Abstract
Background: Detailed nutritional intake data on children with organic acidaemias (OA) (propionic acidaemia (PA), vitamin B12 nonresponsive methylmalonic acidaemia (MMA) and isovaleric acidaemia (IVA)) remains unreported.
Aim and subjects: A review of the longitudinal nutritional intake of 14 children with organic acidaemias (PA n = 8; MMA n = 5; IVA n = 1) dependent on enteral tube feeding (≥90% of energy requirements) from a single treatment centre.
Methods: Nutritional intake (energy, protein, precursor-free l-amino acids, vitamins and minerals), anthropometry and nutritional biochemistry data were collated from diagnosis to current age.
Results: The median energy intake was only 72% (63–137) of the estimated average DH (1991) requirement (EAR), decreasing significantly by 40% between 6 months and 5 years (p < 0.05). Total protein intake met WHO/FAO/UNU (2007) safe intake levels with median (range) precursor-free l-amino acids providing 21% (14–28) of total protein intake. Median mineral intake for sodium was 57% (20–97%), potassium 64% (27–125%) and magnesium 72% (22–116%) and was consistently < RNI for all age points. Fibre median intake was 4 g/day (0–11 g), and fluid intake provided 80% (60–100%) of the requirements for age. Linear growth was poor, and children were overweight for their height (1–10 years: z score median weight +0.6, height −1.2). Nutritional markers consistently indicated that plasma valine concentrations were < target reference ranges in PA and MMA. Iron deficiency anaemia was common in MMA/PA, and in PA, 50% of plasma zinc concentrations were < reference range.
Conclusion: In MMA/PA, energy intake decreases over time, weight gain accelerates, but linear height is poor. There are many nutrient deficiencies which may affect short- and long-term outcome of patients with organic acidaemias. The quality of long-term diet in these conditions deserves more attention.
Keywords: Amino acids organic acidaemias, Energy, Isovaleric acidaemia, Methylmalonic acidaemia, Propionic acidaemia, Protein
Introduction
Neonatal presentation of the classical organic acidaemias (methylmalonic aciduria (MMA), propionic acidaemia (PA), and isovaleric acidaemia (IVA)) is associated with a severe pathogenic phenotype. These children are commonly dependent on nasogastric or gastrostomy tube feeding to provide all their nutritional requirements and prevent catabolism (van der Meer et al. 1994, 1996; Evans et al. 2014). Enteral tube feeding is considered to have a positive effect on morbidity although there are no controlled studies to validate this (North et al. 1995; van der Meer et al. 1994).
Tube feeding enables the ability to optimise nutritional intake by overcoming severe anorexia and feeding difficulties (North et al. 1995), commonly occurring within the first year of life. It also suppresses propiogenic odd chain fatty acid production from lipolysis (Walter and MacDonald 2006; Thompson and Chalmers 1990), ensures even distribution of protein and energy intake and allows administration of medications (Baumgartner and Viardot 1995). Furthermore, hospital admissions may be avoided as minor illness can potentially be managed at home.
Little information is documented on the nutritional intake or composition of enteral formulas for children with organic acidaemias. In MMA/PA although one retrospective cohort study reported longitudinal protein and energy intake of 81 patients (Touati et al. 2006), it included all children rather than those specifically on tube feeds. There are also no disorder-specific feeds designed for tube feeding. In organic acidaemias, module enteral feeds (consisting of many individual ingredients) are given, adapting standard formula designed for general nutritional support. Therefore, formulation of enteral feeds is complex, commonly concentrating on protein amounts rather than energy, fibre, micronutrient and fluid intake.
This retrospective study reviewed the longitudinal dietary intake data of children diagnosed with PA, MMA and IVA dependent on enteral tube feeds (tube feeds supplying ≥90% energy intake) from a single treatment centre.
Methods
Retrospective data was collected from dietary records at 3 monthly intervals up to 2 years of age and then annually (nearest to each subject’s birthday) until present age for each subject. Seventeen nutrients were reviewed (energy, protein from natural and precursor-free l-amino acids, fibre, fat, carbohydrate, essential fatty acids, calcium, magnesium, iron, zinc, selenium, sodium, potassium, vitamin B12, vitamin D and fluid intake). Analysis was conducted using the software program Electronic Manager (EDM 2000™). Each nutrient intake (except protein) was compared as a percentage of the DH (1991) reference nutrient intakes. Energy intake was compared with both the estimated average requirements (EAR) and FAO/WHO/UNU (2001) requirement for energy. Protein intake was compared with the safe levels of protein intake (WHO/FAO/UNU 2007). The Holliday-Segar method was used for calculating maintenance fluid requirements in children (Holliday and Segar 1957).
Anthropometry (weight, height, and BMI z scores) was reported at the time nutritional intake data was estimated. Weight and height were measured in children ≥2 years of age: weight by standing and sitting on Seca electronic scales (accurate to two decimal places) and height by a calibrated stadiometer (accurate to one decimal place). In children <2 years of age, measurements were taken without their clothes on: weight by Seca infant scales (accurate to two decimal places) and supine length on a Seca measuring mat (accurate to one decimal place).
The number of hospital admissions, reason for admission and length of stay were recorded from the time of diagnosis to December 2013. The use of protein-free emergency regimens was also gathered from medical and dietetic records.
All nutritional biochemistry and haematology tests, including plasma zinc, selenium, C-reactive protein, quantitative plasma amino acids, ferritin, haemoglobin and MCV, were documented.
This was registered as an audit project with Birmingham Children’s Hospital Clinical Governance Department. This project was considered by the NHS Health Research Authority decision tool (www.hra-decisiontools.org.uk) as non-research and as such did not require ethical approval. However, each caregiver gave written consent.
Statistics
The nonparametric Wilcoxon signed-rank test was used to compare median results at different age points (aged 6 months, 3 years and 5 years). After 5 years of age, descriptive statistics were used.
Results
Subjects
Fourteen subjects (12 Pakistani Asian, 1 Afro Caribbean, 1 Caucasian) (seven girls, seven boys) all dependent on tube feeding (receiving ≥90% of energy requirements by enteral tube feeds) were included. Eight were diagnosed with PA, five with non-vitamin B12-responsive MMA (three had mut0 activity) and one with IVA. Their present median age at assessment was 6.5 years (range 2–16 years).
The majority were diagnosed within the first week of life (n = 13) (except one child aged 7 months). Two children were diagnosed within 48 h of birth due to previous sibling deaths. All required tube feeds from diagnosis with the exception of three children (one child with MMA diagnosed at 7 months following metabolic encephalopathy, one MMA child at age 9 months with faltering growth and one PA child aged 7 years following metabolic encephalopathy). Twelve children had gastrostomy and two nasogastric feeding tubes. The type and number of feed ingredients used are given in Table 1.
Table 1.
Summary of number and type of feed ingredients in each enteral feed at the time of assessment
| Core ingredients | Ingredient choice 1 | Ingredient choice 2 | Ingredient choice 3 |
|---|---|---|---|
| Natural protein sourcea | Standard whey-/casein-based paediatric tube feeding formula with added fibre (n = 11) | Regular infant whey dominant formula (n = 4) | Standard paediatric tube feeding formula without added fibre (n = 1) |
| Aged 2–10 years: Nutrini multifibre (Nutricia) | Aged 1–2 years: Cow and Gate Infant formula | Aged 2–10 years: Nutrini (Nutricia) | |
| Aged >10 years Tentrini multifibre (Nutricia) | |||
| Precursor-free amino acidsb | MMA/PA precursor-free l-amino acids without vitamins and minerals added (n = 6) | Infant MMA/PA precursor-free formula (n = 6) | Child MMA/PA precursor-free l-amino acids with vitamins and minerals added (n = 1) |
| MMA/PA amino 5 (Vitaflo International) | MMA/PA Anamix Infant (Nutricia) | MMA/PA Maxamaid (Nutricia) | |
| Energy source | Glucose polymer (n = 11) | Infant energy module with added vitamins and minerals (n = 10) | 50% Fat emulsion (n = 1) |
| Maxijul super soluble (Nutricia) | Energivit (Nutricia) | Calogen (Nutricia) | |
| Electrolytes | Potassium chloride (n = 2) | Sodium chloride (n = 1) | |
| Vitamin and minerals supplement | Vitamin and mineral supplement (n = 1) | ||
| Paediatric Seravit (Nutricia) | |||
| Fibre | Soluble fibre only (n = 1) | ||
| Optifibre (Nestle) | |||
| Median (range) number of feed ingredients in each enteral feed (excluding water) | 4 (3 to 5) | ||
a n = 2 children who had two different sources of natural protein in their feed
b n = 1 child with IVA who had no precursor-free amino acid added to feed
Energy Intake (Table A.1 of Appendix; Fig. 1)
Table A.1.
Analysis of median (range) nutrient intake represented as total daily amount or daily intake for subjects on enteral tube feeds from age 3 months to 16 years compared with Dietary Reference Values for Food Energy and Nutrients for the UK (1991) and WHO/FAO/UNU (2007) safe levels of protein intake
| Age of subjects | No of subjects | Energy intake Kcal/day | Energy intake Kcal/kg/day | Energy intake %EAR | Carbohydrate intake g/kg/day | Fat intake g/kg/day | Natural protein intake g day | Intake of precursor-free l-amino acids g/kg/day |
|---|---|---|---|---|---|---|---|---|
| 3 months | 10 | 724 (634–789) | 102 (66–102) | 137 (89–155) | 17 (13–20) | 7 (5–9) | 8.7 (6–11) | 0.3 (0.2–0.4) |
| 6 months | 12 | 761 (620–878) | 100 (70–155) | 112 (96–131) | 12 (8–18) | 5 (3–8) | 11 (6–14) | 0.2 (0–0.2) |
| 9 months | 13 | 775 (648–969) | 87 (64–155) | 99 (79–111) | 10 (7–13) | 5 (3–6) | 11.5 (7–17) | 0.2 (0–0.7) |
| 12 months | 13 | 825 (420–1007) | 82 (63–111) | 94 (49–109) | 9 (7–13) | 4 (3–5) | 11.9 (7–18) | 0.3 (0–1) |
| 15 months | 13 | 844 (427–1008) | 74 (54–115) | 68 (37–87) | 9 (7–16) | 4 (2–5) | 12.2 (7–18) | 0.3 (0–1.1) |
| 18 months | 12 | 920 427–1038) | 81 (59–120) | 75 (37–89) | 10 (6–16) | 4 (2–6) | 13.5 (7–18) | 0.3 (0–1.2) |
| 2 years | 12 | 937 (726–1230) | 72 (48–111) | 76 (59–106) | 10 (7–14) | 3 (2–5) | 13.5 (8–19) | 0.3 (0–1.3) |
| 3 years | 12 | 1038 (806–1465) | 73 (44–98) | 87 (66–123) | 10 (6–14) | 3 (2–4) | 14.6 (9–20) | 0.3 (0–1.4) |
| 4 years | 9 | 1207 (798–1465) | 70 (47–95) | 66 (49–100) | 9 (7–14) | 2 (2–4) | 16.6 (11–24) | 0.3 (0–1.3) |
| 5 years | 9 | 1147 (759–1352) | 52 (45–79) | 67 (44–83) | 8 (7–11) | 2 (1–3) | 18.5 (15–21) | 0.3 (0–1.3) |
| 6 years | 9 | 1202 (916–1435) | 52 (41–83) | 78 (53–93) | 7 (4–12) | 2 (1–6) | 20 (16–39) | 0.4 (0–1.6) |
| 7 years | 6 | 1158 (778–1710) | 51 (37–87) | 63 (45–101) | 7 (5–12) | 2 (1–4) | 17.9 (16–27) | 0.4 (0–1) |
| 8 years | 5 | 1202 (872–1385) | 49 (41–66) | 69 (50–81) | 7 (6–10) | 2 (2–3) | 17.5 (16–20) | 0.2 (0–0.6) |
| 9 years | 5 | 1487 (1132–1628) | 55 (51–65) | 75 (65–89) | 8 (8–9) | 2 (2–3) | 19.2 (18–25) | 0.6 (0–0.7) |
| 10 years | 5 | 1389 (956–1439) | 49 (36–66) | 78 (55–83) | 7 (5–9) | 2 (1–3) | 19.6 (18–27) | 0 (0–0.7) |
| 11 years | 5 | 1257 (965–1445) | 38 (36–60) | 64 (52–68) | 6 (5–9) | 1.4 (1–2) | 20 (20–28) | 0 (0–0.7) |
| 12 years | 4 | 1154 (622–1470) | 38 (20–49) | 66 (34–66) | 6 (3–7) | 1.4 (1–2) | 21 (20–29) | 0 (0.5–0.6) |
| 13 years | 3 | 1390 (529–1447) | 36 (15–43) | 71 (33–78) | 5 (3–6) | 1.5 (0.4–1.7) | 27 (20–34) | 0 (0–6.5) |
| 14 years | 2 | 1430 (1390–1469) | 39 (37–40) | 72 (63–80) | 6 (5–6) | 1.5 (1.4–1.5) | 27 (20–34) | 0 (0–0.6) |
| 15 years | 1 | 1180 | 30 | 43 | 5 | 1.5 | 41 | 0 |
| 16 years | 1 | 920 | 22 | 33 | 3 | 0.9 | 40 | 0 |
Fig 1.
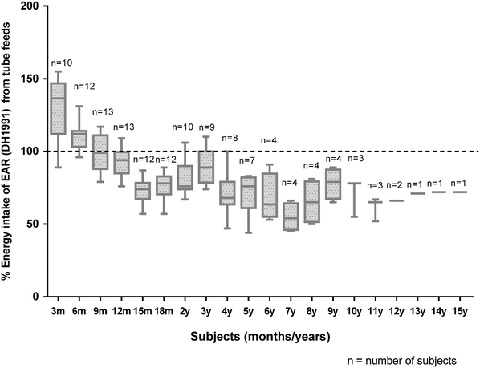
Median energy intake from enteral tube feeds expressed as a percentage of the Estimated Average Requirement (DH 1991) for all ages
Overall, median energy intake was only 72% (range 33–137%) of the EAR (DH 1991). It met EAR in the first 6 months of age only. There was a statistically significant fall in energy intake from 6 months to 3 years by 25% (p < 0.05) and from 6 months to 5 years by 40% (p < 0.05). Energy intakes were also consistently below the FAO/WHO/UNU 2001 recommendations from the age of 2 years. The median (range) percentage energy from fat and carbohydrate remained unchanged. From 3 months to 16 years, fat provided 36% (28–46%) and carbohydrate 53% (45–58%) of energy.
Protein Intake (Table A.2 of Appendix; Fig. 2)
Table A.2.
Analysis of median (range) nutrient intake represented as total daily amount or daily intake for subjects on enteral tube feeds from age 3 months to 16 years compared with Dietary Reference Values for Food Energy and Nutrients for the UK (1991) and WHO/FAO/UNU (2007) safe levels of protein intake
| Age of subjects | No of subjects | Total protein intake g/kg/day | % Total protein intake compared with WHO reference | % Contribution of precursor-free l-amino acids to total protein | B12 μmol/day | B12μmol/L %RNI | Sodium mmol/kg/day | Sodium mmol % RNI |
|---|---|---|---|---|---|---|---|---|
| 3 months | 10 | 2 (1.5–2.3) | 96 (66–132) | 12 (0–25) | 1.7 (0.4–3) | 567 | 1.4 (0.9–1.9) | 76 |
| 6 months | 12 | 2 (1.7–2.2) | 115 (94–147) | 14 (0–500 | 1.7 (1.1–2.5) | 566 | 1.1 (0.9–1.6) | 65 |
| 9 months | 13 | 1.6 (1.2–2.6) | 119 (100–147) | 17 (0–53) | 1.8 (1.1–2.4) | 438 | 1 (0.6–1.2) | 57 |
| 12 months | 13 | 1.5 (1.2–2.3) | 133 (78–155) | 16 (0–55) | 1.9 (1.3–2.6) | 463 | 1 (0.7–1.7) | 58 |
| 15 months | 13 | 1.5 (1–2) | 136 (74–172) | 20 (0–55) | 1.9 (1.5–2.9) | 376 | 1 (0.6–2.1) | 43 |
| 18 months | 12 | 1.5 (1–2) | 136 (90–178) | 21 (0–55) | 1.9 (1.2–2.7) | 380 | 1.1 (0.1–3) | 50 |
| 2 years | 12 | 1.5 (1–2) | 144 (101–193) | 24 (0–54) | 2.6 (1.8–3.2) | 530 | 1.2 (0.7–2.3) | 60 |
| 3 years | 12 | 1.4 (1–2.2) | 145 (113–198) | 26 (0–52) | 2.6 (1.2–3.8) | 510 | 1.3 (1.1–1.9) | 97 |
| 4 years | 9 | 1.3 (1.1–2.3) | 137 (88–205) | 28 (0–57) | 2.7 (2.4–3.9) | 338 | 0.9 (0.9–1.8) | 73 |
| 5 years | 9 | 1.2 (1–2.2) | 167 (111–240) | 28 (0–61) | 3 (1.5–4.7) | 320 | 1.2 (1–2) | 94 |
| 6 years | 9 | 1.3 (1.1–2.6) | 184 (111–228) | 29 (0–50) | 3 (1.5–4) | 375 | 1.3 (0.9–1.5) | 90 |
| 7 years | 6 | 1.3 1–2) | 104 (92–135) | 24 (0–50) | 2.6 (1.5–5) | 155 | 1 (0.8–1.8) | 55 |
| 8 years | 5 | 1.2 (0.8–1.4) | 135 (62–139) | 43 (0–50) | 2.6 (1.5–4.4) | 260 | 1 (0.7–1.4) | 42 |
| 9 years | 5 | 1.2 (0.7–1.8) | 118 (77–147) | 0 (0–49) | 2.3 (1.5-5-4.4) | 230 | 1 (0.8–1.4) | 54 |
| 10 years | 5 | 1 (0.9–1.4) | 126 (70–158) | 0 (0–51) | 2.9 (1.5–5) | 290 | 0.8 (0.7–1.4) | 52 |
| 11 years | 5 | 1 (0.8–1.4) | 82 (49–101) | 0 (0–51) | 3 (1.5–5) | 283 | 0.9 (0.7–1.2) | 40 |
| 12 years | 4 | 1.1 (0.8–1.2) | 72 (52–101) | 0 (0–51) | 4 (2–5) | 333 | 1.1 (1–1.1) | 43 |
| 13 years | 3 | 0.9 (0.8–1.1) | 95 (84–106) | 0 (0–53) | 3 (1–3) | 250 | 1 (1–1.1) | 59 |
| 14 years | 2 | 1.1 (1–1.2) | 95 (84–106) | 0 (0–53) | 3.3 (3–4) | 275 | 0.9 (0.8–0.9) | 45 |
| 15 years | 1 | 1.1 (1–1.1) | 77 | 0 | 3 | 250 | 1 | 59 |
| 16 years | 1 | 1 | 69 | 0 | 2 | 133 | 0.3 | 20 |
Fig 2.
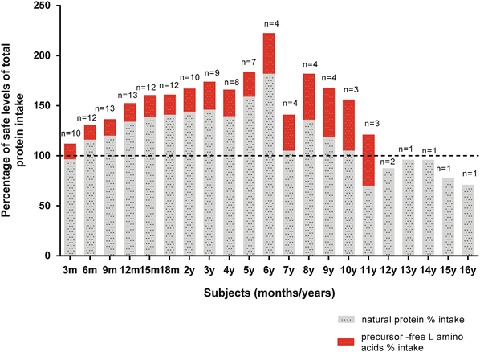
Total protein intake from both natural and precursor-free l-amino acids (with % contribution from precursor-free l-amino acids) expressed as a percentage of the WHO/FAO/UNU 2007 safe levels of protein intake from enteral tube feeds
All children were on modified low-protein tube feeds (standard enteral feeds adapted to lower their protein content). Total protein intake was compared with WHO/UNU (2007) safe levels. Median total protein intake (natural and precursor-free l-amino acids) from tube feeds for all subjects across all ages met or exceeded the minimum safe protein requirements. The precursor-free l-amino acids provided a median of 21% (14–28%) of total protein intake between 6 months and 5 years of age. Between 6 months and 3 years of age, the median contribution of precursor-free l-amino acids to total protein increased by 28% (CI 3.0–52.5) (p = 0.03). From 6 months to age 5 years, the median (range) total protein (natural and precursor-free l-amino acids) intake was 36% (15–67%) > FAO/WHO/UNU (2007) safe levels.
Fluid Intake
The median (range) percentage of fluid intake compared with the Holliday and Segar calculation (1957) was 80% (60–100) for all ages. The fluid intake decreased by a median of 47 mL/kg/day (range −47 to −5) between the ages of 6 months and 3 years.
Fibre Intake (Table A.3 of Appendix)
Table A.3.
Analysis of median (range) nutrient intake represented as total daily amount or daily intake for subjects on enteral tube feeds from age 3 months to 16 years compared with Dietary Reference Values for Food Energy and Nutrients for the UK (1991) and WHO/FAO/UNU (2007) safe levels of protein intake
| Age of subjects | No of subjects | Potassium mmol/kg | Potassium mmol %RNI | Vitamin D μmol/day | Vitamin D % RNI | Fibre intake g/day |
|---|---|---|---|---|---|---|
| 3 months | 10 | 2.6 (1.7–3.9) | 62 | 10 (5–14) | 118 | 3 (0–4) |
| 6 months | 12 | 2.1 (1.7–3.6) | 64 | 11 (7–16) | 153 | 0 (0–5) |
| 9 months | 13 | 1.8 (1.4–2.4) | 91 | 12 (7–15) | 171 | 1 (0–9) |
| 12 months | 13 | 2 (1.5–2.7) | 102 | 12 (9–18) | 171 | 3 (0–5) |
| 15 months | 13 | 1.6 (0.7–2.5) | 90 | 12 (8–19) | 186 | 0 (0–7) |
| 18 months | 12 | 1.7 (0.1–2.3) | 100 | 13 (9–19) | 179 | 0 (0–9) |
| 2 years | 12 | 1.8 (1.2–3) | 105 | 13 (10–22) | 186 | 2 (0–8) |
| 3 years | 12 | 1.7 (1.4–2.2) | 125 | 13a (10–21) | 130 | 4 (0–7) |
| 4 years | 9 | 1.2 (1.2–1.9) | 100 | 13 (11–18) | 130 | 4 (0–8) |
| 5 years | 9 | 1.5 (1–1.9) | 106 | 14 (9–15) | 140 | 0 (0–6) |
| 6 years | 9 | 1.6 (1.1–1.7) | 109 | 14 (9–18) | 140 | 6 (0–12) |
| 7 years | 6 | 1.1 (0.9–2.3) | 49 | 13 (8–24) | 130 | 5 (0–6) |
| 8 years | 5 | 0.9 (0.9–1.3) | 52 | 13 (9–15) | 130 | 5 (4.5–5) |
| 9 years | 5 | 1.3 (0.8–1.8) | 68 | 14 (9–15) | 140 | 4 (0–6) |
| 10 years | 5 | 0.9 (0.7–1.3) | 46 | 15 (9–17) | 150 | 3 (0–6) |
| 11 years | 5 | 1 (0.6–1.3) | 31 | 15 (9–18) | 150 | 6 (5–9) |
| 12 years | 4 | 1 (0.9–1.3) | 47 | 17 (14–21) | 170 | 9 (0–13) |
| 13 years | 3 | 1 (1–1.1) | 53 | 12 (7–19) | 120 | 11 (10–13) |
| 14 years | 2 | 0.9 (0.9–1.3) | 52 | 15 (12–18) | 150 | 9 (6–13) |
| 15 years | 1 | 1 | 53 | 12 | 120 | 0 |
| 16 years | 1 | 0.5 | 27 | 10 | 100 | 0 |
aNo reference values exist for vitamin D for 4–65 years; therefore, value based on 10 μg/day recommended intake for 4–65 years at risk of vitamin D deficiency (Department of Health 1998)
Median (range) fibre intake was only 4 g/day (0–11 g) for all ages. In the UK, there is no dietary reference value for fibre in children, but the Food Standards Agency (Nutrient and Food Based Guideline for Food Institutions 2006) recommends a fibre intake of 18 g/day for children aged ≥5 years.
Mineral Intake (Tables A.1, A.2 and A.3 of Appendix)
The median intake of sodium, potassium and magnesium from age 3 months to 16 years was either consistently below or only narrowly supplied the RNI (DH 1991) (sodium 57% (20–97%), potassium 64% (27–125%) and magnesium 72% (22–116%) of RNI). The median (range) RNI for calcium was 103% (48 to 185%), iron 138% (77–353%) and zinc 143% (84–210%) for all age groups. The selenium intake met the RNI (DH 1991) for subjects ≤10 years of age, but was mainly < RNI after this age.
Long-Chain Polyunsaturated Fatty Acids
The median (range) combined intake of docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) was 226 mg/day (44–282). The median (range) intake of arachidonic acid (AA) was 53 mg/day (16–67). For DHA and EPA, the recommended daily intake is 100–150 mg from 2 to 4 years, 150–200 mg for 4–6 years and 200–250 mg/day from 6 to 10 years (EFSA 2009). The EFSA has not set a recommended intake for AA.
Anthropometric Measurements
Height/length: the median (range) z score from 3 months to 16 years for height/length for age was −1.7 (−0.6 to −3.5) and for weight for age was −1 (+1 to −2.4), and BMI z score was 0.9 (+2.7 to −0.8). All subjects had a negative height z score, and this failed to improve with age for most subjects (n = 9). According to the WHO criteria (de Onis et al. 1993), some subjects (n = 4) were stunted (definition: z score < −2 SD is used to classify low height for age). Height measurements were unavailable for two subjects due to their severe neurological dysfunctions.
Weight: from 3 months to 8 years of age, the weight z score was a median of +0.9 (range −0.8 to 1.1). However, between 7 and 16 years, deliberate dietary intervention controlling energy intake resulting in a lower median weight (range) z score of −1.3 (0.2 to −2.4).
BMI: the BMI z score remained consistently positive with age with a median z score of 0.8 (range −0.2 to 2.2). The rate of overweight and obesity increased throughout the early years.
Nutritional Biochemical Markers (Tables 2 and 3)
Table 2.
Median plasma valine, isoleucine, leucine and threonine concentrations for PA and MMA subjects, compared with the percentage number of samples less than the plasma reference range
| Disorders | Number samples (range) | Median valine μmol/L (range) | % < Reference range (160–350 μmol/L) | Median isoleucine μmol/L (range) | % < Reference range (37–140 μmol/L) | Median leucine μmol/L (range) | % < Reference range (70–170 μmol/L) | Median threonine μmol/L (range) | % < Reference range (67–150 μmol/L) |
|---|---|---|---|---|---|---|---|---|---|
| PA N = 8 | 6 (3–12) | 94 (54–147) | 100% | 32 (18–57) | 63% | 87 (59–141) | 38% | 115 | 25% |
| MMA N = 4a | 5 (5–6) | 100 (160–350) | 100% | 32 (18–51) | 75% | 75 (55–113) | 50% | 109 (68–148) | 0 |
aOne MMA subject had no blood parameters recorded
Table 3.
Median plasma zinc, CRP, selenium, B12, ferritin and haemoglobin concentrations for PA and MMA subjects, compared with the percentage number of samples less than the plasma reference range
| Disordersa | Number samples (range) | Median zinc μmol/L (range) | % < Reference range (11–24) | CRP mg/L (range) | % > Reference range (0–10) | Median selenium μmol/L (range) | % < Reference range (55–115) | Median haemoglobin g/dL (range) | % < Reference range (11–12.5) | Median ferritin μg/L (range) | % < Reference range (4–405) | Median B12 ng/L (range) | % < Reference range (259–823) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PA N = 8 | 8 (1–10) | 11.7 (10.6–16.1) | 50% | 5 (1–10) | 0 | 76.5 (67–105) | 0 | 12 (11–12.5) | 38% | 71 (31–162) | 0 | 878 (583–1349) | 0 |
| MMA N = 5 | 7 (5–7) | 12.1 (11.5–14.4) | 0 | 5 (1–9) | 0 | 68 (52–92) | 20% | 11.2 (10.5–11.8) | 40% | 89 (14–125) | 0 | 1101 (597–1500) | 0 |
aIVA subject n = 1
The median number of subject samples for quantitative plasma amino acids was 5 (range 2–12). In the PA/MMA subjects, all valine concentrations were < plasma target reference ranges. In the PA group, 63% of plasma isoleucine, 38% leucine and 25% threonine were < the reference ranges. In the MMA group, 75% of plasma isoleucine and 50% of leucine concentrations were < reference ranges.
In the PA (but not MMA) group, 50% of plasma zinc concentrations were < the reference range. There was no association between low plasma zinc concentrations and CRP. The % of haemoglobin concentrations < reference range was 38% for the PA group (all had corresponding low MCV concentrations) and 75% for the MMA group (67% had low MCV concentrations). No subject had a ferritin value < reference range.
Number of Times Using Emergency Feeds or on IV Glucose Without Feeds
A protein-free emergency feed was used a median of 2 to 5 times/annually in the first 8 years of life, thereby reducing usual protein intake between 6 and 15 days per year. The number of hospital admissions, requiring at least 24 h on IV glucose without feeds, varied between 2 and 6 times/year.
Medication
All children with MMA, PA and IVA were prescribed l -carnitine. In MMA and PA, metronidazole was given on a cyclical basis usually 7 days in alternate months. Omeprazole, domperidone and ranitidine (n = 8) were given for vomiting, retching and gastro-oesophageal reflux and laxatives (paediatric movicol and lactulose) (n = 8) for constipation. Sodium benzoate and carglumic acid were used intermittently to treat high ammonia concentrations.
Discussion
This longitudinal retrospective study is the first to report the detailed nutritional intake of children with organic acidaemias (MMA, PA and IVA) exclusively dependent on tube feeding. Each subject had a feed designed for their individual requirements, which was complex and composed of a number of individual feed ingredients. Energy intake was below the EAR (DH 1991) and decreased with increasing age, creating feed composition challenges in ensuring that DRVs for all nutrients were achieved. The micronutrients intake was suboptimal (particularly sodium, potassium and magnesium) with the median intake for other nutrients (e.g. calcium and iron) only just providing the RNI. This finding was in contrast to children with chronic disorders on standard enteral feeds (Johnson et al. 2002).
Linear growth was poor, which was possibly due to low energy intake (although weight gain was positive), essential amino acids and zinc deficiencies, mitochondrial dysfunction, renal dysfunction in MMA and frequent hospital admissions interrupting usual feed and nutrient intake. This is not the first study to report low energy intakes (Thomas et al. 2000) and overweight and obesity in PA/MMA, but it is established that resting energy expenditure is lower than predicted in MMA/PA (Hauser et al. 2011; Feillet et al. 2000). Many children in our group (n = 11 of 14) had limited physical activity, and in order to avoid prolonged fasting, they were fed night-time continuous feeds, contributing additional dietary energy. There is concern that low energy intake could lead to metabolic decompensation (Yannicelli et al. 2003), but emergency hospital admissions were commonly due to viral illnesses, seizures, chest infections and metabolic decompensation associated with constipation and vomiting.
A further difficulty in trying to achieve the correct feed formulation is defining the ideal amount and type of protein intake. Children with severe phenotypes have limited protein tolerance. In this study, precursor-free l-amino acids provided a median of 20% of protein intake (MMA/PA only) in the first 5 years of life. The MMA/PA European working group suggests that protein intake should meet safe levels (Baumgartner and Viardot 1995), with precursor-free l-amino acids only being used to supplement natural protein intake if intake is below safe levels (WHO/FAO/UNU 2007). They also suggest that it is important to provide high biological natural protein in order to provide a balanced blend of amino acids, achieving adequate intake for growth and nitrogen balance and preventing accumulation of toxic metabolites (Baumgartner and Viardot 1995). Overall protein deficiency may be associated with poor growth, decreased muscle mass and osteopenia (North et al. 1995; Orwoll 1992), isoleucine deficiency with an acrodermatitis enteropathica-like rash (De Raeve et al. 1994; Lane et al. 2007; Tabanlioglu et al. 2009) and valine deficiency with poor appetite and excess irritability.
Although precursor-free l-amino acids are commonly used in MMA/PA but not IVA, their efficacy has not been fully assessed (Touati et al. 2006) and there is no consensus on the ideal amount of precursor-free l-amino acid that should be given. The amount prescribed in cross-sectional and cohort studies varies between 15% and 50% of total protein intake (Zwickler et al. 2008; Sass et al. 2004; Horster et al. 2009; Touati et al. 2006; van der Meer et al. 1994, 1996), partly influenced by metabolic stability, natural protein intake tolerated, patient age, disorder severity and local practice. Although overuse could lead to valine, leucine and isoleucine deficiency particularly if the major source of natural protein is plant or cereal derived, it is unlikely that the use of precursor-free l-amino acids contributed to low branched-chain amino acids in this study. The natural protein sources consumed from enteral feed were based on whey ± casein sources, and they yielded for each gram of protein between 100 to 110 mg leucine and 50–70 mg of both valine and isoleucine. As each child received in excess of 0.5 g/kg/day natural protein, they comfortably met their safe levels of branched-chain amino acid intake (WHO/FAO/UNU 2007).
Fibre intake was low, with frequent use of laxatives. Optimal fluid intake was not achieved: many failed to drink orally, and when extra fluid was added to feeding regimens, vomiting and retching were common. A combined effect of low fibre intake, use of l-amino acid formula and low fluid intake may be a causal factor of constipation. Other factors such as patient hypomobility and muscle hypotonia may also contribute. In MMA/PA, approximately 20–30% of body propionate formation is from gut flora (Thompson and Chalmers 1990), and so avoidance of constipation is essential. Patients given gut motility agents have consistent reductions in blood ammonia accompanied by reductions in urinary excretion of propionyl glycine and increased free and total carnitine (Prasad et al. 2004). The fibre content of feeds for children with organic acidaemias is given inadequate attention.
Limitations of this retrospective review include the small subject numbers particularly from each subcategory of organic acidaemia, which did not permit meaningful comparison between subject groups. There was also inadequate information on the frequency and use of emergency feeds, insufficient anthropometric measurement for height in children with neurological disabilities and irregular biochemical and haematological nutritional status data.
In conclusion, this study highlights several nutritional concerns in tube-feed-dependent children with organic acidaemias. Despite energy intake decreasing significantly over time, weight gain continues to accelerate, but linear growth is compromised. Essential mineral intake (zinc, magnesium, sodium and potassium) falls below RNI and dietary fibre is inadequately supplemented. Designing appropriate feed formulations that will meet all the nutritional requirements of this group of patients is a challenging but essential task.
Appendix: Detailed Nutrient Intake Analysis for Subjects on Enteral Tube Feeding with Organic Acidaemia
Compliance with Ethics Guidelines
Conflict of Interest
A. Daly – research funding from Vitaflo; financial support from Nutricia and Vitaflo to attend study days and conferences.
S. Evans – a research dietitian funded by Nutricia; financial support from Nutricia and Vitaflo to attend study days and conferences
A. MacDonald – research funding and honoraria from Nutricia, Vitaflo International and Merck Serono, Member of European Nutrition Expert Panel (Merck Serono International), Member of Sapropterin Advisory Board (Merck Serono International), Member of the Advisory Board Element (Danone Nutricia)
S Vijay – has no conflict of interest with nutritional-based companies
S Santra – has no conflict of interest with nutritional-based companies
A Gerrard – has no conflict of interest with nutritional-based companies
Authors’ Roles
All authors were involved in the analysis and interpretation of data, critical revision of the paper for important intellectual content and final approval of the version to be published. Anita MacDonald and Anne Daly were additionally involved in the initial study conception and design; Anne Daly was involved in the collection of data; and Anita MacDonald and Anne Daly in the collation of data and drafting of the initial article
Source of Funding
No funding was obtained for this study.![]()
Footnotes
Competing interests: None declared
References
- Baumgartner ER, Viardot C. Long-term follow-up of 77 patients with isolated methylmalonic acidaemia. J Inherit Metab Dis. 1995;18:138–142. doi: 10.1007/BF00711749. [DOI] [PubMed] [Google Scholar]
- Department of Health (1991) Dietary reference values for food energy and nutrients for the United Kingdom. Report on Health and Social Subjects, No. 41. HMSO, London [PubMed]
- de Onis M, Monteiro C, Akré J, Clugston G. The worldwide magnitude of protein-energy malnutrition: an overview from the WHO Global Database on child growth. Bull World Health Organ. 1993;71:703–712. [PMC free article] [PubMed] [Google Scholar]
- De Raeve L, De Meirleir L, Ramet J, Vandenplas Y, Gerlo E. Acrodermatitis enteropathica-like cutaneous lesions in organic aciduria. J Pediatr. 1994;124:416–420. doi: 10.1016/S0022-3476(94)70364-7. [DOI] [PubMed] [Google Scholar]
- Evans S, Daly A, MacDonald J, Preece MA, Santra VS, Chakrapani A, MacDonald A. The micronutrient status of patients with PKU on dietary treatment: an ongoing challenge. Ann Nutr Metab. 2014;65(1):42–48. doi: 10.1159/000363391. [DOI] [PubMed] [Google Scholar]
- Feillet F, Bodamer OA, Dixon MA, Sequeira S, Leonard JV. Resting energy expenditure in disorders of propionate metabolism. J Pediatr. 2000;136:659–663. doi: 10.1067/mpd.2000.104290. [DOI] [PubMed] [Google Scholar]
- Food Standard Agency Nutrient and Food Based Guideline for Food Institutions 2006 (www.food.gov.uk)
- Hauser NS, Manoli I, Graf JC, Sloan J, Venditti CP. Variable dietary management of methylmalonic acidemia: metabolic and energetic correlations. Am J Clin Nutr. 2011;93:47–56. doi: 10.3945/ajcn.110.004341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holliday MA, Segar WE. The maintenance need for water in parenteral fluid therapy. Pediatrics. 1957;19:823–832. [PubMed] [Google Scholar]
- Horster F, Garbade SF, Zwickler T, Aydin HI, Bodamer OA, Burlina AB, Das AM, De Klerk JB, Dionisi-Vici C, Geb S, et al. Prediction of outcome in isolated methylmalonic acidurias: combined use of clinical and biochemical parameters. J Inherit Metab Dis. 2009;32:630–639. doi: 10.1007/s10545-009-1189-6. [DOI] [PubMed] [Google Scholar]
- Johnson TE, Janes SJ, MacDonald A, Elia M, Booth IW. An observational study to evaluate micronutrient status during enteral feeding. Arch Dis Child. 2002;86:411–415. doi: 10.1136/adc.86.6.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane TN, Mary MD, Sparker MK, Sareeta S, Parker MD. Propionic Acidemia manifesting with low isoleucine generalized exfoliative dermatosis. Pediatr Dermatol. 2007;24:508–510. doi: 10.1111/j.1525-1470.2007.00505.x. [DOI] [PubMed] [Google Scholar]
- North K, Korson M, Yosodha R, Rohr FJ, Brazelton TB, Waisbren SE, Warman ML. Neonatal-onset propionic acidemia:and neurological and developmental profiles and implications for management. J Pediatr. 1995;126:916–922. doi: 10.1016/S0022-3476(95)70208-3. [DOI] [PubMed] [Google Scholar]
- Orwoll ES. The effects of dietary protein insufficiency and excess on skeletal health. Bone. 1992;13:343–350. doi: 10.1016/8756-3282(92)90081-7. [DOI] [PubMed] [Google Scholar]
- Prasad C, Nurko S, Borovoy J, Korson MS. The importance of gut motility in the metabolic control of propionic acidemia. J Pediatr. 2004;144:532–535. doi: 10.1016/j.jpeds.2003.12.044. [DOI] [PubMed] [Google Scholar]
- Sass JO, Hofmann M, Skladal D, Mayatepek E, Schwahn B, Sperl W. Propionic acidemia revisited: a workshop report. Clin Pediatr (Phila) 2004;43:837–843. doi: 10.1177/000992280404300908. [DOI] [PubMed] [Google Scholar]
- Tabanlioglu D, Ersoy-Evans S, Karaduman A. Acrodermatitis enteropathica-like eruption in metabolic disorders: acrodermatitis dysmetabolica is proposed as a better term. Pediatr Dermatol. 2009;26:150–154. doi: 10.1111/j.1525-1470.2008.00803.x. [DOI] [PubMed] [Google Scholar]
- Thomas JA, Bernstein LE, Greene CL, Koeller DM. Apparent decreased energy requirements in children with organic acidemias: preliminary observations. J Am Diet Assoc. 2000;100:1074–1076. doi: 10.1016/S0002-8223(00)00313-8. [DOI] [PubMed] [Google Scholar]
- Thompson GN, Chalmers RA. Increased urinary metabolite excretion during fasting in disorders of propionate metabolism. Pediatr Res. 1990;27:413–416. doi: 10.1203/00006450-199004000-00021. [DOI] [PubMed] [Google Scholar]
- Touati G, Valayannopoulos V, Mention K, de Lonlay P, Jouvet P, Depondt E, Assoun M, Souberbielle JC, Rabier D, Ogier de Baulny H, Saudubray JM. Methylmalonic and propionic acidurias: management without or with a few supplements of specific amino acid mixture. J Inherit Metab Dis. 2006;29:288–298. doi: 10.1007/s10545-006-0351-7. [DOI] [PubMed] [Google Scholar]
- van der Meer SB, Poggi F, Spada M, Bonnefont JP, Ogier H, Hubert P, Depondt E, Rapoport D, Rabier D, Charpentier C, et al. Clinical outcome of long-term management of patients with vitamin B12-unresponsive methylmalonic acidemia. J Pediatr. 1994;125:903–908. doi: 10.1016/S0022-3476(05)82005-0. [DOI] [PubMed] [Google Scholar]
- van der Meer SB, Poggi F, Spada M, Bonnefont JP, Ogier H, Hubert P, Depondt E, Rapoport D, Rabier D, Charpentier C, et al. Clinical outcome and long-term management of 17 patients with propionic acidaemia. Eur J Pediatr. 1996;155:205–210. doi: 10.1007/BF01953939. [DOI] [PubMed] [Google Scholar]
- Walter JH, MacDonald A. The use of amino acid supplements in inherited metabolic disease. J Inherit Metab Dis. 2006;29(2–3):279–280. doi: 10.1007/s10545-006-0357-1. [DOI] [PubMed] [Google Scholar]
- WHO/FAO/UNU (2007) Protein and amino acid requirements in human nutrition. Report of a joint WHO/FAO/UNU expert consultation. World Health Organ Tech Rep Ser 935 [PubMed]
- Yannicelli S, Acosta P, Velazquez A, Hans-Gerog B, Marriage B, Kurczynski T, Miller M, Korson M, Steiner R, Rutledge L, Bernstein L, Chinsky J, Galvin-Parton P, Arnold G. Improved growth and nutrition status in children with methylmalonic or propionic academia fed an elemental medical food. Mol Genet Metab. 2003;80:181–188. doi: 10.1016/j.ymgme.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Zwickler T, Lindner M, Aydin HI, Baumgartner MR, Bodamer OA, Burlina AB, Das AM, DeKlerk JB, Gokcay G, Grunewald S, et al. Diagnostic work-up and management of patients with isolated methylmalonic acidurias in European metabolic centres. J Inherit Metab Dis. 2008;31:361–367. doi: 10.1007/s10545-008-0804-2. [DOI] [PubMed] [Google Scholar]


