of GLUL activity can trigger cell apoptosis. Based on our results, a direct link between nickel exposure, decreased GLUL activity and induced testis abnormalities should be investigated.
MATERIALS AND METHODS
Animals
For histology studies, forty 8-week-old male Wistar rats were randomly divided into four groups. One group was used as control and treated with saline, while the other three groups were administered intraperitoneally with NiSO4 (Sigma, St. Louis, MO) at doses of 1.25, 2.5, or 5.0 mg/kg for 30 days. For nickel concentration and GLUL enzyme activity assay, 36 rats were divided into two groups: a mock treatment control group of 9 animals (3 animals per time point of 10, 20, and 30 days of treatment) and an experimental group of 27 animals (9 animals treated for each time point of 10, 20, and 30 days with 5.0 mg/kg nickel). After treatment, the animals were euthanized, and the testes of the animals were removed for histological and GLUL activity examinations as well as nickel concentration measurement. All animal studies were carried out in accordance with the regulations of the Canadian Council for Animal Care.
Screening of Nickel-Binding Proteins in Rat Testis
Rat testis was homogenized in buffer A (50 mM NaH2-PO4, 300 mM NaCl, 10 mM imidazole, pH 8.0) using a Pellet Pestle® Motor (Kimble Kontes LLC, VWR, Edmonton, AB). The homogenates were prepared by sonication for 2 min at 4°C. The lysate was centrifuged at 10,000 rpm for 10 min at 4°C and the supernatant was collected and pre-cleared by incubating with 10% agarose at 4°C. This was repeated four times. Nickel chelating nitrilotriacetic acid (Ni-NTA) resin (Qiagen, Mississauga, ON) was equilibrated with buffer A and incubated with the precleared supernatant at 4°C for 2 hr. The Ni-NTA resin was then washed extensively with buffer B (50 mM NaH2PO4, 300 mM NaCl, 20 mM imidazole, pH 8.0). The column-bound Ni2+-binding proteins were eluted from Ni-NTA agarose with buffer C (50 mM NaH2-PO4, 300 mM NaCl, 250 mM imidazole, pH 8.0) and resolved by SDS–PAGE. Identification of gel-separated proteins was done by mass spectrometry analysis (MALDI-MS) at the Southern Alberta Mass Spectrometry Centre (University of Calgary).
Protein Quantification
Protein concentrations were determined by the Bradford method using a commercially available reagent (Bio-Rad Laboratories, Mississauga, ON) and bovine serum albumin.
Cloning of Glutamate–Ammonia Ligase cDNA
Rat GLUL mRNA (GenBank accession no. BC072694) was polymerase chain reaction (PCR)-amplified from a rat testis cDNA library (custom-made by Invitrogen, Burlington, ON) using the following forward primer 5′-TCTGGCAGATCTACATGGCCACCTCAGCAAGT-3′ and reverse primer 5′-CAGCGAGATCTGTCGACGTTCTTGTATTGGAAGGG-3′. The PCR product was purified, digested with Bgl II/Sal I, and ligated into the pGEX-5X-1 vector (GE Healthcare, Piscataway, NJ) to yield the recombinant plas-mid pGEX-5X-1-GLUL.
For mapping of the Ni2+-binding domain of GLUL, several primers were designed to make deletion constructs of GLUL by PCR. For mutants A, B, and C, the forward primer was the same as shown above, while the reverse primers were 5′-ATTAGTCGACATGGCCTCCTCAATGCACCTC-3′, 5′-ATTAGTCGACTCCTATCTGGAATTCCCACTG-3′, 5′-ATTAGTCGACCTGGCTGCTCACCATGTCCAT-3′, respectively. The PCR fragments were cloned into the pGEX-5X-1 plasmid.
Expression of Recombinant Protein
E. coli cells were transformed with plasmids encoding GST (pGEX-5x-1), GST–GLUL, GST mutants, and His-tagged GST, respectively, and cultured to log phase (OD600 = 0.6). The expression of the GST or the GST–GLUL fusion protein was induced with 0.15 mM isopropyl-β-D-thiogalactoside (IPTG) and incubated at 37°C for 3 hr. Bacteria were harvested and resuspended in Buffer A. Cells were lysed by sonication for 2 min. The lysate was centrifuged at 10,000 rpm for 10 min at 4°C. The recombinant GST–GLUL protein is not soluble in this buffer, and remained in the resulting pellet. Eight molar urea was used to solubilize the protein, followed by dialysis in buffers containing decreasing concentrations (4, 2, 1, and 0 M) of urea in buffer A at 4°C for 4 hr, respectively.
Nickel-Binding Assay
The recombinant proteins (GST–GLUL and GST–GLUL mutants) were incubated with Ni-NTA agarose for 1 hr at 4°C. Control samples (GST as negative control and His-tagged GST as positive control) were incubated under the same condition. After extensive washing with buffer B, proteins bound to Ni-NTA agarose were eluted with buffer C. Eluted proteins were resolved by SDS–PAGE and stained with Coomassie Blue G-250.
To demonstrate that binding of GLUL to Ni-NTA beads was specific and dependent on nickel, solubilized GST–GLUL protein was incubated for 60 min at 4°C with Ni-NTA beads in the presence of increasing concentrations of NiSO4 (0, 0.125, 0.25, 0.50, 1.0, and 2.0 mM). The Ni-NTA binding assay was performed and analyzed as described above.
Assay for Glutamate–Ammonia Ligase Enzymatic Activity
The enzymatic activity of GLUL was determined using the procedures described previously (Meister, 1985; Minet et al., 1997). In brief, for bacterial recombinant protein, solubilized GST–GLUL was incubated for 30 min at room temperature with different concentration of nickel sulfate at 0, 50, 100, 200, 400, or 800 μM, or with manganese chloride at 0, 12.5, 25, 50, 100, or 200 mM. Seventy to 80 μl of each solution were mixed with 0.8 ml of assay buffer (50 mM imidazole, 50 mM hydroxylamine, 100 mM L-glutamine, 25 mM sodium arsenate dibasic heptahydrate, 0.2 mM ADP, 0.5 mM manganese chloride, pH 6.2) and incubated for 30 min at 37°C. The reactions were terminated with the addition of 0.2 ml of 0.37 M FeCl3, 0.3 M trichloroacetic acid, and 0.6 M HCl. The precipitate was removed by centrifugation. The absorbance of the supernatant was measured with a spectrophotometer at 520 nm. For each assay, blank controls containing the complete incubation mixture lacking ADP and sodium arsenate dibasic heptahydrate were done. A standard curve of L-γ-glutamylhydroxamic acid (Sigma) was prepared in buffer A and incubated in parallel with the experimental samples. The enzyme activity is defined as the amount of L-γ-glutamylhydroxamic acid produced and the unit of GLUL activity is expressed as μM/mg protein/min. In the assay, three parallel samples in every group were used to measure enzyme activity. Enzyme inhibition (%) was calculated using the following equation: Enzyme inhibition (%) =(GLULcontrol – GLULsample)/GLULcontrol – 100%, where GLULcontrol represents the enzyme activity in reaction buffer and GLULsample represents the enzyme activity after treatment with different concentrations of nickel sulfate and manganese chloride.
For determination of GLUL protein activity in rat testis, one testis from each animal was weighed, and homogenized using a Wheaton glass douncer to make a 2% (w/v) tissue suspension in TES buffer (10 mM Tris–HCl, pH 7.4, 0.1 mM EDTA, 10 mM sucrose, and 150 mM NaCl). The suspension was sonicated for 2 min with a micro-tip and spun at 2,000 rpm for 20 min. The supernatant was used for protein concentration measurement and for GLUL enzyme activity assays.
Western Blot Analysis of GLUL in Rat Testis
Western blot analysis was carried out essentially as described previously (Fitzgerald et al., 2006). In short, proteins were boiled in loading buffer, separated on 10% polyacrylamide SDS gels, electrophoretically transferred onto a polyvinylidene fluoride membrane (Amersham Biosciences, GE Healthcare, Piscataway, NJ), blocked overnight at 4°C in blocking buffer (54 mM Tris, pH 7.5, 150 mM NaCl, 0.05% Nonidet P-40, 0.05% Tween-20, 5% nonfat dry milk), and analyzed using primary antibodies followed by HRP-conjugated secondary antibody. Prestained Protein Ladder SM0671 (Fermentas, Burlington, ON) was used as a size marker. ECL solution (GE Healthcare) was used to develop the blot. The antibody to GLUL was purchased from Sigma.
Rat Testis Histology, Immunohistochemistry, Cryo-Sectioning, and Immunofluorescence
A standard protocol was used for preparation and sectioning of testes and hematoxylin and eosin staining. In short, rat testes were cut into two pieces, fixed for 24 hr in 10% formalin, dehydrated through baths of progressively more concentrated ethanol and finally through xylene to remove ethanol. The tissue was then infiltrated with molten paraffin wax, and the embedded sample was cut into 5 μm sections. The sections were rehydrated and stained using the Sigma hematoxylin and eosin staining kit. For mounting, stained sections were dehydrated in 95% and absolute ethanol, cleared in xylene, and mounted onto microscope slides using Cytoseal (EMS, Hatfield, PA).
For cryo-sectioning and immunofluorescence, testis was quick-frozen in OCT compound and sectioned to 5 μm in thickness. The sections were thawed on ice, fixed in cold methanol for 20 min, rinsed with PBS, and stained with rabbit anti-GLUL antibody (Sigma) and a secondary goat-anti-rabbit antibody conjugated with Cy3 fluorescence dye (Sigma). The tissue sections were counter-stained with 4′,6-diamidino-2-phenylindole (DAPI) to indicate cell nuclei of the testis, and were mounted in 90% glycerol containing paraphenylenediamine. The testis sections were examined and photographed under light microscopy, and the images were combined and aligned using Adobe Photoshop.
Determination of Nickel Concentration in Rat Testis
One testis from each rat was weighed, and digested overnight in a 10 ml solution containing four parts of nitric acid and one part of perchloric acid. There was no significant change in either average weight of the testes or the ratio of testis weight and whole body weight. To further solubilize the testis, the digest was subjected to the following sequential treatments: 100°C for 5 hr, 120°C for 3 hr, and 150°C for 1 hr. After this treatment, the testis suspension was allowed to cool down to room temperature and continue for an overnight incubation. The next day, the testis suspension was heated to 180°C for 8 hr. After cooling down, the final volume of the suspension was adjusted to 25 ml with double-distilled water. The content of nickel in the testis suspension was measured using a Graphite Furnace Atomic Absorption Spectrophotometer (Varian, Inc., Santa Clara, CA).
Statistical Analysis
Data are presented as means ± SE. The significance of differences between two groups (control vs. treated) was determined by the Student’s t-test. Data from three and more groups was calculated by the one-way analysis of variance (ANOVA). P <0.05 was considered statistically significant.
Acknowledgments
Contract grant sponsors: China Scholarship Council, Canadian Institutes of Health Research and the Alberta Cancer Board
We thank Dr. Ying Zhang and Ms. Maggie Lu for technical assistance. Y. Sun was supported by funding from the China Scholarship Council. Part of this work was supported by grants from the Canadian Institutes of Health Research and the Alberta Cancer Board (to FAvdH).
Abbreviations
- GLUL
glutamate–ammonia ligase
- GST
glutathione S-transferase
- Ni-NTA
nickel chelating nitrilotriacetic acid
References
- Athar M, Hasan SK, Srivastava RC. Evidence for the involvement of hydroxyl radicals in nickel mediated enhancement of lipid peroxidation: Implications for nickel carcinogenesis. Biochem Biophys Res Commun. 1987;147:1276–1281. doi: 10.1016/s0006-291x(87)80208-5. [DOI] [PubMed] [Google Scholar]
- Barceloux DG. Nickel. J Toxicol Clin Toxicol. 1999;37:239–258. doi: 10.1081/clt-100102423. [DOI] [PubMed] [Google Scholar]
- Benoff S, Cooper GW, Centola GM, Jacob A, Hershlag A, Hurley IR. Metal ions and human sperm mannose receptors. Andrologia. 2000a;32:317–329. doi: 10.1046/j.1439-0272.2000.00401.x. [DOI] [PubMed] [Google Scholar]
- Benoff S, Jacob A, Hurley IR. Male infertility and environmental exposure to lead and cadmium. Hum Reprod Update. 2000b;6:107–121. doi: 10.1093/humupd/6.2.107. [DOI] [PubMed] [Google Scholar]
- Bruske-Hohlfeld I. Environmental and occupational risk factors for lung cancer. Methods Mol Biol. 2009;472:3–23. doi: 10.1007/978-1-60327-492-0_1. [DOI] [PubMed] [Google Scholar]
- Chen CY, Huang YL, Lin TH. Association between oxidative stress and cytokine production in nickel-treated rats. Arch Biochem Biophys. 1998;356:127–132. doi: 10.1006/abbi.1998.0761. [DOI] [PubMed] [Google Scholar]
- Das KK, Dasgupta S. Effect of nickel on testicular nucleic acid concentrations of rats on protein restriction. Biol Trace Elem Res. 2000;73:175–180. doi: 10.1385/BTER:73:2:175. [DOI] [PubMed] [Google Scholar]
- DeMarco V, Dyess K, Strauss D, West CM, Neu J. Inhibition of glutamine synthetase decreases proliferation of cultured rat intestinal epithelial cells. J Nutr. 1999;129:57–62. doi: 10.1093/jn/129.1.57. [DOI] [PubMed] [Google Scholar]
- Doll R. Cancer of the lung and nose in nickel workers. Br J Ind Med. 1958;15:217–223. doi: 10.1136/oem.15.4.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doreswamy K, Shrilatha B, Rajeshkumar T, Muralidhara Nickel-induced oxidative stress in testis of mice: Evidence of DNA damage and genotoxic effects. J Androl. 2004;25:996–1003. doi: 10.1002/j.1939-4640.2004.tb03173.x. [DOI] [PubMed] [Google Scholar]
- Eisenberg D, Gill HS, Pfluegl GM, Rotstein SH. Structure-function relationships of glutamine synthetases. Biochim Biophys Acta. 2000;1477:122–145. doi: 10.1016/s0167-4838(99)00270-8. [DOI] [PubMed] [Google Scholar]
- Fitzgerald C, Sikora C, Lawson V, Dong K, Cheng M, Oko R, van der Hoorn FA. Mammalian transcription in support of hybrid mRNA and protein synthesis in testis and lung. J Biol Chem. 2006;281:38172–38180. doi: 10.1074/jbc.M606010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhardt R, Mecke D. Heterogeneous distribution of glutamine synthetase among rat liver parenchymal cells in situ and in primary culture. EMBO J. 1983;2:567–570. doi: 10.1002/j.1460-2075.1983.tb01464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg A, Stadtman ER. Multienzyme systems. Annu Rev Biochem. 1970;39:429–472. doi: 10.1146/annurev.bi.39.070170.002241. [DOI] [PubMed] [Google Scholar]
- Grimsrud TK, Berge SR, Haldorsen T, Andersen A. Exposure to different forms of nickel and risk of lung cancer. Am J Epidemiol. 2002;156:1123–1132. doi: 10.1093/aje/kwf165. [DOI] [PubMed] [Google Scholar]
- Grimsrud TK, Berge SR, Martinsen JI, Andersen A. Lung cancer incidence among Norwegian nickel-refinery workers 1953–2000. J Environ Monit. 2003;5:190–197. doi: 10.1039/b211722n. [DOI] [PubMed] [Google Scholar]
- Hoey MJ. The effects of metallic salts on the histology and functioning of the rat testis. J Reprod Fertil. 1966;12:461–472. doi: 10.1530/jrf.0.0120461. [DOI] [PubMed] [Google Scholar]
- Hostynek JJ. Sensitization to nickel: Etiology, epidemiology, immune reactions, prevention, and therapy. Rev Environ Health. 2006;21:253–280. doi: 10.1515/reveh.2006.21.4.253. [DOI] [PubMed] [Google Scholar]
- Iscan M, Ada AO, Coban T, Kapucuoglu N, Aydin A, Isimer A. Combined effects of cadmium and nickel on testicular xenobiotic metabolizing enzymes in rats. Biol Trace Elem Res. 2002;89:177–190. doi: 10.1385/BTER:89:2:177. [DOI] [PubMed] [Google Scholar]
- Kasprzak KS, Hernandez L. Enhancement of hydroxylation and deglycosylation of 2′-deoxyguanosine by carcinogenic nickel compounds. Cancer Res. 1989;49:5964–5968. [PubMed] [Google Scholar]
- Kvidera MD, Carey GB. Glutamine synthetase activity in rat epididymis. Proc Soc Exp Biol Med. 1994;206:360–364. doi: 10.3181/00379727-206-43772. [DOI] [PubMed] [Google Scholar]
- Lie-Venema H, de Boer PA, Moorman AF, Lamers WH. Role of the 5′ enhancer of the glutamine synthetase gene in its organ-specific expression. Biochem J. 1997;323:611–619. doi: 10.1042/bj3230611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur AK, Datta KK, Tandon SK, Dikshith TS. Effect of nickel sulphate on male rats. Bull Environ Contam Toxicol. 1977;17:241–248. doi: 10.1007/BF01685557. [DOI] [PubMed] [Google Scholar]
- Meister A. Glutamine synthetase from mammalian tissues. Methods Enzymol. 1985;113:185–199. doi: 10.1016/s0076-6879(85)13030-2. [DOI] [PubMed] [Google Scholar]
- Minet R, Villie F, Marcollet M, Meynial-Denis D, Cynober L. Measurement of glutamine synthetase activity in rat muscle by a colorimetric assay. Clin Chim Acta. 1997;268:121–132. doi: 10.1016/s0009-8981(97)00173-3. [DOI] [PubMed] [Google Scholar]
- Monder C. Metal ion interactions and glutamine synthetase activity. Biochemistry. 1965;4:2677–2686. doi: 10.1021/bi00888a017. [DOI] [PubMed] [Google Scholar]
- Nielsen FH. Ultratrace elements in nutrition. Annu Rev Nutr. 1984;4:21–41. doi: 10.1146/annurev.nu.04.070184.000321. [DOI] [PubMed] [Google Scholar]
- Nielsen FH, Ollerich DA, Fosmire GJ, Sandstead HH. Nickel deficiency in chicks and rats: Effects on liver morphology, function and polysomal integrity. Adv Exp Med Biol. 1974;48:389–403. doi: 10.1007/978-1-4684-0943-7_18. [DOI] [PubMed] [Google Scholar]
- Obone E, Chakrabarti SK, Bai C, Malick MA, Lamontagne L, Subramanian KS. Toxicity and bioaccumulation of nickel sulfate in Sprague–Dawley rats following 13 weeks of sub-chronic exposure. J Toxicol Environ Health A. 1999;57:379–401. doi: 10.1080/009841099157593. [DOI] [PubMed] [Google Scholar]
- Pandey R, Kumar R, Singh SP, Saxena DK, Srivastava SP. Male reproductive effect of nickel sulphate in mice. Biometals. 1999;12:339–346. doi: 10.1023/a:1009291816033. [DOI] [PubMed] [Google Scholar]
- Rotoli BM, Uggeri J, Dall’Asta V, Visigalli R, Barilli A, Gatti R, Orlandini G, Gazzola GC, Bussolati O. Inhibition of glutamine synthetase triggers apoptosis in asparaginase-resistant cells. Cell Physiol Biochem. 2005;15:281–292. doi: 10.1159/000087238. [DOI] [PubMed] [Google Scholar]
- Severa J, Vyskocil A, Fiala Z, Cizkova M. Distribution of nickel in body fluids and organs of rats chronically exposed to nickel sulphate. Hum Exp Toxicol. 1995;14:955–958. doi: 10.1177/096032719501401204. [DOI] [PubMed] [Google Scholar]
- Stangl GI, Kirchgessner M. Nickel deficiency alters liver lipid metabolism in rats. J Nutr. 1996;126:2466–2473. doi: 10.1093/jn/126.10.2466. [DOI] [PubMed] [Google Scholar]
- Tholey G, Bloch S, Ledig M, Mandel P, Wedler F. Chick brain glutamine synthetase and Mn2+–Mg2+interactions. Neurochem Res. 1987;12:1041–1047. doi: 10.1007/BF00970934. [DOI] [PubMed] [Google Scholar]
- Tsirulnikov K, Abuladze N, Newman D, Ryazantsev S, Wolak T, Magilnick N, Koag MC, Kurtz I, Pushkin A. Mouse aminoacylase 3: A metalloenzyme activated by cobalt and nickel. Biochim Biophys Acta. 2009;1794:1049–1057. doi: 10.1016/j.bbapap.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Straaten HW, He Y, van Duist MM, Labruyere WT, Vermeulen JL, van Dijk PJ, Ruijter JM, Lamers WH, Hakvoort TB. Cellular concentrations of glutamine synthetase in murine organs. Biochem Cell Biol. 2006;84:215–231. doi: 10.1139/o05-170. [DOI] [PubMed] [Google Scholar]
- Wattt RK, Ludden PW. Nickel-binding proteins. Cell Mol Life Sci. 1999;56:604–625. doi: 10.1007/s000180050456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whanger PD. Effects of dietary nickel on enzyme activities and mineral contents in rats. Toxicol Appl Pharmacol. 1973;25:323–331. doi: 10.1016/0041-008x(73)90306-2. [DOI] [PubMed] [Google Scholar]
- Xie J, Funakoshi T, Shimada H, Kojima S. Effects of chelating agents on testicular toxicity in mice caused by acute exposure to nickel. Toxicology. 1995;103:147–155. doi: 10.1016/0300-483x(95)03134-2. [DOI] [PubMed] [Google Scholar]
- Yokoi K, Uthus EO, Nielsen FH. Nickel deficiency diminishes sperm quantity and movement in rats. Biol Trace Elem Res. 2003;93:141–154. doi: 10.1385/BTER:93:1-3:141. [DOI] [PubMed] [Google Scholar]