Abstract
Viral neuraminidase inhibitors are widely used as synthetic anti-influenza drugs for the prevention and treatment of influenza. However, drug-resistant influenza A virus variants, including H5N1 highly pathogenic avian influenza viruses (HPAIVs), have been reported. Therefore, the discovery of novel and effective antiviral agents is warranted. We screened the antiviral effects of 11 herbal tea extracts (hibiscus, black tea, tencha, rosehip tea, burdock tea, green tea, jasmine tea, ginger tea, lavender tea, rose tea and oak tea) against the H5N1 HPAIV in vitro. Among the tested extracts, only the hibiscus extract and its fractionated extract (frHibis) highly and rapidly reduced the titers of all H5 HPAIVs and low pathogenic AIVs (LPAIVs) used in the pre-treatment tests of Madin–Darby canine kidney (MDCK) cells that were inoculated with a mixture of the virus and the extract. Immunogold electron microscopy showed that anti-H5 monoclonal antibodies could not bind to the deformed H5 virus particles pretreated with frHibis. In post-treatment tests of MDCK cells cultured in the presence of frHibis after infection with H5N1 HPAIV, the frHibis inhibited viral replication and the expression of viral antigens and genes. Among the plants tested, hibiscus showed the most prominent antiviral effects against both H5 HPAIV and LPAIV.
Keywords: antiviral effect, H5N1, herbal tea extract, hibiscus
In 1997, H5N1 highly pathogenic avian influenza virus (HPAIV) infections occurred in both poultry and humans in Hong Kong. Subsequently, the viruses have spread to many countries and caused severe diseases in both poultry and humans [28]. As of January 2016, 846 people were reported of having H5N1 HPAIV infections, resulting in death in approximately 53% of cases [34]. Thus, the emergence of H5N1 HPAIV has highlighted incredible adverse consequences to both veterinary and human health worldwide. Therefore, effective strategies to ease the burden of H5N1 HPAIV are urgently needed.
Currently, there are two major strategies for influenza control, namely annual vaccination and anti-influenza drug treatment. However, current vaccines cannot completely prevent influenza virus infection, because the vaccines are less effective when their antigenicities do not match with those of circulating viruses undergoing antigenic drift or with those of newly emerging viruses with different antigenicities, such as H5N1 HPAIV. Therefore, the development of efficient antiviral drugs is critical for influenza control, as antiviral drugs are thought to be more effective than annual vaccination, irrespective of the influenza A virus (IAV) subtype.
To date, only two classes of anti-influenza drugs have been investigated: viral M2 ion channel inhibitors (e.g., amantadine) and viral NA inhibitors (e.g., oseltamivir) [7]. However, during the last decade, drug-resistant variants, including H5N1 HPAIV, which have reduced sensitivity to these inhibitors, have been reported [3, 5, 8, 15, 24]. Moreover, the side effects of these drugs have been reported [11, 31]. Therefore, the development of novel, effective and safe anti-influenza drugs that do not promote drug-resistance and possess alternative antiviral mechanisms appears to be essential for influenza control.
Since ancient times, plants and plant derivatives have been used worldwide for medicinal purposes. Although new synthetic drugs to overcome the adverse aspects of current antiviral drugs are being continuously developed, the investigation of antiviral plant-derived materials presents an alternative approach [29]. To date, numerous studies on anti-influenza virus materials derived from plants have been published; however, studies on the anti-H5N1 HPAIV effects of plants and plant derivatives are limited [9, 13, 14, 18, 19, 23, 26, 27].
In this study, we examined the antiviral effects of 11 commercially available herbal teas against H5 subtype HPAIVs and low pathogenic avian influenza viruses (LPAIVs) to contribute to influenza control.
MATERIALS AND METHODS
Viruses: The following H5N1 HPAIV strains were used: Chicken/Yamaguchi/7/04 [17] and Chicken/Miyazaki/K11/07 [35], provided by the National Institute of Animal Health (NIAH; Tsukuba, Japan); Chicken/VN-HT/33/2003, Molly duck/VN-HN/77/07 and Chicken/VN-HT/30/10, provided by the National Institute of Veterinary Research (Hanoi, Vietnam); and Whooper swan/Hokkaido/1/08 [21] and Whooper swan/Hamanaka/11, isolated in our laboratory [4].
The following H5 subtype LPAIV strains were used: Avian/Japan/11OG1083/11 (H5N2) and Avian/Japan/9UO0036/09 (H5N2), isolated in our laboratory [1]; Chicken/Ibaraki/8/05 (H5N2) provided by the NIAH [20]; Duck/Hong Kong/820/80 (H5N3), provided by Dr. Y. Sakoda (Hokkaido University, Sapporo, Japan); and Whistling swan/Shimane/499/83 (H5N3) [25], provided by Dr. T. Ito (Tottori University, Tottori, Japan).
The viruses were propagated in 10-day-old embryonated chicken eggs.
Cell culture and virus titration: Madin–Darby canine kidney (MDCK) cells were cultured as described elsewhere [12]. Viruses were titrated in MDCK cells, and virus titers were quantified as the 50% tissue culture infective dose (TCID50), as described previously [12].
Hemagglutination test: Hemagglutination tests were performed according to the WHO Manual on Animal Influenza Diagnosis and Surveillance [32].
Immunofluorescence assay (IFA): IFA was conducted as previously described [30]. Briefly, virus-infected MDCK cells were fixed with acetone for 10 min and then reacted with a monoclonal antibody (mAb) to IAV nucleoprotein (NP) (clone AA5H, Oxford Biotechnology, Ltd., London, U.K.). FITC-conjugated rabbit anti-mouse IgG (Rockland Immunochemicals Inc., Gilbertsville, PA, U.S.A.) was used as a secondary antibody. Fluorescence was observed using the BZ-9000 BioRevo fluorescence microscope (Keyence Corporation, Osaka, Japan).
Plant extracts: The following 11 herbal teas were tested for antiviral activities: hibiscus (Althaea officinalis), black tea (Camellia sinensis assamica), tencha (Rubus suavissimus), rosehip tea (Rosa rugosa), burdock tea (Arctium lappa), green tea (Camellia sinensis sinensis), jasmine tea (Jasminum officinale), ginger tea (Zingiber officinale), lavender tea (Lavandula spica), rose tea (Rosa odorata) and oak tea (Quercus dentata). Two grams of each herbal tea were boiled in 100-ml ultrapure water for 1 hr (crude extract). In this study, because the hibiscus extract showed high antiviral effects, it was fractionated (frHibis) using a Vivaspin® centrifugal concentrator filter device with an ultrafiltration membrane having a molecular weight cut-off of 3 kDa (Sartorius Lab Instruments GmbH & Co. KG, Göttingen, Germany) and freeze-dried to estimate the molecular weight of the effective component.
Pre-treatment of H5 viruses with the extracts (Pre-treatment test): The H5 subtype HPAIVs and LPAIVs used in this test are shown in Tables 1–3. Briefly, the virus was mixed with an equal volume of the crude extract or frHibis at room temperature. Immediately or 10 min after mixing, the mixture was serially diluted 10-fold with culture medium and titrated in the MDCK cells 5 days post inoculation (dpi), as described above. In effect, a period of 10 sec was required to start the first dilution of the mixture immediately after mixing.
Table 1. Antiviral effects of the crude extracts of 11 teas on Chicken/Yamaguchi/7/04 (pre-treatment test).
| Tea extracts | Infectivity (Log10 TCID50/ml)a) | ||||||
|---|---|---|---|---|---|---|---|
| Treatment time after mixing the virus with the extract | |||||||
| 10 min | Immediately b) | ||||||
| Treated | Untreated | (Log10 reduction)c) | Treated | Untreated | (Log10 reduction) | ||
| Hibiscus | ≤1.5 | 6.1 | (≥4.6)d) | <1.5 | 6.2 | (≥4.7) | |
| Black | 3.0 | 5.5 | (2.5) | 3.2 | 5.5 | (2.3) | |
| Tencha | 4.7 | 6.0 | (1.3) | 5.0 | 6.0 | (1.0) | |
| Rosehip | 4.0 | 6.5 | (2.0) | 5.7 | 6.0 | (0.3) | |
| Burdock | NTe) | NT | 5.5 | 6.2 | (0.7) | ||
| Green | 4.0 | 6.0 | (2.0) | 6.0 | 6.3 | (0.3) | |
| Jasmine | 6.0 | 6.0 | (0.0) | 6.0 | 6.3 | (0.3) | |
| Ginger | 5.5 | 6.2 | (0.7) | NT | NT | ||
| Rose | 3.0 | 6.0 | (3.0) | 5.5 | 6.3 | (0.8) | |
| Lavender | 6.0 | 6.0 | (0.0) | 5.8 | 6.3 | (0.5) | |
| Oak | NT | NT | 5.7 | 6.2 | (0.5) | ||
a) The virus was mixed with the extract (treated) or medium (untreated) and inoculated into the MDCK cells. The virus titers were measured at 5 dpi. b) In effect, a period of 10 sec was required to start the first dilution of the mixture immediately after mixing. c) Log10 reduction in titers was calculated as follows: (extract-untreated virus titers)−(extract-treated virus titers). d) Bold figures of log reduction >2.0 indicate effective reduction; underlined, bold figures >3.0 indicate highly effective reduction. e) NT: not tested.
Table 3. Antiviral effects of frHibis on various H5N1 HPAIVs (pre-treatment test).
| Virus | Treatment time after mixing the virus with the extracta) | |
|---|---|---|
| Log10 reduction in the titerb) | ||
| 10 min | Immediatelyc) | |
| Chicken/Yamaguchi/7/04 | ≥5.0d) | ≥5.0 |
| Whooper swan/Hokkaido/1/08 | ≥5.0 | 3.0 |
| Chicken/Miyazaki/K11/07 | ≥6.3 | ≥7.0 |
| Whooper swan/Hamanaka/11 | ≥5.0 | 3.8 |
| Chicken/VN-HT/33/2003 | ≥5.0 | ≥4.7 |
| Molly duck/VN-HN/77/07 | ≥5.2 | 2.7 |
| Chicken/VN-HT/30/10 | ≥5.0 | 2.0 |
a) The virus was mixed with frHibis (treated) or medium (untreated) and inoculated into the MDCK cells. The virus titers were measured at 5 dpi. b) Log10 reduction in titers as indicated in the legend of Table 1. c) In effect, a period of 10 sec was required to start the first dilution of the mixture immediately after mixing. d) See the description of d) in the legend of Table 1.
Antiviral effect of frHibis on viral replication in MDCK cells (Post-treatment test): MDCK cells were inoculated with 200 TCID50 of Chicken/Yamaguchi/7/04 and adsorbed at 37°C for 1 hr. The inocula were then removed, and the cells were washed three times with phosphate-buffered saline (PBS, pH 7.4). The cells were then overlaid with medium containing frHibis (2.6 and 5.3 mg/ml) and incubated for 4 days. The inoculated cells were observed for the presence of a cytopathic effect (CPE), and HA activities in the culture fluids were tested using a hemagglutination test. IFA was performed to detect NP antigens at 2 dpi, as described above. RNA was extracted from the cells and culture medium at 3 dpi using an RNA extraction kit (Nippon Gene Co., Ltd., Toyama, Japan).
Treatment of cells with the extract prior to virus inoculation: MDCK cells were treated with the crude hibiscus and rosehip extracts or frHibis at 37°C for 1 hr and then washed three times with PBS. The cells were inoculated with Chicken/Yamaguchi/7/04 (200 TCID50) and observed for 5 days. The virus titer was calculated as described above.
Reverse transcription-polymerase chain reaction (RT-PCR): cDNA was synthesized from RNA, which was obtained during the post-treatment tests using random primers and Moloney murine leukemia virus reverse transcriptase (Invitrogen Corporation, Carlsbad, CA, U.S.A.) under the following conditions: 25°C for 10 min, 37°C for 60 min and 65°C for 10 min.
To detect viral matrix (M) and NP genes, RT-PCR was performed using specific primers for the M [33] and NP genes [16].
Electron microscopy (EM) and immunogold EM: Whistling swan/Shimane/499/83 (H5N3) was purified by ultracentrifugation in 30% and 60% sucrose solution [12] and mixed with frHibis at room temperature for 10 min. The treated viruses were loaded on a 400-mesh carbon-coated collodion grid and negatively stained with 2% phosphotungstic acid (PTA, pH 6.4) [6].
For immunogold EM, a grid with frHibis-treated or -untreated viruses, as described above, was blocked for 30 min with 1% bovine serum albumin in PBS and then incubated with anti-H5 HA mAb against A/Vietnam/1203/04 (clone 15A3; Rockland Immunochemicals, Inc.) for 30 min. The grid then was incubated with 10-nm gold-conjugated goat anti-mouse IgG + IgM (H&L) (BBI Solutions, Cardiff, U.K.) for 1 hr, stained with PTA and then observed using a Hitachi HT-7700 electron microscope (Hitachi High-Technologies Corporation, Tokyo, Japan).
Cytotoxicity of frHibis on the MDCK cells: MDCK cells cultured in the presence of frHibis (5.3 mg/ml) at 37°C for 4 days were microscopically observed daily, and live and dead cells were counted using a LUNA-FL™ Dual Fluorescence Cell Counter (Logos Biosystems, Annandale, VA, U.S.A.). Briefly, the cells were trypsinized and then stained with acridine orange and propidium iodide solution. Live cells were stained green, and dead cells were stained red (data not shown).
RESULTS
Pre-treatment of H5 viruses with crude herbal tea extracts and frHibis (Pre-treatment test): Hibiscus tea, black tea, rosehip tea, green tea and rose tea reduced the Chicken/Yamaguchi/7/04 titer by ≥2.0 log10 (Table 1) after 10-min pretreatment, and the hibiscus tea extract showed the strongest antiviral effect (≥4.6 log10 reduction in titer). No significant reduction in the titers was observed when the MDCK cells were inoculated with the virus immediately after mixing with the extracts of rosehip tea, green tea or rose tea, whereas the extracts of hibiscus tea and black tea reduced titers by ≥4.7 and 2.3 log10, respectively.
Five extracts showing antiviral effects against Chicken/Yamaguchi/7/04 were examined for antiviral effect against H5N2 and H5N3 LPAIVs (Table 2). A 10-min treatment with the hibiscus tea extract significantly inactivated each strain and reduced the titers by ≥3.3 log10. The black tea extract reduced the titers of the two H5N2 strains by ≥3.7 log10, whereas the green tea, rosehip tea or rose tea extracts did not produce a significant reduction in the titers.
Table 2. Antiviral effects of the crude extracts on H5N2 and H5N3 LPAIVs (pre-treatment test).
| Virus | 10-min treatment after mixing the virus with the extract | ||||
|---|---|---|---|---|---|
| Log10 reduction in the titera) | |||||
| Hibiscus tea | Green tea | Rosehip tea | Black tea | Rose tea | |
| Avian/Japan/9UO0036/09 | ≥4.0b) | 0.7 | 1.8 | 2.3 | 1.0 |
| Avian/Japan/11OG1083/11 | ≥4.0 | 0.3 | 2.2 | ≥3.7 | 1.8 |
| Chicken/Ibaraki/8/05 | ≥4.7 | NTc) | NT | ≥4.5 | 2.0 |
| Whistling swan/Shimane/499/83 | ≥4.0 | 0.8 | 2.0 | 1.5 | 1.0 |
| Duck/Hong Kong/820/80 | ≥3.3 | NT | NT | 2.0 | 0.5 |
As the hibiscus extract among the tested extracts highly inactivated the H5N1 virus (Chicken/Yamaguchi/7/04) in addition to various LPAIV strains, the antiviral effect of frHibis on various H5N1 HPAIV strains was examined as well. As shown in Table 3, the titers of all strains pretreated with frHibis for 10 min were reduced by ≥5.0 log10. Reduction in the titers by ≥3.0 log10 was observed for all H5N1 strains, except the Vietnamese strains (Molly duck/VN-HN/77/07 and Chicken/VN-HT/30/10), when the MDCK cells were inoculated with the virus immediately after mixing with frHibis. However, 3-min pre-treatment reduced the titers of the Vietnamese strains by ≥5.0 log10 (data not shown).
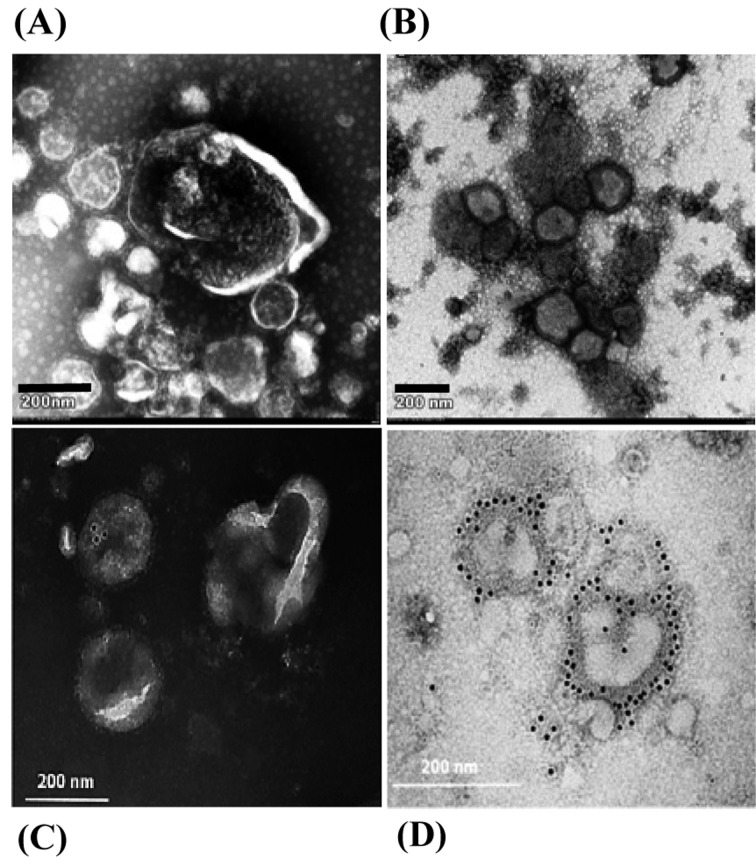
EM and immunogold EM: Abnormal morphologies, such as swelling and deformity, were observed in the viral particles following 10-min pre-treatment with frHibis (Fig. 1A and 1B).
Fig. 1.
Electron microscopy and immunogold electron microscopy. (A) Swelling and deformity of viral structures are observed. Purified H5N3 viruses were pretreated with frHibis for 10 min. (B) Structures of avian influenza virus particles are intact. The purified viruses were pretreated with medium. (C) H5 HA antigen-positive viral particles are not observed. Purified H5N3 viruses pretreated with frHibis for 10 min were incubated with anti-H5 mAb followed by incubation with 10-nm gold-conjugated goat anti-mouse IgG + IgM. (D) H5 HA antigen-positive viral particles are observed. Untreated H5N3 viruses were incubated in the same manner.
Immunogold EM revealed gold particles on the surface of the untreated viral particles, indicating that anti-H5 HA mAb bound to the HA proteins, whereas no particles were found on the surface of the deformed viral particles pretreated with frHibis (Fig. 1C and 1D).
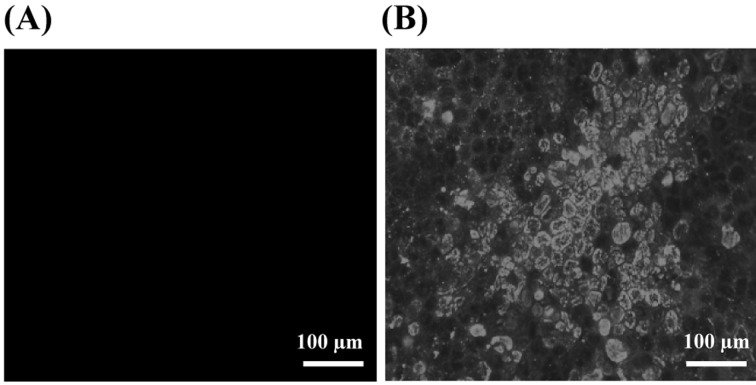
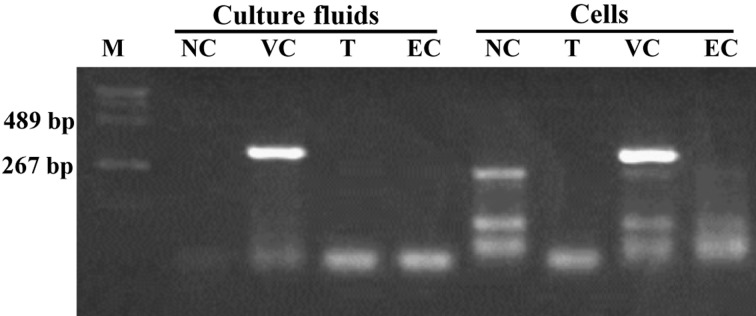
Inhibition of viral replication in the H5N1 virus-infected cells cultured in the presence of frHibis (Post-treatment test): No CPE was observed with 5.3-mg/ml frHibis, and the hemagglutination test was negative for the culture fluids of the inoculated cells (Table 4). In addition, no viral antigens were detected in the virus-inoculated MDCK cells in the presence of 5.3-mg/ml frHibis when examined at 2 dpi (Fig. 2). Furthermore, viral NP (Fig. 3) and M genes were not amplified in the virus-inoculated cells or the culture fluid in the presence of 5.3-mg/ml frHibis at 3 dpi.
Table 4. Detection of CPE and HA activity in the MDCK cells cultured in the presence of frHibis after inoculation with Chicken/Yamaguchi/7/04 (post-treatment test).
| Concentration of frHibis | CPE and HA titera) | ||||
|---|---|---|---|---|---|
| Days post inoculation | |||||
| 1 | 2 | 3 | 4 | ||
| 5.3 mg/ml | CPE | − | − | − | − |
| HA titer | <1:2 | NTb) | <1:2 | <1:2 | |
| 2.6 mg/ml | CPE | + | + | + | + |
| HA titer | <1:2 | NT | 1:32 | 1:32 | |
| None | CPE | + | + | + | + |
| HA titer | <1:2 | NT | 1:32 | 1:64 | |
a) HA titers in the culture fluids of the infected cells were determined. b) NT: not tested.
Fig. 2.
IFA test to detect viral NP antigens in MDCK cells infected with H5N1 and cultured in the presence of frHibis. (A) No viral NP antigen-positive MDCK cells are observed. Cells inoculated with Chicken/Yamaguchi/7/04 were cultured with medium containing 5.3-mg/ml frHibis for 48 hr. (B) Viral NP antigen-positive MDCK cells are observed. The inoculated cells were cultured with medium.
Fig. 3.
RT-PCR assay to detect viral NP genes in MDCK cells inoculated with H5N1 and cultured in the presence of frHibis (5.3 mg/ml) for 72 hr. M: DNA MW standard marker (pHY marker: Takara, Otsu, Japan). NC: cells were cultured with medium. VC: cells were inoculated with 200 TCID50 of Chicken/Yamaguchi/7/04. T: virus-inoculated cells were cultured with medium containing frHibis. EC: cells were cultured with medium containing frHibis.
Treatment of cells with the extract prior to virus inoculation: Chicken/Yamaguchi/7/04 did not replicate in MDCK cells pretreated with the crude hibiscus extract; however, it replicated in those treated with rosehip crude extract (Table 5). Although the virus titers were reduced by 2.0 log10 in cells pretreated with frHibis, no significant reduction was observed, in contrast to that observed in cells pretreated with the crude hibiscus extract.
Table 5. Inhibitory effects of the pre-treatment of the MDCK cells with crude hibiscus and rosehip extracts and frHibis.
| Cell treatment witha) | Infectivity (Log10 TCID50/ml)b) | ||
|---|---|---|---|
| Treated cells | Non-treated cells | (Log10 reduction)c) | |
| Crude extract | |||
| Hibiscus tea | ≤1.5 | 6.7 | (≥5.2) d) |
| Rosehip tea | 5.2 | 6.7 | (1.5) |
| FrHibis | 4.5 | 6.5 | (2.0) |
Cytotoxicity of frHibis on the MDCK cells: No cytotoxicity was observed when cells were cultured for 4 days in the presence of frHibis (5.3 mg/ml). Cell viability was 92%, and the cells were successfully passaged (data not shown).
DISCUSSION
Several studies have been conducted to discover potential anti-influenza agents against H5N1 HPAIV derived from plants. The extracts of Cistus incanus [9], Echinacea purpurea [23], Eugenia jambolana [26] and pomegranate (Punica granatum L.) [27] showed only direct antiviral effects against viral particles. Elderberry (Sambucus nigra L.) extracts reduced the number of foci in infected MDCK cells; however, the effect was limited [14]. Thai herbal plant extracts (Terminalia bellirica, Salacia chinensis, Zingiber montanum and Peltophorum pterocarpum) moderately reduced viral infectivity in MDCK cells simultaneously inoculated with the extract and virus [13]. In contrast, Pelargonium sidoides extracts showed no antiviral effects [18]. Thus, not all plants exhibited high antiviral effects against H5N1 HPAIV. However, our study indicated that in addition to the crude hibiscus extract, the fractionated component (frHibis), which has a low molecular weight (<3 kDa), also exhibited strong antiviral effects against H5N1 HPAIV strains, irrespective of origin (Table 3). Although frHibis was somewhat less effective against the Vietnamese H5N1 strains in a very short treatment time than against the other H5N1 strains, the complete inactivation of the Vietnamese strains was achieved within 3 min of exposure to frHibis (data not shown). This finding suggested that the antiviral effects of frHibis do not significantly differ among the H5N1 strains.
The influenza virus first binds to glycoprotein receptors containing sialic acid on the cell surface via HA and then enters the cell by endocytosis [22]. In the cells treated with frHibis before the viral infection, the cells allowed the virus to enter (Table 5), indicating no adverse effects of hibiscus on the receptors. Unpublished data indicated that the viruses pretreated with hibiscus lose the ability to agglutinate chicken red blood cells (data not shown). Therefore, the results of the antiviral effects of hibiscus observed in the pre-treatment tests strongly suggested that direct contact between the virus and hibiscus is required to abolish the entry of treated viruses into the cells. This interpretation was supported by the observations made by EM and immunogold EM, indicating that the antiviral effect was due to a direct effect against the viral particles as frHibis caused morphological changes to the virus and dysfunction of the viral surface (HA) proteins, indicated by the lack of binding of the anti-H5 HA antibody (Fig. 1). Although either the viral envelope or surface proteins seem to be targeted by hibiscus, further studies are needed to clarify the mechanism underlying this antiviral effect in more detail, for example, identifying the target molecules of hibiscus.
In the post-treatment test, no viral replication was detected in the MDCK cells infected with Chicken/Yamaguchi/7/04 in the presence of frHibis (Table 4). In addition, no viral antigens or viral gene expressions were detected (Figs. 2 and 3). Thus, our findings suggest that viral replication may be inhibited at an early stage by the small molecular weight components contained in frHibis, although frHibis did not inhibit viral binding to the cell membrane receptors (Table 5). However, the crude hibiscus extract inhibited viral binding to the receptors, which may have been affected by other components contained in the crude extract.
In contrast to our results, hibiscus extract prepared using hexane displayed no antiviral effect against H5N1 HPAIV [13]. Although the reason for this discrepancy between these two studies remains unclear, we believe that the different extraction methods may have influenced the results.
Hibiscus is known to contain a high concentration of anthocyanin pigments [2]. Hayashi et al. [10] reported the anti-human influenza virus activity of a red-fleshed potato anthocyanin. However, they speculated that its activity was derived from an additive or synergistic effect with other anthocyanin pigments and/or other coexisting pigments. Therefore, further studies should be conducted to identify the effective components contained in hibiscus and to elucidate potential antiviral mechanisms in more detail.
Our preliminary study showed that, in addition to the H5 subtype, hibiscus inactivated seven other subtypes (data not shown), whereas P. sidoides extracts inactivated human influenza viruses (H1N1 and H3N2) but not H5N1 HPAIV [18]. Thus, hibiscus may be a promising candidate as a potent anti-influenza drug, irrespective of subtype.
Acknowledgments
We would like to thank Mrs. Sachiko Matsuda for her excellent technical support.
REFERENCES
- 1.Abao L. N., Jamsransuren D., Bui V. N., Ngo L. H., Trinh D. Q., Yamaguchi E., Vijaykrishna D., Runstadler J., Ogawa H., Imai K.2013. Surveillance and characterization of avian influenza viruses from migratory water birds in eastern Hokkaido, the northern part of Japan, 2009-2010. Virus Genes 46: 323–329. doi: 10.1007/s11262-012-0868-9 [DOI] [PubMed] [Google Scholar]
- 2.Bahre T. A., Tchouya G. R. F.2016. Comparative study of the anti-oxidant activity of the total polyphenols extracted from Hibiscus Sabdariffa L., Glycine max L. Merr., yellow tea and red wine through reaction with DPPH free radicals. Arab. J. Chem. 9: 1–8. doi: 10.1016/j.arabjc.2014.11.048 [DOI] [Google Scholar]
- 3.Belshe R. B., Smith M. H., Hall C. B., Betts R., Hay A. J.1988. Genetic basis of resistance to rimantadine emerging during treatment of influenza virus infection. J. Virol. 62: 1508–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bui V. N., Ogawa H., Ngo L. H., Baatartsogt T., Abao L. N., Tamaki S., Saito K., Watanabe Y., Runstadler J., Imai K.2013. H5N1 highly pathogenic avian influenza virus isolated from conjunctiva of a whooper swan with neurological signs. Arch. Virol. 158: 451–455. doi: 10.1007/s00705-012-1502-9 [DOI] [PubMed] [Google Scholar]
- 5.Cheung C. L., Rayner J. M., Smith G. J. D., Wang P., Naipospos T. S. P., Zhang J., Yuen K. Y., Webster R. G., Peiris J. S., Guan Y., Chen H.2006. Distribution of amantadine-resistant H5N1 avian influenza variants in Asia. J. Infect. Dis. 193: 1626–1629. doi: 10.1086/504723 [DOI] [PubMed] [Google Scholar]
- 6.Chrystie I. L.1996. Electron microscopy. pp. 91–106. In: Virology Methods Manual. (Mahy, B.W. J. and Kangro H.O. eds.), Academic Press Ltd., London. [Google Scholar]
- 7.De Clercq E.2004. Antiviral drugs in current clinical use. J. Clin. Virol. 30: 115–133. doi: 10.1016/j.jcv.2004.02.009 [DOI] [PubMed] [Google Scholar]
- 8.de Jong M. D., Tran T. T., Truong H. K., Vo M. H., Smith G. J. D., Nguyen V. C., Bach V. C., Phan T. Q., Do Q. H., Guan Y., Peiris J. S., Tran T. H., Farrar J.2005. Oseltamivir resistance during treatment of influenza A (H5N1) infection. N. Engl. J. Med. 353: 2667–2672. doi: 10.1056/NEJMoa054512 [DOI] [PubMed] [Google Scholar]
- 9.Ehrhardt C., Hrincius E. R., Korte V., Mazur I., Droebner K., Poetter A., Dreschers S., Schmolke M., Planz O., Ludwig S.2007. A polyphenol rich plant extract, CYSTUS052, exerts anti influenza virus activity in cell culture without toxic side effects or the tendency to induce viral resistance. Antiviral Res. 76: 38–47. doi: 10.1016/j.antiviral.2007.05.002 [DOI] [PubMed] [Google Scholar]
- 10.Hayashi K., Mori M., Matsutani Knox Y., Suzutan T., Ogasawara M., Yoshida I., Hosokawa K., Tsukui A., Azuma M.2003. Anti-influenza virus activity of a Red-Fleshed potato anthocyanin. Food Sci. Technol. Res. 9: 242–244. doi: 10.3136/fstr.9.242 [DOI] [Google Scholar]
- 11.Hayden F. G., Hoffman H. E., Spyker D. A.1983. Differences in side effects of amantadine hydrochloride and rimantadine hydrochloride relate to differences in pharmacokinetics. Antimicrob. Agents Chemother. 23: 458–464. doi: 10.1128/AAC.23.3.458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Imai K., Ogawa H., Bui V. N., Inoue H., Fukuda J., Ohba M., Yamamoto Y., Nakamura K.2012. Inactivation of high and low pathogenic avian influenza virus H5 subtypes by copper ions incorporated in zeolite-textile materials. Antiviral Res. 93: 225–233. doi: 10.1016/j.antiviral.2011.11.017 [DOI] [PubMed] [Google Scholar]
- 13.Klaywong K., Khutrakul G., Choowongkomon K., Lekcharoensuk C., Petcharat N., Leckcharoensuk P., Ramasoota P.2014. Screening for lead compounds and herbal extracts with potential anti-influenza viral activity. Southeast Asian J. Trop. Med. Public Health 45: 62–74. [PubMed] [Google Scholar]
- 14.Krawitz C., Mraheil M. A., Stein M., Imirzalioglu C., Domann E., Pleschka S., Hain T.2011. Inhibitory activity of a standardized elderberry liquid extract against clinically-relevant human respiratory bacterial pathogens and influenza A and B viruses. BMC Complement. Altern. Med. 11: 16. doi: 10.1186/1472-6882-11-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Le Q. M., Kiso M., Someya K., Sakai Y. T., Nguyen T. H., Nguyen K. H. L., Pham N. D., Ngyen H. H., Yamada S., Muramoto Y., Horimoto T., Takada A., Goto H., Suzuki T., Suzuki Y., Kawaoka Y.2005. Avian flu: isolation of drug-resistant H5N1 virus. Nature 437: 1108. doi: 10.1038/4371108a [DOI] [PubMed] [Google Scholar]
- 16.Lee M. S., Chang P. C., Shien J. H., Cheng M. C., Shieh H. K.2001. Identification and subtyping of avian influenza viruses by reverse transcription-PCR. J. Virol. Methods 97: 13–22. doi: 10.1016/S0166-0934(01)00301-9 [DOI] [PubMed] [Google Scholar]
- 17.Mase M., Tsukamoto K., Imada T., Imai K., Tanimura N., Nakamura K., Yamamoto Y., Hitomi T., Kira T., Nakai T., Kiso M., Horimoto T., Kawaoka Y., Yamaguchi S.2005. Characterization of H5N1 influenza A viruses isolated during the 2003-2004 influenza outbreaks in Japan. Virology 332: 167–176. doi: 10.1016/j.virol.2004.11.016 [DOI] [PubMed] [Google Scholar]
- 18.Michaelis M., Doerr H. W., Cinatl J., Jr2011. Investigation of the influence of EPs® 7630, a herbal drug preparation from Pelargonium sidoides, on replication of a broad panel of respiratory viruses. Phytomedicine 18: 384–386. doi: 10.1016/j.phymed.2010.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michaelis M., Geiler J., Naczk P., Sithisarn P., Leutz A., Doerr H. W., Cinatl J., Jr2011. Glycyrrhizin exerts antioxidative effects in H5N1 influenza A virus-infected cells and inhibits virus replication and pro-inflammatory gene expression. PLoS ONE 6: e19705. doi: 10.1371/journal.pone.0019705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okamatsu M., Saito T., Yamamoto Y., Mase M., Tsuduku S., Nakamura K., Tsukamoto K., Yamaguchi S.2007. Low pathogenicity H5N2 avian influenza outbreak in Japan during the 2005-2006. Vet. Microbiol. 124: 35–46. doi: 10.1016/j.vetmic.2007.04.025 [DOI] [PubMed] [Google Scholar]
- 21.Okamatsu M., Tanaka T., Yamamoto N., Sakoda Y., Sasaki T., Tsuda Y., Isoda N., Kokumai N., Takada A., Umemura T., Kida H.2010. Antigenic, genetic, and pathogenic characterization of H5N1 highly pathogenic avian influenza viruses isolated from dead whooper swans (Cygnus cygnus) found in northern Japan in 2008. Virus Genes 41: 351–357. doi: 10.1007/s11262-010-0530-3 [DOI] [PubMed] [Google Scholar]
- 22.Palese P., Shaw M. L.2007. Orthomyxoviridae: The viruses and their replication. pp. 1646–1650. In: Fields Virology (Knipe, D.M. and Howley, P.M. eds.), Lippincott Williams & Wilkins, a Wolters Kluwer Business, Philadelphia. [Google Scholar]
- 23.Pleschka S., Stein M., Schoop R., Hudson J. B.2009. Anti-viral properties and mode of action of standardized Echinacea purpurea extract against highly pathogenic avian influenza virus (H5N1, H7N7) and swine-origin H1N1 (S-OIV). Virol. J. 6: 197. doi: 10.1186/1743-422X-6-197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sheu T. G., Deyde V. M., Okomo-Adhiambo M., Garten R. J., Xu X., Bright R. A., Butler E. N., Wallis T. R., Klimov A. I., Gubareva L. V.2008. Surveillance for neuraminidase inhibitor resistance among human influenza A and B viruses circulating worldwide from 2004 to 2008. Antimicrob. Agents Chemother. 52: 3284–3292. doi: 10.1128/AAC.00555-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shinya K., Awakura T., Shimada A., Silvano F. D., Umemura T., Otsuki K.1995. Pathogenesis of pancreatic atrophy by avian influenza a virus infection. Avian Pathol. 24: 623–632. doi: 10.1080/03079459508419102 [DOI] [PubMed] [Google Scholar]
- 26.Sood R., Swarup D., Bhatia S., Kulkarni D. D., Dey S., Saini M., Dubey S. C.2012. Antiviral activity of crude extracts of Eugenia jambolana Lam. against highly pathogenic avian influenza (H5N1) virus. Indian J. Exp. Biol. 50: 179–186. [PubMed] [Google Scholar]
- 27.Sundararajan A., Ganapathy R., Huan L., Dunlap J. R., Webby R. J., Kotwal G. J., Sangster M. Y.2010. Influenza virus variation in susceptibility to inactivation by pomegranate polyphenols is determined by envelope glycoproteins. Antiviral Res. 88: 1–9. doi: 10.1016/j.antiviral.2010.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Swayne D. E., Suarez D. L., Sims L. D.2013. Influenza. pp. 181–218 In: Swayne, D. E. Diseases of Poultry, 13th ed. (Glisson, J. R., McDougald, L. R., Nolan, L. K., Suarez, D. L. and Nair, V. eds.), Wiley-Blackwell, Amesterdam. [Google Scholar]
- 29.Uchide N., Ohyama K., Toyoda H.2010. Current and future anti-influenza virus drugs. Open Antimicrob. Agents J 2: 34–48. doi: 10.2174/1876518101002020034 [DOI] [Google Scholar]
- 30.Von Bülow V., Biggs P. M.1975. Differentiation between strains of Marek’s disease virus and turkey herpesvirus by immunofluorescence assays. Avian Pathol. 4: 133–146. doi: 10.1080/03079457509353859 [DOI] [PubMed] [Google Scholar]
- 31.Welliver R., Monto A. S., Carewicz O., Schatteman E., Hassman M., Hedrick J., Jackson H. C., Huson L., Ward P., Oxford J. S., Oseltamivir Post Exposure Prophylaxis Investigator Group2001. Effectiveness of oseltamivir in preventing influenza in household contacts: a randomized controlled trial. JAMA 285: 748–754. doi: 10.1001/jama.285.6.748 [DOI] [PubMed] [Google Scholar]
- 32.World Health Organization (WHO)2002. WHO Manual on Animal Influenza Diagnosis and Surveillance. Available online at http://www.who.int/csr/resources/publications/influenza/whocdscsrncs20025rev.pdf.
- 33.World Health Organization (WHO)2007. Recommendations and laboratory procedures for detection of avian influenza A (H5N1) virus in specimens from suspected human cases. http://www.who.int/influenza/resources/documents/RecAIlabtestsAug07.pdf?ua=1.
- 34.World Health Organization (WHO)2016. Cumulative number of confirmed human cases of avian influenza A (H5N1) reported to WHO. Available online at http://www.who.int/influenza/human_animal_interface/H5N1_cumulative_table_archives/en/.
- 35.Yamamoto Y., Nakamura K., Okamatsu M., Yamada M., Mase M.2008. Avian influenza virus (H5N1) replication in feathers of domestic waterfowl. Emerg. Infect. Dis. 14: 149–151. doi: 10.3201/eid1401.071036 [DOI] [PMC free article] [PubMed] [Google Scholar]