Abstract
The analysis of (6R)-5,6,7,8-tetrahydrobiopterin (BH4) and neopterin in cerebrospinal fluid (CSF) is often used to identify defects in the pterin biosynthetic pathway affecting monoamine metabolism that can lead to pediatric neurotransmitter diseases. Low levels of BH4 and neopterin alone may not be sufficient to determine the defect, and further testing is often required. We have developed a sensitive liquid chromatography tandem mass spectrometry (LC-MS/MS) method for determination of BH4, 7,8-dihydrobiopterin (BH2), neopterin, and sepiapterin in CSF, which provides a more comprehensive evaluation of the pterin pathway. The method utilizes labeled stable isotopes as internal standards and allows for a fast 10-minute analysis by LC/MS/MS over a linear working range of 3 to 200 nmol/L. Total analytical imprecision is less than 14.4% for all pterin metabolites. Accuracy for BH4 and neopterin was determined by comparing data obtained by an alternative method using HPLC with EC and fluorescence detection. Excellent correlation was demonstrated for BH4 (r = 0.9646, 1/slope = 0.9397; n = 28; concentration range 3 to 63 nmol/L) and neopterin (r = 0.9919, 1/slope = 0.9539; n = 13; concentration range 5 to 240 nmol/L). CSF specimens from patients diagnosed with inborn errors of sepiapterin reductase (SR), 6-pyruvoyl-tetrahydropterin synthase (PTPS), dihydropteridine reductase (DHPR), and guanosine triphosphate cyclohydrolase (GTPCH) have been analyzed, and distinct pterin metabolite patterns were consistent with the initial diagnosis. This method differentiates patients with DHPR and SR deficiency from other pterin defects (GTPCH and PTPS) and will be useful for the diagnosis of specific defects in the pterin biosynthetic pathway.
Introduction
A variety of rare inherited inborn errors related to (6R)-5,6,7,8-tetrahydrobiopterin (BH4) metabolism have been described, often referred to as pediatric neurotransmitter disease. These disorders may be associated with or without hyperphenylalaninemia (HPA) and can lead to low or decreased homovanillic acid and 5-hydroxyindoleacetic acid (Blau et al. 2001; Fig. 1). Some BH4 disorders are detected by neonatal newborn screening since they present with HPA and are caused by enzyme deficiencies of guanosine triphosphate cyclohydrolase (GTPCH, OMIM 600225; MIM 233910; EC 3.5.4.16)-autosomal recessive form (Niederwieser 1984), pterin-4-α-carbinolamine dehydratase (PCD, OMIM 126090; EC 4.2.1.96) (Thöny et al. 1998), 6-pyruvoyl-tetrahydropterin synthase (PTPS, OMIM 261640; EC 4.2.3.12), and dihydropteridine reductase (DHPR, OMIM 261630; EC 1.5.1.34) (Kaufman et al. 1975). The neurological symptoms associated with these disorders can vary and include mental disability, convulsions, dystonia, oculogyric crises, drowsiness, irritability, and swallowing difficulties. However, other inborn errors of pterin metabolism that do not present with HPA have been described and include GTPCH-autosomal dominant form (MIM 128230), initially described as Segawa disease (Segawa et al. 1976) and sepiapterin reductase (SR, OMIM 182125; EC 1.1.1.153) deficiency (Bonafe et al. 2001). Initial screening for these defects relies on analysis of pterins and monoamine neurotransmitter metabolites in cerebrospinal fluid (CSF).
Fig. 1.

Pterin and monoamine metabolism. Abbreviations: GTP guanosine triphosphate, GTPCH GTP cyclohydrolase, PTPS 6-pyruvoyl-tetrahydropterin synthase, SR sepiapterin reductase, BH4 (6R,S)-5,6,7,8-tetrahydrobiopterin, BH2 7,8-dihydrobiopterin, DHPR dihydropteridine reductase, PCD pterin-4-α-carbinolamine dehydratase, CR carbonyl reductase, DHFR dihydrofolate reductase, qBH2 quinine-dihydrobiopterin, TH tyrosine hydroxylase, TPH tryptophan hydroxylase, PAH phenylalanine hydroxylase, AADC aromatic amino acid decarboxylase, L-DOPA L-3,4-dihydroxyphenylalanine, 5-HTP 5-hydroxytryptophan, 3-OMD 3-o-methyldopa, DA dopamine, 5-HT serotonin, HVA homovanillic acid, 5-HIAA 5-hydroxyindoleacetic acid
Numerous methodologies have been employed to determine pteridines, including radioenzymatic assay (Guroff et al. 1967), radioimmunoassay (Nagatsu et al. 1981), HPLC with fluorescence (Stea et al. 1979; Fukushima and Nixon 1980a), HPLC with electrochemistry (Lunte and Kissinger 1983; Bräutigam et al. 1982), and HPLC or UPLC with electrochemistry and fluorescence (Hyland 1985; Guibal et al. 2014). One of the first HPLC methods developed relied on the highly fluorescent characteristics of the fully oxidized pteridines and the differential oxidation of reduced biopterin states (Fukushima and Nixon 1980b; Fukushima et al 1978). This allowed an indirect method of detecting both BH4 and 7,8-dihydrobiopterin (BH2) through the measurement of total biopterin. This method has been applied to detect BH4 and BH2 in tissues, urine, plasma, and dried blood spots (Schmidt et al. 2006; Antonozzi et al. 1988).
We here describe a method using stable isotope dilution liquid chromatography tandem mass spectrometry (LC-MS/MS) to quantitate BH4, BH2, neopterin, and sepiapterin in CSF that can be applied as a screening method for the differentiation of inborn errors of pterin metabolism.
Materials and Methods
Standards and 15N-labeled stable isotope internal standards, BH4, BH2, neopterin, sepiapterin, 15N-BH4, 15N-BH2, and 15N-neopterin, were obtained from Schircks Laboratories (Switzerland). Formic acid, heptafluorobutyric acid, ammonium acetate, and dithiothreitol (DTT) were obtained from Fluka and Optima LC-MS grade methanol from Fisher Scientific. Diethylenetriaminepentaacetic acid (DETAPAC) was purchased from Sigma. Stock standards for each pterin metabolite and internal standard were prepared as 1 mmol/L solutions in water containing 0.2% DTT and stored at −80°C. Microtiter plates used for were purchased from NUNC.
Sample Collection
CSF samples were collected in CSF collection kits provided by the neuropharmacology laboratory at the Baylor Research Institute. Specimens used for accuracy validation of CSF BH4 and neopterin were performed on tube #3 of 5 which contained DTT and DETAPAC. After collection, samples were frozen and shipped by overnight courier on dry ice and are stored at −80°C until analysis. Blood-contaminated CSF samples are rejected, since hemoglobin from erythrocytes may cause auto-oxidation of pterins. This can be avoided by centrifuging prior to freezing, and the clear CSF transferred to another vial and frozen.
Analysis of Amine Neurotransmitters in CSF
CSF amine neurotransmitters were analyzed by HPLC with electrochemical detection by a previously published method (Hyland et al. 1986).
Analysis of BH4 and Neopterin in CSF by HPLC with Electrochemical and Fluorescence
CSF BH4 and neopterin were analyzed by HPLC with electrochemical and fluorescence detection by a previously published method (Hyland 1985).
Analysis of BH4, BH2, Sepiapterin, and Neopterin in CSF by LC-MS/MS
Sample Preparation
The calibration curve was prepared in water containing 0.2% DTT over a range of 3 to 200 nmol/L for each pterin analyte. Internal standard concentrations of 15N-BH4, 15N-BH2, and 15N-neopterin were prepared in water containing 0.2% DTT at a final concentration of 100 nmol/L each. Sample preparation for CSF pterin analysis consisted of combining 210 μL of 100 nmol/L internal standard solution with 30 μL of blank, standard, control, or sample in 1.5 mL Eppendorf tubes, mixed by vortex and loaded in a microtiter plate for analysis.
LC-MS/MS
The LC-MS/MS analysis was performed on an AB Sciex 5500QTRAP mass spectrometer (Foster City, CA, USA) coupled with a Shimadzu ultrahigh pressure Nexera chromatograph system (Kyoto, Japan). Nitrogen gas was supplied by an AB-3G gas generator (Peak Scientific) and was used as the drying, nebulizing, curtain, and collision gas. The MS/MS experiments were performed under positive electrospray ionization (+ESI) with multiple-reaction monitoring (MRM) using a Turbolon Spray electrospray source operating at a voltage of 1.1 kV and desolvation temperature of 700°C. All pterin standards exhibited intense protonated molecular ions under + ESI conditions. The parameters for ion selection and collision-activated fragmentation of the molecular ions of pterin standards and 15N-labeled isotopes were optimized by continuous infusion of pure compounds (1 μmol/L) at a flow rate of 10 μL/min. The selected precursor and fragment ions used for the measurement of unlabeled and labeled pterins are summarized in Table 1. The mass spectrometer was operated under unit resolution. The LC-MS/MS data was acquired and processed using Analyst 1.5.2 software (AB Sciex).
Table 1.
MS/MS parameters of pterins and internal standards (declustering potential (DP); collision energy (CE); and collision exit potential (CXP))
| Analyte | Retention time (min) | MRM transition | |||||
|---|---|---|---|---|---|---|---|
| Q1 (m/z) | Q3 (m/z) | Dwell time (ms) | DP (V) | CE (V) | CXP (V) | ||
| BH4 | 5.9 | 242.2 | 166.0 | 80 | 66 | 25 | 16 |
| 15N-BH4 | 5.9 | 243.1 | 167.0 | 80 | 66 | 25 | 13 |
| BH2 | 5.7 | 240.1 | 164.9 | 80 | 51 | 29 | 14 |
| 15N-BH2 | 5.7 | 241.1 | 197.0 | 80 | 66 | 19 | 14 |
| Neopterin | 5.2 | 254.1 | 206.1 | 80 | 40 | 23 | 10 |
| 15N-Neopterin | 5.2 | 255.1 | 206.1 | 80 | 40 | 23 | 10 |
| Sepiapterin | 5.9 | 238.1 | 165.1 | 80 | 40 | 27 | 10 |
Liquid chromatography was performed on a Shimadzu Nexera system by reversed-phase HPLC (EZfaast 250 × 2 mm 4 μm AAA-MS column, with a 4 × 2 mm SecurityGuard column, Phenomenex, CA, USA) equilibrated at 40°C. The system consisted of a binary gradient: Eluent A (water containing 0.1% formic acid and 0.1% heptafluorobutyric acid) and Eluent B (methanol containing 0.1% formic acid). The flow rate was 0.20 mL/min, and 10 μL of processed sample was injected for analysis. The LC gradient was optimized to retain the pterin compounds on the column while eluting ion-suppressing moieties. The initial composition of the gradient was 95% A and increased linearly to 25% A during the first 4 min. The concentration of Eluent B was increased to 100% at 4.1 min and held until 5.0 min. At 5.1 min eluents were returned to initial conditions for equilibration. Total analytical analysis, including column re-equilibration, was 10 minutes. Eluent flow from the column was diverted to waste at the beginning and end of each run and was only directed to the source for the period from 5.0 to 7.5 min.
Assay Validation (Precision, Accuracy, and Recovery)
The method was validated by investigation of linearity, method precision studies (intra- and inter-day), accuracy, detection limit, and recovery. CSF was spiked with external pterin standards to produce a bi-level quality control (QC) used for both intra- and inter-day precision studies. Intraday precision (n = 10) was evaluated by analysis of bi-level QC material acquired within a single analytical run. Inter-day precision (n = 20) was evaluated by repeated analysis of bi-level QC material analyzed in duplicate over a period of 20 different days. Precision was determined as the relative standard deviation (RSD %). Recovery of pterins was performed by spiking CSF with water, 50 or 200 nM of each analyte. Determination of accuracy was performed only for BH4 and neopterin by comparing results obtained by HPLC with electrochemical and fluorescence detection currently used in our laboratory for these metabolites only.
Results
The collision-induced dissociation spectra of the protonated molecular ions of each pterin standard are given in Fig. 2. Representative chromatograms for the analytes in CSF are also shown in Fig. 2. The precursor-product transitions for each analyte and internal standard are shown: m/z 242.2 → 166.0 (BH4), 240.1 → 164.9 (BH2), 254.1 → 206.1 (neopterin), 238.1 → 165.1 (sepiapterin), 243.1 → 167.0 (15N-BH4), 241.1 → 197.0 (15N-BH2), and 255.1 → 206.1 (15N-neopterin) (Table 1). Monitoring of a second transition was not possible because there were no other unique fragments of significant intensity. In order to minimize ion suppression, this LC method was developed to retain analytes and internal standards later in chromatographic run. In preliminary ESI both positive mode and negative mode were tested. Positive ESI produced greater signal intensity for all analytes.
Fig. 2.
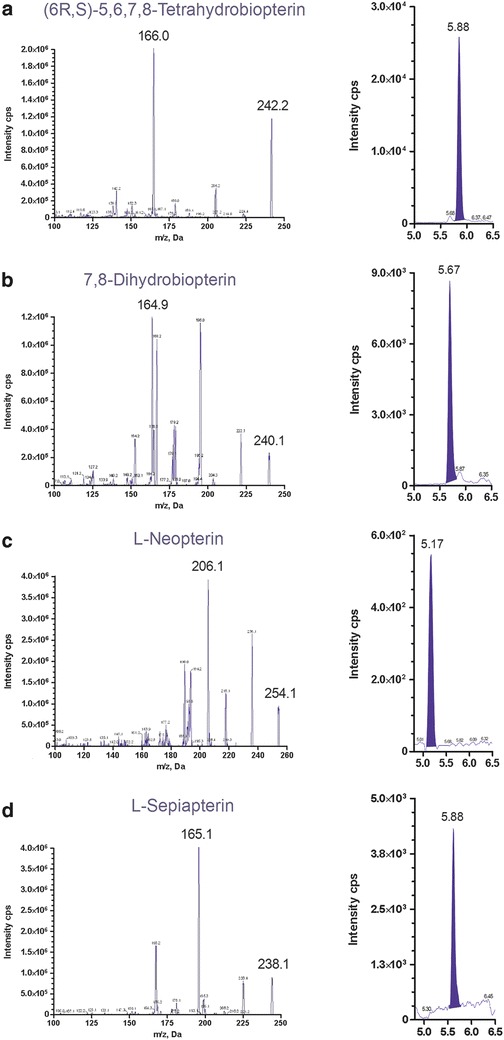
Product ion spectra of each pterin and a representative chromatograph of each metabolite in CSF. (a, BH4 = 51 nM; b, BH2 = 39 nM; c, neopterin = 20 nM; and d, sepiapterin = 23 nM). Spectra were generated with + ESI-MS/MS by infusion (10 μL/min) of pure standards (1 μM/L each)
Assay Performance and Validation
Linear regression analysis showed linearity for all analytes over their calibration range (data not shown). The calibration curves for BH4, BH2, sepiapterin, and neopterin were linear over a concentration range of 3 to 200 nmol/L. Intra-assay precision (n = 10) was assessed in spiked bi-level CSF with coefficient of variation (CVs) for all analytes <6% (Table 2). The inter-assay precision (n = 20) was assessed by analysis of duplicates of bi-level quality controls over a period of 20 different days. The CVs for BH4, BH2, and neopterin were <10% (Table 2). The inter-assay precision for sepiapterin was higher than the other pterins since 15N-BH2 was used as the internal standard since labeled sepiapterin is unavailable (Table 2). Recovery of 50 and 200 nM spikes of BH4, BH2, and neopterin ranged from 98 to 112%, whereas recovery for sepiapterin was 85 to 75% (data not shown).
Table 2.
Assay precision, linearity, and limit of detection (LOD)
| Analyte (nmol/L) | Internal standard | Intra-assay (n = 10) | Inter-assay (n = 20) | Linearity | LOD | ||
|---|---|---|---|---|---|---|---|
| Level 1 (CV%) | Level 2 (CV%) | Level 1 (CV%) | Level 2 (CV%) | ||||
| BH4 | 15N-BH4 | 55.1 ± 1.7 (3.0) | 156.1 ± 7.2 (4.6) | 50.2 ± 2.9 (5.9) | 149.0 ± 6.2 (4.2) | 3–200 | 1 |
| BH2 | 15N-BH2 | 41.0 ± 1.8 (4.4) | 170.9 ± 5.9 (3.5) | 38.6 ± 3.2 (8.2) | 152.7 ± 12.8 (8.4) | 3–200 | 1 |
| Neopterin | 15N-Neopterin | 35.6 ± 2.0 (5.6) | 160.2 ± 7.6 (4.7) | 29.1 ± 2.8 (9.5) | 141.3 ± 10.3 (7.3) | 3–200 | 1 |
| Sepiapterin | 15N-BH2 | CV% (5.3) | CV% (4.3) | CV% (14.4) | CV% (12.5) | 3–200 | 1 |
Data expressed as nmol/L, mean ± standard deviation (coefficient of variation, %)
Method Comparison (BH4 and Neopterin)
Accuracy of the LC-MS/MS method was determined by comparing BH4 and neopterin concentrations in CSF to values obtained by HPLC with electrochemical and fluorescence detection (Hyland 1985). CSF neopterin could not be directly compared between the two methods due to the electrochemical cell oxidizing reduced forms of neopterin completely to neopterin. The LC-MS/MS method only detects native oxidized neopterin and not any of the reduced forms. In order to obtain native oxidized neopterin on the HPLC with electrochemical and fluorescence method, the electrochemical cell was switched off, and this allowed only the native oxidized neopterin present in the CSF to be quantified by fluorescence. Method comparison data of BH4 (HPLC with electrochemical and LC-MS/MS) and neopterin (HPLC fluorescence and LC-MS/MS) is shown in Fig. 3.
Fig. 3.
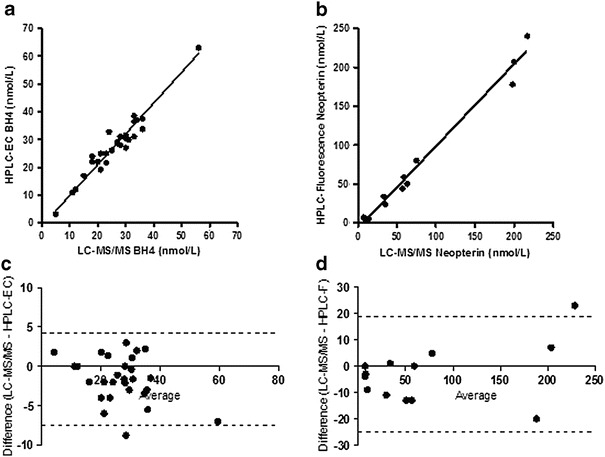
Pearson correlation coefficients (95% CLs) for BH4 and neopterin (a and b) were r = 0.9646 (0.9240, 0.9837) and r = 0.9919 (0.972, 0.998)%, respectively. Deming regression analysis for BH4 (a) yielded an intercept (95% CLs) of 1.05 (−1.16, 1.65) nmol/L and a 1/slope of 0.9397; neopterin (b) yielded an intercept of (95% CLs) of −7.17 (−2.86, −11.48) nmol/L and a 1/slope of 0.9539. Bland–Altman proportional bias analyses (c and d) relative bias (95% CLs) for BH4 and neopterin were −1.6 (4.1, −7.4)% and −2.9 (18.8, −24.5)%, respectively. All data expressed as nmol/L
Analysis of Pterins in Known Cases of BH4 Deficiencies
CSF specimens from subjects with pterin enzyme deficiencies, confirmed by genomic DNA analysis, having GTPCH-autosomal dominant PTPS, SR, and DHPR, were analyzed by LC-MS/MS. Pterin metabolite results for each specific enzyme deficiency, with data on monoamine neurotransmitter metabolites, are shown in Table 3. In some cases CSF samples were obtained either prior to treatment or following L-dopa therapy. As expected low BH4 levels were detected in subjects with GTPCH-autosomal dominant form and PTPS, whereas BH2 levels were elevated in patients with SR and DHPR deficiency. Elevated concentrations of sepiapterin were detected in two patients with SR deficiency and were undetectable (<1 nmol/L) in the other pterin disorders. Sepiapterin was undetectable in CSF from other subjects (n = 20) without any defects in pterin metabolism and having monoamine metabolite concentrations within the normal reference range (data not shown). The patient with PTPS deficiency presented with an increase in neopterin and with decreased BH4 and BH2 concentrations in CSF.
Table 3.
Differentiation of pterin defects through LC-MS/MS analysis
| Subject ID | Age | BH4 | BH2 | Neopterin | Sepiapterin | 5-HIAA | HVA | 3-OMD |
|---|---|---|---|---|---|---|---|---|
| Sepiapterin reductase deficiency | ||||||||
| Subject 1 | 8 years | 16 | 42.8 H | 2 | 7 H | 3 L | 49 L | 50 |
| Subject 1 | 9 years | 22 | 55.4 H | 4 | 6 H | 9 L | 81 L | 208* H |
| Subject 2 | 6 years | 24 | 46.5 H | 4 | 9 H | 4 L | 67 L | 27 |
| Subject 2 | 7 years | 31 | 58.1 H | 5 | 11 H | 10 L | 106 L | 205* H |
| Dihydropteridine reductase deficiency | ||||||||
| Subject 3 | 4 months | 27 | 54.0 H | 7 | <1 | 82 L | 281 L | 1835* H |
| Subject 3 | 11 months | 22 | 48.0 H | 17 | <1 | 39 L | 171 L | 453* H |
| 6-Pyruvoyl-tetrahydropterin synthase deficiency | ||||||||
| Subject 4 | 11 years | <1 L | 1 L | 29 H | <1 | 119 | 199 | 1533* H |
| Subject 4 | 11 years | 2 L | 3 | 27 H | <1 | 90 | 199 | 1870* H |
| G TP Cyclohydrolase deficiency – autosomal dominant | ||||||||
| Subject 5 | 36 years | 3 L | 3 | <1 | <1 | NA | NA | NA |
| Reference range | ||||||||
| 2–6 months | 23–98 | 3–18 | 0–18 | <1 | 179–711 | 450–1,132 | 0–300 | |
| 6 months–2 years | 20–58 | 3–18 | 0–18 | <1 | 129–520 | 294–1,115 | 0–300 | |
| 2–5 years | 20–58 | 3–18 | 0–18 | <1 | 74–345 | 233–928 | 0–150 | |
| 5–10 years | 20–58 | 3–18 | 0–18 | <1 | 66–338 | 218–852 | 0–100 | |
| 10–15 years | 9–40 | 3–18 | 0–18 | <1 | 67–189 | 167–563 | 0–100 | |
| >15 years | 9–32 | 3–18 | 0–18 | <1 | 67–140 | 145–852 | 0–100 | |
Data expressed as nmol/L. (L = below reference range; H = above reference range; * denotes L-dopa therapy)
Subjects 1 and 2 are siblings with confirmed SR deficiency having a homozygous 587A>G (Y196G) mutation. Subject 3 has a confirmed DHPR deficiency with a homozygous IVS7-2 A>G mutation. Subject 4 is assumed PTPS deficiency based on biopterin profile and clinical symptoms. Subject 5 has a confirmed GTPCH-1 deficiency with a Q180R mutation
NA not available
Discussion
An LC-MS/MS method was developed and validated for the simultaneous determination of four important pterin metabolites in CSF BH4, BH2, neopterin, and sepiapterin. The sample preparation procedure was optimized for increased accuracy and ease of use as a screening procedure for defects in pterin metabolism, which lead to pediatric neurotransmitter diseases. This method was further validated by analysis of CSF from subjects with known defects in pterin metabolism. Results showed the expected pattern of changes in pterin metabolites in subjects (Table 3) with defects in GTPCH-autosomal dominant (low BH4 and low BH2), SR (increased sepiapterin, increased BH2, and normal BH4), PTPS (increased neopterin, low BH4, and low BH2), and DHPR (increased BH2). Detection of neopterin is limited to native oxidized neopterin, which may not be reliable to discriminate GTPCH-autosomal dominant cases. However, elevated levels can distinguish patients that have PTPS deficiency or in cases of macrophage activation due to infection and inflammation.
Previous reports in which metabolites were measured in subjects with suspected disorders of pterin metabolism have relied on either multiple analytical analyses or differential oxidation of BH4 and BH2 to obtain a more complete understanding of the state of pterin synthesis. For example, shortly after the discovery of SR deficiency resulting in decreased BH4 metabolism (Bonafe et al. 2001), a HPLC-fluorescence method was reported to measure sepiapterin alone in CSF of patients suspected of having this disorder (Zorzi et al. 2002). Two recently published methods demonstrated the utility of LC-MS/MS for the determination of pterins (Fismen et al 2012; Kim et al 2012). The study by Fismen et al. (2012) first described the use of 15N-labeled stable isotopes to quantify different oxidative states of biopterin forms without differential oxidation. However, these pterins were only measured in human umbilical vein endothelial cells and did not include sepiapterin and neopterin. The study by Kim et al. (2012) described the quantitative analysis of BH4 and dopamine in rat brain extracts only, using LC-MS/MS and stable isotopes as internal standards.
The LC-MS/MS method described in this study is rapid and precise and requires minimal sample preparation for the analysis of CSF samples The high sensitivity provided by positive ESI LC-MS/MS eliminates the need for derivatization, and the HPLC separation is sufficient to avoid the analytes of interest co-eluting with matrix components that can cause ion suppression. The ability to quantitate BH4, BH2, sepiapterin, and neopterin in a single method will allow differentiation of pterin defects, particularly identifying those with DHPR and SR deficiency from other pterin defects (GTPCH and PTPS), by detecting elevated BH2 and the presence of sepiapterin in the case of SR deficiency. The pterin metabolite profile provided by this method will be beneficial in ascertaining the specific pterin defect involved and the correct course for follow-up testing. This method offers a significant improvement in screening patients and will lead to prompt diagnosis and management of inborn errors of pterin metabolism as well as therapeutic monitoring.
Acknowledgements
We would like to thank the following doctors for providing CSF from subjects with inborn errors of BH4 metabolism. Dr. Jose Abdenur and Dr. Richard Chang (Children’s Hospital of Orange County, Orange, CA); Dr. Klaas Wierenga (University of Oklahoma Health Sciences Center, Oklahoma City, OK); and Dr. Kathryn Swoboda (Pediatric Motor Disorders Research Program, Department of Neurology, University of Utah, Salt Lake City, UT).
Synopsis
Newly developed LC-MS/MS method for determination of CSF pterins will improve diagnostic differentiation of patients with inborn errors of pterin metabolism.
Compliance with Ethics Guidelines
Conflict of Interest
Erland Arning and Teodoro Bottiglieri declare that they have no conflict of interest.
Informed Consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Appropriate informed consents were obtained from all subjects or attending pediatric neurologists. All biochemical data was obtained through the routine analysis required for regular clinical care of each subject, as approved by the attending pediatric neurologist.
Animal Rights
This article does not contain any studies with animal subjects performed by the any of the authors.
Details of the Contributions of Individual Authors
Article contributions by Erland Arning: conception and design, analysis, interpretation of data, drafting, and revising manuscript. Article contributions by Teodoro Bottiglieri: conception and design, interpretation of data, and revising manuscript. Erland Arning is the guarantor for this article and accepts full responsibility for the work and conduct of the study, had access to the data, and controlled the decision to publish.
Footnotes
Competing interests: None declared
An erratum to this chapter is available at 10.1007/8904_2014_372
An erratum to this chapter can be found at http://dx.doi.org/10.1007/8904_2014_372
Contributor Information
Erland Arning, Email: erlanda@baylorhealth.edu.
Collaborators: Matthias R. Baumgartner, Marc Patterson, Shamima Rahman, Verena Peters, Eva Morava, and Johannes Zschocke
References
- Antonozzi I, Carducci C, Vestri L, Pontecorvi A, Moretti F. Rapid and sensitive method for high-performance liquid chromatographic analysis of pterins in biological fluids. J Chromatogr A. 1988;459:319–324. doi: 10.1016/S0021-9673(01)82042-2. [DOI] [PubMed] [Google Scholar]
- Blau N, Thöny B, Cotton RGH, Hyland K. Disorders of tetrahydrobiopterin and related biogenic amines. In: Scriver CR, Beaudet AL, Sly WS, Valle D, Vogelstein B, editors. The metabolic and molecular bases of inherited disease. New York: McGraw-Hill; 2001. pp. 1725–1776. [Google Scholar]
- Bonafe L, Thöny B, Penzien JM, Czarnecki B, Blau N. Mutations in the sepiapterin reductase gene cause a novel tetrahydrobiopterin-dependent monoamine-neurotransmitter deficiency without hyperphenylalaninemia. Am J Hum Genet. 2001;69:269–277. doi: 10.1086/321970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bräutigam M, Dreesen R, Herken H. Determination of reduced biopterins by high pressure liquid chromatography and subsequent electrochemical detection. Hoppe Seylers Z Physiol Chem. 1982;363(3):341–343. [PubMed] [Google Scholar]
- Fismen L, Eide T, Djurhuus R, Svardal AM. Simultaneous quantification of tetrahydrobiopterin, dihydrobiopterin and biopterin by liquid chromatography coupled electrospray tandem mass spectrometry. Anal Biochem. 2012;430:163–170. doi: 10.1016/j.ab.2012.08.019. [DOI] [PubMed] [Google Scholar]
- Fukushima T, Nixon JC. Chromatographic analysis of pteridines. Meth Enzymol. 1980;66:429–436. doi: 10.1016/0076-6879(80)66485-4. [DOI] [PubMed] [Google Scholar]
- Fukushima T, Nixon JC. Analysis of reduced forms of biopterin in biological tissues and fluids. Anal Biochem. 1980;120:176–188. doi: 10.1016/0003-2697(80)90336-X. [DOI] [PubMed] [Google Scholar]
- Fukushima T, Kobayashi K, Eto I, Shiota T. A differential microdetermination for various forms of biopterin. Anal Biochem. 1978;89:71. doi: 10.1016/0003-2697(78)90727-3. [DOI] [PubMed] [Google Scholar]
- Guibal P, Lévêque N, Doummar D, Giraud N, Roze E, Rodriguez D, Couderc R, Billette De Villemeur T, Moussa F (2014) Simultaneous determination of all forms of biopterin and neopterin in cerebrospinal fluid. ACS Chem Neurosci 5(7):533–541 [DOI] [PMC free article] [PubMed]
- Guroff G, Rhoads CA, Abramowitz A. A simple radioisotope assay for phenylalanine hydroxylase cofactor. Anal Biochem. 1967;21(2):273–278. doi: 10.1016/0003-2697(67)90189-3. [DOI] [PubMed] [Google Scholar]
- Hyland K. Estimation of tetrahydro, dihydro and fully oxidized pterins by high-performance liquid chromatography using sequential electrochemical and fluorometric detection. J Chromatogr Biomed Appl. 1985;343:35–41. doi: 10.1016/S0378-4347(00)84565-X. [DOI] [PubMed] [Google Scholar]
- Hyland K, Howell DW, Smith I (1986) An isocratic high-performance liquid chromatographic system for the investigation of abnormalities of neurotransmitter amine, biopterin, and aromatic amino acid metabolism in cerebrospinal fluid using sequential coulometric electrochemical and fluorescence detection. In: Joseph MH, Fillenz M, Macdonald IA, Marsden C (eds) Monitoring neurotransmitter release during behaviour. Ellis Howard, UK, pp 233-238
- Kaufman S, Holtzman N, Milstein S, Butler IJ, Krumholz A. Phenylketonuria due to a deficiency of dihydropteridine reductase. N Engl J Med. 1975;293:785–789. doi: 10.1056/NEJM197510162931601. [DOI] [PubMed] [Google Scholar]
- Kim HR, Kim TH, Hong SH, Kim HG. Direct detection of tetrahydrobiopterin (BH4) and dopamine in rat brain using liquid chromatography coupled electrospray tandem mass spectrometry. Biochem Biophys Res Commun. 2012;419(4):632–637. doi: 10.1016/j.bbrc.2012.02.064. [DOI] [PubMed] [Google Scholar]
- Lunte CE, Kissinger PT. The determination of pterins in biological samples by liquid chromatography/electrochemistry. Anal Biochem. 1983;129(2):377–386. doi: 10.1016/0003-2697(83)90565-1. [DOI] [PubMed] [Google Scholar]
- Nagatsu T, Yamaguchi T, Kato T, Sugimoto T, Matsuura S, Akino M, Tsushima S, Nakazawa N, Ogawa H. Radioimmunoassay for biopterin in body fluids and tissues. Anal Biochem. 1981;110(1):182–189. doi: 10.1016/0003-2697(81)90133-0. [DOI] [PubMed] [Google Scholar]
- Niederwieser A, Blau N, Wang M, Joller P, Atarés M, Cardesa-Garcia J. GTP cyclohydrolase I deficiency, a new enzyme defect causing hyperphenylalaninemia with neopterin, biopterin, dopamine, and serotonin deficiencies and muscular hypotonia. Eur J Pediatr. 1984;141(4):208–214. doi: 10.1007/BF00572762. [DOI] [PubMed] [Google Scholar]
- Schmidt H, Tegeder I, Geisslinger G (2006) Determination of neopterin and biopterin by liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS) in rat and human plasma, cell extracts and tissue homogenates. http://www.nature.com/protocolexchange/protocols/86
- Segawa M, Hosaka A, Miyagawa F, Nomura Y, Imai H. Hereditary progressive dystonia with marked diurnal fluctuation. Adv Neurol. 1976;14:215–233. [PubMed] [Google Scholar]
- Stea B, Halpern R, Smith R. Separation of unconjugated pteridines by high-pressure cation-exchange liquid chromatography. J Chromatogr. 1979;188(2):363–375. doi: 10.1016/S0021-9673(00)81259-5. [DOI] [PubMed] [Google Scholar]
- Thöny B, Neuheiser F, Kierat L, Blaskovics M, Arn PH, Ferreira P, Rebrin I, Ayling J, Blau N (1998) Hyperphenylalaninemia with high levels of 7-biopterin is associated with mutations in the PCBD gene encoding the bifunctional protein pterin-4a-carbinolamine dehydratase and transcriptional coactivator (DCoH). Am J Hum Genet 62(6):1302–1311 [DOI] [PMC free article] [PubMed]
- Zorzi G, Redweik U, Trippe H, Penzien JM, Thöny B, Blau N. Detection of Sepiapterin in CSF of Patients with Sepiapterin Reductase Deficiency. J Neurochem. 2002;80(2):362–364. doi: 10.1046/j.0022-3042.2001.00710.x. [DOI] [PubMed] [Google Scholar]


