Abstract
Background: Pompe disease (PD) is a disorder of lysosomal glycogen storage. The introduction of enzyme replacement therapy (ERT) has shifted the focus of care from survival to quality of life. The presence of lower urinary tract symptoms (LUTS) and incontinence has not been previously described in children with PD.
Methods: Children with PD followed in the Duke Lysosomal Storage Disease Clinic completed a validated bladder control symptom score (BCSS) and additional questions regarding urinary tract infections (UTIs), giggle, and stress incontinence. Descriptive statistics were used to discriminate urinary symptoms between gender, age, and different types of PD.
Results: Sixteen of 23 children (aged 4–14 years) seen in our clinic participated. Seven were girls; ten had classic infantile PD, two atypical infantile PD, and four childhood presentation late-onset PD (LOPD). When stratified by PD subtype, median BCSS was worst for the classic PD subtype followed by atypical PD and LOPD. Daytime urinary incontinence accompanied by constipation was noted in six. Eight reported urinary incontinence with laughing: giggle incontinence in six and stress incontinence in two. Four girls reported a history of UTI. Longitudinal follow-up in 11 patients showed stable BCSS in six, improvement in three, and worsening in two. Worsening corresponded with changes in bowel function and improvement with increase in ERT dose or treatment of constipation.
Conclusions: LUTS and incontinence are common in children with PD with greater symptoms noted with infantile-type PD. Improved bowel function and increase in ERT dose may lead to improvements in BCSS.
Electronic supplementary material
The online version of this chapter (doi:10.1007/8904_2015_492) contains supplementary material, which is available to authorized users.
Keywords: Incontinence, Infantile-type Pompe disease, Lower urinary tract symptoms, Urinary tract infections
Introduction
Pompe disease (PD) or type II glycogen storage disease is a potentially lethal, autosomal recessive disorder first identified in 1932 by Dutch pathologist J.C. Pompe (Pompe 1932; van Gijn and Gijselhart 2011). It is the result of mutations in the alpha-1,4-glucosidase (GAA) gene which leads to a deficiency of the enzyme acid alpha-glucosidase (also known as acid maltase) that breaks down lysosomal glycogen. Due to this deficiency, glycogen builds up in lysosomes, most seriously affecting cardiac, skeletal, and smooth muscle cells. The incidence of all forms of PD is reported as 1 in 40,000 (Martiniuk et al. 1998; Chien et al. 2008; Byrne et al. 2011) with geographic and ethnic variation ranging from 1 in 14,000 to 1 in 156,000 (Lin et al. 1987; Ausems et al. 1999; Kishnani et al. 2006). With the introduction of newborn screening, incidence as high as 1 in 8,657 has been reported (Kemper 2013).
PD is a continuum; one extreme is the classic infantile-onset type with symptom onset before 6 months of age including cardiomyopathy. Another is the nonclassic (atypical) infantile type with symptom onset before 12 months but typically without cardiomyopathy. Late-onset (LOPD) type has symptom onset after 12 months of age and represents the rest of the disease continuum (Byrne et al. 2011). Left untreated, classic infantile-onset PD patients die within two years of life succumbing to cardiac and respiratory failure (Kishnani et al. 2006). However, enzyme replacement therapy (ERT) with alglucosidase alfa which became available in 2006, has led to dramatic improvements in survival rates (Prater et al. 2012). With long-term survivors now reaching later childhood and adolescence, the focus of care is shifting toward improving the quality of life and understanding the new natural history of treated disease.
The goal of this study was to prospectively collect validated patient questionnaires to evaluate LUTS and urinary and fecal incontinence in a relatively large pediatric PD clinic population. We were interested in looking at association between these symptoms and type of PD, ambulatory status, and other surrogates of disease severity.
Methods
Participants and Recruitment
Children with PD across the disease continuum were recruited from the Duke Lysosomal Storage Disease Clinic under a protocol approved by the Duke Institutional Review Board. Our study included patients aged 4 years or older (presumably after toilet training is complete) and younger than 18 years. Parents or legal guardians of the participants were introduced to study protocols at clinic visits, and informed consent was obtained. Verbal consent was obtained from participants aged 6–12 years, and written assent obtained from individuals 12–18 years of age.
Data Collection and Questionnaire
Demographics, treatment data, and pulmonary assessment by pulmonary function tests were extracted from medical records. Urinary and bowel symptoms were evaluated using a validated instrument to assess pediatric voiding dysfunction (bladder control symptom score [BCSS])(Afshar et al. 2009). This 13-question, 52-point questionnaire captures data on the quantity and frequency of daytime urinary incontinence, frequency, urgency, holding maneuvers, dysuria, nocturia, enuresis, constipation, and encopresis. A score ≥11 has an 80% sensitivity and 91% specificity to diagnose voiding dysfunction and was used in this study to define voiding dysfunction (Afshar et al. 2009). This questionnaire has previously been used in evaluation of a variety of dysfunctional elimination syndromes and vesicoureteral reflux (Drzewiecki et al. 2012; Ching et al. 2015). Questionnaires were completed by parents or legal guardians together with their children at clinic visits, by mail, or over the phone. A supplementary questionnaire of eighteen questions was developed after noting specific symptoms in this clinic population not captured by the validated questionnaire (Appendix 2). All data was collected prior to treatment of LUTS unless otherwise specified. Lower extremity functional assessment was performed by a physical therapist using the Vignos Scale (1–10) with 1 being the best (Appendix 3) (Personius et al. 1994).
Statistical Analysis
Descriptive statistics were used to compare prevalence of LUTS and urinary incontinence. All normally distributed variables were expressed as mean (± standard deviation), and all continuous, non-normal variables were expressed as median (interquartile range). Categorical variables were listed as number (percentage). Fisher’s exact and Kruskal-Wallis tests were used to compare characteristics between patients with and without voiding dysfunction. All analyses were performed in STATA 13.1 (StataCorp, College Station, TX, USA).
Results
Twenty-three consecutive children with PD aged 4 years and older were seen during the study period. A 4-year-old girl who was ventilator dependent was excluded due to severity of disease impacting toilet training. Six chose not to participate in the study, leaving 16 patients who provided data. The cohort included nine boys and seven girls with an age range of 4–14 years (median 9 ± 3 years). All patients had confirmed diagnosis of PD by enzyme deficiency and two mutations in the GAA gene. They were all cross-reactive immunological material (CRIM)-positive with low antibody titers. Titers ranged from no titers to 12,800 (median 1,600) after a median of 110 months (range 14–188) on ERT. PD subtype, respiratory status, and ambulatory status are noted in Table 1. All patients were on ERT.
Table 1.
Patient characteristics stratified by PD subtype
| Total | Classic infantile PD | Nonclassic infantile PD | Late-onset PD | |
|---|---|---|---|---|
| N | 16 | 10 | 2 | 4 |
| Age, mean ± SD | 9 ± 3 | 8 ± 3 | 8 ± 1 | 12 ± 3 |
| Age, range | 4–14 | 4–12 | 7–9 | 8–13 |
| Female (%) | 7 (44) | 4 (40) | 0 (0) | 3 (75) |
| Use of BiPaP N (%) | 2 (12) | 1 (10) | 0 (0) | 1 (25) |
| Ambulatory assistance, N (%) | 5 (31) | 5 (50) | 0 (0) | 0 (0) |
| Lower extremity functional score, N (%) | ||||
| 1 | 5 (31) | 1 (10) | 1 (50) | 3 (75) |
| 2 | 2 (13) | 1 (10) | 1 (50) | 0 (0) |
| 3 | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 6 | 1 (6.2) | 1 (10) | 0 (0) | 0 (0) |
| Missing | 8 (50) | 7 (70) | 0 (0) | 1 (25) |
| Number of years on ERT, median (range) | 8 (1–15) | 10 (5–15) | 8 (8–9) | 5 (1–7) |
| Six-minute walk test, median % of normal (IQR) | 72% (48–80) | 40% (25–60) | 65% (56–73) | 79% (76–80) |
| Missing (%) | 6 (38) | 5 (50) | 0 (0) | 1 (25) |
SD standard deviation, IQR interquartile range, PD Pompe disease
The median BCSS for the entire cohort was 9.5 (IQR 4–16.5). Seven of sixteen (44%) patients had score ≥11 to meet the definition of voiding dysfunction. Four of seven girls (57%) and three of nine boys (33%) had score ≥11. Six of these patients had classic infantile PD, and one had nonclassic infantile subtype. All seven patients were subsequently referred to pediatric urology for treatment of their symptoms.
None of the LOPD patients had voiding dysfunction (scores ≥11), while 6/10 (60%) with classic infantile PD and 1/2 (50%) with nonclassic PD had voiding dysfunction (Fig. 1, Table 2). Median BCSS for classic infantile PD was 13 (IQR 6–17), for nonclassic infantile PD 10 (IQR 5–14), and for LOPD patients 4 (IQR 3–4) (Table 3).
Fig. 1.
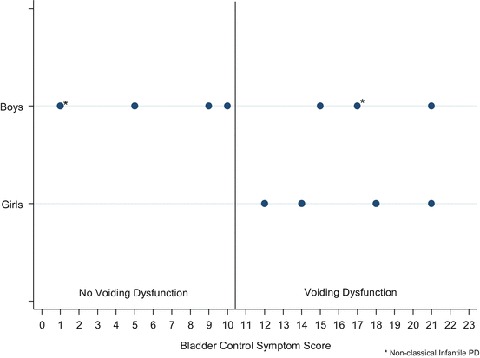
Total BCSS distribution among patients with classic infantile PD and nonclassic infantile PD (represented by asterisk) using 11 as a cutoff for voiding dysfunction
Table 2.
Patient characteristics stratified by voiding dysfunction symptoms
| Total | No voiding dysfunction (score <11) | Voiding dysfunction (score ≥11) | p-value | |
|---|---|---|---|---|
| Total (%) | 16 | 9 (56) | 7 (44) | |
| Male, N (%) | 9 | 6 (67) | 3 (43) | 0.3 |
| Classic infantile-type PD, N (%) | 10 | 4 (40) | 6 (60) | 0.6 |
| Nonclassic infantile-type PD, N (%) | 2 | 1 (50) | 1 (50) | 0.5 |
| Late-onset PD, N (%) | 4 | 4 (100) | 0 (0) | <0.01 |
| Use of BiPaP, N (%) | 2 | 2 (22) | 0 (0) | 0.3 |
| Wheelchair dependence, N (%) | 5 | 2 (22) | 3 (43) | 0.4 |
| Lower extremity functional score, N (%) | ||||
| 1 | 5 (31) | 5 (56) | 0 (0) | 0.04 |
| 2 | 2 (13) | 0 (0) | 2 (29) | |
| 3 | 0 (0) | 0 (0) | 0 (0) | |
| 6 | 1 (6) | 1 (11) | 0 (0) | |
| Missing | 8 (50) | 3 (33) | 5 (71) | |
| Six-minute walk test, median % of normal (IQR) | 72% (48–80) | 80% (79–81) | 44% (32–54) | 0.01 |
IQR interquartile range, PD Pompe disease, BiPaP bi-level positive airway pressure
Table 3.
Urinary symptoms (BCSS) stratified by PD subtype
| Total | Classic infantile PD | Nonclassic infantile PD | Late-onset PD | |
|---|---|---|---|---|
| Total, N | 16 | 10 | 2 | 4 |
| Any daytime incontinence “wet,” N (%) | 6 (38) | 5 (50) | 1 (50) | 0 (0) |
| Frequency of daytime incontinence, N (%) | ||||
| None | 9 (56) | 4 (40) | 1 (50) | 4 (100) |
| 1 day a week | 1 (6) | 1 (10) | 0 (0) | 0 (0) |
| 2–3 days a week | 4 (25) | 3 (30) | 1 (50) | 0 (0) |
| 4–5 days a week | 1 (6) | 1 (10) | 0 (0) | 0 (0) |
| Every day | 1 (6) | 1 (10) | 0 (0) | 0 (0) |
| Quantity of daytime incontinence, N (%) | ||||
| None (0) | 9 (56) | 4 (40) | 1 (50) | 4 (100) |
| Almost dry (1) | 1 (6.2) | 1 (10) | 0 (0) | 0 (0) |
| Damp (2) | 4 (25) | 3 (30) | 1 (50) | 0 (0) |
| Wet (3) | 1 (6.2) | 1 (10) | 0 (0) | 0 (0) |
| Soaked (4) | 1 (6.2) | 1 (10) | 0 (0) | 0 (0) |
| Frequency (I use the bathroom more than 5–6 times a day, score of ≥2), N (%) | 7 (44) | 5 (50) | 1 (50) | 1 (25) |
| Urgency (I have to rush to the bathroom at least half the time, score of ≥2), N (%) | 5 (31) | 4 (40) | 1 (50) | 0 (0) |
| Holding maneuvers (I hold my pee by crossing my legs at least half the time, score of ≥2), N (%) | 4 (25) | 4 (40) | 0 (0) | 0 (0) |
| Dysuria (it hurts when I pee at least half the time, score of ≥2), N (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Enuresis (I wet my bed at night at least half the time, score of ≥2), N (%) | 3 (18) | 3 (30) | 0 (0) | 0 (0) |
| Nocturia (I wake up to pee at night at least half the time, score of ≥2), N (%) | 5 (31) | 3 (30) | 1 (50) | 1 (25) |
| Intermittency (when I pee it stops and starts at least half the time, score of ≥2), N (%) | 2 (12) | 2 (20) | 0 (0) | 0 (0) |
| Hesitancy (I have to push or wait for my pee to start at least half the time, score of ≥2), N (%) | 1 (6) | 1 (10) | 0 (0) | 0 (0) |
| Constipation (I have a bowel movement every other day or less frequent, score of ≥2), N (%) | 2 (12) | 1 (10) | 0 (0) | 1 (25) |
| Hard stools (my stool is hard at least half of the time, score of ≥2), N (%) | 5 (31) | 4 (40) | 1 (50) | 0 (0) |
| Encopresis (I have bowel accidents in my underwear, score ≥1), N (%) | 5 (31) | 5 (50) | 0 (0) | 0 (0) |
| Total score, median (IQR) | 10 (4–17) | 13 (6–17) | 10 (5–14) | 4 (3–4) |
We also looked at the association between ambulatory status and BCSS. In those patients fully or partially wheelchair dependent (n = 5), BCSS was higher with median score of 14 (IQR 9–21) compared to those not using wheelchairs (n = 9) with a median score of 5 (IQR 4–15); however, this was not statistically significant (p = 0.3). When looking at surrogates for disease severity, we had information for all patients on the 6-min walk test. Median percent of normal distance in the 6-min walk was significantly lower in patients with voiding dysfunction (BCCS ≥11) compared to those without voiding dysfunction (44% versus 80%, p = 0.01) (Table 2).
Overall, infantile type of PD was associated with worse LUTS and urinary incontinence (Table 2). Age at which ERT was started, ambulatory status, and dependence on bi-level positive airway pressure (BiPaP) were not associated with LUTS.
Urinary symptoms were each examined individually (Table 3). Daytime urinary incontinence was noted in three boys (33%) and three girls (43%), all of whom had infantile PD. LUTS of urgency, frequency, enuresis, nocturia, intermittency, and hesitancy were seen exclusively in children with daytime incontinence. Likewise, symptoms of constipation such as hard stools, infrequent bowel movements, and encopresis were seen almost exclusively in those with urinary incontinence. Boys tended to have lower scores with a median of 9 (4–15), whereas girls had a median score of 12 (IQR 4–18, p = 0.6). When children with LOPD were excluded, the gender difference was more striking with boys having a median score of 9.5 (IQR 3–16.5) versus 16 (IQR 13–19.5) in girls (Fig. 1).
The additional questionnaire was completed by 11 patients: seven boys and four girls (Appendix 2). The girls reported being toilet trained at ages 1.5, 3, 3, and 4 years, whereas the four boys responding were all toilet trained at age 2. Incontinence with laughing was particularly notable. Three girls reported this to be a small amount (1) or medium amount (2). This occurred 1–2 times per week (2) or less than weekly (1). Five boys reported this to be a small amount (1), medium amount (1), and large amount (3). This occurred more frequently than three times per week in two boys and occurred with tickling or laughing hard. This appeared to be true giggle incontinence in six and was stress incontinence in one boy and one girl who had also reported leaking small amounts with other Valsalva activities, such as sneezing. In all, three children, two boys and one girl, reported leak with Valsalva activities. All children that reported giggle incontinence had infantile-type PD, and five of six patients with daytime urinary incontinence also reported giggle incontinence. Two girls also reported either loss of stool (1) or flatus (1) with laughing. Two boys and three girls reported a small amount of post-void dribbling. Finally, no boys and four girls reported a history of UTIs (p = 0.02), with two reporting multiple UTIs.
Voiding dysfunction was also noted in patients who started ERT at a younger age. Among patients with infantile PD, we found no difference in presence of voiding dysfunction by age at which ERT was started (Appendix 1). Eleven patients completed the BCSS questionnaire on multiple clinic visits over an 18-month period: five on three separate visits each and six on two separate visits each. Median change in score was 3 points (IQR 2–5). Three had a decrease in total score of 4, 5, and 8 points, and two had an increase in total score of 6 and 10 points. These changes in total scores correlated with points related to constipation which has been shown to negatively impact bladder function. Among six patients who had an increase in ERT dosage during the study period, three (50%) demonstrated an improvement in BCSS, and two (33%) reported subjective improvement in symptoms. Two (33%) reported worsening of the BCSS with increase in dosage. These were also accompanied by worsening constipation.
Discussion
This exploratory study is the first to examine LUTS and urinary incontinence in children with PD (classic, atypical, and LOPD) and in infantile-type PD patients of any age. Symptoms of voiding dysfunction were noted in nearly half of our cohort. This is not surprising since PD affects organs containing smooth muscle cells with a vacuolar myopathy secondary to glycogen accumulation. This accumulation has been demonstrated in the bladder by autopsy reports (Hobson-Webb et al. 2012). Case series have correlated the pathological findings with clinical symptoms of urinary and fecal incontinence. In 1991, Chancellor et al. initially described exercise-induced urinary incontinence in a 78-year-old LOPD patient (Chancellor et al. 1991). Bernstein et al. described three LOPD patients aged 16, 34, and 52: two with fecal incontinence and one with nocturnal urinary incontinence (Bernstein et al. 2010). Remiche et al. described a cohort of 20 LOPD patients with a mean age of 44.8 years with fecal incontinence in 25% and urinary incontinence in 5% (Remiche et al.). Karabul et al. found urinary and bowel incontinence occurring at a higher frequency in adults with Pompe disease in comparison to age- and gender-matched controls (Karabul et al. 2014). Furthermore, our recently reported case series of 35 adults with PD outlined phenotypes of micturition disorders in LOPD (McNamara et al. 2015). We have previously described low anal sphincter tone and fecal incontinence in infantile-onset PD (Tan et al. 2013).
The introduction of alglucosidase alfa in 2006 has led to dramatic improvements in survival and clinical outcomes in these patients with treatment reducing the risk of death by 99% (Kishnani et al. 2007). Retrospective reviews have shown improvement in cardiac and lung function and gross motor function in long-term survivors (Prater et al. 2012). Persistent deficiencies in hearing loss, risk of arrhythmias, dysphagia, osteopenia, fecal incontinence, and other long-term complications have been addressed (Jones et al. 2010; van Gelder et al. 2012; Tan et al. 2013). However, we are unaware of any published data on LUTS and urinary incontinence in children with PD.
Urinary incontinence is an often undertreated condition that can greatly affect quality of life (Jones et al. 2010; van Gelder et al. 2012; Tan et al. 2013; Thibodeau et al. 2013). Ninety percent of children achieve daytime urinary continence by the age of 5 (Hunskaar et al. 2003). By age 7 years, only 2–3% of children in the general population report daytime incontinence, 10% nocturnal enuresis, and 1–3% fecal incontinence (Schultz-Lampel et al. 2011). In comparison, 50% of our children with infantile PD (classic and atypical) experienced some degree of daytime urinary incontinence. While robust epidemiological data is unavailable in this patient population, we previously reported that the prevalence of urinary incontinence in our adult LOPD patients is at 54% (McNamara et al. 2015). We found no urinary incontinence in children with LOPD. Development of incontinence is common in adult women; but the increasing prevalence of urinary incontinence in adults as they age, particularly in middle-aged men, could be due to PD-related changes in the bladder and sphincter.
Giggle incontinence, or enuresis risoria, is a condition that usually affects females without other LUTS and is defined by complete bladder emptying during or immediately after laughing (Neveus et al. 2006). Hypothesized to be caused by detrusor instability, the incidence of giggle incontinence is estimated between 8 and 24% in children ages 5–12 years (Chandra et al. 2002). In comparison, 73% of our cohort reported giggle incontinence. While female predominant in a non-PD population, 71% of the boys with PD, in addition to 75% of the girls, reported some degree of giggle incontinence.
In a non-PD population, the reported incidence of UTIs is between 3 and 6.6% in girls and 1 and 1.8% in boys less than 11 years of age (Winberg et al. 1974; Marild and Jodal 1998). In our cohort of infantile-type PD, four of four of girls reported at least one UTI before the age of 10; no boys reported a UTI. This could be overreported since we did not have outside urinalysis and culture data for confirmation and voided urine collections are notoriously inaccurate in girls. The prevalence of voiding dysfunction was higher in girls (57%) when compared to boys (33%). This finding mirrors the findings of LUTS in adults with LOPD in which 61% of women and 39% of men reported urinary symptoms (McNamara et al. 2015). In our pediatric cohort, nocturnal enuresis was noted in 42% of female patients and fecal incontinence in 43%, which were higher than their male counterparts (0% nocturnal enuresis and 22% fecal incontinence). The rate of fecal incontinence in girls was similar to that noted in adult (45%) (McNamara et al. 2015). While healthy girls typically have more LUTS than boys, enuresis is typically more common in healthy boys. Explanations of these different findings in children with PD and the potential for these to change as the children transition to adulthood are unknown and demand further study.
The close relationship between constipation and bladder dysfunction is well known (Loening-Baucke 1993; Clayden and Wright 2007). The prevalence of constipation in children worldwide is reported between 0.7 and 29.6% (Mugie et al. 2011). Our PD patients had a relatively higher prevalence of constipation with 31% of our cohort reporting hard stools more than half the time and 31% reporting fecal incontinence. Fifty percent of the infantile PD patients with daytime urinary incontinence reported decreased frequency of bowel movements, 60% reported hard stools, and 67% reported fecal incontinence. As in normal children, we noted an inverse relationship between the frequency and consistency of bowel movements and the findings of daytime incontinence and BCSS. Aggressive treatment of constipation and pelvic floor physical therapy has been shown to improve voiding dysfunction in normal children (De Paepe et al. 2000), and this should be the first step in management of these problems in children with PD. Timed voiding or voiding volitionally at scheduled intervals, avoiding bladder irritants like coffee, and other bladder training techniques may also be employed to help treat PD patients with LUTS.
Normal bladder function requires the coordinated interplay of the pontine micturition center; sympathetic, parasympathetic, and somatic nerves; detrusor smooth muscle; and striated sphincteric muscles. Glycogen accumulation as evidenced by vacuolar changes in the urinary bladder and glycogen accumulation in the motor and spinal cord neurons are the presumed mechanism for myogenic and neurogenic dysfunction in PD patients (Hobson-Webb et al. 2012). The same effect upon the striated muscles of the voluntary urethral and anal sphincter would be presumed. Though the exact etiology of LUTS, urinary incontinence, and constipation in PD patients remains unexplained, it is probably a combination of neurogenic and myogenic factors.
ERT is the mainstay of treatment for PD (Kishnani et al. 2007). Baseline cardiomyopathy has been observed to improve after initiation of ERT (Prater et al. 2012). However, ptosis, hypotonia, osteopenia, hypernasal speech, hearing loss, arrhythmia risk, and dysphagia have been shown to persist with long-term ERT in survivors (Prater et al. 2012). Patients show variable reduction in glycogen load when treated with ERT, but this is closely correlated with clinical outcomes (Thurberg et al. 2006). In a small series, there was improvement in gastrointestinal function and urinary incontinence after ERT initiation in adults and children (Remiche 2012; Bernstein et al. 2010). Due to our small sample size, we are unable to make conclusions in the infantile PD cohort. However, the impact of increase in ERT dose is under study at our center. About half of our patients reported subjective improvements in symptoms with increases in ERT dosage, and these patients also demonstrated improvement in voiding function as measured by BCSS. Constipation was a large confounder that was difficult to control when assessing this relationship.
These data must be interpreted within the context of the small sample size. Selection bias could be a further limitation since 30% of the patients from our clinic did not participate in the study. Of the participants, we did not have supplementary questionnaires and longitudinal follow-up from all. Like any survey, ours is subject to recall bias by the parents, legal guardians, and patients themselves. However, we noted that individual patient scores did not vary greatly between surveys. This was designed as a descriptive study; therefore, long-term follow-up to examine the natural history of LUTS and their management in this patient population was not undertaken.
The gold standard for diagnosing a urinary tract infection is a positive urinalysis and urine culture from a catheterized specimen, so false-positive results are common in girls. Since many of our patients receive primary care from distant locales, we had to rely on patient-/legal guardian-reported occurrence of UTI without clinical confirmation. We did not address patient perceptions and quality of life assessment. Understanding how much patients and caregivers are bothered by LUTS and urinary incontinence offers a future direction for investigation.
Conclusion
LUTS and urinary incontinence were found to be relatively common in children with PD, but only to a mild-moderate degree. Giggle incontinence was surprisingly noted in multiple children. Higher BCSS was seen in children with classic and nonclassic infantile PD, and LUTS were not noted in children with LOPD. Constipation was a frequently associated condition. An increase in ERT dose led to improvement in LUTS in two patients. LUTS and incontinence should be treated in children with PD in a similar fashion to other children with voiding dysfunction, starting with querying for and management of constipation.
Electronic Supplementary Material
Abbreviations
- BCSS
Bladder control symptom score
- ERT
Enzyme replacement therapy
- LOPD
Late-onset Pompe disease
- LUTS
Lower urinary tract symptoms
- PD
Pompe disease
- UTIs
Urinary tract infections
Take-Home Message
Lower urinary tract symptoms, urinary incontinence, and constipation were found to be relatively common in children with Pompe disease and were worse in children with classic and nonclassic infantile Pompe disease that those with LOPD.
Author Contributions
Dr. Divya Ajay – (a) conception and design, or analysis and interpretation of data, and (b) drafting the article or revising it critically for important intellectual content
Dr. Erin McNamara – (b) drafting the article or revising it critically for important intellectual content
Ms. Stephanie Austin – (b) drafting the article or revising it critically for important intellectual content
Dr. John Wiener – (a) conception and design, or analysis and interpretation of data, and (b) drafting the article or revising it critically for important intellectual content
Dr. Priya Kishnani – (a) conception and design, or analysis and interpretation of data, and (b) drafting the article or revising it critically for important intellectual content
Compliance with Ethics Guidelines
Conflict of Interest
Dr. Divya Ajay reports no disclosures.
Dr. Erin McNamara reports no disclosures; she is supported by grant number T32 HS000063 from the Agency for Healthcare Research and Quality (AHRQ).
Ms. Stephanie Austin reports no disclosures.
Dr. John Wiener reports no disclosures.
Dr. Priya Kishnani has received research/grant support and honoraria from Genzyme Corporation and is a member of the Pompe and Gaucher Disease Registry Advisory Board for Genzyme Corporation. She has also received honoraria from Amicus Therapeutics.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all patients for being included in the study.
Funding sources: None
Footnotes
Competing interests: None declared
References
- Afshar K, Mirbagheri A, Scott H, MacNeily AE. Development of a symptom score for dysfunctional elimination syndrome. J Urol. 2009;182:1939–1943. doi: 10.1016/j.juro.2009.03.009. [DOI] [PubMed] [Google Scholar]
- Ausems MG, Verbiest J, Hermans MP, et al. Frequency of glycogen storage disease type II in The Netherlands: implications for diagnosis and genetic counselling. Eur J Hum Genet. 1999;7:713–716. doi: 10.1038/sj.ejhg.5200367. [DOI] [PubMed] [Google Scholar]
- Bernstein DL, Bialer MG, Mehta L, Desnick RJ. Pompe disease: dramatic improvement in gastrointestinal function following enzyme replacement therapy. A report of three later-onset patients. Mol Genet Metab. 2010;101:130–133. doi: 10.1016/j.ymgme.2010.06.003. [DOI] [PubMed] [Google Scholar]
- Byrne BJ, Kishnani PS, Case LE, et al. Pompe disease: design, methodology, and early findings from the Pompe Registry. Mol Genet Metab. 2011;103:1–11. doi: 10.1016/j.ymgme.2011.02.004. [DOI] [PubMed] [Google Scholar]
- Chancellor AM, Warlow CP, Webb JN, Lucas MG, Besley GT, Broadhead DM. Acid maltase deficiency presenting with a myopathy and exercise induced urinary incontinence in a 68 year old male. J Neurol Neurosurg Psychiatry. 1991;54:659–660. doi: 10.1136/jnnp.54.7.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra M, Saharia R, Shi Q, Hill V. Giggle incontinence in children: a manifestation of detrusor instability. J Urol. 2002;168:2184–2187. doi: 10.1016/S0022-5347(05)64350-9. [DOI] [PubMed] [Google Scholar]
- Chien YH, Chiang SC, Zhang XK, et al. Early detection of Pompe disease by newborn screening is feasible: results from the Taiwan screening program. Pediatrics. 2008;122:e39–e45. doi: 10.1542/peds.2007-2222. [DOI] [PubMed] [Google Scholar]
- Ching CB, Lee H, Mason MD, et al. Bullying and lower urinary tract symptoms: why the pediatric urologist should care about school bullying. J Urol. 2015;193:650–654. doi: 10.1016/j.juro.2014.08.103. [DOI] [PubMed] [Google Scholar]
- Clayden G, Wright A. Constipation and incontinence in childhood: two sides of the same coin? Arch Dis Child. 2007;92:472–474. doi: 10.1136/adc.2007.115659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Paepe H, Renson C, Van Laecke E, Raes A, Vande Walle J, Hoebeke P. Pelvic-floor therapy and toilet training in young children with dysfunctional voiding and obstipation. BJU Int. 2000;85:889–893. doi: 10.1046/j.1464-410x.2000.00664.x. [DOI] [PubMed] [Google Scholar]
- Drzewiecki BA, Thomas JC, Pope JC, Adams MC, Brock JW, Tanaka ST. Observation of patients with vesicoureteral reflux off antibiotic prophylaxis: physician bias on patient selection and risk factors for recurrent febrile urinary tract infection. J Urol. 2012;188:1480–1484. doi: 10.1016/j.juro.2012.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson-Webb LD, Proia AD, Thurberg BL, Banugaria S, Prater SN, Kishnani PS. Autopsy findings in late-onset Pompe disease: a case report and systematic review of the literature. Mol Genet Metab. 2012;106:462–469. doi: 10.1016/j.ymgme.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Hunskaar S, Burgio K, Diokno A, Herzog AR, Hjalmas K, Lapitan MC. Epidemiology and natural history of urinary incontinence in women. Urology. 2003;62:16–23. doi: 10.1016/S0090-4295(03)00755-6. [DOI] [PubMed] [Google Scholar]
- Jones HN, Muller CW, Lin M, et al. Oropharyngeal dysphagia in infants and children with infantile Pompe disease. Dysphagia. 2010;25:277–283. doi: 10.1007/s00455-009-9252-x. [DOI] [PubMed] [Google Scholar]
- Karabul N, Skudlarek A, Berndt J, et al. Urge incontinence and gastrointestinal symptoms in adult patients with pompe disease: a cross-sectional survey. JIMD Rep. 2014;17:53–61. doi: 10.1007/8904_2014_334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemper AR (2013) Evidence report: newborn screening for Pompe disease. In: Book evidence report: newborn screening for Pompe disease. http://www.hrsa.gov/advisorycommittees/mchbadvisory/heritabledisorders/nominatecondition/reviews/pompereport2013.pdf. Accessed 23 Apr 2015
- Kishnani PS, Hwu WL, Mandel H, Nicolino M, Yong F, Corzo D. A retrospective, multinational, multicenter study on the natural history of infantile-onset Pompe disease. J Pediatr. 2006;148:671–676. doi: 10.1016/j.jpeds.2005.11.033. [DOI] [PubMed] [Google Scholar]
- Kishnani PS, Corzo D, Nicolino M, et al. Recombinant human acid [alpha]-glucosidase: major clinical benefits in infantile-onset Pompe disease. Neurology. 2007;68:99–109. doi: 10.1212/01.wnl.0000251268.41188.04. [DOI] [PubMed] [Google Scholar]
- Lin CY, Hwang B, Hsiao KJ, Jin YR. Pompe’s disease in Chinese and prenatal diagnosis by determination of alpha-glucosidase activity. J Inherit Metab Dis. 1987;10:11–17. doi: 10.1007/BF01799482. [DOI] [PubMed] [Google Scholar]
- Loening-Baucke V. Constipation in early childhood: patient characteristics, treatment, and long-term follow up. Gut. 1993;34:1400–1404. doi: 10.1136/gut.34.10.1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marild S, Jodal U (1998) Incidence rate of first-time symptomatic urinary tract infection in children under 6 years of age. Acta Paediatrica (Oslo, Norway: 1992) 87:549–552 [DOI] [PubMed]
- Martiniuk F, Chen A, Mack A, et al. Carrier frequency for glycogen storage disease type II in New York and estimates of affected individuals born with the disease. Am J Med Genet. 1998;79:69–72. doi: 10.1002/(SICI)1096-8628(19980827)79:1<69::AID-AJMG16>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- McNamara ER, Austin S, Case L, Wiener JS, Peterson AC, Kishnani PS (2015) Expanding our understanding of lower urinary tract symptoms and incontinence in adults with Pompe disease. JIMD Rep 20:5–10. doi:10.1007/8904_2014_381 [DOI] [PMC free article] [PubMed]
- Mugie SM, Benninga MA, Di Lorenzo C. Epidemiology of constipation in children and adults: a systematic review. Best Pract Res Clin Gastroenterol. 2011;25:3–18. doi: 10.1016/j.bpg.2010.12.010. [DOI] [PubMed] [Google Scholar]
- Neveus T, von Gontard A, Hoebeke P, et al. The standardization of terminology of lower urinary tract function in children and adolescents: report from the Standardisation Committee of the International Children's Continence Society. J Urol. 2006;176:314–324. doi: 10.1016/S0022-5347(06)00305-3. [DOI] [PubMed] [Google Scholar]
- Personius KE, Pandya S, King WM, Tawil R, McDermott MP. Facioscapulohumeral dystrophy natural history study: standardization of testing procedures and reliability of measurements. The FSH DY Group. Phys Therapy. 1994;74:253–263. doi: 10.1093/ptj/74.3.253. [DOI] [PubMed] [Google Scholar]
- Pompe JC. Over idiopathische hypertrophie van het hart. Ned Tijdschr Geneeskd. 1932;76:304. [Google Scholar]
- Prater SN, Banugaria SG, DeArmey SM, et al. The emerging phenotype of long-term survivors with infantile Pompe disease. Genet Med. 2012;14:800–810. doi: 10.1038/gim.2012.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remiche G, Herbaut AG, Ronchi D et al (2012) Incontinence in late-onset Pompe disease: an underdiagnosed treatable condition. Eur Neurol 68(2):75–78 [DOI] [PubMed]
- Schultz-Lampel D, Steuber C, Hoyer PF, Bachmann CJ, Marschall-Kehrel D, Bachmann H. Urinary incontinence in children. Deutsches Ärzteblatt Int. 2011;108:613–620. doi: 10.3238/arztebl.2011.0613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan QK, Cheah SM, Dearmey SM, Kishnani PS. Low anal sphincter tone in infantile-onset Pompe disease: an emerging clinical issue in enzyme replacement therapy patients requiring special attention. Mol Genet Metab. 2013;108:142–144. doi: 10.1016/j.ymgme.2012.11.013. [DOI] [PubMed] [Google Scholar]
- Thibodeau BA, Metcalfe P, Koop P, Moore K. Urinary incontinence and quality of life in children. J Pediatr Urol. 2013;9:78–83. doi: 10.1016/j.jpurol.2011.12.005. [DOI] [PubMed] [Google Scholar]
- Thurberg BL, Lynch Maloney C, Vaccaro C et al (2006) Characterization of pre- and post-treatment pathology after enzyme replacement therapy for Pompe disease. Laboratory investigation 86:1208–1220 [DOI] [PubMed]
- van Gelder CM, van Capelle CI, Ebbink BJ, et al. Facial-muscle weakness, speech disorders and dysphagia are common in patients with classic infantile Pompe disease treated with enzyme therapy. J Inherit Metab Dis. 2012;35:505–511. doi: 10.1007/s10545-011-9404-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gijn J, Gijselhart JP (2011) [Pompe and his disease]. Nederlands tijdschrift voor geneeskunde 155:A2878 [PubMed]
- Winberg J, Andersen HJ, Bergstrom T, Jacobsson B, Larson H, Lincoln K (1974) Epidemiology of symptomatic urinary tract infection in childhood. Acta Paediatr Scand Suppl 1–20 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


