Abstract
Background
Positive esophageal proximal resection margin (ERM+) following esophagectomy was considered as incomplete or R1 resection. The clinicopathological data and long-term prognosis of esophageal cancer (EC) patients with ERM+ after esophagectomy were still unknown. Therefore, the aim of this study was to assess the clinical significance of ERM+ and its therapeutic option.
Methods
From November 2008 to December 2014, 3,594 patients with histologically confirmed EC underwent radical resection in our department. Among them there were 37 patients (1.03%) who had ERM+. ERM+ was defined as carcinoma or atypical hyperplasia (severe or moderate) at the residual esophageal margin in our study. For comparison, another 74 patients with negative esophageal proximal resection margin (ERM−) were propensity-matched at a ratio of 1:2 as control group according to sex, age, tumor location and TNM staging. The relevant prognostic factors were investigated by univariate and multivariate regression analysis.
Results
In this large cohort of patients, the rate of ERM+ was 1.03%. The median survival time was 35.000 months in patients with ERM+, significantly worse than 68.000 months in those with ERM− (Chi-square =4.064, P=0.044). Survival in patients with esophageal residual atypical hyperplasia (severe or moderate) was similar to those with esophageal residual carcinoma. Survival rate in stage I–II was higher than that in stage III–IV (Chi-square =27.598, P=0.000) in ERM−; But there was no difference between the two subgroups of patients in ERM+. Furthermore, in those patients with ERM+, survival was better in those who having adjuvant therapy, compared to those without adjuvant therapy (Chi-square =5.480, P=0.019). And the average survival time which was improved to a well situation for ERM+ patients who have adjuvant therapy was 68.556 months which is comparable to average survival time (65.815 months) of ERM− for those patients who are at earlier stages.
Conclusions
ERM+ after esophagectomy nowadays is of low incidence but still an important prognostic factor for patients with EC. Survival of ERM+ patients who have adjuvant therapy was improved to a well situation which is comparable to overall survival (OS) rate of ERM− for those patients who are at earlier stages.
Keywords: Esophageal carcinoma (EC), positive resection margin, prognosis, therapy
Introduction
Esophageal cancer (EC) is the eighth most common malignancy and the fifth leading cause of cancer in males worldwide (1,2). Each year, about 300,000 people died from EC on earth and the half of those patients were Chinese (3). The prognosis of EC patients is much poor with a 5-year overall survival (OS) rate of 15–39.2% (4). Surgical resection has been the standard treatment for localized EC. Some studies have reported that the prognosis of EC is associated with several clinicopathologic characteristics, including pathological stage, lymph node metastases, lymphovascular invasion, and positive esophageal proximal resection margin (ERM+) status (3-5).
ERM+ following esophagectomy was considered as incomplete or R1 resection. The clinicopathological data and long-term prognosis of EC patients with ERM+ after esophagectomy were still seldom analyzed. The incidence of ERM+ was reported mostly between 2.9% to 12%, even up to 31.3% reported in certain study (6). Microscopic identification of residual tumor at the proximal or distal esophageal proximal resection margin (termed as R1) increases the risk of recurrence and disease-related mortality. In order to reduce the incidence of ERM+, many studies (1) had explored the therapeutic strategies including preoperative evaluation, preoperative neoadjuvant therapy and postoperative adjuvant treatment and salvage esophagectomy. Markar et al. (7) showed that neoadjuvant chemoradiotherapy did significantly improve survival of patients with ERM+, and reduced distant tumor recurrence but failed to significantly influence locoregional control of the disease. Song et al. (8) showed that patients with margin involvement yielded long term survival by postoperative radiotherapy, suggesting a potential role of postoperative radiotherapy, especially for patients with margin involvement. Morita et al. (9) showed that salvage esophagectomy should be considered carefully for recurrent cancer patients in whom complete resection can be achieved.
However, each research above has different localization. These issues remain to be resolved, such as: risk of ERM+, importance of remedial measures and significance of postoperative radiotherapy and chemotherapy. These questions badly needed strong evidence to elucidate. Therefore, we conducted this retrospective study aiming to explore these questions. As a retrospective study, we did not do special pertinence measures according to these patients, only to analysis current situation. The aim of this study is to assess the clinical significance of ERM+ and its therapeutic option.
Methods
Patients
The patients’ data for this study was collected from the EC database of our hospital from November 2008 to December 2014. This study was approved by the Ethics Committee of West China Hospital (No. 201649). In that period, 3,594 patients with histologically confirmed EC underwent radically intended resection in our department. ERM+ was defined as carcinoma or atypical hyperplasia (severe or moderate) at the residual esophageal margin after surgery. All patients with ERM+ had been followed-up in this study. For comparison, we had successful followed up 3,308 patients (93.0%) in the other patients with negative esophageal proximal resection margins (ERM−). From these data, we chose suitable patients to comparing with patients with ERM+ by specific propensity matching method as a ratio of 1:2 (10).
All the patients enrolled in the study met the following criteria: (I) pathologically confirmed thoracic squamous cell carcinoma; (II) all pathological specimens were examined by two independent pathologists, at least one being a senior gastrointestinal pathologist; (III) patient’s demographic and tumor related data were collected; (IV) and complete information was available for stage grouping. ERM+ was defined as carcinoma or atypical hyperplasia (severe or moderate) at the residual esophageal margin in our study.
Follow-up
All patients were followed up by telephone or interview at a 3-month interval for the first postoperative 2 years, at 6-month intervals for the following 3 years, and then annually thereafter. Survival time was measured from the operation date to the date of death or last follow-up. The last general follow-up of survivors was done at the end of December 2015.
Statistical analysis
Statistical analysis was carried out by using SPSS version 16.0 software. Continuous variables are expressed as the mean ± standard deviation and categorical variables as percentage. A Mann-Whitney U test was used for intergroup comparisons of continuous variables, whereas a Chi-square test was used to compare categorical data. OSs, data had been presented as the median for survival, were estimated using the Kaplan-Meier method. The log rank test was used to compare survival curves. On the other hand, we carried out a propensity score matching analysis to compensate for the differences in baseline characteristics between the ERM+ and ERM− groups in the assessment of relevant outcomes. We matched each ERM+ recipient to two ERM− recipients with similar covariates, represented as propensity scores. Variables used in our model for estimating propensity scores included sex, age, operation time and TNM staging. All statistical tests were 2-sided with the threshold of significance set at a P<0.05.
Results
Patient characteristics and results of propensity-matched
In this study, there were 37 patients who had ERM+. For comparison, 74 patients with ERM− were propensity-matched at a ratio of 1:2 as control group according to sex, age, operation time and TNM staging (Table 1).
Table 1. Clinical characteristics of patients in propensity score matched.
| Factor | ERM+ (n=37) (%) | ERM− (n=74) (%) | P value |
|---|---|---|---|
| Age (years) (mean ± SD) | 59.5±8.5 | 60.6±8.3 | 0.784 |
| Sex | 0.859 | ||
| Male | 31 (83.8) | 61 (82.4) | |
| Female | 6 (16.2) | 13 (17.6) | |
| Pathological stage | 0.738 | ||
| I | 8 (21.6) | 16 (21.6) | |
| II | 19 (51.4) | 31 (41.9) | |
| III | 8 (21.6) | 20 (27.0) | |
| IV | 2 (5.4) | 7 (9.5) | |
| Adjuvant therapy | 0.635 | ||
| Yes | 10 (27.0) | 16 (21.6) | |
| No | 27 (73.0) | 58 (78.4) | |
| Lymph node metastasis | 0.232 | ||
| Yes | 19 (51.4) | 29 (39.2) | |
| No | 18 (48.6) | 45 (60.8) | |
| Pathological type | – | ||
| Severe or moderate | 17 (45.9) | – | |
| Carcinoma | 20 (54.1) | – | |
| Operation time (months) (mean ± SD) | 45.3±22.6 | 43.2±21.7 | 0.626 |
Data are expressed as n (%) unless otherwise specified. ERM+, positive esophageal proximal resection margin; ERM−, negative esophageal proximal resection margin.
Result of univariate analysis
In this large cohort of patients, 3,594 patients with histologically confirmed EC underwent radical intent resection in our department. Among them there were 37 patients who had ERM+. The rate of ERM+ was 1.03%. The median survival time was 35 months in patients with ERM+, significantly worse than the survival time of 68 months in those with ERM− (Chi-square =4.064, P=0.044) (Figure 1).
Figure 1.
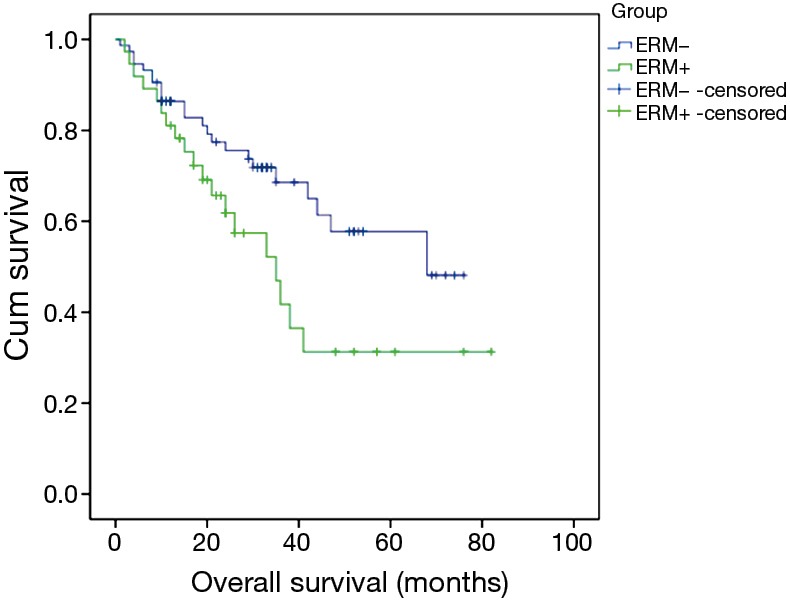
Survival analysis of ERM+ & ERM−: the median survival time was 35 months in ERM+ and 68 months in ERM− (Chi-square =4.064, P=0.044). ERM+, positive esophageal proximal resection margin; ERM−, negative esophageal proximal resection margin.
In this study, patients with ERM+ were divided into two subgroups [carcinoma: ERM+ 1, atypical hyperplasia (severe or moderate): ERM+ 2]. There was no survival difference between these two groups (Figure 2).
Figure 2.
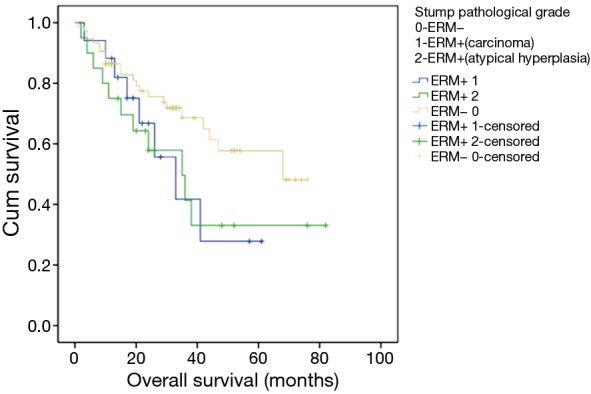
Survival analysis according to pathological staging system: overall survival by stump pathological staging system (ERM− 0 versus ERM+ 1, ERM+ 2: P>0.05; ERM+ 1 versus ERM+ 2: P>0.05). ERM+, positive esophageal proximal resection margin; ERM−, negative esophageal proximal resection margin.
Survival in ERM+ with adjuvant therapy had significant longer survival than those without adjuvant therapy (Chi-square =5.480, P=0.019); to understand the survival of ERM+, we also do some cross comparison. The average survival time of ERM+ patients who have adjuvant therapy comparable to average survival time of ERM− patients in stage I–II (68.556 versus 65.815 months) (Figure 3).
Figure 3.
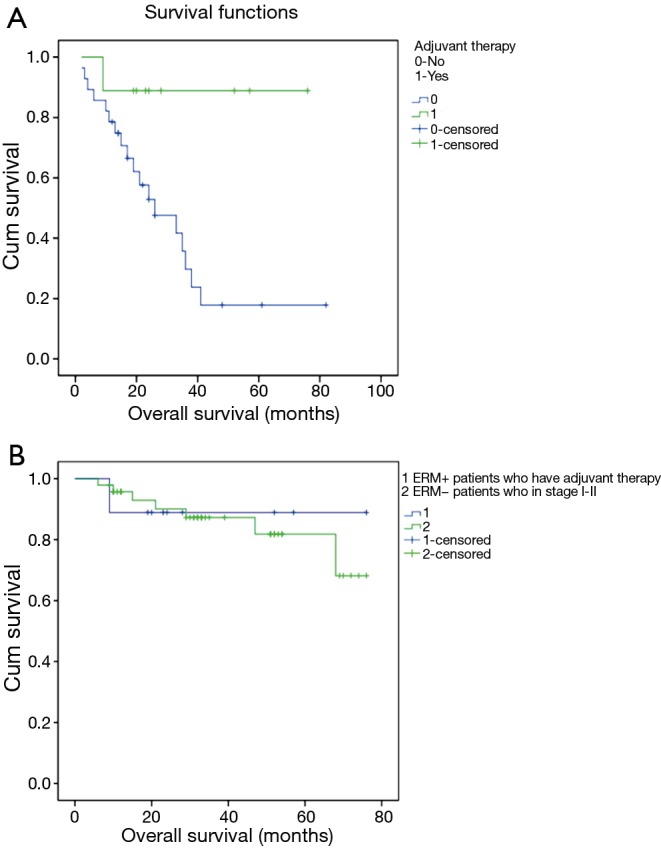
(A) Survival analysis according to adjuvant therapy in ERM+: overall survival by adjuvant therapy staging system (ERM+ 1 versus ERM+ 0: Chi-square =5.480, P=0.019); (B) the average survival time of ERM+ patients who have adjuvant therapy comparable to average survival time of ERM− patients in stage I–II (68.556 versus 65.815 months). ERM+, positive esophageal proximal resection margin; ERM−, negative esophageal proximal resection margin.
From these data, lymph node metastasis did not significantly influence the survival of patients with patients with ERM+; however, we saw a contrary result for patients with ERM− (Chi-square =18.197, P=0.000) (Figure 4).
Figure 4.
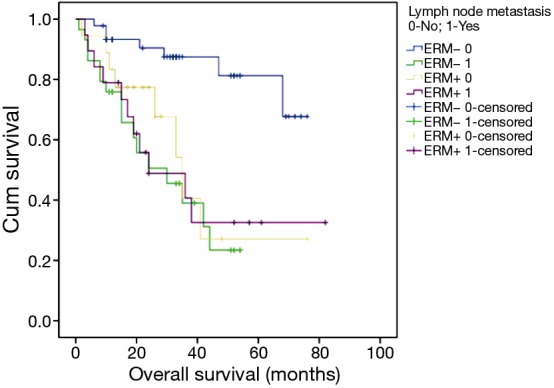
Survival analysis according to adjuvant therapy: overall survival by lymph node metastasis staging system (ERM− 0 versus ERM− 1: Chi-square =18.197, P=0.000; ERM+ 0 versus ERM+ 1: P>0.05; ERM− 0 versus ERM+ 1: P<0.05). ERM+, positive esophageal proximal resection margin; ERM−, negative esophageal proximal resection margin.
Survival rate in stage I–II was higher than that in stage III–IV (Chi-square =27.598, P=0.000) in ERM−; but there was no difference between the same two subgroups of patients in ERM+ (Figure 5).
Figure 5.
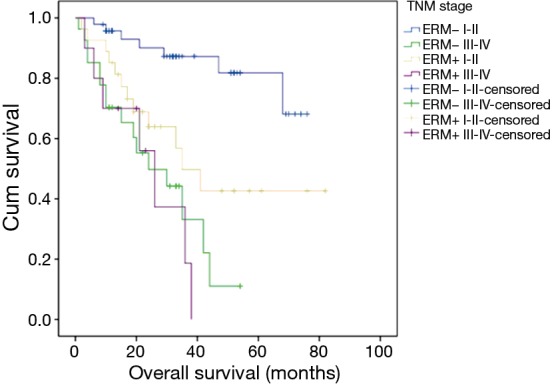
Survival analysis according to adjuvant therapy: overall survival by TNM staging system (ERM− I–II versus ERM− III–IV: Chi-square =27.598, P=0.000; ERM+ I–II versus ERM+ III–IV: P>0.05; ERM− I–II versus ERM+ I–II: P<0.05). ERM+, positive esophageal proximal resection margin; ERM−, negative esophageal proximal resection margin.
Result of multivariate analysis
In this study, ERM+ is an important prognostic factor (P=0.003) for patients and adjuvant therapy (P=0.001) too. However, no significance was found about lymph node metastasis, stump histological grade in this multivariate analysis about patients with ERM+ (Table 2, Figure 6).
Table 2. Result of multivariate analysis about patients with ERM+.
| Item | B | SE | Wald | Df | P | Exp(B) | 95.0% Exp(B) | |
|---|---|---|---|---|---|---|---|---|
| Lower bound | Upper bound | |||||||
| Group | −4.054 | 1.387 | 8.541 | 1 | 0.003 | 0.017 | 0.001 | 0.263 |
| Adjuvant therapy | 1.016 | 0.293 | 12.016 | 1 | 0.001 | 2.762 | 1.555 | 4.905 |
| Lymph node metastasis | 0.074 | 0.391 | 0.035 | 1 | 0.851 | 1.076 | 0.500 | 2.315 |
| Stump pathological grade | −0.049 | 0.493 | 0.010 | 1 | 0.921 | 0.952 | 0.362 | 2.504 |
ERM+, positive esophageal proximal resection margin; B, regression coefficient; SE, standard of error; Wald, χ2 value; P, P value; Exp(B), relative risk.
Figure 6.
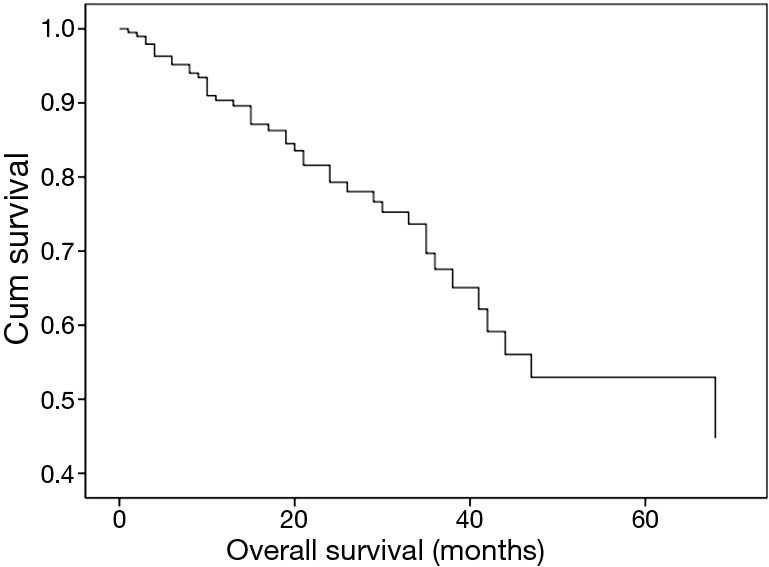
Survival function of multivariate analysis about patients with ERM+. ERM+, positive esophageal proximal resection margin.
Discussion
EC is found to have geographic differences in occurrence rate. China has a high incidence of EC in the world, with 15 million people died of EC each year (11). The 5-year survival rate of patients with EC after esophagectomy is low although many progresses have been made in treating EC. The main factors affecting the long-term survival of patients with EC were postoperative local recurrence and distant metastasis. A number of studies (11-13) have shown that the main prognostic factors for postoperative EC patients’ local control and survival is the depth and breadth of invasion as well as the type of the esophagectomy. ERM+, also an important factor, followed by esophagectomy was considered as imcomplete or R1 resection. ERM+ is inevitable in certain surgery. In this study, 3,594 patients with histologically confirmed EC underwent radical resection in our department. Among them there were 37 patients with ERM+. The rate of ERM+ was 1.03%. This lower incidence can be accepted digitally. The median survival time was 35 months in the ERM+, significantly lower than survival time of 68 months in the ERM− (χ2=4.064, P=0.044). Obviously, there was a difference statistically significant between the two groups. Therefore, ERM+ in nowadays is rare but still an important prognostic factor for patients with EC undergoing esophagectomy. Cao et al. (14) and Watanabe et al. (15) reported that resection margin invasion were independent predictors of a poor prognosis. A large multicenter European study (7) provided evidence to support the notion that R1 resection margin, as microscopic residual tumor present at the vertical or circumferential resection margins of the surgical specimen, was a prognostic indication of aggressive tumor biology with a poor long-term prognosis. In this study, the median survival reached 35 months which seems not to be so poor status and not an evil result for patients with ERM+. We have also analyzed the situation of these patients in the subgroup in further.
In the past half century, many scholars had reported that increasing the length of resection even total esophagectomy could decrease the occurrence of residual stump lesions (16). However, it can not be achieved to resect the enough length because of the location of EC. So we need to understand the influences of various factors, such as ERM+, stump pathological grade, adjuvant therapy, lymph node metastasis, TNM stage and age, for the EC recurrence and survival. For example, the main factors influencing the survival of EC patients with ERM+ were the depth of tumor infiltration, histological classification, lymph node metastasis and radical or conservative resection (17). Another example showed that tumor length, number of metastatic lymph nodes, stump pathological grade and treatment modality were important prognostic factors for patients with ERM+ (14). And invasion depth, stump pathological grade and treatment modality were important influencing factors for locoregional control.
Positive esophageal stump after esophagectomy were diagnosed with carcinoma or atypical hyperplasia (severe or moderate) of residual esophageal stump. Therefore, we grouped these cases into two subgroups, as patients who were diagnosed with carcinoma: ERM+ 1, and patients who were diagnosed with atypical hyperplasia (severe or moderate): ERM+ 2, for patients with ERM+. In this study, Survival in patients ERM+ 1 was similar with ERM+ 2. In Cao’s research (14), grade III atypical hyperplasia and carcinoma were classified as a group because of their similarity. As we know, it just needed a formation evolution and a little time from moderately and severe atypical hyperplasia cells to carcinoma cells. The conversion process was short in the whole period prognosis due to the high progressing speed of EC. Therefore, there was no difference in the comparison of these two subgroups whose survival is below to patients with ERM−.
Chemotherapy, radiotherapy or chemoradiotherapy were considered to be necessary for patients with high risk of distant metastasis and local recurrence, especially to these patients with ERM+. This strategy can improve the OS of these patients. Zheng et al. (18) reported 3-year survival rates of patients with and without salvage treatment were 53.2% and 7.8%, respectively (P=0.027). Three-year survival rate of patients with salvage radiotherapy was 56.0%. Gilbert et al. (19) and Rice (20) reported the role of adjuvant systemic therapy in patients with an isolated microscopically ERM+ merits further evaluation. In ERM+, survival rate with adjuvant therapy was significantly higher than those without adjuvant therapy (Chi-square =5.480, P=0.019); survival remained in a situation comparable to OS rate of ERM− for those patients who are at earlier stages and improved for those patients or who have adjuvant therapy.
The AJCC TNM staging system has been widely used to stratify patients and select treatment strategies. Due to a relatively small sample size, we only divided the cases into two subgroups (stage I–II and stage III–IV). Survival rate in stage I–II was higher than that in stage III–IV (Chi-square =27.598, P=0.000) in ERM−; but there was no difference between the two subgroups of patients in ERM+. Another same phenomenon was that lymph node metastasis did not significantly influence the survival of patients in ERM+; However, we saw a different result for patients in ERM− (Chi-square =18.197, P=0.000). Some research suggests that advanced T and N stage are the risk factors of residual cancer after esophagectomy in the patients with squamous cell carcinoma of the esophagus (18). Other research suggest that positive nodal status (P=0.002; HR 2.730) were independent predictors of a poor prognosis (21). Combined with our research, depth of tumor invasion and lymph node metastasis are the important component for determining tumor stage. Although these two factors are the main determinants causing different survival in ERM− but they did not show the same effect in ERM+. This may suggest that ERM+ plays more important role for determining survival than those factors of T and N stage.
In addition, it should be mentioned that age is not the key contraindication for the operation along with the improvement of surgical technique. Whether ERM+ or ERM−, there was no significant difference comparing patients with an age of ≥60 years with those with an age of <60 years in our study. This suggests that age is not an important factor in survival.
In conclusion, ERM+ in nowadays is rare but still an important prognostic factor for patients undergoing esophagectomy for cancer. In patients with ERM+, survival remains in a situation comparable to OS rate of ERM− for those patients who are at earlier stages and improved for those patients who have adjuvant therapy.
Acknowledgements
None.
Ethical Statement: The study was approved by the Ethics Committee of West China Hospital (No. 201649).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. 10.3322/caac.20107 [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012;62:10-29. 10.3322/caac.20138 [DOI] [PubMed] [Google Scholar]
- 3.Zhou ZG, Gao XS, Qiao XY, et al. Literature analysis of radiotherapy for esophageal cancer in China. Chin J Cancer 2010;29:873-81. 10.5732/cjc.010.10165 [DOI] [PubMed] [Google Scholar]
- 4.Kelsen DP, Winter KA, Gunderson LL, et al. Long-term results of RTOG trial 8911 (USA Intergroup 113): a random assignment trial comparison of chemotherapy followed by surgery compared with surgery alone for esophageal cancer. J Clin Oncol 2007;25:3719-25. 10.1200/JCO.2006.10.4760 [DOI] [PubMed] [Google Scholar]
- 5.Okada N, Fujii S, Fujita T, et al. The prognostic significance of the positive circumferential resection margin in pathologic T3 squamous cell carcinoma of the esophagus with or without neoadjuvant chemotherapy. Surgery 2016;159:441-50. 10.1016/j.surg.2015.06.044 [DOI] [PubMed] [Google Scholar]
- 6.Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin 2005;55:74-108. 10.3322/canjclin.55.2.74 [DOI] [PubMed] [Google Scholar]
- 7.Markar SR, Gronnier C, Duhamel A, et al. Significance of Microscopically Incomplete Resection Margin After Esophagectomy for Esophageal Cancer. Ann Surg 2016;263:712-8. 10.1097/SLA.0000000000001325 [DOI] [PubMed] [Google Scholar]
- 8.Song S, Chie EK, Kim HJ, et al. Role of postoperative radiotherapy for microscopic margin involvement in the squamous cell carcinoma of esophagus. Cancer Res Treat 2013;45:202-9. 10.4143/crt.2013.45.3.202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morita M, Kumashiro R, Hisamatsu Y, et al. Clinical significance of salvage esophagectomy for remnant or recurrent cancer following definitive chemoradiotherapy. J Gastroenterol 2011;46:1284-91. 10.1007/s00535-011-0448-0 [DOI] [PubMed] [Google Scholar]
- 10.Khera R, Cram P, Vaughan-Sarrazin M, et al. Use of Mechanical Circulatory Support in Percutaneous Coronary Intervention in the United States. Am J Cardiol 2016;117:10-6. 10.1016/j.amjcard.2015.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lerut T, Moons J, Coosemans W, et al. Postoperative complications after transthoracic esophagectomy for cancer of the esophagus and gastroesophageal junction are correlated with early cancer recurrence: role of systematic grading of complications using the modified Clavien classification. Ann Surg 2009;250:798-807. 10.1097/SLA.0b013e3181bdd5a8 [DOI] [PubMed] [Google Scholar]
- 12.Harvin JA, Lahat G, Correa AM, et al. Neoadjuvant chemoradiotherapy followed by surgery for esophageal adenocarcinoma: significance of microscopically positive circumferential radial margins. J Thorac Cardiovasc Surg 2012;143:412-20. 10.1016/j.jtcvs.2011.10.044 [DOI] [PubMed] [Google Scholar]
- 13.Ninomiya I, Osugi H, Fujimura T, et al. Thoracoscopic esophagectomy with extended lymph node dissection in the left lateral position: technical feasibility and oncologic outcomes. Dis Esophagus 2014;27:159-67. 10.1111/dote.12071 [DOI] [PubMed] [Google Scholar]
- 14.Cao Z, Ye Q, Qian X, et al. End-to-end anastomosis after segmental esophagectomy for early stage cervical esophageal carcinoma. Ann Thorac Surg 2013;95:1815-7. 10.1016/j.athoracsur.2012.11.063 [DOI] [PubMed] [Google Scholar]
- 15.Watanabe M, Baba Y, Yoshida N, et al. Outcomes of preoperative chemotherapy with docetaxel, cisplatin, and 5-fluorouracil followed by esophagectomy in patients with resectable node-positive esophageal cancer. Ann Surg Oncol 2014;21:2838-44. 10.1245/s10434-014-3684-8 [DOI] [PubMed] [Google Scholar]
- 16.Valentí V, Fares S, Reynolds N, et al. Open and laparoscopic transhiatal oesophagectomy for cancer of the oesophagus: analysis of resection margins and lymph nodes. Cir Esp 2008;83:24-7. [DOI] [PubMed] [Google Scholar]
- 17.Qiu ZJ. Long-term results of patients with positive margin after resection of esophageal and gastric cardiac cancers. Zhonghua Zhong Liu Za Zhi 1990;12:366-7. [PubMed] [Google Scholar]
- 18.Zheng Z, Cai J, Yin J, et al. Transthoracic versus abdominal-transhiatal resection for treating Siewert type II/III adenocarcinoma of the esophagogastric junction: a meta-analysis. Int J Clin Exp Med 2015;8:17167-82. [PMC free article] [PubMed] [Google Scholar]
- 19.Gilbert S, Martel AB, Seely AJ, et al. Prognostic significance of a positive radial margin after esophageal cancer resection. J Thorac Cardiovasc Surg 2015;149:548-55; discussion 555. 10.1016/j.jtcvs.2014.10.040 [DOI] [PubMed] [Google Scholar]
- 20.Rice TW. Esophageal nightmare: cancer recurrence after definitive chemoradiation. Is salvage esophagectomy possible? Semin Thorac Cardiovasc Surg 2013;25:83-6. 10.1053/j.semtcvs.2013.05.003 [DOI] [PubMed] [Google Scholar]
- 21.Lin CS, Cheng CT, Liu CY, et al. Radical Lymph Node Dissection in Primary Esophagectomy for Esophageal Squamous Cell Carcinoma. Ann Thorac Surg 2015;100:278-86. 10.1016/j.athoracsur.2015.02.053 [DOI] [PubMed] [Google Scholar]


