Abstract
Background
Thymic epithelial tumors (TET) are rare neoplasms with inconsistent treatment strategies. When researching for molecular pathways to find new therapies, the correlation between specific molecular markers and outcome has only rarely been investigated. The aim of this study was to investigate the correlation between survival, metastatic potential and invasiveness of aggressive subtypes of TET and immunohistochemical markers.
Methods
Overall survival (OS), disease-free survival (DFS), progression-free survival (PFS) and metastasis-free survival (MFS) of patients with WHO type B2/B3 mixed type thymoma (MT), thymoma type B3 (B3) and thymic carcinoma (TC), undergoing surgery [1998–2013] were determined. Tumor specimens were stained using a tissue microarray (TMA) (CD117, CD5, p63, p40, p21, p27, p53, Bcl-2, Ki67, podoplanin, synaptophysin, PTEN and Pax8). Invasive behavior of primary tumors and the presence of extrathoracic metastases were assessed.
Results
We found in 23 patients included into this study (four MT, ten B3, nine TC) that (I) p21 expression in the cytoplasm significantly correlated with a decrease of OS (P=0.016), PFS (P=0.034) and MFS (P=0.005); (II) MFS was significantly shorter when the combination of p21-low p27-low p53-high was present (P=0.029); and (III) nuclear p27 (P=0.042), Ki-67 (P=0.024) and podoplanin (P=0.05) expression correlated with the presence of extrathoracic metastases.
Conclusions
The main finding of this study is that cytoplasmic p21 expression negatively influences the outcome of malignant TETs and correlates with metastatic activity. Additionally, selected immunohistochemical markers correlate with the distant metastatic potential of TETs. These results may contribute to the stratification of diagnosis and improvement of treatment strategies for thymic malignancies.
Keywords: Thymoma, thymic carcinoma (TC), immunohistochemistry, metastases
Introduction
Thymic epithelial tumors (TET) are rare neoplasms arising from epithelial cells of the thymus whose cause of development remains unknown until today (1). Due to the rarity of TETs, treatment strategy is not yet standardized and mostly based on retrospective reviews (1-3). The pathological classification of TETs has been the subject of discussion over many years. The revised World Health Organization (WHO) classification and the staging system according to Masaoka with modifications by Koga et al. are the ones commonly employed and have been shown to be of prognostic relevance (4). The WHO classification stratifies TETs into six entities according to the morphology of the tumor cells and the quantity of non-tumoral lymphocytes: A, AB, B1, B2, B3 and thymic carcinoma (TC). TCs are further classified into several subtypes including squamous cell carcinoma, basaloid carcinoma, mucoepidermoid carcinoma and neuroendocrine carcinoma. TCs account for 15–20% of TETs, and are aggressive tumors with frequent invasion into adjacent organs and metastatic spread, mostly into lung, bone and liver (5). All thymoma subtypes can present in advanced stages and exhibit malignant behavior, although some of them more frequently (6,7). The Masaoka-Koga staging system indicates the invasiveness of the tumor, TETs with stage I being completely encapsulated and with stage IVB showing lymphatic or hematogenous metastasis (8). The prognosis of TETs is variable with a 5- and 10-year overall survival (OS) of 95% and 91% for thymoma and 60% and 40% for TC, respectively (9,10).
In the past few years, the molecular pathways involved in the development of TETs have been extendedly researched. The growing knowledge about tumor biology has led to clinical trials testing molecularly targeted therapies, most of them with disappointing results (1,11). Tumorigenesis is a result of genetic changes and abnormal protein expression in the cell. These molecular alterations may be visible with genomic analysis or immunohistochemical staining. Several studies have investigated the diagnostic relevance of molecular markers, but only few have analyzed the influence on invasion and metastasis (12-17). Also, many researchers excluded patients with TCs or aggressive forms of thymoma with metastatic spread (4,10,16,18,19).
The prediction of the clinical behavior of TETs in biopsies or surgical specimens is of clinical relevance, especially for planning individualized therapeutic options. The aim of this study therefore was to investigate the prognostic value of a versatile panel of protein markers in a relatively large number of patients with aggressive forms of TET, with special attention to invasiveness, metastatic potential and survival.
Methods
Patients and TET specimens
For this retrospective study, we perused the clinical records of all patients who had surgery for WHO type B2/B3 mixed type thymoma (MT), thymoma type B3 (B3) and TC between 1998 and 2013 at our clinic. MTs were only included if the tumors contained more than 50% type B3, in order to filter aggressive specimens of this group. Tumor size was measured as the greatest diameter of the largest lesion in a CT or PET/CT scan before surgery, or retrieved from the gross pathological report of the resected tumor specimen. The study was approved by the institutional review board and the local ethics committee (KEK ZH 29-2009). Data on gender, age, symptoms, presence of paraneoplastic syndromes, preoperative biopsy, tumor size, treatment modality, invasion of surrounding organs, completeness of resection, metastatic spread and follow-up information (recurrence, progression, death) of these patients were obtained.
All cases were pathologically reviewed and subtyped according to the revised WHO classification and Masaoka-Koga staging system by a pathologist experienced in intrathoracic tumors (A Soltermann) (5-8). Complete resection (R0) was defined as macroscopically and microscopically clear surgical margins, incomplete resections were classified as either R1 (microscopically positive margins) or R2 (grossly residual tumor). Mainly, primary tumor specimens were statistically analyzed, tumor specimens of recurrent or metastatic disease were, if available, analyzed separately. The patients were followed up as long as possible, censored on January 20, 2015, and if information was missing, the patient or his general practitioner was contacted for additional information.
Construction of tissue microarrays (TMAs) and immunohistochemical staining
A set of three TMAs with quadruple punches per specimen was prepared with a semiautomatic tissue arrayer (Beecher Instruments, Sun Prairie, WI, USA) as previously described (20). TMA blocks were sectioned and stained with H&E (hematoxylin and eosin) for morphologic assessment. The used antibodies and detection kits are listed in Table 1. All stainings were performed with automated staining instruments (Ventana BenchMark, Ventana, Tucson, AZ, USA) and according to vendor’s instructions. Negative and positive controls were included in all studies.
Table 1. Used antibodies and detection kits for immunohistochemical staining.
| Antibody specificity | Clone | Company | Dilution | Detection kit |
|---|---|---|---|---|
| CD117: polyclonal rabbit anti-human c-kit | Polyclonal | Dako A/S | 1:100 | OptiViewKit |
| CD5: mouse anti-human CD5 | 4C7 | Novocastra | 1:20 | OptiViewKit |
| p63: mouse anti-p63 | 4A4 | Ventana | Prediluted | OptiViewKit |
| p40: mouse anti-p40 | Polyclonal | Zytomed Systems | 1:50 | OptiViewKit |
| p21 (C-19): rabbit-anti-p21 | Polyclonal | Santa Cruz Biotechnology, Inc. | 1:50 | ChromoMapKit |
| p27: rabbit anti-p27 | Polyclonal | Santa Cruz Biotechnology, Inc. | 1:60 | OptiViewKit |
| p53: mouse anti-human p53 protein | DO-7 | Dako A/S | 1:80 | OptiViewKit |
| Bcl-2: mouse anti-Bcl-2 | 124 | Ventana | Prediluted | OptiViewKit |
| Ki-67: rabbit anti-Ki-67 | 30-9 | Ventana | Prediluted | OptiViewKit |
| Podoplanin: mouse anti-human podoplanin | D2-40 | Dako A/S | 1:50 | OptiViewKit |
| Synaptophysin: mouse anti-synaptophysin | 27G12 | Novocastra | 1:50 | OptiViewKit |
| PTEN: rabbit anti-PTEN | D4.3 | Cell Signaling Technology | 1:50 | UltraViewKit |
| Pax8: rabbit anti-paired box 8 | Polyclonal | Protein Tech Group, Inc. | 1:400 | OptiViewKit |
The evaluation of the TMAs was conducted in a blinded manner by using an established pathological scoring system (H-score): intensity of staining was scored as 0= negative, 1= weak, 2= moderate or 3= strong, and for each intensity, the frequency was indicated in percent (in steps of 10). The H-score was then calculated as the sum of: 1× frequency of weak staining +2× frequency of moderate staining +3× frequency of strong staining. The score per tumor tissue sample was calculated as the average of the four punches and could range between 0 and 300. No H-score was calculated for CD117, podoplanin and synaptophysin; they were simply scored as either positive (at least five tumor cells per visual field) or negative. Proliferative activity was assessed as total proliferation index by counting Ki-67 positive cells in three representative visual fields (containing approximately 100–200 cells each) in percent. p21, p27 and Pax8 were evaluated separately for nuclear and cytoplasmic staining, whereas for p53, p63, p40 and Ki-67 nuclear staining, for Bcl-2, PTEN and synaptophysin cytoplasmic staining, and for CD117, CD5 and podoplanin membranous staining was considered positive. Low staining of p21 and p27 was defined as staining of less than 5% of tumor cell nuclei, high staining of p53 as staining of more than 5% of tumor cell nuclei as described elsewhere (18,19).
Statistical analysis
Primary endpoint of this study was OS, due to the otherwise low number of patients and resulting low statistical power. Secondary endpoints were disease-free survival (DFS), progression-free survival (PFS), metastasis-free survival (MFS), invasion and extrathoracic metastasis. OS was defined as the date of diagnosis until death of any cause, DFS was calculated from the date after successful treatment (date of complete resection or date at the end of adjuvant therapy after R0 resection) until clinical or pathological evidence of recurrence of the tumor, and PFS was defined as the time from the beginning of the treatment until progression of the tumor (according to RECIST criteria) or death for patients with incomplete resection status. MFS was defined as the date of diagnosis until the occurrence of radiologically suspected or histologically confirmed metastases. Patients with no event were censored at the last date of follow-up.
All data were statistically analyzed using IBM SPSS Version 22 for Windows (SPSS Inc., Chicago, IL, USA). Impact on survival and on development of metastasis was analyzed using the Kaplan-Meier Method with the Log rank test for comparison of the groups, and the Cox regression for scale variables. Correlations among clinical parameters and staining patterns, as well as among different staining patterns themselves were made using the Spearman Rank correlation, the Mann-Whitney-U test and cross tabulations (with Fisher’s exact test). A P value of less than 0.05 was considered statistically significant, a P value of less than 0.1 defined as trend towards significance or marginal significance.
Results
Patient characteristics
A total number of 34 patients with MT, B3 and TC were included in this study. Due to incomplete clinical data or pathological material, or predominantly type B2 MT, eleven patients were excluded, thus 23 patients were left for statistical analysis. For one patient, only the tumor specimen of the recurrent disease (after radiotherapy) could be used for pathological evaluation and immunohistochemical staining, because the primary surgery was in 1984 and pathological material not available anymore. The treatment of all other patients was naïve, except for two patients being treated either with cortisone and azathioprine or cortisone and pyridostigmine. The clinical data is summarized in Table 2. The histological subtypes of TC included squamous cell carcinoma (n=4), basaloid carcinoma (n=3), and neuroendocrine carcinoma (n=2). Paraneoplastic syndromes were diagnosed in patients with MT or B3 only. All patients except two were treated with surgical excision of the tumor: 16 patients with resection of the thymus (thymectomy) and five with resection of the intrathymic tumor only, meaning that part of the thymus was left in the patient. The tumor specimens of the remaining two patients were biopsied and then treated with palliative chemotherapy in one patient and radiotherapy in the other patient. Eleven patients received extended surgery: pleurectomy was performed in three patients, complete pneumonectomy in two, wedge resection of lung tissue in eight and partial resection and reconstruction of great vessels in five patients. Selected cases additionally received neoadjuvant or adjuvant chemotherapy and/or radiotherapy.
Table 2. Patient and disease characteristics, and treatment modalities.
| Characteristics | Median/n (%) | IQR/n (%) | NA |
|---|---|---|---|
| Age at diagnosis | 49 years | IQR 17 years | |
| Gender | Male 14 (60.9) | Female 9 (39.1) | |
| Smoking history | Yes 10 (43.5) | No 13 (56.5) | |
| Range of pack years | 3–70 | ||
| Tumor size at diagnosis | 68.5 mm | IQR 55.5 mm | |
| Paraneoplastic syndrome | Yes 8 (34.8) | No 15 (65.2) | |
| Myasthenia gravis | 8 | ||
| Good syndrome | 1 | ||
| Positive ANA, anti ds-DNA, anti Scl-70 antibodies | 1 | ||
| WHO type | |||
| B2/B3 | 4 (17.4) | ||
| B3 | 10 (43.5) | ||
| C | 9 (39.1) | ||
| Masaoka-Koga stage | 1 | ||
| IIA | 8 (34.8) | ||
| IIB | 3 (13.0) | ||
| III | 5 (21.7) | ||
| IVA | 3 (13.0) | ||
| IVB | 3 (13.0) | ||
| Preoperative biopsy | Yes 8 (34.8) | No 14 (60.9) | 1 |
| Neoadjuvant chemotherapy | Yes 4 (17.4) | No 19 (82.6) | |
| ADOC | 2 | ||
| PAC | 2 | ||
| Surgical method | 1 | ||
| Open | 12 (52.2) | ||
| Minimally invasive | 7 (30.4) | ||
| Combined | 3 (13.0) | ||
| Surgical procedure | |||
| Thymectomy | 16 (69.6) | ||
| Resection of intrathymic tumor | 5 (21.7) | ||
| Biopsy | 2 (8.7) | ||
| Residual disease | |||
| R0 | 12 (52.2) | ||
| R1 | 8 (34.8) | ||
| R2 (including biopsy) | 3 (13.0) | ||
| Intraoperatively observed macroscopic invasion | Yes 14 (60.9) | No 7 (30.4) | 2 |
| Lung | 8 | ||
| Pleura | 6 | ||
| Pericardium | 8 | ||
| Vessels | 6 | ||
| Metastatic spread | Yes 13 (56.5) | No 10 (43.5) | |
| Pleura | 8 | ||
| Lung | 8 | ||
| Lymph nodes | 8 | ||
| Bone | 2 | ||
| Brain | 1 | ||
| Adjuvant therapy | Yes 13 (56.5) | No 10 (43.5) | |
| Chemotherapy | 1 | ||
| Radiotherapy | 12 |
IQR, interquartile range; NA, not assessable; ADOC, cisplatin doxorubicin vincristine cyclophosphamide; PAC, cisplatin doxorubicin cyclophosphamide ; ANA, anti-nuclear antibody; anti ds-DNA, anti-double stranded DNA antibody; anti Scl-70, anti-topoisomerase I antibody.
Immunohistochemistry (IHC) of CD117, CD5, p63 and p40
CD117 and CD5 support the diagnosis of TC, therefore may predict a worse prognosis, whereas in thymoma they are mostly negative (12). p63 has been described as a useful marker for thymic epithelial cells and is involved in the survival and differentiation of epithelial cells. p40 reacts only with three of the six isoforms of p63 (21,22).
CD117 staining was evaluable in 19 specimens, whereof seven were positive, all of which were TCs. CD5 was expressed in all tumor specimens (20/20) with a median H-score of 168.75 [interquartile range (IQR) 88.125], as well as p63 [18/18, median H-score (H) 245, IQR 63.13] and p40 (22/22, H 218.75, IQR 76.75). There was no noticeable change in expression of these markers in metastatic or relapse specimens, except for one case, where the primary tumor was weakly positive for p40 (H 67.5) and the relapse completely lacked p40 expression (Figure 1A,B).
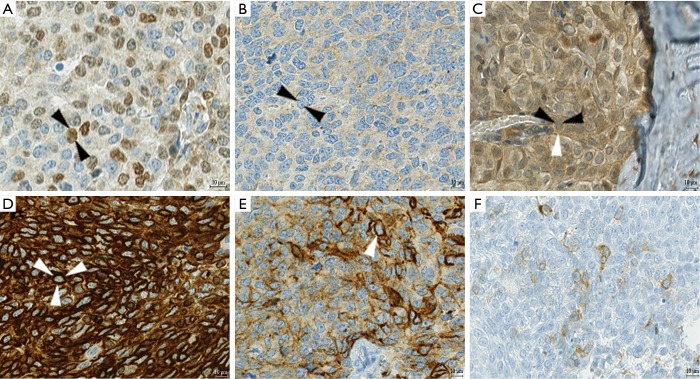
Figure 1.
Examples of immunohistochemical staining. (A,B) In a case of basaloid TC, the primary tumor showed scattered nuclear expression of p40 (dark brown, black arrows mark the outline of one nucleus) whereas the relapse specimen of the same patient was negative for p40; (C) p21 was evaluated for nuclear and cytoplasmic staining separately; in this case of a B3, p21 was negative in the nucleus (black arrows), but moderately positive in the cytoplasm (white arrow); (D) Bcl-2, an oncogene often positive in TCs, shows strong cytoplasmic expression in this case of squamous TC (white arrows mark the cell membrane of a tumor cell); (E) only one case of TC expressed podoplanin (white arrow marks the cell membrane), a marker for lymphatic endothelium which can also be positive in thymic epithelial tumor cells; (F) neuroendocrine markers are also expressed in non-neuroendocrine TCs, like in this case with weak cytoplasmic expression of synaptophysin in a basaloid TC. TC, thymic carcinoma.
IHC of tumor suppressors and oncogenes (p21, p27, p53, Bcl-2, Ki-67)
These proteins are involved in controlling apoptosis and proliferation, and are frequently deregulated in tumor cells. Expression of nuclear p21 was generally weak (H 15, IQR 25) and completely negative in 4 out of 22 cases, whereas cytoplasmic staining was stronger (H 113.75, IQR 48.13) and positive in all cases (22/22) (Figure 1C). p27 expression in 21 specimens was similarly strong in nucleus and cytoplasm (H 180, IQR 117.5; H 160, IQR 77.5, respectively), and no case was negative. p53 was positive in all cases (22/22) with a median H-score of 122.5 (IQR 36.88). The combination of low p21, low p27 and high p53 was seen in one patient, whereas p21 low and p53 high was present in ten of 22 patients.
Bcl-2 was negative in 12 of 21 cases, nine cases were positive with a median H-score of 280 (IQR 76.25). Bcl-2 expression was observed in eight TC and one MT (Figure 1D). Total proliferation index assessed with Ki-67 staining was median 20% (IQR 26.25) in 22 patients.
IHC of podoplanin, synaptophysin, PTEN and Pax8
Podoplanin as a marker for lymphatic endothelium and often positive in TETs has been shown to be predictive for lymph node metastasis and poor clinical outcome in a variety of malignant tumors (16). Pax8, a nuclear transcription factor usually only expressed during organogenesis, may be reactivated and deregulated in certain tumors. Its influence on invasion and metastasis has not been investigated yet (23). Furthermore, synaptophysin as a marker for neuroendocrine differentiation, and PTEN, a tumor suppressor frequently downregulated in tumors, are grouped in this section (24,25). Only one of 20 evaluated cases moderately expressed podoplanin (Figure 1E). Synaptophysin was positive in 7/21 specimens, whereof two were B3 and five were TC (basaloid n=2, squamous n=3) (Figure 1F). One of the two neuroendocrine carcinomas (NC) included in our study was negative for synaptophysin on the TMA and the other was not evaluable due to damaged sections; but both specimens were positive on the original histological slides. The relapse specimen of one of the primarily synaptophysin-positive B3 was negative for synaptophysin. H-score of PTEN was assessed for 21 patients, which were all positive (H 132.5, IQR 46.25). Nuclear and cytoplasmic staining of Pax8 was positive in all of 20 evaluated cases, with a median H-score of 197.5 (IQR 162.5) and 185 (IQR 75), respectively. The results of all immunohistochemical stainings are summarized in the supplemental digital content 1 (Table S1).
Survival analysis
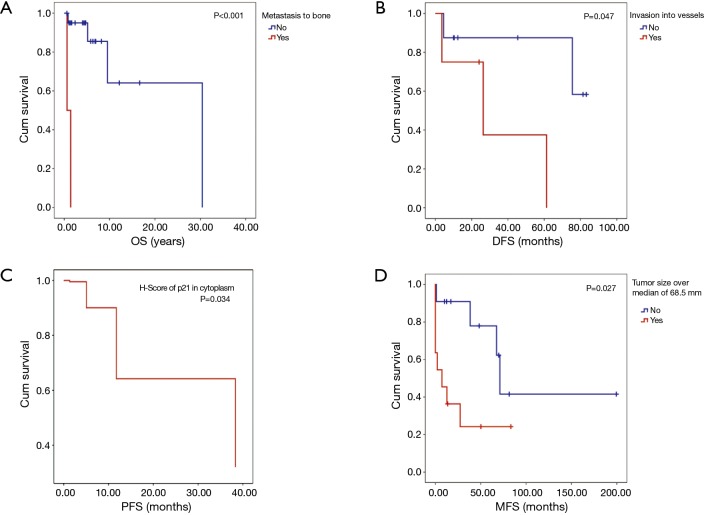
Median follow-up was 53.64 months (range, 7–365 months). At the time of analysis, 17 patients were alive and six had died. One-, 5- and 10-year OS were 91%, 86% and 58%, respectively. From the twelve patients with complete resection, five patients had recurrent disease. One- and 5-year DFS were 83% and 69%, respectively. From the remaining eleven patients with incomplete resection, five patients had progressive disease, and 1- and 5-year PFS were 72% and 60%, respectively. The significant results of the univariate analysis of the impact of clinical factors and staining pattern on OS, DFS and PFS are summarized in Table 3. The complete data are shown in Tables S2,S3. Figure 2A shows the significantly decreased OS if metastases to bone occurred. WHO type (C < B3 < B2/B3), subtype of TC (neuroendocrine < squamous < basaloid), Masaoka stage (III + IV < II and IV < II + III) and metastasis to pleura showed a trend towards shorter OS.
Table 3. Survival analysis (significant results).
| Characteristics | Significant P value (<0.05) | |||
|---|---|---|---|---|
| OS | DFS | PFS | MFS | |
| WHO type (C vs. B3 + B2/B3) | 0.021 | |||
| Masaoka-Koga stage | 0.036 | <0.001 | ||
| Masaoka-Koga stage (III + IV vs. II) | 0.009 | <0.001 | ||
| Masaoka-Koga stage (IV vs. II + III) | 0.023 | <0.001 | ||
| Tumor size over median (68.5 mm) | 0.027 | |||
| Surgical approach (minimally invasive vs. open + combined) | 0.031 | |||
| Surgical procedure (biopsy vs. resection of intrathymic tumor vs. thymectomy) | <0.001 | 0.002 | 0.026 | |
| Invasion into lung (yes vs. no) | 0.045 | |||
| Invasion into pleura (yes vs. no) | 0.023 | 0.001 | ||
| Invasion into vessels (yes vs. no) | 0.047 | 0.024 | ||
| Metastasis (yes vs. no) | 0.014 | |||
| Metastasis to pleura (yes vs. no) | 0.002 | |||
| Metastasis to lung (yes vs. no) | 0.008 | |||
| Metastasis to lymph nodes (yes vs. no) | 0.034 | |||
| Metastasis to bone (yes vs. no) | <0.001 | <0.001 | ||
| p21 cytoplasm (yes vs. no) | 0.016 | 0.034 | 0.005 | |
| p21 low, p27 low, p53 high (yes vs. no) | 0.029 | |||
OS, overall survival; DFS, disease-free survival; PFS, progression-free survival; MFS, metastasis-free survival.
Figure 2.
Survival curves. (A) Patients with metastases to bone show significantly worse overall survival (OS) in 23 cases with malignant thymic epithelial tumors (TET); (B) disease-free survival (DFS) was significantly worse for patients with intraoperatively observed macroscopic invasion into vessels, in 12 cases with completely resected malignant TETs; (C) cytoplasmatically stained p21 (assessed as scale variable by H-score) had significant impact on progression-free survival (PFS), shown by this Cox regression survival curve at mean of H-score of p21 in cytoplasm of 11 cases with incompletely resected malignant TETs; (D) patients with tumors larger than the median of the study group (>68.5 mm) show significantly worse metastasis-free survival (MFS) in 22 analyzed cases with malignant TETs.
Figure 2B shows a negative impact on DFS by invasion into vessels. Synaptophysin positive tumor specimens had a trend towards worse DFS. Figure 2C shows the significantly shorter PFS for H-score of p21 in cytoplasm. A trend towards shorter PFS was seen for tumor size larger than the median of the study group (68.5 mm), metastasis and metastasis to lung.
Correlations between clinical parameters and immunohistochemistry
Higher age at diagnosis correlated significantly with a higher total proliferation index (P=0.013) and H-score of Pax8 in cytoplasm (P=0.032), age at diagnosis (P=0.020), H-score of CD5 (P=0.005), H-score of p21 in nucleus and cytoplasm (P=0.003 and P=0.030, respectively), H-score of p27 in nucleus and cytoplasm (P=0.007 and P=0.027, respectively), H-score of Bcl-2 (P<0.001), total proliferation index (P=0.022) and H-score of Pax8 in cytoplasm (P=0.012) were higher in patients with TC compared to MT and B3. H-score of PTEN was lower in patients with TC, but not statistically significant (P=0.076).
Patients with Masaoka stage III or IV had a higher H-score of p21 in cytoplasm than patients with stage II (P=0.018) and patients with Masaoka stage IV had larger tumors compared to patients with stage II and III (P=0.046). H-score of p63 was lower (P=0.097) and H-score of p21 in cytoplasm was higher (P=0.096) in patients with Masaoka stage IV compared to stage II and III, but not significantly. The correlations between the 13 different stainings are summarized in Table S4.
Impact on metastatic activity and invasive behavior
Results of the univariate analysis testing the influence of clinical factors and staining patterns on the development of metastases are shown in Tables S2,S3, significant results are summarized in Table 3. Figure 2D shows the significantly shorter MFS for tumor size larger than the median.
A non-significant trend towards shorter MFS was seen for patients without paraneoplastic syndrome, subtype of TC (neuroendocrine < basaloid < squamous), invasion and H-score of p21 in nucleus.
Extrathoracic metastasis was significantly correlated with R2 resection (P=0.034), higher H-score of p27 in nucleus (P=0.042), higher total proliferation index (P=0.024) and podoplanin expression (P=0.050).
Intraoperatively observed macroscopic invasion was significantly associated with greater tumor size (P=0.030) and marginally significant with higher H-score of p21 in cytoplasm (P=0.100).
Discussion
Immunohistochemical staining pattern and its impact on survival, invasive behavior and metastatic potential of TETs has gained increased research interest (12). In this study, we found that positive staining for p21 in the cytoplasm of tumor cells significantly correlated with a decreased OS, PFS and MFS. Furthermore, strong positivity for p27 in the nucleus, high total proliferation index, and expression of podoplanin were significantly related to an increased presence of extrathoracic metastases. In contrast, none of the tested markers correlated with intraoperatively observed macroscopic invasion of surrounding tissue and organs. In addition to the immunohistochemical stainings, a particular set of clinical factors showed significantly worse survival, such as WHO type, Masaoka stage, and metastasis to pleura, lung, lymph nodes and bone, some of which have been investigated in previous studies with comparable results (18,19,26-28) .
Located in the nucleus, p21 and p27 are known to act as tumor suppressors and arrest cell cycle progression, and they are frequently downregulated in tumor cells (29,30). On the other hand, they are exported from the nucleus into the cytoplasm through complex mechanisms, where they can have a contrary, oncogenic function, e.g., inhibit apoptosis or promote invasion (29-31). Most previous studies have ignored cytoplasmic staining of p21, p27 and Pax8 (18,19,23). To the best of our knowledge, this is the first study to also investigate cytoplasmic positivity of Pax8 in TETs, and cytoplasmic p21 and p27 have been evaluated in only one study so far (32). The relevance of cytoplasmic Pax8 staining is still unclear, but it has also been described in other neoplasms such as pulmonary neuroendocrine tumors (33). In gastric cancer, strong cytoplasmic p21 expression significantly correlated with lymph node metastasis, distant metastasis, advanced TNM stage, depth of invasion and OS (29). In our study, cytoplasmic expression of p21 negatively influenced survival and significantly correlated with WHO type, Masaoka stage and marginally significant with intraoperatively observed invasion. As for p27, Omatsu et al. demonstrated that cytoplasmic p27 was increased with the increase in malignancy of TET (only abstract available) and Chen et al. found a significant correlation between cytoplasmic p27 expression and worse OS and disease-specific survival of melanoma patients (30,32). We could confirm a stronger cytoplasmic p27 expression in more malignant TETs, but no impact on survival was seen. Nuclear export inhibitors, such as Selinexor, are currently being tested against different cancers in phase I/II human clinical trials and may be a promising targeted therapy option also for patients suffering from TETs (31).
The combination of low p21, low p27 and high p53 expression has been shown to significantly decrease DFS and predict the response to neoadjuvant chemotherapy in patients with thymoma (18,19). In our study, only one patient had this combination, but still a significant impact on MFS was seen. The influence of combined low p21 and high p53 on survival was additionally investigated in our study, but no correlation was found. We found remarkably strong nuclear expression of p21 and p27 compared to other studies. This discrepancy may be explained by the different stainings used: we employed rabbit polyclonal antibodies, whereas Mineo et al. used mouse monoclonal antibodies (18,19). Moreover, the cytoplasmic overexpression of p21 and p27 seems to be an important step in the development of TETs, rather than their nuclear downregulation. Unfortunately, patients with TCs were excluded in both of Mineo’s studies.
Synaptophysin is a commonly used marker to assess neuroendocrine differentiation (24). We found seven specimens to be positive for synaptophysin, all of which were not NC, but had basaloid or squamous differentiation or were thymomas type B3. Lauriola et al. have discovered early that TCs can express neuroendocrine markers, although they morphologically do not look like the neuroendocrine tumor type (24). These tumors should be graded as carcinomas with neuroendocrine differentiation, not as NCs (12). NCs have been shown to have a worse prognosis than other subtypes of TCs and are frequently excluded in studies (26-28). The two NCs in our study exhibited an exceedingly aggressive behavior, they were inoperable and could only be biopsied, both were Masaoka stage IV, and had a marginally significant lower OS and MFS.
The majority of our patients suffered from invasive tumors and metastatic disease which significantly influenced survival. There are only few studies that have investigated the correlation of immunohistochemical stainings with invasion and metastasis, for example: Ki-67 expression is associated with MFS and invasiveness (13,14). VEGF is more frequently and higher expressed in invasive thymomas (14,15). Podoplanin is more often expressed in higher stages of thymoma and is positive in metastasized tumor cells in lymph nodes. Unfortunately, in this study TCs were excluded (16). Additionally, TCs with activated STAT3 are more invasive and more often exhibit lymph node metastases (17). A comparison with the results of our study is difficult due to the divergent patient population and different evaluated stainings, but the correlation of podoplanin expression and the presence of extrathoracic metastases may be an interesting topic for future investigations.
Table S4 summarizes the correlation-coefficients and corresponding P values of the 13 evaluated stainings. Interestingly, many stainings showed a significant correlation. However, our study has the following undoubtable drawbacks: firstly, the number of patients is too limited to allow the performance of an extended statistical analysis, including multivariate analysis. Significant results in this study may sometimes rely on few patients, thus the results should be interpreted critically. Secondly, WHO type B2/B3, B3 and TC were analyzed together, which may modify outcome. Regarding the first limitation, this study, compared to others, still comprises a relatively large number of patients with aggressive subtypes of TETs (14-16,18,19). Fortunately, large international databases have recently been created in order to increase the availability of data of this rare tumor (26). As for the second downside, type B3 thymomas were classified as well-differentiated TC before the WHO classification was released, but they are still mentioned under this synonyme (5,34). Furthermore, Ströbel et al. outlined the close relation of B3 and thymic squamous cell carcinoma on the background of partially alike genetic abnormalities (35). Therefore, we decided to include both B3 and TC in our study. We discovered that particular staining patterns and OS significantly varied among WHO types; however, DFS, PFS and MFS were similar, and many patients with MT and B3 had invasive tumors and even developed extrathoracic metastases.
In summary, we found in this study previously undescribed correlations between immunohistochemically stainable proteins and survival and metastatic potential of aggressive subtypes of TETs. These findings can contribute to an improvement of the yet not standardized treatment strategy of these rare tumors.
Acknowledgements
None.
Table S1. Results of immunohistochemical staining.
| Marker | Positive N | Negative N | Median H-score | IQR | Total N |
|---|---|---|---|---|---|
| CD117 | 7 | 12 | NA | NA | 19 |
| CD5 | 20 | 0 | 168.75 | 88.13 | 20 |
| p63 | 18 | 0 | 245.00 | 63.13 | 18 |
| p40 | 22 | 0 | 218.75 | 76.75 | 22 |
| p21n | 18 | 4 | 15.00 | 25.00 | 22 |
| p21c | 22 | 0 | 113.75 | 48.13 | 22 |
| p27n | 21 | 0 | 180.00 | 117.50 | 21 |
| p27c | 21 | 0 | 160.00 | 77.50 | 21 |
| p53 | 22 | 0 | 122.50 | 36.88 | 22 |
| Bcl-2 | 9 | 12 | 280.00 | 76.25 | 21 |
| Ki-67 | 22 | 0 | 20%* | 26.25 | 22 |
| Podoplanin | 1 | 19 | NA | NA | 20 |
| Synaptophysin | 7 | 14 | NA | NA | 21 |
| PTEN | 21 | 0 | 132.50 | 46.25 | 21 |
| Pax8n | 20 | 0 | 197.50 | 162.50 | 20 |
| Pax8c | 20 | 0 | 185.00 | 75.00 | 20 |
*, indication of median total proliferation index in percent instead of H-score. n, nuclear staining; c, cytoplasmic staining; IQR, interquartile range; NA, not assessable.
Table S2. Survival analysis of clinical parameters.
| Characteristics | P value | |||
|---|---|---|---|---|
| OS | DFS | PFS | MFS | |
| Gender | 0.63 | 0.47 | 0.712 | 0.82 |
| Age at diagnosis over median (49 y) | 0.87 | 0.63 | 0.39 | 0.38 |
| Smoking | 0.87 | 0.80 | 0.52 | 0.94 |
| Paraneoplastic syndrome | 0.37 | 0.19 | 0.80 | 0.053 |
| WHO type (C vs. B3 vs. B2/B3) | 0.055 | 0.40 | 0.28 | 0.24 |
| WHO type (C vs. B3 + B2/B3) | 0.021* | 0.29 | 0.29 | 0.34 |
| Subtype of WHO type C (neuroendocrine vs. squamous vs. basaloid) | 0.051 | 0.12 | 0.24 | 0.089 |
| Masaoka-Koga stage | 0.23 | 0.036* | 0.29 | <0.001* |
| Masaoka-Koga stage (III + IV vs. II) | 0.076 | 0.009* | 0.30 | <0.001* |
| Masaoka-Koga stage (IV vs. II + III) | 0.064 | 0.023* | 0.28 | <0.001* |
| Tumor size over median (68.5 mm) | 0.39 | 0.29 | 0.091 | 0.027* |
| Surgical approach (minimally invasive vs. open + combined) | 0.031* | 0.52 | 0.27 | 0.83 |
| Surgical procedure (biopsy vs. resection of intrathymic tumor vs. thymectomy) | <0.001* | 0.12 | 0.002* | 0.026* |
| Invasion | 0.67 | 0.16 | 0.95 | 0.066 |
| Invasion into lung | 0.98 | 0.14 | 0.22 | 0.045* |
| Invasion into pleura | 0.16 | 0.023* | 0.72 | 0.001* |
| Invasion into pericardium | 0.50 | 0.71 | 0.86 | 0.87 |
| Invasion into vessels | 0.22 | 0.047* | 0.28 | 0.024* |
| Residual disease (R2 vs. R1 vs. R0) | 0.23 | – | – | 0.86 |
| Metastasis | 0.37 | 0.014* | 0.074 | – |
| Metastasis to pleura | 0.056 | 0.45 | 0.002* | – |
| Metastasis to lung | 0.28 | 0.008* | 0.099 | – |
| Metastasis to lymph nodes | 0.38 | 0.034* | 0.55 | – |
| Metastasis to bone | <0.001* | – | <0.001* | – |
| Metastasis to brain | 0.44 | 0.23 | – | – |
*, significant P value (P<0.05). OS, overall survival; DFS, disease-free survival; PFS, progression-free survival; MFS, metastasis-free survival.
Table S3. Survival analysis of immunohistochemical staining.
| Marker | OS | DFS | PFS | MFS |
|---|---|---|---|---|
| CD117 | P=0.17 | P=0.29 | P=0.42 | P=0.67 |
| CD5 | HR 1.016; CI: 0.983–1.050; P=0.35 | HR 1.005; CI: 0.985–1.026; P=0.596 | HR 1.012; CI: 0.975–1.050; P=0.54 | HR 1.002; CI: 0.987–1.016; P=0.83 |
| p63 | HR 0.988; CI: 0.961–1.016; P=0.41 | HR 0.990; CI: 0.965–1.017; P=0.47 | HR 1.006; CI: 0.962–1.051; P=0.81 | HR 0.995; CI: 0.975–1.015; P=0.60 |
| p40 | HR 0.997; CI: 0.986–1.008; P=0.58 | HR 0.992; CI: 0.979–1.005; P=0.23 | HR 1.002; CI: 0.988–1.017; P=0.76 | HR 0.996; CI: 0.998–1.004;P=0.36 |
| p21 nucleus | HR 1.029; CI: 0.990–1.080; P=0.15 | HR 1.025; CI: 0.975–1.078; P=0.34 | HR 1.127; CI: 0.962–1.319; P=0.14 | HR 1.009; CI: 1.000–1.018; P=0.053 |
| p21 cytoplasm | HR 1.044; CI: 1.008–1.081; P=0.016* | HR 1.041; CI: 0.989–1.096; P=0.12 | HR 1.047; CI: 1.004–0.093; P=0.034* | HR 1.017; CI: 1.005–1.029; P=0.005* |
| p27 nucleus | HR 1.022; CI: 0.998–1.046; P=0.078 | HR 1.013; CI: 0.992–1.035; P=0.23 | HR 1.018; CI: 0.996–1.042; P=0.11 | HR 1.003; CI: 0.993–1.013; P=0.62 |
| p27 cytoplasm | HR 1.014; CI: 0.993–1.036; P=0.20 | HR 1.008; CI: 0.985–1.032; P=0.49 | HR 1.024; CI: 0.998–1.052; P=0.078 | HR 1.003; CI: 0.991–1.015; P=0.59 |
| p53 | HR 1.016; CI: 0.998–1.034; P=0.083 | HR 1.008; CI: 0.991–1.024; P=0.38 | HR 0.999; CI: 0.979–1.019; P=0.92 | HR 1.004; CI: 0.993–1.015; P=0.45 |
| p21 low, p27 low, p53 high | – | – | – | P=0.029* |
| p21 low, p53 high | P=0.68 | P=0.52 | P=0.20 | P=0.18 |
| Bcl-2 | HR 1.007; CI: 0.997–1.018; P=0.18 | HR 1.004; CI: 0.996–1.012; P=0.36 | HR 1.003; CI: 0.993–1.012; P=0.60 | HR 1.001; CI: 0.996–1.006; P=0.64 |
| Ki-67 | HR 1.037; CI: 0.976–1.101; P=0.24 | HR 1.006; CI: 0.947–1.068; P=0.85 | HR 1.081; CI: 0.995–1.174; P=0.064 | HR 1.005; CI: 0.967–1.046; P=0.79 |
| Podoplanin | P=0.41 | P=0.23 | – | P=0.38 |
| Synaptophysin | P=0.24 | P=0.10 | P=0.85 | P=0.91 |
| PTEN | HR 0.985; CI: 0.955–1.016; P=0.34 | HR 1.004; CI: 0.989–1.020; P=0.60 | HR 1.005; CI: 0.963–1.049; P=0.80 | HR 1.000; CI: 0.988–1.012; P=0.99 |
| Pax8 nucleus | HR 1.0; CI: 0.984–1.016; P=0.98 | HR 1.008; CI: 0.992–1.024; P=0.34 | HR 1.004; CI: 0.988–1.020; P=0.67 | HR 1.002; CI: 0.994–1.010; P=0.58 |
| Pax8 cytoplasm | HR 1.008; CI: 0.987–1.030; P=0.45 | HR 1.010; CI: 0.992–1.029; P=0.28 | HR 0.995; CI: 0.958–1.034; P=0.80 | HR 0.999; CI: 0.987–1.012; P=0.90 |
*, significant P value (P<0.05) and 95% confidence interval. OS, overall survival; DFS, disease-free survival; PFS, progression-free survival; MFS, metastasis-free survival; HR, hazard ratio; CI, 95% confidence interval; P, P value.
Table S4. Correlations among immunohistochemical stainings (first line: correlation coefficient; second line: correspondent P value).
| Correlations | CD117 | CD5 | p63 | p40 | p21n | p21c | p27n | p27c | p53 | Bcl-2 | Ki67 | Podoplanin | Synaptophysin | PTEN | Pax8n | Pax8c |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CD117 | 1 | Pos | Neg | Neg | Pos | Pos | Pos | Pos | Pos | Pos | Pos | Pos | Pos | Pos | ||
| 0.005 | 0.892 | 0.446 | 0.090 | 0.090 | 0.022 | 0.047 | 0.190 | <0.001* | 0.105 | 0.368 | 0.045* | 0.069 | 0.353 | 0.018* | ||
| CD5 | Pos | 1 | 0.049 | 0.058 | 0.445 | 0.467 | 0.534 | 0.463 | 0.149 | 0.583 | 0.477 | Pos | Pos | −0.384 | 0.403 | 0.580 |
| 0.005* | 0.846 | 0.807 | 0.049* | 0.038* | 0.015* | 0.040* | 0.530 | 0.007* | 0.033* | 0.487 | 0.104 | 0.095 | 0.078 | 0.007* | ||
| p63 | Neg | 0.049 | 1 | 0.816 | 0.026 | 0.364 | 0.514 | 0.560 | 0.112 | 0.138 | 0.173 | Neg | Pos | 0.577 | 0.588 | 0.497 |
| 0.892 | 0.846 | <0.001* | 0.918 | 0.137 | 0.029* | 0.016* | 0.657 | 0.584 | 0.492 | 0.101 | 0.389 | 0.012* | 0.010* | 0.036* | ||
| p40 | Neg | 0.058 | 0.816 | 1 | −0.134 | 0.219 | 0.352 | 0.287 | 0.145 | 0.208 | 0.275 | Neg | Neg | 0.465 | 0.518 | 0.416 |
| 0.446 | 0.807 | <0.001* | 0.553 | 0.328 | 0.117 | 0.208 | 0.520 | 0.365 | 0.216 | 0.099 | 0.709 | 0.034* | 0.019* | 0.068 | ||
| p21n | Pos | 0.445 | −0.026 | −0.134 | 1 | 0.538 | 0.429 | 0.462 | 0.272 | 0.815 | 0.469 | Pos | Pos | −0.319 | 0.228 | 0.333 |
| 0.010* | 0.049* | 0.918 | 0.553 | 0.010* | 0.052 | 0.035* | 0.220 | <0.001* | 0.028* | 0.253 | 0.546 | 0.159 | 0.334 | 0.152 | ||
| p21c | Pos | 0.467 | 0.364 | 0.219 | 0.538 | 1 | 0.543 | 0.570 | 0.285 | 0.518 | 0.381 | Pos | Pos | 0.056 | 0.449 | 0.483 |
| 0.090 | 0.038* | 0.137 | 0.328 | 0.010* | 0.011* | 0.007* | 0.198 | 0.016 | 0.080 | 0.543 | 0.116 | 0.809 | 0.047* | 0.031* | ||
| p27n | Pos | 0.534 | 0.514 | 0.352 | 0.429 | 0.543 | 1 | 0.806 | 0.155 | 0.624 | 0.511 | Pos | Pos | −0.104 | 0.815 | 0.887 |
| 0.022* | 0.015* | 0.029* | 0.117 | 0.052 | 0.011* | <0.001 | 0.503 | 0.002* | 0.018* | 0.140 | 0.136 | 0.654 | <0.001* | <0.001* | ||
| p27c | Pos | 0.463 | 0.560 | 0.287 | 0.462 | 0.570 | 0.806 | 1 | 0.171 | 0.580 | 0.426 | Pos | Pos | 0.093 | 0.590 | 0.666 |
| 0.047* | 0.040* | 0.016* | 0.208 | 0.035* | 0.007* | <0.001* | 0.458 | 0.006* | 0.054 | 0.435 | 0.192 | 0.690 | 0.006 | 0.001 | ||
| p53 | Pos | 0.149 | 0.112 | 0.145 | 0.272 | 0.285 | 0.155 | 0.171 | 1 | 0.346 | −0.008 | Pos | Pos | 0.167 | 0.289 | 0.261 |
| 0.190 | 0.530 | 0.657 | 0.520 | 0.220 | 0.198 | 0.503 | 0.458 | 0.125 | 0.972 | 0.340 | 0.332 | 0.470 | 0.217 | 0.266 | ||
| Bcl-2 | Pos | 0.583 | 0.138 | 0.208 | 0.815 | 0.518 | 0.624 | 0.580 | 0.346 | 1 | 0.548 | Pos | Pos | −0.271 | 0.391 | 0.605 |
| <0.001* | 0.007* | 0.584 | 0.365 | <0.001* | 0.016 | 0.002 | 0.006* | 0.125 | 0.010* | 0.377 | 0.115 | 0.235 | 0.088 | 0.005* | ||
| Ki67 | Pos | 0.477 | 0.173 | 0.275 | 0.469 | 0.381 | 0.511 | 0.426 | −0.008 | 0.548 | 1 | Pos | Neg | −0.022 | 0.419 | 0.552 |
| 0.105 | 0.033* | 0.492 | 0.216 | 0.028* | 0.080 | 0.018* | 0.054 | 0.972 | 0.010* | 0.113 | 0.365 | 0.926 | 0.066 | 0.012* | ||
| Podoplanin | Pos | Neg | Neg | Pos | Pos | Pos | Pos | Pos | Pos | Pos | 1 | 1 | Neg | Pos | Pos | |
| 0.368 | 0.487 | 0.101 | 0.099 | 0.253 | 0.543 | 0.140 | 0.435 | 0.340 | 0.377 | 0.113 | 0.259 | 0.260 | 0.134 | |||
| Synaptophysin | Pos | Pos | Neg | Pos | Pos | Pos | Pos | Pos | Pos | Neg | 1 | 1 | Neg | Pos | Pos | |
| 0.045* | 0.104 | 0.389 | 0.709 | 0.546 | 0.116 | 0.136 | 0.192 | 0.332 | 0.115 | 0.365 | 0.654 | 0.663 | 0.183 | |||
| PTEN | Neg | −0.384 | 0.577 | 0.465 | −0.319 | 0.056 | −0.104 | 0.093 | 0.167 | −0.271 | −0.022 | Neg | Neg | 1 | 0.126 | −0.029 |
| 0.069 | 0.095 | 0.012* | 0.034* | 0.159 | 0.809 | 0.654 | 0.690 | 0.470 | 0.235 | 0.926 | 0.259 | 0.654 | 0.595 | 0.904 | ||
| Pax8n | Pos | 0.403 | 0.588 | 0.518 | 0.228 | 0.449 | 0.815 | 0.590 | 0.289 | 0.391 | 0.419 | Pos | Pos | 0.126 | 1 | 0.877 |
| 0.353 | 0.078 | 0.010* | 0.019* | 0.334 | 0.047* | <0.001* | 0.006* | 0.217 | 0.088 | 0.066 | 0.260 | 0.663 | 0.595 | <0.001 | ||
| Pax8c | Pos | 0.580 | 0.497 | 0.416 | 0.333 | 0.483 | 0.887 | 0.666 | 0.261 | 0.605 | 0.552 | Pos | Pos | −0.029 | 0.877 | 1 |
| 0.018* | 0.007* | 0.036* | 0.068 | 0.152 | 0.031* | <0.001 | 0.001 | 0.266 | 0.005* | 0.012* | 0.134 | 0.183 | 0.904 | <0.001 |
*, significant P value (<0.05). n, nuclear staining; c, cytoplasmic staining; Pos, positive correlation in the Mann-Whitney-U-test; Neg, negative correlation in the Mann-Whitney-U-test.
Ethical Statement: The study was approved by the institutional review board and the local ethics committee (KEK ZH 29-2009).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Serpico D, Trama A, Haspinger ER, et al. Available evidence and new biological perspectives on medical treatment of advanced thymic epithelial tumors. Ann Oncol 2015;26:838-47. 10.1093/annonc/mdu527 [DOI] [PubMed] [Google Scholar]
- 2.Falkson CB, Bezjak A, Darling G, et al. The management of thymoma: a systematic review and practice guideline. J Thorac Oncol 2009;4:911-9. 10.1097/JTO.0b013e3181a4b8e0 [DOI] [PubMed] [Google Scholar]
- 3.Ruffini E, Van Raemdonck D, Detterbeck F, et al. Management of thymic tumors: a survey of current practice among members of the European Society of Thoracic Surgeons. J Thorac Oncol 2011;6:614-23. 10.1097/JTO.0b013e318207cd74 [DOI] [PubMed] [Google Scholar]
- 4.Roden AC, Yi ES, Jenkins SM, et al. Modified Masaoka stage and size are independent prognostic predictors in thymoma and modified Masaoka stage is superior to histopathologic classifications. J Thorac Oncol 2015;10:691-700. 10.1097/JTO.0000000000000482 [DOI] [PubMed] [Google Scholar]
- 5.Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol 2015;10:1243-60. 10.1097/JTO.0000000000000630 [DOI] [PubMed] [Google Scholar]
- 6.Marx A, Chan JK, Coindre JM, et al. The 2015 World Health Organization Classification of Tumors of the Thymus: Continuity and Changes. J Thorac Oncol 2015;10:1383-95. 10.1097/JTO.0000000000000654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marx A, Ströbel P, Badve SS, et al. ITMIG consensus statement on the use of the WHO histological classification of thymoma and thymic carcinoma: refined definitions, histological criteria, and reporting. J Thorac Oncol 2014;9:596-611. 10.1097/JTO.0000000000000154 [DOI] [PubMed] [Google Scholar]
- 8.Detterbeck FC, Nicholson AG, Kondo K, et al. The Masaoka-Koga stage classification for thymic malignancies: clarification and definition of terms. J Thorac Oncol 2011;6:S1710-6. 10.1097/JTO.0b013e31821e8cff [DOI] [PubMed] [Google Scholar]
- 9.Ahmad U, Yao X, Detterbeck F, et al. Thymic carcinoma outcomes and prognosis: results of an international analysis. J Thorac Cardiovasc Surg 2015;149:95-100. 10.1016/j.jtcvs.2014.09.124 [DOI] [PubMed] [Google Scholar]
- 10.Safieddine N, Liu G, Cuningham K, et al. Prognostic factors for cure, recurrence and long-term survival after surgical resection of thymoma. J Thorac Oncol 2014;9:1018-22. 10.1097/JTO.0000000000000215 [DOI] [PubMed] [Google Scholar]
- 11.Lamarca A, Moreno V, Feliu J. Thymoma and thymic carcinoma in the target therapies era. Cancer Treat Rev 2013;39:413-20. 10.1016/j.ctrv.2012.11.005 [DOI] [PubMed] [Google Scholar]
- 12.Weissferdt A, Moran CA. Immunohistochemistry in the diagnosis of thymic epithelial neoplasms. Appl Immunohistochem Mol Morphol 2014;22:479-87. 10.1097/PAI.0b013e3182a53856 [DOI] [PubMed] [Google Scholar]
- 13.Roden AC, Yi ES, Jenkins SM, et al. Diagnostic significance of cell kinetic parameters in World Health Organization type A and B3 thymomas and thymic carcinomas. Hum Pathol 2015;46:17-25. 10.1016/j.humpath.2014.10.001 [DOI] [PubMed] [Google Scholar]
- 14.Kaira K, Oriuchi N, Imai H, et al. L-type amino acid transporter 1 (LAT1) is frequently expressed in thymic carcinomas but is absent in thymomas. J Surg Oncol 2009;99:433-8. 10.1002/jso.21277 [DOI] [PubMed] [Google Scholar]
- 15.Tomita M, Matsuzaki Y, Edagawa M, et al. Correlation between tumor angiogenesis and invasiveness in thymic epithelial tumors. J Thorac Cardiovasc Surg 2002;124:493-8. 10.1067/mtc.2002.124389 [DOI] [PubMed] [Google Scholar]
- 16.Tateyama H, Sugiura H, Yamatani C, et al. Expression of podoplanin in thymoma: its correlation with tumor invasion, nodal metastasis, and poor clinical outcome. Hum Pathol 2011;42:533-40. 10.1016/j.humpath.2010.08.012 [DOI] [PubMed] [Google Scholar]
- 17.Chang KC, Wu MH, Jones D, et al. Activation of STAT3 in thymic epithelial tumours correlates with tumour type and clinical behaviour. J Pathol 2006;210:224-33. 10.1002/path.2041 [DOI] [PubMed] [Google Scholar]
- 18.Mineo TC, Ambrogi V, Mineo D, et al. Long-term disease-free survival of patients with radically resected thymomas: relevance of cell-cycle protein expression. Cancer 2005;104:2063-71. 10.1002/cncr.21433 [DOI] [PubMed] [Google Scholar]
- 19.Mineo TC, Mineo D, Onorati I, et al. New predictors of response to neoadjuvant chemotherapy and survival for invasive thymoma: a retrospective analysis. Ann Surg Oncol 2010;17:3022-9. 10.1245/s10434-010-1134-9 [DOI] [PubMed] [Google Scholar]
- 20.Schweizer MS, Schumacher L, Rubin MA. Constructing tissue microarrays for research use. Curr Protoc Hum Genet 2004;Chapter 10:Unit 10.7. [DOI] [PubMed] [Google Scholar]
- 21.Dotto J, Pelosi G, Rosai J. Expression of p63 in thymomas and normal thymus. Am J Clin Pathol 2007;127:415-20. 10.1309/2GAYKPDDM85P2VEW [DOI] [PubMed] [Google Scholar]
- 22.Chilosi M, Zamò A, Brighenti A, et al. Constitutive expression of DeltaN-p63alpha isoform in human thymus and thymic epithelial tumours. Virchows Arch 2003;443:175-83. 10.1007/s00428-003-0857-4 [DOI] [PubMed] [Google Scholar]
- 23.Weissferdt A, Moran CA. Pax8 expression in thymic epithelial neoplasms: an immunohistochemical analysis. Am J Surg Pathol 2011;35:1305-10. 10.1097/PAS.0b013e3182260735 [DOI] [PubMed] [Google Scholar]
- 24.Lauriola L, Erlandson RA, Rosai J. Neuroendocrine differentiation is a common feature of thymic carcinoma. Am J Surg Pathol 1998;22:1059-66. 10.1097/00000478-199809000-00003 [DOI] [PubMed] [Google Scholar]
- 25.Leonard MK, Kommagani R, Payal V, et al. ΔNp63α regulates keratinocyte proliferation by controlling PTEN expression and localization. Cell Death Differ 2011;18:1924-33. 10.1038/cdd.2011.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ruffini E, Detterbeck F, Van Raemdonck D, et al. Tumours of the thymus: a cohort study of prognostic factors from the European Society of Thoracic Surgeons database. Eur J Cardiothorac Surg 2014;46:361-8. 10.1093/ejcts/ezt649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weissferdt A, Moran CA. Thymic carcinoma, part 1: a clinicopathologic and immunohistochemical study of 65 cases. Am J Clin Pathol 2012;138:103-14. 10.1309/AJCP88FZTWANLRCB [DOI] [PubMed] [Google Scholar]
- 28.Ruffini E, Detterbeck F, Van Raemdonck D, et al. Thymic carcinoma: a cohort study of patients from the European society of thoracic surgeons database. J Thorac Oncol 2014;9:541-8. 10.1097/JTO.0000000000000128 [DOI] [PubMed] [Google Scholar]
- 29.Huang Y, Wang W, Chen Y, et al. The opposite prognostic significance of nuclear and cytoplasmic p21 expression in resectable gastric cancer patients. J Gastroenterol 2014;49:1441-52. 10.1007/s00535-013-0900-4 [DOI] [PubMed] [Google Scholar]
- 30.Chen G, Cheng Y, Zhang Z, et al. Prognostic significance of cytoplasmic p27 expression in human melanoma. Cancer Epidemiol Biomarkers Prev 2011;20:2212-21. 10.1158/1055-9965.EPI-11-0472 [DOI] [PubMed] [Google Scholar]
- 31.Gravina GL, Senapedis W, McCauley D, et al. Nucleo-cytoplasmic transport as a therapeutic target of cancer. J Hematol Oncol 2014;7:85. 10.1186/s13045-014-0085-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Omatsu M, Kunimura T, Mikogami T, et al. Cyclin-dependent kinase inhibitors, p16 and p27, demonstrate different expression patterns in thymoma and thymic carcinoma. Gen Thorac Cardiovasc Surg 2014;62:678-84. 10.1007/s11748-014-0437-3 [DOI] [PubMed] [Google Scholar]
- 33.Sangoi AR, Ohgami RS, Pai RK, et al. PAX8 expression reliably distinguishes pancreatic well-differentiated neuroendocrine tumors from ileal and pulmonary well-differentiated neuroendocrine tumors and pancreatic acinar cell carcinoma. Mod Pathol 2011;24:412-24. 10.1038/modpathol.2010.176 [DOI] [PubMed] [Google Scholar]
- 34.Kirchner T, Schalke B, Buchwald J, et al. Well-differentiated thymic carcinoma. An organotypical low-grade carcinoma with relationship to cortical thymoma. Am J Surg Pathol 1992;16:1153-69. 10.1097/00000478-199212000-00003 [DOI] [PubMed] [Google Scholar]
- 35.Ströbel P, Marx A, Zettl A, et al. Thymoma and thymic carcinoma: an update of the WHO Classification 2004. Surg Today 2005;35:805-11. 10.1007/s00595-005-3047-y [DOI] [PubMed] [Google Scholar]