Abstract
Objective: This study was undertaken to analyse serially the effects of decoppering therapy on the clinical features, disability and MRI brain including DTI metrics in patients with Wilson’s disease.
Methods and Results: Thirty-five patients with clinically and serologically confirmed neuropsychiatric form of Wilson’s disease (WD) on decoppering therapy were followed for a minimum duration of 1 year with serial assessment of their clinical features, disability status and serial MR imaging of the brain including DTI. The cohort included 18 treatment-naïve patients and 17 patients already on decoppering therapy (M/F = 2.18:1). The mean age at which they underwent baseline assessment for this study was 18.6 ± 7.6 years, and follow-up assessment was done after a mean duration of 23.5 ± 8.8 months (range, 12 to 45 months). Along with the overall clinical improvement noted at follow-up, the disability assessed using Chu staging and MSEADL showed significant reduction in the number of patients with severe disability and the mean NSS reducing from 9.74 to 6.37 (p = 0.002). The mean MRI scores showed significantly reduced disease burden from a baseline score of 5.9 (±4.2) to 4.9 (±4.7) in follow-up scans (p < 0.05). Voxel-wise comparison of serial DTI metrics on TBSS (tract-based spatial statistics) analysis showed that the entire cohort had significant (p < 0.05) improvement in all the four parameters (MD, FA, DA and RD) indicated by a decrease in MD, DA and RD values and increase in FA values. Comparison of whole-brain white matter DTI measures between pre- and posttreatment did not show any significant difference (p < 0.05).
Conclusion: Patients with Wilson’s disease on decoppering therapy showed clinical improvement accompanied with improvement in DTI metrics. Quantitative DTI metrics may be used as surrogate markers of clinical status following initiation of medical therapy in Wilson’s disease.
Keywords: DTI, MRI, Wilson’s disease
Introduction
Wilson’s disease (WD) is a rare disease of copper metabolism which shows neurological symptoms as a presenting feature in 40% of cases (Walshe 1962; Ala et al. 2007). Increased copper deposition in various brain structures causes cellular injury resulting in various clinical manifestations like motor and behavioural disturbances. The disease is diagnosed based on clinical features, the presence of KF ring along with biochemical markers including low serum level of ceruloplasmin, increased serum copper concentration and increased urinary copper excretion (Ala et al. 2007). MRI features in Wilson’s disease have been well described in a number of studies (Prayer et al. 1990; Starosta-Rubinstein et al. 1987; Magalhaes et al. 1994; Sinha et al. 2006; van Wassenaer et al. 1996). The disease predominantly involves deep grey matter structures, corpus callosum and brainstem. Involvement of cerebral cortex and hemispheric white matter (WM) is relatively less common. White matter abnormalities are usually seen in the frontal lobes and are associated with cortical abnormalities. The cerebellum is relatively spared (Sinha et al. 2006).
Diffusion tensor imaging (DTI) is a well-established technique for studying structural properties of neural tissue and is based on the properties of water diffusion (Le Bihan et al. 2001; Stejskal and Tanner 1965). In previous studies, abnormalities of diffusion have been noted in WD patients (Sener 2003a, b; Favrole et al. 2006). It has been found useful in studying WD patients in presymptomatic stage (Favrole et al. 2006). In our previous study, we found diffusion measurements are sensitive for detecting abnormalities in normal-appearing WM as well, and they also show correlation with the disability scores of patients (Jadav et al. 2013). However, in previous studies, effects of treatment on DTI measurements have not been evaluated. Effects of decoppering treatment on MRI have been studied using conventional MRI and MR spectroscopy (Sinha et al. 2007a, b; Kim et al. 2006; Tarnacka et al. 2008). Conventional MRI findings show improvement with prolonged treatment but are more often subjective. MRS has been found useful for serial evaluation; however, only a small part of the brain can be sampled by routinely available spectroscopy methods. Whole-brain spectroscopy is a good alternative, but it usually takes a very long time and is technically challenging.
To the best of our knowledge, the effect of decoppering therapy on DTI metrics has not been studied previously. We hypothesize that patients with clinical improvement will show improvement in their WM DTI metrics. Therefore, in this study, we aimed to evaluate the effect of decoppering therapy on clinical features, disability scores, conventional MRI and DTI metrics.
Patients and Methods
Patient Selection
The study included 35 patients with WD recruited from the outpatient services of the Department of Neurology at a tertiary care centre for neuropsychiatric patients. The diagnosis of WD was based on clinical features, the presence of corneal KF ring on slit lamp examination, low serum total copper and ceruloplasmin levels and elevated 24-h urinary copper excretion (Table 1). The details including demographics, history, family pedigree, duration of illness and phenotypic features were noted at the time of initial evaluation. Patients had a neuropsychiatric form of WD, and there was no active hepatic abnormality at the time of initial evaluation, though six patients had past history of subclinical liver dysfunction (elevated liver enzymes). During follow-up, none developed clinical evidence of liver dysfunction. The clinical severity and disability status were scored using the neurological symptom score (NSS), Chu staging and Modified Schwab and England Activities of Daily Living (MSEADL) scores (Schwab and England 1960; Chu 1986; Meenakshi-Sundaram et al. 2002). Ethical approval for the study was obtained from the NIMHANS institutional ethics committee. Written informed consent was obtained from the patients.
Table 1.
Details of demographic and clinical features of patients with Wilson’s disease
| Demographic/clinical parameters | Values |
|---|---|
| M/F | 24:11 |
| Treatment status | |
| On treatment (%) | 18 (51.4) |
| Drug naïve (%) | 17 (48.6) |
| Age at baseline MRI (years) | 18.6 ± 7.64 (R:8–37) |
| Age at follow-up MRI (years) | 20.31 ± 7.58 (R:10–38) |
| Time period between MRIs (months) | 23.48 ± 8.79 (R:12–45) |
| Involuntary movements (%) | 20 (57.1) |
| Slowness of activities (%) | 25 (71.4) |
| Dysphagia (%) | 21 (60) |
| Walking difficulty (%) | 21 (60) |
| Behavioural disturbance (%) | 19 (54.3) |
| Seizure (%) | 6 (17.1) |
| KF ring (%) | 32 (91.4) |
| Dysarthria (%) | 25 (71.4) |
| Bradykinesia (%) | 12 (34.3) |
| Tremor (%) | 14 (40) |
| Dystonia (%) | 16 (45.7) |
| Chorea (%) | 4 (11.4) |
| Athetosis (%) | 2 (5.7) |
| Myoclonus (%) | 2 (5.7) |
| Rigidity (%) | 10 (28.6) |
| Spasticity (%) | 1 (2.9) |
| Cerebellar signs (%) | 7 (20) |
Data Collection and Analysis
Their demographic and phenotypic characteristics were noted, and functional assessment was done using disability and impairment scales. Based on the treatment profile at initial evaluation, the cohort included 18 patients who were treatment naive and 17 patients on decoppering treatment for varying periods before the baseline MRI scan. Patients were then followed up longitudinally for a minimum of 1 year with recommended decoppering therapy. Subjects underwent clinical evaluation and MRI of the brain again after a minimum of one year of decoppering therapy with routine and DTI sequences utilizing similar parameters. Routine investigations to rule out hepatic, renal, haematological and other organ involvement were performed at baseline and follow-up. Analysis of appearance or disappearance of signal changes with decoppering was performed.
Disability and Impairment Assessment
For serially assessing disability and impairment in ADL, three scales were applied at baseline and follow-up, viz. Modified Schwab and England Activities of Daily Living scores (MSEADL) (Schwab and England 1960), Chu staging (Chu 1986) and neurological symptom score (NSS) (Meenakshi-Sundaram et al. 2002).
MRI
MRI was obtained on a Philips Achieva MRI scanner with a superconducting magnet of 3.0 tesla field strength using a 32-channel head coil; standard protocols and methodology were utilized. Those who required sedation for MRI were administered midazolam or propofol. Conventional MR sequences used for the evaluation included T1-weighted (T1W), T2-weighted (T2W) and fluid-attenuated inversion recovery (FLAIR). T1-weighted images were acquired in axial plane (TR/TE = 650/14 ms, NEX-1). T2-weighted (TR/TE = 6,000/120 ms, NEX-1) images were acquired in axial and coronal planes. Fluid-attenuated inversion recovery (FLAIR) (TR/TE/TI = 11,000/120/2,600 ms, NEX-1) sequences were done in axial plane. The slice thickness was 5 mm for all the conventional imaging sequences. The DTI data was obtained from all individuals using single-shot spin-echo echo planar sequence. Imaging parameters included sensitivity-encoding (SENSE) reduction factor, 2.5; TR, 5 s; TE, 65 ms; acquired resolution, 2.2 mm isotropic; 32 noncoplanar gradient directions with b value of 800 s/mm2; and 2 repetitions. Scanning time per diffusion sequence acquisition was approximately 9 min.
Conventional MRI
MRI data was reviewed using a structured assessment for the presence or absence of signal intensity changes within the cerebral tissue on both DWI and FLAIR images. Severity of signal abnormalities on FLAIR images was analysed in those regions usually involved in WD (basal ganglia, thalamus, midbrain, pons, medulla, cerebellum and cerebral WM).
MRI Scoring
This grading system by King et al. (1996) provides a score with zero being a normal scan and a higher number in a scan with severe or marked changes. Structures assessed for grading included frontal, parietal, occipital and cerebellar WM and cortical changes.
DTI Analysis
Data analysis was carried out using FMRIB Software Library tools (www.fmrib.ox.ac.uk/fsl) version 4.1.6. Raw DTI images were preprocessed using “eddy current correction”, to correct for distortions due to the gradient directions applied. Fractional anisotropy (FA) and mean diffusivity (MD) maps were generated using DTIFit, part of FMRIB’s Diffusion Toolbox (http://www.fmrib.ox.ac.uk/fsl/fdt) that fits a diffusion tensor model at each voxel. All image analysis was performed using FSL 5.2. Group comparison of DTI data was performed by standard procedure of tract-based spatial statistics (TBSS). FA, AD, MD and RD maps were generated using FMRIB’s Diffusion Toolbox, after preprocessing the DTI data. Preprocessing included eddy current correction and motion correction (by linear registering to the non-diffusion-weighted image using FLIRT). Group-wise voxel-based statistical analysis of FA was performed using TBSS. Here, individual subject’s skull-stripped FA images were aligned to the MNI152 standard space using the nonlinear registration method, followed by the creation of group mean FA skeleton by thinning mean FA volumes (FA > 0.2 overlaid with the mean FA image). The mean FA skeleton represents the centres of all tracts common to the entire group of subjects. Each subject’s aligned FA data were then projected onto the mean FA skeleton, and the resulting data was fed into voxel-wise paired sample testing. A voxel-by-voxel permutation nonparametric test (5,000 permutations) was used to assess group-related differences using threshold-free cluster enhancement, which avoids using an arbitrary threshold for the initial cluster formation. In addition to FA, MD, AD and RD were also compared using TBSS in an analogous fashion. The null distribution was built up over 5,000 permutations, and significance was tested at p < 0.05 corrected for multiple comparisons. A similar process of nonlinear registration and voxel-wise comparison was followed for determining the differences in MD, RD and DA maps. Results were expressed at p < 0.051 (family-wise error corrected) (Smith et al. 2006; Smith and Nichols 2009).
The whole-brain and whole-brain white matter DTI metrics such as FA, MD, RD and DA were extracted from each subject’s aligned FA data and projected skeletonized DTI image files, respectively, which were generated during the TBSS processing pipeline using command line operations as implemented in the FSL software programme.
Statistics
Data analysis was done using SPSS. Groups were compared using Student’s t-test/Fisher’s exact probability test. Follow-up data was analysed using paired t-test and McNemar’s test.
Results
Demography and Clinical Features
Thirty-five patients of WD with predominant neurological features underwent evaluation initially. The details are mentioned in Table 1. There were 24 males and 11 females. The mean age at onset of disease of this cohort was 15.7 ± 6.6 years (median age, 14; range, 7–31 years). The mean age at which they underwent baseline MRI of the brain for this study was 18.6 ± 7.6 years, and they underwent a follow-up MRI of the brain at a mean age of 20.3 ± 7.6 years. The follow-up MRI was performed after 23.5 ± 8.8 months (range, 12–45 months) of follow-up.
None of the patients worsened during the follow-up period of almost 2 years: 32 patients (91.4%) had an improving course, and three (8.6%) had an unchanged course. Seventeen (48.6%) patients were drug naïve (n = 17), while 18 (51.4%) patients were already on decoppering therapy at the time of recruitment. All patients were given decoppering treatment with penicillamine and zinc individualized based on other organ involvement and tolerance. Twenty-four patients (68.6%) were both on D-penicillamine and zinc therapy, while 11 of them (31.4%) were only on zinc therapy.
Though there was improvement in the severity, the same symptoms dominated the clinical features during follow-up also. Corneal KF rings, noted in 32 patients initially, were detectable in 22 patients during follow-up. Sunflower cataract in one patient and arthritis in two patients were noted during baseline evaluation.
Disability Status
The Chu staging showed worse functional status in five patients (stage 3) and mild to moderate involvement (Chu stages 1 and 2) in 30 patients. At follow-up, the number of patients with mild to moderate involvement increased to 33, and only two patients had severely impaired functional status based on Chu staging. A 100% MSEADL score denoting no impairment was observed in 12 patients at baseline which increased to 21 at the time of follow-up scan. The NSS which includes assessment of 14 neurological features assigns a total score of 46 for the worst disability and 0 for a normal person. There was statistically significant improvement in mean NSS scores at baseline and follow-up from 9.74 to 6.37 (p = 0.002) indicating improvement in disease burden.
Neuroimaging
The mean MRI score at baseline was 5.9 (±4.2) which reduced to 4.9 (±4.7) in follow-up scans which signify decreased disease burden as observed in routine T2W/FLAIR sequences (p < 0.05).
TBSS Analysis (p < 0.05)
The serial DTI metrics showed that the entire cohort had significant (p < 0.05) improvement in all the four parameters (MD, FA, DA and RD). The improvement of DTI metrics was indicated by a decrease in MD, DA and RD values and increase in FA values. TBSS analysis comparing initial study and follow-up study showed multiple scattered areas with increased FA and decreased diffusivity distributed in both supra- and infratentorial white matter (Figs. 1 and 2).
Fig. 1.
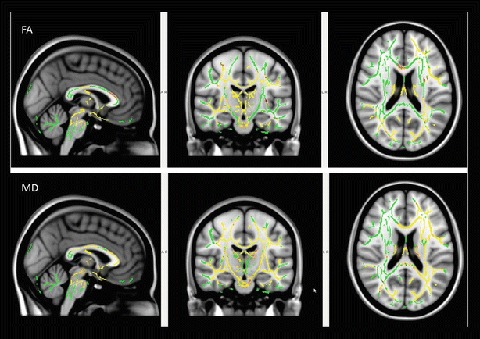
TBSS analysis (t-test) comparing the initial and follow-up DTI imaging in patients with Wilson’s disease. Voxels demonstrating significantly increased FA and decreased MD in the follow-up MRI are shown as yellow red in colour (FWE corrected p < 0.05). Results are shown overlaid on the MNI152-T1 template and the mean FA skeleton (green). FA fractional anisotropy, MD mean diffusivity, FWE family-wise error, TBSS tract-based spatial statistics
Fig. 2.

TBSS analysis (t-test) comparing the initial and follow-up DTI imaging in patients with Wilson’s disease. Voxels demonstrating significantly decreased AD and RD in the follow-up MRI are shown as yellow red in colour. Results are shown overlaid on the MNI152-T1 template and the mean FA skeleton (green) (FWE corrected p < 0.05). RD radial diffusivity, AD axial diffusivity, FWE family-wise error, TBSS tract-based spatial statistics
Whole-brain DTI metric comparison between pretreatment and posttreatment studies showed no significant differences in the anisotropy and diffusivity measures (p < 0.05). Mean FA, MD, RD and AD values of the study cohort are tabulated in Table 2.
Table 2.
Whole-brain WM mean values of fractional anisotropy and diffusivity measurements
| Posttreatment | Standard deviation | Pretreatment | Standard deviation | |
|---|---|---|---|---|
| Mean_FA | 0.42 | 0.02 | 0.43 | 0.04 |
| Mean_MD | 0.82 | 0.03 | 0.82 | 0.08 |
| Mean_RD | 0.61 | 0.03 | 0.61 | 0.09 |
| Mean_DA | 1.23 | 0.02 | 1.23 | 0.05 |
Discussion
In this longitudinal study, the changes in the WM integrity were studied using DTI in a cohort of patients with Wilson’s disease (WD) who were receiving decoppering treatment for Wilson’s disease. We found clinical improvement along with partial resolution of imaging changes in the conventional MRI. The DTI showed increased anisotropy and decreased diffusivity on follow-up imaging. Improvement in DTI metrics was paralleled by improvement in disability scores.
The DWI in patients of WD have shown both increased and decreased diffusivity in the focal lesions (Sener 2003a, b; Favrole et al. 2006; Jadav et al. 2013). Underlying pathological findings in WD include demyelination, inflammation, gliosis and spongiosis. Meenakshi-Sundaram et al. (2002) and Favrole et al. (2006) reported normal to decreased diffusivity in presymptomatic subjects, and increased diffusivity was seen in the symptomatic individuals. This suggests changes in the diffusivity pattern with evolution of disease. In our previous study, we found increased diffusivity and decreased FA not only in the focal lesions but also in the normal-appearing hemispheric WM. Further correlation was observed between the disability scores and the DTI measurements (Jadav et al. 2013).
Treatment and its effects on clinical scores and MRI appearance have also been studied previously, and it was found that clinical disability scores and MRI appearance showed significant improvement following treatment. However, it was noted that patients who had extensive gliosis, diffuse white matter changes and significant atrophy showed poor response to the treatment (Kim et al. 2006). In another study, serial imaging was done for evaluating the pontine signal changes, and authors found significant resolution of these signal abnormalities after the start of decoppering therapy (Sinha et al. 2007a). Larnaout et al. reported one case which had extensive signal abnormalities of the subcortical WM at the initiation of therapy after 5 years of treatment that showed significant resolution of these pathological signal changes (Larnaout et al. 2008). However, most of these studies relied on serial changes in the signal abnormalities usually seen in WD patients. In a quantitative MRS study, authors reported significant improvement in the NAA/Cr ratio in patients who showed neurological improvement following treatment with decoppering therapy. Neurological deterioration was mirrored by fall in Glx/Cr and NAA/Cr ratios, while hepatic deterioration caused decreased mI/Cr and increased Glx/Cr ratios (Tarnacka et al. 2008). In our study, none of the patients reported significant neurological deterioration during the period of serial evaluation.
To the best of our knowledge, there are no studies which looked at the effect of treatment on DTI metrics in Wilson’s disease patients. Pathological basis of improvement in WM DTI indices in WD remains speculative. Gliosis in WD possibly represents the irreversible WM damage. Reversible abnormalities include mitochondrial dysfunction, inflammatory changes and myelin disturbances. Reversible mitochondrial dysfunction is supported by the previous MRS study where increase in NAA/Cr ratio was noted following initiation of treatment. Presence of neuroinflammation has been reported to be present in the rat model of copper toxicity (Tarnacka et al. 2008). Demyelination and inflammatory changes have also been noted in the previous pathology study of Meenakshi-Sundaram et al. (2002). Some of these pathological changes possibly reverse following initiation of the decoppering therapy thus resulting in the radiological improvement in the form of increased NAA in MRS and increased FA and reduced diffusivity in DTI. This has been seen in the form of improved clinical disability scores as well as increased FA values and decreased diffusivity values. Another possible mechanism for these changes in the DTI metrics may be improvement in the underlying subclinical hepatic dysfunction.
Some of the limitations of the study include small sample size and variable follow-up duration. Another aspect which needs to be explained is the lack of significant improvement in the whole-brain white matter DTI metrics, while TBSS analysis showed areas with significant improvement. This discrepancy may be explained by understanding the fact that a large number of voxels are not showing any significant difference on voxel-wise analysis and they may be nullifying the effect of significant voxels when whole-brain WM metrics are considered together. Similarly, certain voxels of the brain might be showing worsening DTI metrics in spite of treatment like in the areas of significant gliosis, and these might be negating the effect of improving trends shown by other voxels. However, the use of sophisticated analytical methods like histogram analysis of whole-brain WM and use of parameters like kurtosis and skewness indices can show changes which may not be detectable by global means of anisotropy and diffusivity. In conclusion, our study shows clinical improvement which is paralleled by improvement in the disability scores and DTI metrics. Quantitative DTI metrics may be used as surrogate markers of clinical status following initiation of medical therapy in Wilson’s disease.
Compliance with Ethics Guidelines
Conflict of Interest
A. Lawrence declares that he has no conflict of interest.
J. Saini declares that he has no conflict of interest.
S. Sinha declares that he has no conflict of interest.
S. Rao declares that he has no conflict of interest.
M. Naggappa declares that she has no conflict of interest.
P.S. Bindu declares that she has no conflict of interest.
A.B. Taly declares that he has no conflict of interest.
Contribution Declaration by Authors
“We declare that all the authors have been involved in (a) conception and design or analysis and interpretation of data and (b) drafting the article or revising it critically for important intellectual content”.
Details of the Contributions of Individual Authors
A. Lawrence: planning, conduct and reporting of the work
J. Saini: planning, conduct and reporting of the work
S. Sinha: planning, conduct and reporting of the work
S. Rao: reporting of the work
M. Naggappa: conduct and reporting of the work
P.S. Bindu: reporting of the work
A.B. Taly: planning, conduct and reporting of the work
Informed Consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all patients for being included in the study.
Animal Rights
This article does not contain any studies with animal subjects performed by any of the authors.
Financial Disclosure
None.
Footnotes
Competing interests: None declared
References
- Ala A, Walker AP, Ashkan K, Dooley JS, Schilsky ML. Wilson’s disease. Lancet. 2007;369:397–408. doi: 10.1016/S0140-6736(07)60196-2. [DOI] [PubMed] [Google Scholar]
- Chu NS. Sensory evoked potentials in Wilson’s disease. Brain. 1986;109:491–507. doi: 10.1093/brain/109.3.491. [DOI] [PubMed] [Google Scholar]
- Favrole P, Chabriat H, Guichard JP, Woimant F. Clinical correlates of cerebral water diffusion in Wilson disease. Neurology. 2006;66:384–389. doi: 10.1212/01.wnl.0000196482.71636.7d. [DOI] [PubMed] [Google Scholar]
- Jadav R, Saini J, Sinha S, Bagepally B, Rao S, Taly AB. Diffusion Tensor Imaging (DTI) and its clinical correlates in drug naïve Wilson’s disease. Metabolic Brain Dis. 2013;28:455–462. doi: 10.1007/s11011-013-9407-1. [DOI] [PubMed] [Google Scholar]
- Kim TJ, Kim IO, Kim WS, et al. MR imaging of the brain in Wilson disease of childhood: findings before and after treatment with clinical correlation. AJNR Am J Neuroradiol. 2006;27:1373–1378. [PMC free article] [PubMed] [Google Scholar]
- King AD, Walshe JM, Kendall BE, Chinn RJ, Paley MN, Wilkinson ID, Halligan S, Hall-Craggs MA. Cranial MR changes in Wilson’s disease. Am J Roentgenol. 1996;167(6):1579–1584. doi: 10.2214/ajr.167.6.8956601. [DOI] [PubMed] [Google Scholar]
- Larnaout A, Ammar N, Mourad Z, Naji S, Hentati F. Wilson's disease: appreciable improvement of sub-cortical white matter abnormalities after copper chelating treatment: five years follow-up. Neuropediatrics. 2008;39:176–178. doi: 10.1055/s-0028-1085464. [DOI] [PubMed] [Google Scholar]
- Le Bihan D, Mangin JF, Poupon C, et al. Diffusion tensor imaging: concepts and applications. J Magn Reson Imaging. 2001;13:534–546. doi: 10.1002/jmri.1076. [DOI] [PubMed] [Google Scholar]
- Magalhaes AC, Caramelli P, Menezes JR, et al. Wilson’s disease: MRI with clinical correlation. Neuroradiology. 1994;36:97–100. doi: 10.1007/BF00588068. [DOI] [PubMed] [Google Scholar]
- Meenakshi-Sundaram S, Taly AB, Kamath V, Arunodaya GR, Rao S, Swamy HS. Autonomic dysfunction in Wilson’s disease – a clinical and electrophysiological study. Clin Auton Res. 2002;12:185–189. doi: 10.1007/s10286-002-0038-6. [DOI] [PubMed] [Google Scholar]
- Prayer L, Wimberger D, Kramer J, Grimm G, Oder W, Imhof H. Cranial MRI in Wilson’s disease. Neuroradiology. 1990;32:211–214. doi: 10.1007/BF00589114. [DOI] [PubMed] [Google Scholar]
- Schwab R, England A (1960) Projection technique for evaluating surgery in Parkinson’s disease. In: Gillingham F, Donaldson I (eds) Theme symposium on Parkinson’s disease, 1 edn. E&S Livingston, London
- Sener RN. Diffusion MR imaging changes associated with Wilson disease. AJNR Am J Neuroradiol. 2003;24:965–967. [PMC free article] [PubMed] [Google Scholar]
- Sener RN. Diffusion MRI findings in Wilson’s disease. Comput Med Imaging Graph. 2003;27:17–21. doi: 10.1016/S0895-6111(02)00047-2. [DOI] [PubMed] [Google Scholar]
- Sinha S, Taly AB, Ravishankar S, et al. Wilson's disease: cranial MRI observations and clinical correlation. Neuroradiology. 2006;48:613–621. doi: 10.1007/s00234-006-0101-4. [DOI] [PubMed] [Google Scholar]
- Sinha S, Taly AB, Ravishankar S, Prashanth LK, Vasudev MK. Central pontine signal changes in Wilson’s disease: distinct MRI morphology and sequential changes with de-coppering therapy. J Neuroimaging. 2007;17:286–291. doi: 10.1111/j.1552-6569.2007.00120.x. [DOI] [PubMed] [Google Scholar]
- Sinha S, Taly AB, Prashanth LK, Ravishankar S, Arunodaya GR, Vasudev MK. Sequential MRI changes in Wilson's disease with de-coppering therapy: a study of 50 patients. Br J Radiol. 2007;80:744–749. doi: 10.1259/bjr/48911350. [DOI] [PubMed] [Google Scholar]
- Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Starosta-Rubinstein S, Young AB, Kluin K, et al. Clinical assessment of 31 patients with Wilson’s disease. Correlations with structural changes on magnetic resonance imaging. Arch Neurol. 1987;44:365–370. doi: 10.1001/archneur.1987.00520160007005. [DOI] [PubMed] [Google Scholar]
- Stejskal EO, Tanner JE. Spin diffusion measurements: spin echoes in the presence of a time‐dependent field gradient. J Chem Phys. 1965;42:288–292. doi: 10.1063/1.1695690. [DOI] [Google Scholar]
- Tarnacka B, Szeszkowski W, Golebiowski M, Czlonkowska A. MR spectroscopy in monitoring the treatment of Wilson’s disease patients. Mov Disord. 2008;23:1560–1566. doi: 10.1002/mds.22163. [DOI] [PubMed] [Google Scholar]
- van Wassenaer-van Hall HN, van den Heuvel AG, Algra A, Hoogenraad TU, Mali WP. Wilson disease: findings at MR imaging and CT of the brain with clinical correlation. Radiology. 1996;198:531–536. doi: 10.1148/radiology.198.2.8596862. [DOI] [PubMed] [Google Scholar]
- Walshe JM. Wilson’s disease. The presenting symptoms. Arch Dis Child. 1962;37:253–256. doi: 10.1136/adc.37.193.253. [DOI] [PMC free article] [PubMed] [Google Scholar]


