Abstract
Background
Primary pulmonary artery sarcoma (PPAS) is a rare tumor that mimics pulmonary thromboembolism (PE). Similarities to PE can delay the diagnosis and misguide the treatment of PPAS. This study aimed to evaluate tumor characteristics and outcome predictors among those diagnosed with PPAS and misdiagnosed as PE.
Methods
From 1991–2010, 10 PPAS cases were available from the Cleveland Clinic (CC) institutional database and another 381 cases were reported in the literature. Patient characteristics, tumor subtypes, diagnostic testing & timing, interventions and clinical outcomes were analyzed. We also noted effects of misdiagnosis as PE and clinical outcome as a result of inappropriate intervention.
Results
Among 391 confirmed cases of PPAS, the mean age at diagnosis was 52±14 years; 55% were male. The median duration of symptoms prior to diagnosis was 100 [interquartile range (IQR), 30–210] days. Nearly half (47%) of PPAS were originally misdiagnosed as PE including 39% that received thrombolytic and/or anticoagulation therapy. For every doubling of time from symptom onset to diagnosis, the odds of death increased by 46% (OR: 1.46, 95% CI: 1.21–1.82; P<0.001). The odds of death (OR: 2.66, 95% CI: 1.58–4.54; P=0.0003) and occurrence of distant metastasis (OR: 2.30, 95% CI: 1.30–4.15; P=0.049) were increased among those who did not receive chemotherapy but chemotherapy did not impact local recurrence. Those with complete resection had a better survival.
Conclusions
PPAS has a radiological appearance similar to PE, which makes accurate and timely diagnosis challenging. More rapid diagnosis may lead to earlier, appropriate surgical treatment and improved outcomes, when combined with adjuvant treatment.
Keywords: Pulmonary artery sarcoma (PAS), pulmonary embolism
Introduction
Primary pulmonary artery sarcoma (PPAS) is an uncommon malignancy arising from the mesenchymal cells of the pulmonary artery (PA). Although PPAS is rare, the radiographic characteristics frequently simulate pulmonary thromboembolism (PE)—a relatively common condition affecting 112 in 100,000 US adults (1). PPAS causes an intraluminal filling defect of the PA that resembles PE on computed tomography angiography (CTA), magnetic resonance angiography (MRA), or transesophageal echocardiography (TEE) (Figure 1) (2). Aggressive, occlusive tumor growth within the PA predisposes to a poor prognosis, including right ventricular failure and sudden death. Without treatment, the survival of PPAS has been estimated to be as brief as six weeks (3).
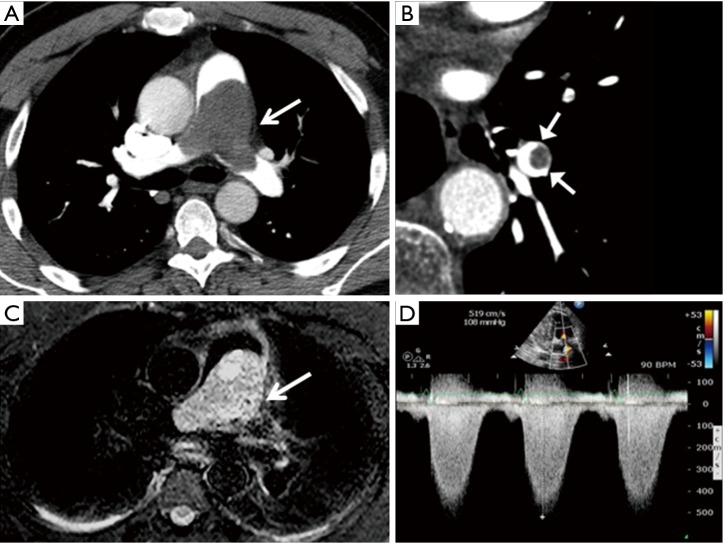
Figure 1.
Diagnostic features of primary pulmonary artery sarcoma (PPAS). (A) An axial section of computed tomography angiography (CTA) demonstrates subtotal obstruction of the main pulmonary artery. Despite the nearly occlusive nature of tumor, this young patient presented with a progressive history of dyspnea over 2–3 weeks; (B) extension of mass is observed within a secondary branch of the pulmonary artery; (C) magnetic resonance angiography (MRA) from the same patient demonstrates local tissue invasion; (D) continuous wave Doppler from transthoracic echocardiography confirming pulmonary hypertension provides an estimated right ventricular systolic pressure >100 mmHg prior to surgical resection.
While PPAS occurs less commonly than PE, misdiagnosis and underreporting is potentially important. In one series, 3–4% of patients discharged with chronic thromboembolic PE were later diagnosed with PPAS (4). Early surgical diagnosis and treatment may improve survival by avoiding treatment delays as well as unnecessary and potentially hazardous antithrombotic medications. Advances in imaging technology may improve diagnostic capability (5). Regardless, preemptive diagnosis remains challenging as the clinical presentation of PPAS is often overshadowed by the heightened awareness and much higher prevalence of PE.
The purpose of this analysis was to characterize the presenting features and natural history of PPAS as well as management strategies from our institution and the available literature. Secondly, we sought to determine those factors associated with outcome in this condition, including misdiagnosis as PE and inappropriate interventions as a result. We hypothesized that early accurate diagnosis may be associated with better outcomes, including survival.
Methods
Study design
The primary objective of this observational analysis was to perform a comprehensive characterization of patients with PPAS, including those from the literature and a cohort from the Cleveland Clinic (CC). Clinical features at diagnosis, histology types, treatment strategies, and long-term outcomes were recorded. The characteristics, which can help this condition to differentiate it from PE were also sought, as well as outcomes as a result of incorrect diagnosis.
Patient population
After Cleveland Clinic Institutional Review Board approval (IRB #11-642), we identified ten patients with biopsy-confirmed PPAS from 1991–2010 from our institutional pathology database. Data were retrospectively retrieved from the medical records of our institution. A literature search for case reports or case series of pulmonary artery sarcoma (PAS) was performed in OVID and MEDLINE using the keywords “Sarcoma”, “Pulmonary artery” and “Primary”. The search was limited to English language articles from 1991 to 2010. In some instances, more than one case reports and case series were published by the same author, from the same health center. We carefully adjudicated the duplicate reports so as to include individual cases only once. The resulting literature search provided 381 individual cases of PPAS among both case reports/series and clinical studies (see Supplementary for full list of references). In total, 391 cases of PPAS were included for this analysis.
Definitions and study variables
PPAS was represented by a diverse spectrum of histology within our population and among published reports. Thirteen unique histology types were reported. Among the literature cohort, 20 cases had no histology type reported.
Clinical features at the time of diagnosis included age, gender, and symptoms. Ten cases available in the literature had no reported presenting symptoms. The time from symptom onset to the diagnosis of PPAS was available among 207 patients (53%). Diagnostic modalities included bronchoscopic fine needle aspiration biopsy, surgical biopsy based upon diagnostic imaging or exploratory surgery. The exploratory surgery was often performed for presumed pulmonary embolism with anticipated thromboembolectomy.
The duration of follow-up after diagnosis was reported with 333 cases (85%). Vital status was available among 357 patients (91%). Other details of clinical outcomes included the presence of distant metastases (n=89) and local tumor recurrence (n=34). Distant metastasis was defined as tumor exhibiting non-contiguous spread outside of the PA to another non-adjacent organ. Local recurrence of tumor meant cancer recurred at or near the same site as the primary tumor after a period of time during which the cancer could not be detected.
The reported treatments included thrombolytic therapy, anticoagulation, definitive or exploratory surgery, palliative surgery, radiotherapy and chemotherapy. Details of surgical procedures were available in 353 patients, excluding those diagnosed at autopsy (20 patients). Surgical treatment compromised of partial resection or palliative debulking surgery, and definitive surgeries such as complete resection including concurrent pneumonectomy or lobectomy, thromboembolectomy and pulmonary endarterectomy. Information regarding adjunct oncologic treatment was available in 296 (80%) patients.
Statistical analysis
Continuous variables were summarized as mean with corresponding standard deviation or median with corresponding IQR. Comparisons of continuous data were performed with Welch two-sample t-test or Pearson’s product-moment correlation test. Categorical variables were summarized as percentages. Comparisons of categorical data were performed with chi-squared test or analysis of variance (ANOVA). Two binary covariates had missing data: receipt of anticoagulation prior to PPAS diagnosis (6.9% missing) and reported presumption of pulmonary embolism prior to PPAS diagnosis (7.2% missing). Missing data for these covariates were imputed to the null for multivariable analysis. The log-rank test was used for comparison between groups. Among the 357 cases with reported outcome, only 333 had duration of follow-up information available. Therefore, only these 333 cases were studied in survival and other time to event analysis.
Logistic regression was used to model associations between clinical factors and mortality. Baseline clinical characteristics, tumor histology, symptom type, symptom duration preceding diagnosis, diagnostic modality, and treatment status were considered in a comprehensive logistic regression model to estimate the odds of mortality. To avoid over-fitting of data, a stepwise model path with an analysis of deviance function (step AIC function) was used to select variables in a stepwise fashion (MASS package), whereby an initial model of 50 variables was simplified to a final model of 10 variables. A two-sided alpha level of 0.05 was used for all superiority testing. All data were analyzed and figures generated using R software (Version 2.13.1, The R Foundation for Statistical Computing, 2011) (6).
Results
Study population
Clinical characteristics by literature (n=381) and CC (n=10) data sources are summarized in Table 1. Characteristics of patients from both data sources were largely similar. Overall, 55% of the cohort was male. The median age at diagnosis was 52 (IQR, 41–62; range, 14–94) years. Comparing the two major data sources, there was no significant difference in median survival between those treated at CC [650 days (IQR, 274–2,353)] and cases obtained from the literature [510 days (IQR, 240–1,050)] (log-rank, P=0.42). Survival was not significantly influenced by gender (P=0.88).
Table 1. Presenting characteristics of patients diagnosed with pulmonary artery sarcoma.
| Patient characteristic | CCF (n=10) | Literature (n=381) | P value |
|---|---|---|---|
| Age (mean, SD) | 47.0 (11.3) | 51.7 (14.3) | 0.23 |
| Male gender | 8 (80%) | 206 (54%) | 0.12 |
| Histology type | |||
| Leiomyosarcoma | 1 (10%) | 79 (21%) | 0.69 |
| Spindle cell sarcoma | 2 (20%) | 59 (15%) | 0.66 |
| Fibrous histiocytoma | 0 | 44 (12%) | – |
| Undifferentiated | 0 | 45 (12%) | – |
| Angiosarcoma | 3 (30%) | 25 (7%) | 0.03 |
| Myxosarcoma | 0 | 23 (6%) | – |
| Myofibroblastic sarcoma | 1 (10%) | 20 (5%) | 0.43 |
| Osteosarcoma | 2 (20%) | 16 (4%) | 0.07 |
| Chondrosarcoma | 0 | 18 (5%) | – |
| Pleomorphic sarcoma | 0 | 11 (3%) | – |
| Fibrosarcoma | 0 | 10 (3%) | – |
| Rhabdomyosarcoma | 1 (10%) | 9 (2%) | 0.23 |
| Liposarcoma | 0 | 2 (1%) | – |
| Not reported | 0 | 20 (5%) | – |
| Symptom duration preceding diagnosis (n=207) (median days, IQR) | 33 [15–60] | 120 [35–240] | 0.009 |
| Duration of reported follow-up (n=333) (median days, IQR) | 650 [274–2,353] | 510 [240–1,050] | 0.25 |
| Vital status available (n=357) | 10 (100%) | 347 (91%) | 0.96 |
| Death reported during follow-up (n=199) | 5 (50%) | 194 (56%) | 0.96 |
| Time to death among cases with mortality (n=176) (median days, IQR) | 360 [180–645] | 528 [267–771] | 0.04 |
IQR, interquartile range.
Presenting symptoms
Presenting symptoms of PPAS are listed in Table S1. Shortness of breath was the most common presenting symptom (74%). Other symptoms included chest pain, cough, hemoptysis, weight loss, fatigue, dizziness, and fever. Clinical signs of digital clubbing, right heart failure or murmur were reported only in a minority of cases. Overall, the median duration of symptoms prior to diagnosis of tumor was 100 (IQR, 31–210) days among 207 patients with available data. A delay in diagnosis was associated with an increased risk of death. For every doubling in duration with undiagnosed symptoms, the odds of death increased by 46% (OR: 1.46, 95% CI: 1.21–1.82; P<0.001).
Diagnosis
A wide variety of diagnostic imaging modalities, including CTA, MRA, transthoracic echocardiogram (TTE), TEE, positron emission tomography (FDG-PET), and pulmonary angiography were used to evaluate patients (Table S1). FDG-PET scanning was utilized for diagnosis in 6% of cases; interestingly, two of these 21 patients had false-negative scans for tumor. Overall, PPAS was diagnosed at autopsy among 20 (5%) patients.
Exploratory thoracotomy leading to diagnosis of PPAS was performed among 50 (13%) patients; 13 of these patients died during follow-up. This observation was confirmed in multivariable regression analysis (Table 2). Patients diagnosed incidentally during exploratory surgery had a higher odds of death reported during follow-up (OR: 6.26, 95% CI: 1.67–27.9, P=0.010). Alternatively, 15% of patients were suspected to have a PA tumor by noninvasive imaging and were subsequently confirmed by surgical biopsy. Such patients suspected to have tumor preoperatively had a reduced odds of death (OR: 0.44, 95% CI: 0.19–0.95, P=0.041) (Table 2).
Table 2. Odds of mortality as estimated by multivariable logistic regression using clinical characteristics of diagnosis, clinical presentation, and treatment.
| Clinical characteristic | Odds of mortality | 95% CI | P value |
|---|---|---|---|
| Diagnostic modality | |||
| Surgical biopsy | 0.44 | 0.19–0.95 | 0.041 |
| Exploratory surgery | 6.26 | 1.67–27.9 | 0.010 |
| Thromboembolectomy (for presumed pulmonary embolism) | 1.48 | 0.85–2.60 | 0.17 |
| Symptoms | |||
| Chest pain | 1.56 | 0.96–2.57 | 0.073 |
| Signs | |||
| Right heart failure | 0.55 | 0.21–1.40 | 0.21 |
| Murmur | 0.33 | 0.08–1.14 | 0.09 |
| Digital clubbing | 7.69 | 1.26–149.5 | 0.06 |
| Treatment status | |||
| No chemotherapy | 2.66 | 1.58–4.54 | 0.0003 |
| Distant metastases reported | 2.30 | 1.30–4.15 | 0.005 |
| Recurrent tumor | 2.37 | 1.04–5.93 | 0.049 |
Histology types of PPAS
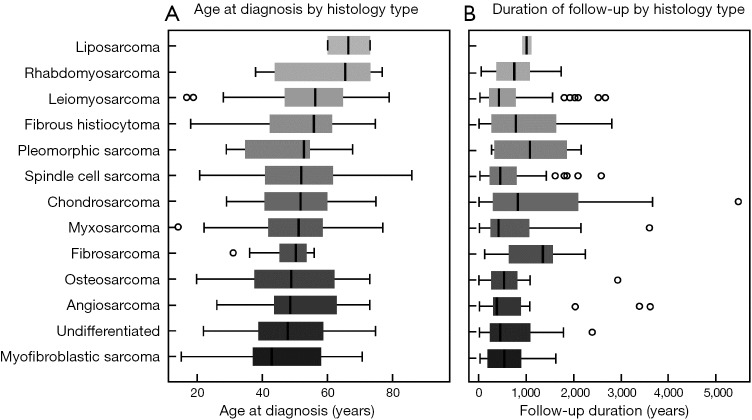
Of the 13 reported histopathology types, leiomyosarcoma (n=80) was the most common, accounting for 20% of all tumors (Table 1). The median age at presentation was lowest for myofibroblastic sarcoma and undifferentiated sarcoma at 43 and 48 years, respectively (Figure S1). Rhabdomyosarcoma and liposarcoma were associated with the oldest ages at presentation at 66 and 67 years (median), respectively.
The median time from symptom onset to diagnosis was 100 days (IQR, 31–210), although varied significantly across tumor types. Myofibroblastic sarcoma and leiomyosarcoma tended to present early after symptom onset at a median time of 40 and 43 days, respectively. Myxosarcoma had a much more latent presentation, with diagnosis occurring at a median 517 days. Following diagnosis, angiosarcoma, myxosarcoma, and leiomyosarcoma tended to have the shortest duration of follow-up (Figure S2). Gender was not associated with histology type (P=0.10). Among the literature cohort, 20 cases did not have histology type reported.
PPAS vis-à-vis PE
Diagnosis of PE commonly preceded a later confirmation of PPAS. Based on imaging or clinical criteria, 47% of patients were initially considered to have PE, including 39% that received thrombolytic and/or anticoagulation therapy. In fact, 22% underwent thromboembolectomy for presumed PE and 5% were diagnosed at autopsy who received treatment for PE. However, preoperative use of thrombolytic or anticoagulant medication, as with treatment for presumed PE, did not predict mortality in multivariate analysis (Table 2). Additionally, 45 (11%) subjects were diagnosed with chronic thromboembolic pulmonary hypertension (CTPEH) then later confirmed as having PPAS.
Treatment strategies of PPAS
The details of surgical procedure were available of 353 PPAS subjects, including those underwent thromboembolectomy (86 patients). Thirty-one (9%) patients did not undergo any surgery, mostly because of evidence of distant metastasis. Total 46 (13%) underwent partial resection or palliative debulking and 155 (44%) complete resection; in addition to pulmonary endarterectomy in 32 (9%) patients. Among those who underwent complete resection, 87 (56%) and 34 (22%) underwent concomitant pneumonectomy and lobectomy respectively. Three patients underwent Heart-Lung transplantation in order to achieve complete removal of PPAS. However two of them later died due to occurrence of distant metastasis (7,8).
Chemotherapy was reportedly used in 108 (28%) cases and included a variety of regimens. Among 90 cases, an anthracycline agent (i.e., doxorubicin, epirubicin, +/– cisplatin) was used with or without an alkylating agent (n=76) (i.e., dacarbazine, ifosfamide, cyclophosphamide). Other agents reported also included vincristine, paclitaxel, interleukin-2, mitomycin, methotrexate, gemcitabine, etoposide, and sunitinib.
Outcomes
Vital status was available among 357 cases (91%) at the time of case publication or follow-up. However, only 333 also had a reported duration of follow-up after diagnosis with a median survival of 528 (IQR, 240–1,050) days. As expected, survivors (n=157, 47%) had a significantly longer median follow-up duration [870 days (IQR, 360–1,560 days)] compared to those who died (n=176, 53%) [360 days (IQR, 180–660)] (P<0.001). In fact, with every doubling of follow-up duration the odds of death was reduced by 43% (OR: 0.57, 95% CI: 0.42–0.75).
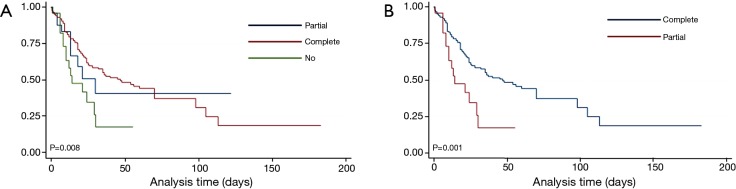
Patients who underwent definitive surgeries had a significantly improved survival compared to partial or no resection (P=0.008; Figure 2A). This survival difference was principally driven by the significant difference between patients with partial and complete resection (P=0.001; Figure 2B) in our analysis. The heart-lung transplantations, done to achieve complete cure, were unsuccessful because of later occurrence of distant metastasis.
Figure 2.
Estimated survival following surgical interventions in patients diagnosed with PA sarcoma. (A) Comparison of three groups of patients—who did not undergo any surgery against those who had a partial resection or debulking surgery and those who underwent a definitive surgery i.e., complete resection, pulmonary endarterectomy (P=0.008); (B) estimated survival in PA sarcoma patients following partial resection vis-à-vis complete resection (P=0.001).
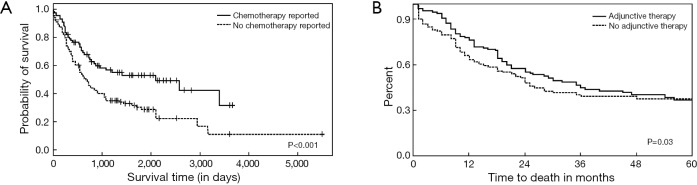
Patients lacking treatment involving chemotherapy were over 2.5-times more likely to die during follow-up (OR: 2.66, 95% CI: 1.58–4.54) (Table 2). Furthermore, those that received chemotherapy had a significantly improved median survival of 2100 (95% CI: 870–3,300) days versus 660 (95% CI: 540–840) days, respectively (P<0.001) (Figure S3).
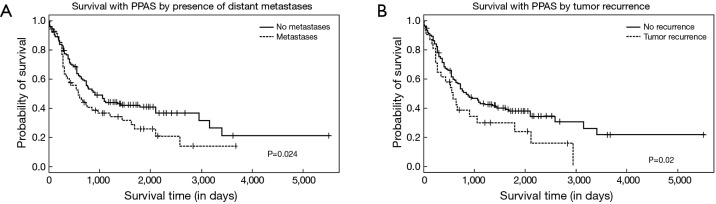
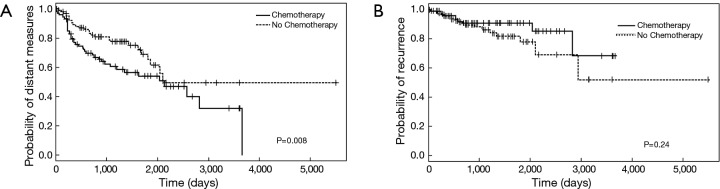
Distant metastases were noted in 89 patients (23%); their presence was associated with over two-time increase in mortality during follow-up (OR: 2.30, 95% CI: 1.30–4.15). Compared to those with metastases, those without metastatic disease had an improved median survival of 900 (95% CI: 720–1,410) days versus 570 (95% CI: 360–960) days, respectively (P=0.024) (Figure 3A). Local recurrence was reported in 34 cases (9%) and was associated with an over two-fold increase in mortality (OR: 2.37, 95% CI: 1.04–5.93). Compared to those with recurrence, those without tumor recurrence trended toward an improved median survival of 840 (95% CI: 720–1,110) days versus 570 (95% CI: 270–1,050) days, respectively (P=0.051) (Figure 3B). Those patients who did not receive chemotherapy had a significantly higher chance of having distant metastasis (P=0.008) (Figure 4A) but chemotherapy did not significantly impact local recurrence (P=0.24; Figure 4B).
Figure 3.
Estimated survival after diagnosis of PA sarcoma, stratified by presence of distant metastases (A) and by local tumor recurrence (B). PA, pulmonary artery.
Figure 4.
Probability of occurrence of distant metastasis (A) and local recurrence (B) in PA sarcoma patients stratified by chemotherapy. Chemotherapy significantly reduced the probability of distant metastasis but not the probability of local recurrence. PA, pulmonary artery.
Discussion
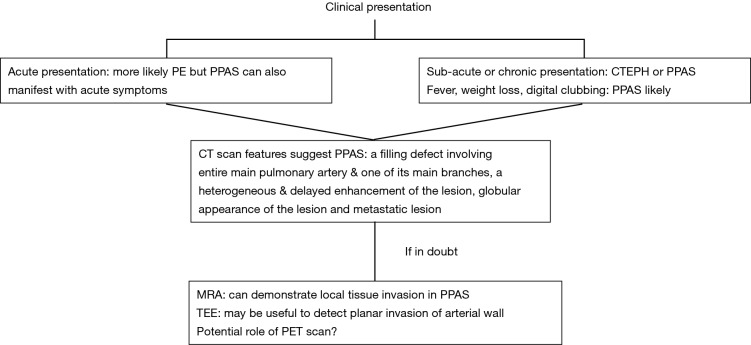
The first case of PPAS was described by Mandelstamm in 1923 (9). Since then, the narrative of PPAS remains limited to case reports and small case series owing to the ostensibly rare nature of this condition. Our analysis suggests that many of cases of PPAS share features similar to that of PE, including shortness of breath, chest pain, cough, and hemoptysis. Perhaps the greatest distinction between the two conditions is the duration of symptoms preceding diagnosis. The symptoms of PE are often dramatic and acute, whereas the median duration of symptoms for those diagnosed with PPAS was 100 days. This difference is highlighted in the dramatic obstruction of right ventricular outflow seen in Figure 1. A pronounced obstruction of the PA would likely result in profound hypotension or shock, particularly if developed acutely, as with saddle PE. As such an obstruction was hemodynamically tolerated; suspicion should be raised for an alternate diagnosis, including PPAS. However, given the chronicity of obstruction and elevated right ventricular outflow tract (RVOT) pressure, CTEPH is often considered as a possible diagnosis. We have created a flow-diagram illustrating these differentiating characteristics in Figure 5.
Figure 5.
Proposed flow-chart highlighting characteristics in clinical presentation and imaging work-up which may help to differentiate PPAS from PE. PPAS, primary pulmonary artery sarcoma; PE, pulmonary thromboembolism.
Average age of presentation has remained consistent in different series (10-12). While older patients tended to have a significantly shorter duration of symptoms, age was not a predictor of survival in PPAS. These results may indicate that older patients, relative to a younger group, tend to have their symptoms more aggressively evaluated, thereby facilitating a more rapid diagnosis. Younger subjects with greater duration of illness had higher mortality in our cohort. Although information bias (i.e., lead-time and length biases) may confound our analysis, these results may also suggest young patients enjoy the greatest benefit with timely diagnosis of PPAS.
The histological subtypes reported in current analysis differ slightly from those reported elsewhere (13). The histological sub-classification of sarcoma has evolved over time, with modifications to PPAS nomenclature modified by the World Health Organization (WHO) (14). We documented the histological patterns as reported. Nonetheless, in our study leiomyosarcoma was the most common histological type (20%) followed by spindle cell sarcoma; the two most frequent histopathology types described in the literature (2,15).
We noted several interesting features based on histological variants of PPAS. Liposarcoma had a tendency of occurring at an older age, whereas myofibroblastic variant presented at a younger age. Myxosarcoma and angiosarcoma histology type tended to have shorter duration of illness. Tavora and colleagues described improved survival in patients with myofibroblastic sarcoma and early diagnosis less than 40 years of age in a series of 43 patients, with only one patient having a chondrosarcoma (16). Additionally, in a small series by Huo and colleagues, leiomyosarcoma had the best survival and rhabdomyosarcoma had the worst prognosis (17). However, the association of histology type with mortality has not been consistently demonstrated across the studies (12,18). Similarly, we found no significant mortality association across histology types in our study.
PPAS was correctly suspected based on diagnostic imaging in only 36% of patients prior to attempted biopsy or surgical intervention. Accurate diagnosis by imaging led to directed surgical tissue confirmation and treatment with improved clinical outcomes. The majority misdiagnosed had potentially inappropriate interventions and delayed oncologic treatment. However, initial erroneous interventions, such as thrombolytic therapy/anticoagulation or planned thromboembolectomy for presumed PE, did not impact mortality by multivariable analysis. Nevertheless, our study showed that delayed diagnosis, even by days, may affect survival. Therefore, misidentification of PA tumor as PE may have significant clinical relevance.
Characteristic patterns of PPAS have been described based upon the CT scan appearance, which helps to distinguish it from PE or CTEPH. PPAS may be distinguished from PE as a filling defect involving the entirety of the main PA and one of its main branches, a heterogeneous and delayed enhancement of the lesion and globular appearance of the lesion, as seen in Figure 1 (10). PPAS may also be suspected based on an atypical or sometimes dramatic radiographic features inconsistent with PE, as with metastatic nodules in the lung, leading to the correct diagnosis. In other instances, the distinction between tumor and thrombus is less clear. Interestingly, 5% of patients were noted to have a concurrent burden of large thrombus surrounding the tumor on histopathology.
TTE aided in an initial correct diagnosis in 46 (12%) cases. TEE was a particularly helpful tool, able to identify planar invasion of tumor into the arterial wall. FDG-PET scan is a valuable tool differentiating the two conditions. It detected tumor in nearly all the subjects, yet two of the PPAS case had false negative studies. In our review, FDG-PET scan is infrequently used (5%). This is possibly due to the fact many of the cases reported before FDG-PET scan became widely available, more so due to higher propensity to diagnose PE in these patients. The PET has a very low sensitivity to detect PE, so may not be ideal screening tool, especially given its cost and availability (19). But having a high specificity, it helps to differentiate the two conditions, particularly if there is a concurrent clot burden around the tumor (20).
Interestingly, our analysis showed that adjunctive therapy did improve survival and occurrence of distant metastasis but did not affect local recurrence. To our knowledge, no previous study has looked into this association. We found, there is a wide variability in different chemotherapy regimens that the patients received. As a result, it was difficult to derive any conclusion, in terms if efficacy of individual chemotherapeutic drugs. Overall, we observed a significant association of mortality with subjects of younger age having a greater duration of illness. It is notable that the survival in our analysis is higher than described in earlier epidemiological analysis of this condition (3,5,10,11), presumably due to early detection of the tumor by more widespread use of imaging. More recently, Tavora and coworkers reported improved median survival in their PPAS cohort (16). The survival in the present review is comparable to their analysis but less than described by Blackmon and colleagues (13). This is due to many factors affecting survival including patient population and use of adjunctive therapy.
Limitations
The present study has several important limitations. This is a retrospective observational analysis obtained from several data sources in the literature spanning nearly 20 years. Reporting bias and differences in data collection certainly influence this analysis, although given the rare nature of PPAS these limitations are expected. Missing data on anticoagulation use and presumed PE, prior to confirmed PPAS diagnosis, required imputation for regression analysis.
Data was also incomplete regarding surgical intervention in 38 patients and in regards to adjunct oncologic treatment, 296 patients had this information available, which may have confounded the outcome analysis. We could not extract enough information regarding surgical resection techniques such as transmural resection and endoluminal desobliteration. The survival analysis in our study was based on those patients with complete follow up information, which raises a possibility of selection bias. Despite these limitations, this analysis remains the largest systematic analysis of the presentation and determinants of survival in this rare condition.
Conclusions
Our analysis shows, PPAS is commonly mistaken for pulmonary thromboembolic disease. Although misdiagnosis in itself does not increase risk of mortality, significant delay in diagnosis does appear to negatively impact outcome. PPAS should be considered in patients with atypical symptoms, inadequate response to thrombolytic therapy and a characteristic pattern on initial imaging. Although PPAS can be challenging to treat, tumors confirmed early and treated with surgery (complete resection) and chemotherapy was observed to have the best long-term outcomes in terms of survival as well as occurrence of distant metastasis.
Acknowledgements
We acknowledge the contribution of Clare Prendergast, MA in writing this manuscript.
Figure S1.
Age at diagnosis of pulmonary artery sarcoma, stratified by histology type—the median age at PPAS diagnosis was 52 (IQR, 41–62) years and ranged from 14–94 years. Overall, there was a non-significant relationship of tumor type by age. PPAS, primary pulmonary artery sarcoma; IQR, interquartile range.
Figure S2.
Duration of follow-up after diagnosis of pulmonary artery sarcoma-histology type and follow-up duration following diagnosis were reported among 333 PPAS cases. The various histology types are ordered by median follow-up duration. Outliers outside of the 95% confidence intervals are represented by open circles. PPAS, primary pulmonary artery sarcoma.
Figure S3.
Estimated survival after diagnosis of PA sarcoma, stratified by reported treatment with chemotherapy. PA, pulmonary artery.
Table S1. Presenting symptoms reported, clinical signs, and diagnostic modalities used among patients diagnosed with pulmonary artery sarcoma.
| Presenting symptoms/signs | No. reported (n) | Frequency reported (%) |
|---|---|---|
| Symptom | ||
| Shortness of breath | 288 | 74 |
| Chest pain | 123 | 31 |
| Cough | 89 | 23 |
| Hemoptysis | 57 | 15 |
| Weight loss | 41 | 10 |
| Fatigue | 38 | 10 |
| Dizziness | 33 | 8 |
| Fever | 13 | 3 |
| None reported | 10 | 3 |
| Clinical sign | ||
| Right heart failure | 22 | 6 |
| Murmur | 13 | 3 |
| Digital clubbing | 9 | 2 |
| Diagnostic modality | ||
| Computed tomography | 119 | 30 |
| Magnetic resonance imaging | 85 | 22 |
| Echocardiography | 46 | 12 |
| Positron emission tomography | 18 | 5 |
| Pulmonary angiography | 23 | 7 |
| Thromboembolectomy, presumed PE | 85 | 22 |
| Surgical biopsy, PAS suspected | 58 | 15 |
| Exploratory surgery | 50 | 13 |
| Autopsy | 20 | 5 |
| Modality not reported | 60 | 15 |
PE, pulmonary embolism; PAS, pulmonary artery sarcoma.
Ethical Statement: The study was approved by Cleveland Clinic Institutional Review Board (No. 11-642).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Wiener RS, Schwartz LM, Woloshin S. Time trends in pulmonary embolism in the United States: evidence of overdiagnosis. Arch Intern Med 2011;171:831-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Djordjevic I, Pejcic T, Rancic M, et al. Difficulties in establishing a timely diagnosis of pulmonary artery sarcoma misdiagnosed as chronic thrombo-embolic pulmonary disease: a case report. J Med Case Rep 2009;3:64. 10.1186/1752-1947-3-64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krüger I, Borowski A, Horst M, et al. Symptoms, diagnosis, and therapy of primary sarcomas of the pulmonary artery. Thorac Cardiovasc Surg 1990;38:91-5. 10.1055/s-2007-1014001 [DOI] [PubMed] [Google Scholar]
- 4.Jamieson SW, Auger WR, Fedullo PF, et al. Experience and results with 150 pulmonary thromboendarterectomy operations over a 29-month period. J Thorac Cardiovasc Surg 1993;106:116-26. [PubMed] [Google Scholar]
- 5.Austin BA, Griffin BP. Pulmonary artery intimal sarcoma: a brief case series. J Am Soc Echocardiogr 2008;21:978.e5-7. 10.1016/j.echo.2007.10.013 [DOI] [PubMed] [Google Scholar]
- 6.R Development Core Team. R: A language and environment for statistical computing. In: Computing RFfS. editor. Vienna, Austria, 2011. [Google Scholar]
- 7.Talbot SM, Taub RN, Keohan ML, et al. Combined heart and lung transplantation for unresectable primary cardiac sarcoma. J Thorac Cardiovasc Surg 2002;124:1145-8. 10.1067/mtc.2002.126495 [DOI] [PubMed] [Google Scholar]
- 8.Keel SB, Bacha E, Mark EJ, et al. Primary pulmonary sarcoma: a clinicopathologic study of 26 cases. Mod Pathol 1999;12:1124-31. [PubMed] [Google Scholar]
- 9.Mandelstamm M. Über primäre Neubildungen des Herzens. Virchows Arch 1923;245:43-54. 10.1007/BF01992097 [DOI] [Google Scholar]
- 10.Cox JE, Chiles C, Aquino SL, et al. Pulmonary artery sarcomas: a review of clinical and radiologic features. J Comput Assist Tomogr 1997;21:750-5. 10.1097/00004728-199709000-00018 [DOI] [PubMed] [Google Scholar]
- 11.Jin T, Zhang C, Feng Z, et al. Primary pulmonary artery sarcoma. Interact Cardiovasc Thorac Surg 2008;7:722-4. 10.1510/icvts.2008.177816 [DOI] [PubMed] [Google Scholar]
- 12.Parish JM, Rosenow EC, 3rd, Swensen SJ, et al. Pulmonary artery sarcoma. Clinical features. Chest 1996;110:1480-8. 10.1378/chest.110.6.1480 [DOI] [PubMed] [Google Scholar]
- 13.Blackmon SH, Rice DC, Correa AM, et al. Management of primary pulmonary artery sarcomas. Ann Thorac Surg 2009;87:977-84. 10.1016/j.athoracsur.2008.08.018 [DOI] [PubMed] [Google Scholar]
- 14.Fletcher CD. The evolving classification of soft tissue tumours: an update based on the new WHO classification. Histopathology 2006;48:3-12. 10.1111/j.1365-2559.2005.02284.x [DOI] [PubMed] [Google Scholar]
- 15.Mattoo A, Fedullo PF, Kapelanski D, et al. Pulmonary artery sarcoma: a case report of surgical cure and 5-year follow-up. Chest 2002;122:745-7. 10.1378/chest.122.2.745 [DOI] [PubMed] [Google Scholar]
- 16.Tavora F, Miettinen M, Fanburg-Smith J, et al. Pulmonary artery sarcoma: a histologic and follow-up study with emphasis on a subset of low-grade myofibroblastic sarcomas with a good long-term follow-up. Am J Surg Pathol 2008;32:1751-61. 10.1097/PAS.0b013e31817d7fd0 [DOI] [PubMed] [Google Scholar]
- 17.Huo L, Lai S, Gladish G, et al. Pulmonary artery angiosarcoma: a clinicopathologic and radiological correlation. Ann Diagn Pathol 2005;9:209-14. 10.1016/j.anndiagpath.2005.04.012 [DOI] [PubMed] [Google Scholar]
- 18.Burke AP, Virmani R. Sarcomas of the great vessels. A clinicopathologic study. Cancer 1993;71:1761-73. [DOI] [PubMed] [Google Scholar]
- 19.Le Roux PY, Robin P, Delluc A, et al. Performance of 18F fluoro-2-désoxy-D-glucose positron emission tomography/computed tomography for the diagnosis of venous thromboembolism. Thromb Res 2015;135:31-5. 10.1016/j.thromres.2014.10.008 [DOI] [PubMed] [Google Scholar]
- 20.Flavell RR, Behr SC, Brunsing RL, et al. The incidence of pulmonary embolism and associated FDG-PET findings in IV contrast-enhanced PET/CT. The incidence of pulmonary embolism and associated FDG-PET findings in IV contrast-enhanced PET/CT. Acad Radiol 2014;21:718-25. 10.1016/j.acra.2014.02.013 [DOI] [PubMed] [Google Scholar]
- Flaherty G, McCarthy P, Mortimer G. Pulmonary artery leiomyosarcoma: an unusual cause of shortness of breath. Ir J Med Sci 2011;180:275-8. 10.1007/s11845-009-0451-0 [DOI] [PubMed] [Google Scholar]
- Tueller C, Fischer Biner R, Minder S, et al. FDG-PET in diagnostic work-up of pulmonary artery sarcomas. Eur Respir J 2010;35:444-6. 10.1183/09031936.00114708 [DOI] [PubMed] [Google Scholar]
- Singla Long S, Johnson PT, Hruban RH, et al. CT features of pulmonary artery sarcoma: critical aid to a challenging diagnosis. Emerg Radiol 2010;17:153-5. 10.1007/s10140-009-0812-z [DOI] [PubMed] [Google Scholar]
- Scheidl S, Taghavi S, Reiter U, et al. Intimal sarcoma of the pulmonary valve. Ann Thorac Surg 2010;89:e25-7. 10.1016/j.athoracsur.2010.01.053 [DOI] [PubMed] [Google Scholar]
- Oberson M, Pawelczak CS, Meincke F. Paraneoplastic thrombus or relapse of a pulmonary artery sarcoma? Thorax 2010;65:941-2. 10.1136/thx.2009.124693 [DOI] [PubMed] [Google Scholar]
- Mendiz O, Lev G, Valdivieso L, et al. Lifesaving kissing stent for pulmonary trunk stenosis due to primary angiosarcoma. Ann Vasc Surg 2010;24:1135.e9-12. 10.1016/j.avsg.2010.05.018 [DOI] [PubMed] [Google Scholar]
- Koch A, Mechtersheimer G, Tochtermann U, et al. Ruptured pseudoaneurysm of the pulmonary artery--rare manifestation of a primary pulmonary artery sarcoma. Interact Cardiovasc Thorac Surg 2010;10:120-1. 10.1510/icvts.2009.219840 [DOI] [PubMed] [Google Scholar]
- Kim JB, Kim SH, Lim SY, et al. Primary angiosarcoma of the pulmonary trunk mimicking pulmonary thromboembolism. Echocardiography 2010;27:E23-6. 10.1111/j.1540-8175.2009.01059.x [DOI] [PubMed] [Google Scholar]
- Kaderli AA, Baran I, Sağ S, et al. A rare reason for pulmonary hypertension: primary sarcoma of the pulmonary artery. Heart Surg Forum 2010;13:E28-30. 10.1532/HSF98.20091123 [DOI] [PubMed] [Google Scholar]
- Ferentinos P, Rizos E, Christodoulou C, et al. Multiple pulmonary thromboembolism and severe depression. Gen Hosp Psychiatry 2010;32:560.e5-7. 10.1016/j.genhosppsych.2010.04.003 [DOI] [PubMed] [Google Scholar]
- Abul Y, Eryuksel E, Karakurt S, et al. A malign mesenchymal tumor (sarcoma) of the pulmonary artery presenting as a form of acute thromboembolism: educational case. Clin Respir J 2010;4:e1-3. 10.1111/j.1752-699X.2010.00186.x [DOI] [PubMed] [Google Scholar]
- Abunasser J, Colucci J, Bandyopadhyay T. Not pulmonary embolism! Conn Med 2009;73:277-80. [PubMed] [Google Scholar]
- Akomea-Agyin C, Dussek JE, Anderson DR, et al. Pulmonary artery sarcoma mimicking pulmonary embolism: successful surgical intervention. Ann Thorac Surg 1996;61:1536-8. 10.1016/0003-4975(95)01196-X [DOI] [PubMed] [Google Scholar]
- Akram K, Silverman ME, Voros S. A unique case of pulmonary artery leiomyosarcoma. J Natl Med Assoc 2006;98:1995-7. [PMC free article] [PubMed] [Google Scholar]
- Alsoufi B, Slater M, Smith PP, et al. Pulmonary artery sarcoma mimicking massive pulmonary embolus: a case report. Asian Cardiovasc Thorac Ann 2006;14:e71-3. 10.1177/021849230601400424 [DOI] [PubMed] [Google Scholar]
- Anderson MB, Kriett JM, Kapelanski DP, et al. Primary pulmonary artery sarcoma: a report of six cases. Ann Thorac Surg 1995;59:1487-90. 10.1016/0003-4975(95)00149-F [DOI] [PubMed] [Google Scholar]
- Athanassiadi K, Grothusen C, Mengel M, et al. Primary leiomyosarcoma of the pulmonary artery: Is aggressive treatment justified for a long survival? J Thorac Cardiovasc Surg 2006;132:435-6. 10.1016/j.jtcvs.2006.02.057 [DOI] [PubMed] [Google Scholar]
- Austin BA, Griffin BP. Pulmonary artery intimal sarcoma: a brief case series. J Am Soc Echocardiogr 2008;21:978.e5-7. 10.1016/j.echo.2007.10.013 [DOI] [PubMed] [Google Scholar]
- Bacha EA, Wright CD, Grillo HC, et al. Surgical treatment of primary pulmonary sarcomas. Eur J Cardiothorac Surg 1999;15:456-60. 10.1016/S1010-7940(99)00045-7 [DOI] [PubMed] [Google Scholar]
- Bakaeen FG, Jaroszewski DE, Rice DC, et al. Outcomes after surgical resection of cardiac sarcoma in the multimodality treatment era. J Thorac Cardiovasc Surg 2009;137:1454-60. 10.1016/j.jtcvs.2008.11.026 [DOI] [PubMed] [Google Scholar]
- Blackmon SH, Rice DC, Correa AM, et al. Management of primary pulmonary artery sarcomas. Ann Thorac Surg 2009;87:977-84. 10.1016/j.athoracsur.2008.08.018 [DOI] [PubMed] [Google Scholar]
- Bode-Lesniewska B, Zhao J, Speel EJ, et al. Gains of 12q13-14 and overexpression of mdm2 are frequent findings in intimal sarcomas of the pulmonary artery. Virchows Arch 2001;438:57-65. 10.1007/s004280000313 [DOI] [PubMed] [Google Scholar]
- Bressler EL, Nelson JM. Primary pulmonary artery sarcoma: diagnosis with CT, MR imaging, and transthoracic needle biopsy. AJR Am J Roentgenol 1992;159:702-4. 10.2214/ajr.159.4.1529830 [DOI] [PubMed] [Google Scholar]
- Burke AP, Virmani R. Sarcomas of the great vessels. A clinicopathologic study. Cancer 1993;71:1761-73. [DOI] [PubMed] [Google Scholar]
- Casullo J, Lisbona A, Palayew MJ. General case of the day. Primary sarcoma of the pulmonary artery (chondrosarcoma). Radiographics 1992;12:401-4. 10.1148/radiographics.12.2.1561430 [DOI] [PubMed] [Google Scholar]
- Choi EY, Yoon YW, Kwon HM, et al. A case of pulmonary artery intimal sarcoma diagnosed with multislice CT scan with 3D reconstruction. Yonsei Med J 2004;45:547-51. 10.3349/ymj.2004.45.3.547 [DOI] [PubMed] [Google Scholar]
- Chong S, Kim TS, Chung MP, et al. Unilateral usual interstitial pneumonia associated with sarcoma of the pulmonary artery. AJR Am J Roentgenol 2007;189:W221-3. 10.2214/AJR.05.1011 [DOI] [PubMed] [Google Scholar]
- Chong S, Kim TS, Kim BT, et al. Pulmonary artery sarcoma mimicking pulmonary thromboembolism: integrated FDG PET/CT. AJR Am J Roentgenol 2007;188:1691-3. 10.2214/AJR.05.0874 [DOI] [PubMed] [Google Scholar]
- Choong CK, Lawton JS, Moon MR, et al. Failure of medical therapy for pulmonary "thromboembolic" disease: beware the unsuspected primary sarcoma of the pulmonary artery. J Thorac Cardiovasc Surg 2004;128:763-5. 10.1016/j.jtcvs.2004.03.049 [DOI] [PubMed] [Google Scholar]
- Croitoru AG, Klein MJ, Galla JD, et al. Primary pulmonary artery leiomyosarcoma. Cardiovasc Pathol 2003;12:166-9. 10.1016/S1054-8807(02)00184-9 [DOI] [PubMed] [Google Scholar]
- Delany SG, Doyle TC, Bunton RW, et al. Pulmonary artery sarcoma mimicking pulmonary embolism. Chest 1993;103:1631-3. 10.1378/chest.103.5.1631 [DOI] [PubMed] [Google Scholar]
- Dennie CJ, Veinot JP, McCormack DG, et al. Intimal sarcoma of the pulmonary arteries seen as a mosaic pattern of lung attenuation on high-resolution CT. AJR Am J Roentgenol 2002;178:1208-10. 10.2214/ajr.178.5.1781208 [DOI] [PubMed] [Google Scholar]
- Dimitrakakis G, Zilidis G, Buchalter M, et al. Pulmonary artery sarcoma--a challenging diagnosis: a case report. Heart Surg Forum 2006;9:E897-9. 10.1532/HSF98.20061112 [DOI] [PubMed] [Google Scholar]
- Eng J, Murday AJ. Leiomyosarcoma of the pulmonary artery. Ann Thorac Surg 1992;53:905-6. 10.1016/0003-4975(92)91468-O [DOI] [PubMed] [Google Scholar]
- Disler L, Manga P. Primary leiomyosarcoma of the pulmonary trunk. Int J Cardiol 1992;35:412-4. 10.1016/0167-5273(92)90242-U [DOI] [PubMed] [Google Scholar]
- Djordjevic I, Pejcic T, Rancic M, et al. Difficulties in establishing a timely diagnosis of pulmonary artery sarcoma misdiagnosed as chronic thrombo-embolic pulmonary disease: a case report. J Med Case Rep 2009;3:64. 10.1186/1752-1947-3-64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dornas AP, Campos FT, Rezende CJ, et al. Intimal sarcoma of the pulmonary artery: a differential diagnosis of chronic pulmonary thromboembolism. J Bras Pneumol 2009;35:814-8. 10.1590/S1806-37132009000800015 [DOI] [PubMed] [Google Scholar]
- Dumont P, Diot P, Aupart MR, et al. Leiomyosarcoma of the pulmonary artery. Ann Thorac Surg 1998;66:2089-91. 10.1016/S0003-4975(98)01070-4 [DOI] [PubMed] [Google Scholar]
- Esaki M, Kagawa K, Noda T, et al. Primary cardiac leiomyosarcoma growing rapidly and causing right ventricular outflow obstruction. Intern Med 1998;37:370-5. 10.2169/internalmedicine.37.370 [DOI] [PubMed] [Google Scholar]
- Farsad M, Pernter P, Triani A, et al. Thromboembolism in pulmonary artery sarcoma. Clin Nucl Med 2009;34:239-40. 10.1097/RLU.0b013e31819a1f7b [DOI] [PubMed] [Google Scholar]
- Fegbeutel C, Strüber M, Becker JU, et al. Recurrent sarcoma originating from the pulmonary artery 6 years after extensive thoracic resection. J Thorac Cardiovasc Surg 2008;136:1093-5. 10.1016/j.jtcvs.2008.02.006 [DOI] [PubMed] [Google Scholar]
- Ferdinande B, Herijgers P, Debiec-Rychter M, et al. An unusual presentation of a tumour of the heart. Eur Heart J 2009;30:1942. 10.1093/eurheartj/ehp201 [DOI] [PubMed] [Google Scholar]
- Fernández-Golfín C, Escribano P, Cortina J, et al. Management of primary pulmonary artery sarcoma: experience of a single center. Angiology 2008;59:636-9. 10.1177/0003319707305981 [DOI] [PubMed] [Google Scholar]
- Flexman AM, Del Vicario G, Schwarz SK. Hemodynamic collapse under anesthesia in a patient with pulmonary artery sarcoma. Can J Anaesth 2009;56:604-8. 10.1007/s12630-009-9118-6 [DOI] [PubMed] [Google Scholar]
- Fujii H, Osako M, Otani H, et al. Primary pulmonary artery sarcoma. Jpn Circ J 1998;62:379-81. 10.1253/jcj.62.379 [DOI] [PubMed] [Google Scholar]
- Funabashi N, Komuro I. Images in clinical medicine. Heart-shaped tumor in pulmonary trunk. N Engl J Med 2005;352:608. 10.1056/NEJMicm040835 [DOI] [PubMed] [Google Scholar]
- Furest I, Marín M, Escribano P, et al. Intimal sarcoma of the pulmonary artery: a rare cause of pulmonary hypertension. Arch Bronconeumol 2006;42:148-50. [DOI] [PubMed] [Google Scholar]
- Gaumann A, Bode-Lesniewska B, Zimmermann DR, et al. Exploration of the APC/beta-catenin (WNT) pathway and a histologic classification system for pulmonary artery intimal sarcoma. A study of 18 cases. Virchows Arch 2008;453:473-84. 10.1007/s00428-008-0671-0 [DOI] [PubMed] [Google Scholar]
- George I, Shah JN, Bacchetta M, et al. Stentless bioprosthesis in a valved conduit: implications for pulmonary reconstruction. Ann Thorac Surg 2009;88:2022-4. 10.1016/j.athoracsur.2009.04.145 [DOI] [PubMed] [Google Scholar]
- Girard N, Triby-Moreau C, Benabidallah S, et al. Pulmonary artery sarcoma, a paradigm of orphan thoracic oncology. Presse Med 2009;38:1167-70. 10.1016/j.lpm.2008.12.026 [DOI] [PubMed] [Google Scholar]
- Goldblum JR, Rice TW. Epithelioid angiosarcoma of the pulmonary artery. Hum Pathol 1995;26:1275-7. 10.1016/0046-8177(95)90205-8 [DOI] [PubMed] [Google Scholar]
- Gómez-Caro A, Martinez E, Rodríguez A, et al. Cryopreserved arterial allograft reconstruction after excision of thoracic malignancies. Ann Thorac Surg 2008;86:1753-61; discussion 1761. [DOI] [PubMed]
- Govender D, Pillay SV. Right pulmonary artery sarcoma. Pathology 2001;33:243-5. 10.1080/00313020120038593 [DOI] [PubMed] [Google Scholar]
- Halank M, Jakob C, Kolditz M, et al. Intimal pulmonary artery sarcoma presenting as severe dyspnea and right heart insufficiency. Onkologie 2010;33:313-6. 10.1159/000313861 [DOI] [PubMed] [Google Scholar]
- Head HD, Flam MS, John MJ, et al. Long-term palliation of pulmonary artery sarcoma by radical excision and adjuvant therapy. Ann Thorac Surg 1992;53:332-4. 10.1016/0003-4975(92)91345-A [DOI] [PubMed] [Google Scholar]
- Heid F, Guth S, Mayer E, et al. Extracorporeal circulation and cardiac arrest in an awake patient: a safe approach for single lung pulmonary artery stenting? Ann Thorac Surg 2006;82:746-7. 10.1016/j.athoracsur.2005.11.065 [DOI] [PubMed] [Google Scholar]
- Henzler T, Gill IS, Krissak R, et al. Unexpected diagnosis of pseudoaneurysmal pulmonary artery sarcoma in a patient with acute chest pain. J Thorac Oncol 2009;4:1438-9. 10.1097/JTO.0b013e3181b4c480 [DOI] [PubMed] [Google Scholar]
- Herlihy JP, Loyalka P, Jayaraman G, et al. Extracorporeal membrane oxygenation using the TandemHeart System's catheters. Tex Heart Inst J 2009;36:337-41. [PMC free article] [PubMed] [Google Scholar]
- Hirose T, Ishikawa N, Hamada K, et al. A case of intimal sarcoma of the pulmonary artery treated with chemoradiotherapy. Intern Med 2009;48:245-9. 10.2169/internalmedicine.48.1560 [DOI] [PubMed] [Google Scholar]
- Hiroshima K, Uruma T, Ishibashi M, et al. A case of primary sarcoma of the pulmonary artery. Acta Pathol Jpn 1992;42:755-9. [DOI] [PubMed] [Google Scholar]
- Hou Y, Shen Z, Gao W, et al. Pulmonary artery intimal sarcoma: case report. J Card Surg 2010;25:29-31. 10.1111/j.1540-8191.2009.00926.x [DOI] [PubMed] [Google Scholar]
- Hu XP, Xu JP, Liu NN. Primary pulmonary artery sarcoma: surgical management and differential diagnosis with pulmonary embolism and pulmonary valve stenosis. J Card Surg 2009;24:613-6. 10.1111/j.1540-8191.2009.00853.x [DOI] [PubMed] [Google Scholar]
- Huang SS, Huang CH, Yang AH, et al. Images in cardiovascular medicine. Solitary pulmonary artery intima sarcoma manifesting as pulmonary embolism and subacute cor pulmonale. Circulation 2009;120:2269-70. 10.1161/CIRCULATIONAHA.109.899724 [DOI] [PubMed] [Google Scholar]
- Huo L, Lai S, Gladish G, et al. Pulmonary artery angiosarcoma: a clinicopathologic and radiological correlation. Ann Diagn Pathol 2005;9:209-14. 10.1016/j.anndiagpath.2005.04.012 [DOI] [PubMed] [Google Scholar]
- Huo L, Moran CA, Fuller GN, et al. Pulmonary artery sarcoma: a clinicopathologic and immunohistochemical study of 12 cases. Am J Clin Pathol 2006;125:419-24. 10.1309/9H8RHUV1JL1WE0QF [DOI] [PubMed] [Google Scholar]
- Huwer H, Ozbek C, Waldmann R, et al. Sarcoma of the pulmonary trunk and the main pulmonary arteries. J Surg Oncol 2008;97:476-8. 10.1002/jso.20962 [DOI] [PubMed] [Google Scholar]
- Ishiguro T, Kasahara K, Matsumoto I, et al. Primary pulmonary artery sarcoma detected with a pulmonary infarction. Intern Med 2007;46:601-4. 10.2169/internalmedicine.46.6292 [DOI] [PubMed] [Google Scholar]
- Ito K, Kubota K, Morooka M, et al. Diagnostic usefulness of 18F-FDG PET/CT in the differentiation of pulmonary artery sarcoma and pulmonary embolism. Ann Nucl Med 2009;23:671-6. 10.1007/s12149-009-0292-y [DOI] [PubMed] [Google Scholar]
- Iversen S, Hake U, Schmiedt W, et al. Resection of central primary pulmonary artery sarcoma. Eur J Cardiothorac Surg 1991;5:603-7. 10.1016/1010-7940(91)90228-C [DOI] [PubMed] [Google Scholar]
- Jin T, Zhang C, Feng Z, et al. Primary pulmonary artery sarcoma. Interact Cardiovasc Thorac Surg 2008;7:722-4. 10.1510/icvts.2008.177816 [DOI] [PubMed] [Google Scholar]
- Johansson L, Carlén B. Sarcoma of the pulmonary artery: report of four cases with electron microscopic and immunohistochemical examinations, and review of the literature. Virchows Arch 1994;424:217-24. 10.1007/BF00193503 [DOI] [PubMed] [Google Scholar]
- Kacl GM, Bruder E, Pfammatter T, et al. Primary angiosarcoma of the pulmonary arteries: dynamic contrast-enhanced MRI. J Comput Assist Tomogr 1998;22:687-91. 10.1097/00004728-199809000-00003 [DOI] [PubMed] [Google Scholar]
- Kanjanauthai S, Kanluen T, Ray C. Pulmonary artery sarcoma masquerading as saddle pulmonary embolism. Heart Lung Circ 2008;17:417-9. 10.1016/j.hlc.2007.08.003 [DOI] [PubMed] [Google Scholar]
- Kaplinsky EJ, Favaloro RR, Pombo G, et al. Primary pulmonary artery sarcoma resembling chronic thromboembolic pulmonary disease. Eur Respir J 2000;16:1202-4. 10.1183/09031936/00/16612020 [DOI] [PubMed] [Google Scholar]
- Kathiravel Y, Westwood D, Macemon J, et al. An international surgical collaboration for the management of pulmonary artery sarcoma: a New Zealand experience. N Z Med J 2007;120:U2609. [PubMed] [Google Scholar]
- Kim HK, Choi YS, Kim K, et al. Surgical treatment for pulmonary artery sarcoma. Eur J Cardiothorac Surg 2008;33:712-6. 10.1016/j.ejcts.2008.01.025 [DOI] [PubMed] [Google Scholar]
- Kim JH, Gutierrez FR, Lee EY, et al. Primary leiomyosarcoma of the pulmonary artery: a diagnostic dilemma. Clin Imaging 2003;27:206-11. 10.1016/S0899-7071(02)00568-5 [DOI] [PubMed] [Google Scholar]
- Komanapalli C, Alsoufi B, Shen I, et al. Recurrent pulmonary artery sarcoma. J Card Surg 2006;21:587-9. 10.1111/j.1540-8191.2006.00309.x [DOI] [PubMed] [Google Scholar]
- Kondo Y, Muto A, Nishibe T, et al. Primary pulmonary artery sarcoma: difficult differential diagnosis from chronic pulmonary thromboembolism. Ann Vasc Surg 2007;21:505-7. 10.1016/j.avsg.2006.08.007 [DOI] [PubMed] [Google Scholar]
- Kotooka N, Nagaya N, Tanaka R. Pulmonary artery sarcoma. Heart 2003;89:1388. 10.1136/heart.89.12.1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P, Cheah FK, Huang J, et al. A suspicious clot. Thorax 2009;64:1011. 10.1136/thx.2008.111211 [DOI] [PubMed] [Google Scholar]
- Lee S, Park IK, Cho SH, et al. Images in cardiovascular medicine. Leiomyosarcoma involving main and left pulmonary artery treated surgically with homograft replacement and concomitant left pneumonectomy. Circulation 2007;116:e559-61. 10.1161/CIRCULATIONAHA.107.698092 [DOI] [PubMed] [Google Scholar]
- Levy E, Korach A, Amir G, et al. Undifferentiated sarcoma of the pulmonary artery mimicking pulmonary thromboembolic disease. Heart Lung Circ 2006;15:62-3. 10.1016/j.hlc.2005.06.009 [DOI] [PubMed] [Google Scholar]
- Madu EC, Taylor DC, Durzinsky DS, et al. Primary intimal sarcoma of the pulmonary trunk simulating pulmonary embolism. Am Heart J 1993;125:1790-2. 10.1016/0002-8703(93)90780-D [DOI] [PubMed] [Google Scholar]
- Manso L, Alvarez E, Quintela M, et al. Primary pulmonary artery sarcoma: report of three cases and review of the literature. Clin Lung Cancer 2007;8:277-81. 10.3816/CLC.2007.n.007 [DOI] [PubMed] [Google Scholar]
- Maruo A, Okita Y, Okada K, et al. Surgical experience for the pulmonary artery sarcoma. Ann Thorac Surg 2006;82:2014-6. 10.1016/j.athoracsur.2006.07.018 [DOI] [PubMed] [Google Scholar]
- Mattoo A, Fedullo PF, Kapelanski D, et al. Pulmonary artery sarcoma: a case report of surgical cure and 5-year follow-up. Chest 2002;122:745-7. 10.1378/chest.122.2.745 [DOI] [PubMed] [Google Scholar]
- Mayer E, Kriegsmann J, Gaumann A, et al. Surgical treatment of pulmonary artery sarcoma. J Thorac Cardiovasc Surg 2001;121:77-82. 10.1067/mtc.2001.111423 [DOI] [PubMed] [Google Scholar]
- Mayer F, Aebert H, Rudert M, et al. Primary malignant sarcomas of the heart and great vessels in adult patients--a single-center experience. Oncologist 2007;12:1134-42. 10.1634/theoncologist.12-9-1134 [DOI] [PubMed] [Google Scholar]
- Mazzucco A, Luciani GB, Bertolini P, et al. Primary leiomyosarcoma of the pulmonary artery: diagnostic and surgical implications. Ann Thorac Surg 1994;57:222-5. 10.1016/0003-4975(94)90407-3 [DOI] [PubMed] [Google Scholar]
- Meckel S, Buitrago-Téllez C, Herrmann R, et al. Stenting for pulmonary artery stenosis due to a recurrent primary leiomyosarcoma. J Endovasc Ther 2003;10:141-6. 10.1177/152660280301000127 [DOI] [PubMed] [Google Scholar]
- Minakata K, Konishi Y, Matsumoto M, et al. Primary leiomyosarcoma of the pulmonary artery mimicking massive pulmonary thromboembolism. Jpn Circ J 2000;64:783-4. 10.1253/jcj.64.783 [DOI] [PubMed] [Google Scholar]
- Miura S, Meirmanov S, Nakashima M, et al. Intimal sarcoma of the pulmonary artery: report of an autopsy case. Pathol Res Pract 2005;201:469-74. 10.1016/j.prp.2005.06.001 [DOI] [PubMed] [Google Scholar]
- Nakahira A, Ogino H, Sasaki H, et al. Long-term survival of a pulmonary artery sarcoma produced by aggressive surgical resection and adjuvant chemoradiotherapy. Eur J Cardiothorac Surg 2007;32:388-90. 10.1016/j.ejcts.2007.04.019 [DOI] [PubMed] [Google Scholar]
- Nakajima J, Morota T, Matsumoto J, et al. Pulmonary intimal sarcoma treated by a left pneumonectomy with pulmonary arterioplasty under cardiopulmonary bypass: report of a case. Surg Today 2007;37:496-9. 10.1007/s00595-006-3432-1 [DOI] [PubMed] [Google Scholar]
- Noh HW, Park KJ, Sun JS, et al. Primary pulmonary malignant fibrous histiocytoma mimics pulmonary artery aneurysm with partial thrombosis: various radiologic evaluations. Eur Radiol 2008;18:1653-7. 10.1007/s00330-008-0922-0 [DOI] [PubMed] [Google Scholar]
- Okada K, Okada M, Yamamoto S, et al. Successful resection of a recurrent leiomyosarcoma of the pulmonary trunk. Ann Thorac Surg 1993;55:1009-12. 10.1016/0003-4975(93)90139-9 [DOI] [PubMed] [Google Scholar]
- Okano Y, Satoh T, Tatewaki T, et al. Pulmonary artery sarcoma diagnosed using intravascular ultrasound images. Thorax 1999;54:748-9. 10.1136/thx.54.8.748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omura A, Tobe S, Yoshida K, et al. Surgical treatment for recurrent pulmonary artery sarcoma. Gen Thorac Cardiovasc Surg 2008;56:28-31. 10.1007/s11748-007-0183-x [DOI] [PubMed] [Google Scholar]
- Ozbek C, Emrecan B, Calli AO, et al. Intimal sarcoma of the pulmonary artery with retrograde extension into the pulmonic valve and right ventricle. Tex Heart Inst J 2007;34:119-21. [PMC free article] [PubMed] [Google Scholar]
- Pagni S, Passik CS, Riordan C, et al. Sarcoma of the main pulmonary artery: an unusual etiology for recurrent pulmonary emboli. J Cardiovasc Surg (Torino) 1999;40:457-61. [PubMed] [Google Scholar]
- Panfeng X, Zheying Z, Jie W, et al. Successful diagnosis of pulmonary artery sarcoma by contrast-enhanced computed tomography. J Thorac Oncol 2008;3:772-3. 10.1097/JTO.0b013e31817c7428 [DOI] [PubMed] [Google Scholar]
- Parish JM, Rosenow EC, 3rd, Swensen SJ, et al. Pulmonary artery sarcoma. Clinical features. Chest 1996;110:1480-8. 10.1378/chest.110.6.1480 [DOI] [PubMed] [Google Scholar]
- Park BJ, Bacchetta M, Bains MS, et al. Surgical management of thoracic malignancies invading the heart or great vessels. Ann Thorac Surg 2004;78:1024-30. 10.1016/j.athoracsur.2004.02.043 [DOI] [PubMed] [Google Scholar]
- Penel N, Taieb S, Ceugnart L, et al. Report of eight recent cases of locally advanced primary pulmonary artery sarcomas: failure of Doxorubicin-based chemotherapy. J Thorac Oncol 2008;3:907-11. 10.1097/JTO.0b013e318180720d [DOI] [PubMed] [Google Scholar]
- Pewarchuk JA, Nassaralla CL, Midthun DE. A. 39-year-old woman with cough, chest pressure, and worsening dyspnea. Chest 2007;131:934-7. 10.1378/chest.06-1175 [DOI] [PubMed] [Google Scholar]
- Ramos SG, Salvatti LG, Cipriano FG, et al. Pulmonary artery sarcoma and chronic thromboembolism. Pathol Res Pract 2008;204:139-41. 10.1016/j.prp.2007.10.002 [DOI] [PubMed] [Google Scholar]
- Ramp U, Gerharz CD, Iversen S, et al. Sarcoma of the pulmonary artery: report of two cases and a review of the literature. J Cancer Res Clin Oncol 1992;118:551-6. 10.1007/BF01225272 [DOI] [PubMed] [Google Scholar]
- Rashid A, Molloy S, Lehovsky J, et al. Metastatic pulmonary intimal sarcoma presenting as cauda equina syndrome: first report of a case. Spine (Phila Pa 1976) 2008;33:E516-20. 10.1097/BRS.0b013e31817cf7ef [DOI] [PubMed] [Google Scholar]
- Salamat SM, Landy HJ, O'Sullivan MJ. Pulmonary artery sarcoma in a pregnant woman: report of a case. Obstet Gynecol 1994;84:668-9. [PubMed] [Google Scholar]
- Sandhu A, Yates TJ, Kuriakose P. Pulmonary artery sarcoma mimicking a pulmonary embolism. Indian J Cancer 2008;45:27-9. 10.4103/0019-509X.40643 [DOI] [PubMed] [Google Scholar]
- Scheffel H, Stolzmann P, Plass A, et al. Primary intimal pulmonary artery sarcoma: a diagnostic challenge. J Thorac Cardiovasc Surg 2008;135:949-50. 10.1016/j.jtcvs.2007.11.041 [DOI] [PubMed] [Google Scholar]
- Schuler PK, Weber A, Bode PK, et al. MRI of intimal sarcoma of the pulmonary arteries. Circ Cardiovasc Imaging 2009;2:e37-9. 10.1161/CIRCIMAGING.108.840793 [DOI] [PubMed] [Google Scholar]
- Shehatha J, Saxena P, Clarke B, et al. Surgical management of extensive pulmonary artery sarcoma. Ann Thorac Surg 2009;87:1269-71. 10.1016/j.athoracsur.2008.08.030 [DOI] [PubMed] [Google Scholar]
- Simpson WL, Jr, Mendelson DS. Pulmonary artery and aortic sarcomas: cross-sectional imaging. J Thorac Imaging 2000;15:290-4. 10.1097/00005382-200010000-00010 [DOI] [PubMed] [Google Scholar]
- Sogabe O, Ohya T. Right ventricular failure due to primary right ventricle osteosarcoma. Gen Thorac Cardiovasc Surg 2007;55:19-22. 10.1007/s11748-006-0053-y [DOI] [PubMed] [Google Scholar]
- Stella F, Davoli F, Brandolini J, et al. Pulmonary artery leiomyosarcoma successfully treated by right pneumonectomy. Asian Cardiovasc Thorac Ann 2009;17:513-5. 10.1177/0218492309348631 [DOI] [PubMed] [Google Scholar]
- Talbot SM, Taub RN, Keohan ML, et al. Combined heart and lung transplantation for unresectable primary cardiac sarcoma. J Thorac Cardiovasc Surg 2002;124:1145-8. 10.1067/mtc.2002.126495 [DOI] [PubMed] [Google Scholar]
- Tanaka H, Hasegawa S, Egi K, et al. Thorac Cardiovasc Surg 2001;122:1039-40. 10.1067/mtc.2001.116196 [DOI] [PubMed] [Google Scholar]
- Tanaka I, Masuda R, Inoue M, et al. Primary pulmonary-artery sarcoma. Report of a case with complete resection and graft replacement, and review of 47 surgically treated cases reported in the literature. Thorac Cardiovasc Surg 1994;42:64-8. 10.1055/s-2007-1016459 [DOI] [PubMed] [Google Scholar]
- Tavora F, Miettinen M, Fanburg-Smith J, et al. Pulmonary artery sarcoma: a histologic and follow-up study with emphasis on a subset of low-grade myofibroblastic sarcomas with a good long-term follow-up. Am J Surg Pathol 2008;32:1751-61. 10.1097/PAS.0b013e31817d7fd0 [DOI] [PubMed] [Google Scholar]
- Terra RM, Fernandez A, Bammann RH, et al. Pulmonary artery sarcoma mimicking a pulmonary artery aneurysm. Ann Thorac Surg 2008;86:1354-5. 10.1016/j.athoracsur.2008.04.005 [DOI] [PubMed] [Google Scholar]
- Timmers L, Bové T, De Pauw M. Intimal sarcoma of the pulmonary artery: a report of two cases. Acta Cardiol 2009;64:677-9. 10.2143/AC.64.5.2042701 [DOI] [PubMed] [Google Scholar]
- Totaro M, Miraldi F, Ghiribelli C, et al. Cardiac angiosarcoma arising from pulmonary artery: endovascular treatment. Ann Thorac Surg 2004;78:1468-70. 10.1016/S0003-4975(03)01502-9 [DOI] [PubMed] [Google Scholar]
- Tschirch FT, Del Grande F, Marincek B, et al. Angiosarcoma of the pulmonary trunk mimicking pulmonary thromboembolic disease. A case report. Acta Radiol 2003;44:504-7. [DOI] [PubMed] [Google Scholar]
- Tsunezuka Y, Oda M, Takahashi M, et al. Primary chondromatous osteosarcoma of the pulmonary artery. Ann Thorac Surg 2004;77:331-4. 10.1016/S0003-4975(03)00761-6 [DOI] [PubMed] [Google Scholar]
- Uchida A, Tabata M, Kiura K, et al. Successful treatment of pulmonary artery sarcoma by a two-drug combination chemotherapy consisting of ifosfamide and epirubicin. Jpn J Clin Oncol 2005;35:417-9. 10.1093/jjco/hyi106 [DOI] [PubMed] [Google Scholar]
- Uemura S, Watanabe M, Iwama H, et al. Extensive primary cardiac liposarcoma with multiple functional complications. Heart 2004;90:e48. 10.1136/hrt.2004.036707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varriale P, Chryssos B. Pulmonary artery sarcoma: another cause of sudden death. Clin Cardiol 1991;14:160-4. 10.1002/clc.4960140213 [DOI] [PubMed] [Google Scholar]
- Viana-Tejedor A, Mariño-Enríquez A, Sánchez-Recalde A, et al. Intimal sarcoma of the pulmonary artery: diagnostic value of different imaging techniques. Rev Esp Cardiol 2008;61:1363-5. 10.1016/S0300-8932(08)75752-X [DOI] [PubMed] [Google Scholar]
- Widera E, Sulica R. Pulmonary artery sarcoma misdiagnosed as chronic thromboembolic pulmonary hypertension. Mt Sinai J Med 2005;72:360-4. [PubMed] [Google Scholar]
- Yamada N, Kamei S, Yasuda F, et al. Primary leiomyosarcoma of the pulmonary artery confirmed by catheter suction biopsy. Chest 1998;113:555-6. 10.1378/chest.113.2.555 [DOI] [PubMed] [Google Scholar]
- Yamada N, Minato N, Ikeda K, et al. Surgical treatment of primary pulmonary artery tumor: two cases of malignant fibrous histiocytoma and leiomyosarcoma. Jpn J Thorac Cardiovasc Surg 2003;51:557-61. 10.1007/s11748-003-0124-2 [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Yamamoto F, Ishibashi K, et al. Primary sarcoma of the right ventricle: surgical and adjuvant therapy. Gen Thorac Cardiovasc Surg 2009;57:421-5. 10.1007/s11748-009-0403-7 [DOI] [PubMed] [Google Scholar]
- Yokoi S, Iizasa T, Hiroshima K, et al. Pulmonary artery leiomyosarcoma expressing Epstein-Barr virus in an immunocompetent individual. Ann Thorac Surg 2006;81:1897-9. 10.1016/j.athoracsur.2005.05.083 [DOI] [PubMed] [Google Scholar]
- Zerkowski HR, Hofmann HS, Gybels I, et al. Primary sarcoma of pulmonary artery and valve: multimodality treatment by chemotherapy and homograft replacement. J Thorac Cardiovasc Surg 1996;112:1122-4. 10.1016/S0022-5223(96)70120-4 [DOI] [PubMed] [Google Scholar]