Abstract
We evaluated the anti-inflammatory effect of tyrosol (Tyr) on endotoxin-induced uveitis (EIU) in rats. EIU was induced in male Lewis rats by subcutaneous injection of lipopolysaccharide (LPS). Tyr (10, 50 or 100 mg/kg) was intravenously injected 2 hr before, simultaneously and 2 hr after LPS injection. The aqueous humor (AqH) was collected 24 hr after LPS injection; the infiltrating cell number, protein concentration, and tumor necrosis factor (TNF)-α, prostaglandin (PG)-E2 and nitric oxide (NO) levels were determined. Histopathologic examination and immunohistochemical studies for nuclear factor (NF)-κB, inhibitor of κB (IκB)-α, cyclooxygenase (COX)-2 and inducible NO synthase (iNOS) in the iris–ciliary body (ICB) were performed at 3 or 24 hr after LPS injection. To further clarify the anti-inflammatory effects, RAW264.7 macrophages were stimulated with LPS in the presence or absence of Tyr. Tyr reduced, in a dose-dependent manner, the infiltrating cell number, protein concentration, and TNF-α, PGE2 and NO levels in AqH and improved histopathologic scores of EIU. Tyr also inhibited LPS-induced COX-2 and iNOS expression, IκB-α degradation and nuclear translocation of activated NF-κB in ICB. Tyr significantly suppressed inflammatory mediator production in the culture medium and COX-2 and iNOS expression and activated NF-κB translocation in LPS-stimulated RAW264.7 cells. These results suggest that Tyr suppresses ocular inflammation of EIU by inhibiting NF-κB activation and subsequent proinflammatory mediator production.
Keywords: anti-inflammatory effect, endotoxin-induced uveitis, nuclear factor-κB, tyrosol
Endotoxin-induced uveitis (EIU) is an animal model of acute anterior segment inflammation induced by an injection of lipopolysaccharide (LPS) that is extracted from gram-negative bacterial cells [34]. This model is characterized by leukocyte infiltration and protein leakage into the anterior chamber mainly by disruption of the blood–aqueous barrier (BAB). In EIU, leukocyte infiltration and protein leakage into the aqueous humor (AqH) peak at 24 hr after LPS injection [7, 12]. Although EIU pathogenesis remains unclear, the evidence indicates that the elevated inflammatory cytokine expression, including tumor necrosis factor (TNF)-α, interleukin (IL)-1β and IL-6, plays an important role in EIU development [12,13,14, 16]. Prostaglandin (PG)-E2 and nitric oxide (NO) derived from cyclooxygenase (COX)-2 and inducible NO synthase (iNOS), respectively, are involved in EIU development [5, 17].
Nuclear factor (NF)-κB is a family of important transcription factors that upregulate pro-inflammatory cytokine gene expression [3]. There are five subunits in the NF-κB family [p50, p65 (RelA), c-Rel, p52 and RelB] in mammals. The most common form of NF-κB is the p65/p50 heterodimers. The NF-κB dimer is normally present in the cytoplasm in an inactive form bound to its suppressor protein, which is called the inhibitor of κB (IκB)-α [24, 38]. Extracellular signals, such as LPS, induce ubiquitylation and subsequent proteolytic IκB-α degradation, which results in nuclear translocation of activated NF-κB. The active form of NF-κB binds to specific sequences of target genes involved in inflammation and innate immune response [32, 44]. NF-κB activation is enhanced in iris–ciliary body (ICB) of EIU rats [18, 23], and it plays a pivotal role in EIU pathogenesis by upregulation of inflammatory mediator gene expression [39].
Tyrosol (Tyr), 2-(4-Hydroxyphenyl) ethanol, is a phenolic compound abundantly found in olive oil, white wine and plant extracts [2, 37, 41]. Tyr has numerous biological activities, including antioxidant and neuro- and cardio-protective effects [8, 14, 27, 36], and exhibits anti-inflammatory properties. Tyr treatment inhibited TNF-α, IL-1β and IL-6 production by LPS-stimulated human peripheral blood mononuclear cells [6] and decreased PGE2 and NO levels produced by phorbol ester-stimulated RAW264.7 macrophages [27]. The mechanisms underlying these properties of Tyr may be associated with the inhibition of NF-κB activation [11, 27]. Tyr shows potential anti-inflammatory activities; however, its effect and mechanisms in the ocular tissue remain unclear.
In the present study, we investigated the effect of Tyr on anterior segment inflammation and mechanisms associated with the NF-κB pathway using a rat EIU model and LPS-stimulated RAW264.7 macrophages.
MATERIALS AND METHODS
Animals: Male Lewis rats (7 weeks, 170–180 g) were purchased from Japan SLC, Inc. (Hamamatsu, Japan). Rats were randomly divided to a control group, a LPS group and a LPS + Tyr group (Tyr group) in the same number. There were 29 rats for AqH collection in each group; 8 of 29 rats were used for cell counting and protein concentration assay, and the other rats for measurement of inflammatory mediator levels. Eight other rats were used for histologic examination. EIU was induced by subcutaneous injection with 200 µg of LPS from Salmonella Typhimurium (Sigma-Aldrich Co. LLC, St. Louis, MO, U.S.A.) diluted in 0.2 ml of sterilized saline. Two hr before, simultaneously and 2 hr after LPS injection, animals were injected intravenously with 10, 50 or 100 mg/kg of Tyr (188255; Sigma-Aldrich Co. LLC) diluted in 0.8 ml of sterilized saline under isoflurane (Dainippon Sumitomo Pharma Co., Ltd., Osaka, Japan) anesthesia. Animals in the control and LPS groups were treated with 0.8 ml of sterilized saline in the same way as the Tyr group. All animals were cared for in accordance with the Association for Research in Vision and Ophthalmology (ARVO) Statement, and with Animal Care and Use committee of Kitasato University (approval no. 09–109) for the use of animals in ophthalmic and vision research.
Infiltrating cell number and protein concentration in AqH: Twenty-four hr after LPS injection, rats were euthanized under deep isoflurane anesthesia, and AqH (20–25 µl/rat) was collected immediately from both eyes with a 30-gauge needle under a surgical microscope (OME-1000; Olympus Optical Co., Ltd., Tokyo, Japan). AqH samples were stored in ice water until tested. The infiltrating cell number and protein concentration were measured on the day of sample collection.
To count infiltrating cell number, AqH samples in the LPS and Tyr groups were diluted 10-fold with sterilized saline, whereas those in the control group were not diluted. Then, AqH samples were mixed in an equivalent amount of Türk’s stain solution, and infiltrating cell number was counted with a hemocytometer (Bürker-Türk hemocytometer; Erma Inc., Tokyo, Japan) under an optical microscope. The cell number per microliter was obtained by averaging the results of four grid areas of the hemocytometer. Immediately after counting the cell number, AqH samples were centrifuged at 2,500 rpm for 5 min at 4°C to obtain the supernatants. The supernatants in the Tyr and LPS groups were diluted 100-fold with sterilized saline, while those in the control group were diluted 5-fold for measuring the protein concentration. The total protein concentration in AqH was measured with a bicinchoninic acid (BCA) protein assay kit (Pierce, Rockford, IL, U.S.A.), and an MTP-300 microplate reader (Corona Electric Co., Ltd., Hitachinaka, Japan) was used to measure the absorbance spectrophotometrically at 540 nm.
Histopathologic evaluation: Rats were euthanized 24 hr after LPS injection. Both eyes were enucleated immediately under a surgical microscope and fixed in 4% paraformaldehyde for 12 hr at 4°C, and then, the eyes were embedded in paraffin. Sagittal sections (3 µm) were cut near the optic nerve head and stained with hematoxylin and eosin (H&E). The histopathologic evaluation of the anterior chamber, ICB, vitreous and retina in the EIU rats was scored (Grade, 0 to 3) using a light microscope [21]. Grade 0 represents no infiltrating cells; grade 1 represents mild cell infiltration; grade 2 represents moderate cell infiltration; and grade 3 represents severe cell infiltration in the ocular tissues.
TNF-α, PGE2 and NO Levels in AqH: Twenty-four hr after LPS injection, rats were euthanized, and AqH from both eyes was immediately collected. TNF-α and PGE2 levels in AqH were measured by ELISA kits (TNF-α: RTA00; R&D Systems Inc., Minneapolis, MN, U.S.A.; PGE2: KGE004B; R&D Systems Inc.) in accordance with each instruction manual (n=7). The absorbance was measured with the microplate reader at 450 nm (dominant wavelength) and 540 nm (correction wavelength). The total NO produced in AqH was measured by a Colorimetric Nitric Oxide Assay Kit (NB98; Oxford Biomedical Research Inc., Oxford, MI, U.S.A.) in accordance with the instruction manual (n=7). The absorbance was measured using the microplate reader at 540 nm.
Immunohistochemical studies for NF-κB, IκB-α, COX-2 and iNOS in ICB: At 3 or 24 hr after LPS injection, rats were euthanized, and both eyes were enucleated. The sections from the eyes embedded in paraffin were dewaxed with Clear Plus (Falma Co., Inc., Tokyo, Japan) and rehydrated with ethanol. Antigen retrieval was performed by immersing sections in 10 mM sodium citrate buffer solution and heating in an autoclave for 10 and 5 min at 120°C for the detection of NF-κB and the other parameters, respectively. The tissue samples were incubated with primary antibodies against NF-κB p65 (1:200; sc-372; Santa Cruz Biotechnology Inc., Dallas, TX, U.S.A.), IκB-α (1:200; sc-371; Santa Cruz Biotechnology Inc.), COX-2 (1:50; sc-1747; Santa Cruz Biotechnology Inc.) and iNOS (1:200; sc-650; Santa Cruz Biotechnology Inc.) overnight at 4°C. The sections were then gently washed with 10 mM phosphate-buffered saline (PBS) and incubated with a biotinylated secondary antibody (Nichirei Biosciences Inc., Tokyo, Japan) for 30 min at room temperature. After washing with 10 mM PBS, the sections were incubated with peroxidase-labeled streptavidin (Nichirei Biosciences Inc.) for 40 min at room temperature, and then, the antibody reaction products were observed with 3,3′-diaminobenzidine tetrahydrochloride (DAB) substrate solution (Nichirei Biosciences Inc.). After that, the sections for the detection of IκB-α, COX-2 and iNOS were counterstained with hematoxylin.
Cell culture: RAW264.7, a mouse leukemic monocyte/macrophage cell line, was obtained from the American Type Culture Collection (Rockville, MD, U.S.A.) and cultured in RPMI-1640 medium supplemented with 2 mM L-glutamine, 10% heat-inactivated fetal bovine serum and 1% antibiotics (100 U/ml penicillin and 100 µg/ml streptomycin) at 37°C in 5% CO2. Cells with a passage number less than 20 were used.
Cell viability: Cell Counting Kit-8 (Dojindo Molecular Technologies Inc., Kumamoto, Japan) was used to assay the viability of the RAW264.7 cells. RAW264.7 cells were seeded into 96-well plates in triplicate and precultured for 24 hr. Then, cells were treated with 10, 50 or 100 µM of Tyr for 48 hr and subsequently cultured with kit reagent (10 µl/well) for 3 hr. Cell viability was determined by measuring the absorbance at 450 nm with the microplate reader. The absorbance was measured with the microplate reader at 450 nm (dominant wavelength) and 540 nm (correction wavelength).
TNF-α, PGE2 and NO Levels in the culture medium: RAW264.7 cells were pretreated with 10, 50 or 100 µM of Tyr for 24 hr and subsequently incubated with 1 µg/ml LPS for 24 hr without exchanging with fresh medium. After that, the cell culture supernatant was collected to measure TNF-α, PGE2 and NO levels. TNF-α and PGE2 levels in the supernatant were measured by ELISA kits (TNF-α: MTA00B; R&D Systems Inc.; PGE2: 514010; Cayman Chemical Co., Ann Arbor, MI, U.S.A.) in accordance with each instruction manual. The absorbance was measured with the microplate reader at 450 nm (dominant wavelength) and 540 nm (correction wavelength). The total level of nitrate/nitrite in AqH was measured using a NO2/NO3 colorimetric assay kit (NK05; Dojindo Molecular Technologies, Inc.). The absorbance was measured using the microplate reader at 540 nm.
Western blot analysis for COX-2 and iNOS: After treatment, cells were washed three times with PBS and lysed in 200 µl lysis buffer (1% NP-40; 50 mM Tris-HCl, pH 7.5; 150 mM NaCl; 10% glycerol; 1 mM phenylmethylsulfonyl fluoride; and 5 mM protease/phosphatase inhibitor cocktail; Cell Signaling Technology, Inc., Madrid, Spain) for 20 min on ice. The plates were scraped, and the whole cell extracts were obtained by centrifugation at 14,000 ×g for 5 min at 4°C. Then, the protein concentration of each sample was determined using the BCA protein assay kit, and the rest of each of the samples was diluted in the same amount of sodium dodecyl sulfate (SDS) buffer (0.125 M Tris-HCl [pH 6.8], 5% SDS, 10% 2-mercaptoethanol, 20% glycerol and 0.1% bromophenol blue) and heated for 4 min at 95°C. The samples were stored at 4°C until use. Equal amounts (1 µg) of protein were separated on 8% SDS–polyacrylamide gels and transferred to polyvinylidene difluoride (PVDF) membranes (Immobilon®-P Transfer Membranes; Merck Millipore Co., Bedford, MA, U.S.A.). The membranes were blocked with PVDF Blocking Reagent (TOYOBO Co., Ltd., Osaka, Japan) diluted 10-fold with distilled water for 1 hr and incubated with primary antibodies against COX-2 (1:200; sc-1747; Santa Cruz Biotechnology Inc.), iNOS (1:200; sc-650; Santa Cruz Biotechnology Inc.) and α-tubulin (1:100; RB-9281-P1; Thermo Fisher Scientific Inc., Waltham, MA, U.S.A.) for 1 hr at room temperature. After washing the membranes three times with tris-buffered saline with tween 20 (TBS-T) solution for 5 min each, the membranes were incubated with a horseradish peroxidase-conjugated secondary antibody (1:20,000; NA934V; GE Healthcare UK, Ltd., Chalfont St. Giles, U.K.) for 1 hr at room temperature. The membranes were washed three times with TBS-T again and incubated with ECL Plus Western Blotting Detection Reagents (GE Healthcare U.K., Ltd.). The bands were visualized by a chemiluminescenes detection device (FluorChemTM; Alpha Innotech Co., San Leandro, CA, U.S.A.), and the band intensity was analyzed by AlphaEase FCTM software (Alpha Innotech Co.). The target protein absolute intensity was normalized to α-tubulin absolute intensity. SDS-polyacrylamide gel electrophoresis (PAGE) and western blot analysis were performed three times with similar trends.
Immunocytochemical studies for NF-κB: RAW264.7 cells were seeded into four-well plates with cover glass in the bottom of the wells and precultured for 1 hr. Cells were then pretreated with 10, 50 or 100 µM of Tyr for 24 hr and subsequently stimulated with LPS for 30 min. After incubation, cells were washed twice with PBS, and fixed and permeabilized with 100% methanol (Kanto Kagaku, Tokyo, Japan) for 20 min at −20°C. Then, cells were washed once with TBS-T for 1 min and blocked with 3% goat serum (Nichirei Biosciences Inc.) diluted in TBS-T for 30 min at room temperature. After blocking, cells were incubated with anti-NF-κB p65 antibody (1:200; sc-372; Santa Cruz Biotechnology Inc.) diluted in 1% goat serum/TBS-T for 1 hr at 4°C. Cells were then washed four times with TBS-T for 5 min each and subsequently incubated with Alexa Fluor–conjugated secondary antibody (1:1,000; 4412S; Cell Signaling Technology Inc.) for 1 hr at room temperature in the dark. Then, cells were washed four times and once with TBS-T and distilled water for 5 min each, respectively. Cell nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI) (VECTASHIELD Antifade Mounting Medium with DAPI; Vector Laboratories Inc., Burlingame, CA, U.S.A.). Images were captured using a fluorescence microscope (ECLIPSE E600; Nikon Co., Tokyo, Japan).
Statistical analysis: The results are expressed as the means ± standard deviation (S.D.). Statistical analysis was performed with StatMate III (ATMS Co., Ltd., Tokyo, Japan). To compare the data of two independent groups, t-tests were used. One-way analysis of variance (ANOVA) followed by Tukey’s post hoc test and Kruskal–Wallis test followed by Newman–Keuls post hoc test were used to compare the data of more than three independent groups. P<0.05 was considered statistically significant.
RESULTS
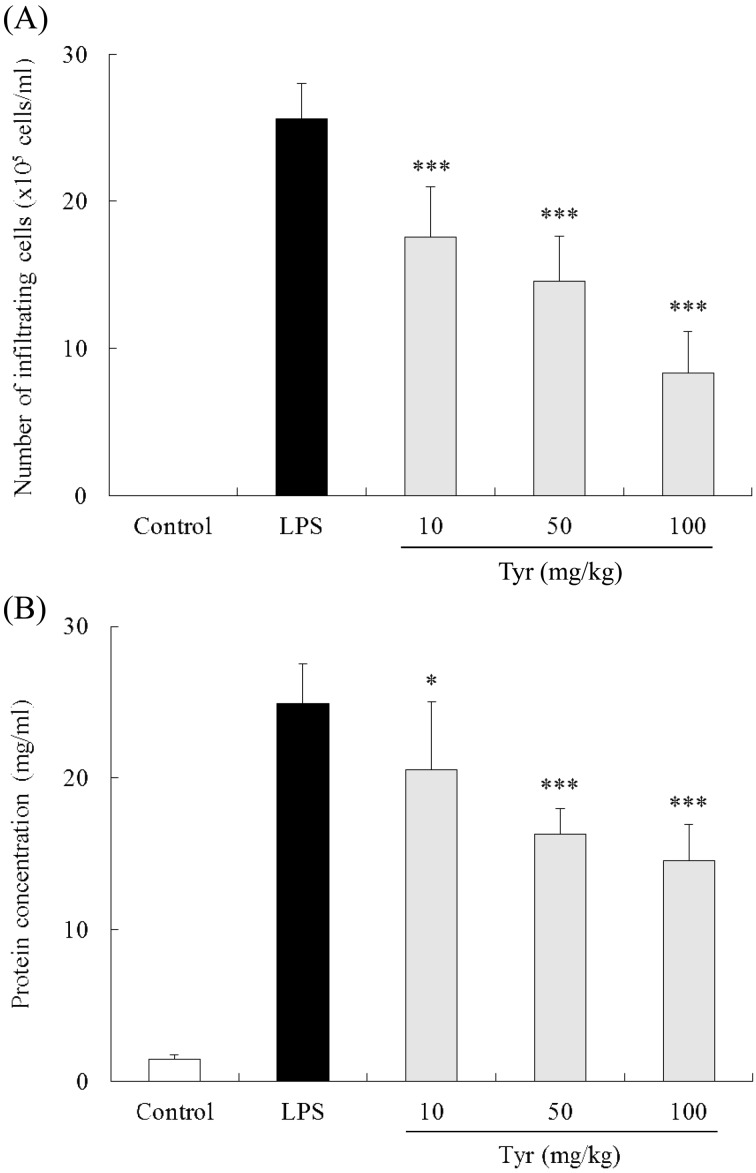
Effect of Tyr on cellular infiltration and protein concentration in AqH: Twenty-four hr after LPS injection, the cell number in AqH of untreated EIU rats was 25.6 ± 2.4 × 105 cells/ml (n=8), while that in the control group was not detected. Treatment with 10, 50 and 100 mg/kg of Tyr significantly reduced the cell number in AqH (10 mg/kg: 17.6 ± 3.4 × 105 cells/ml, P<0.001; 50 mg/kg: 14.1 ± 3.9 × 105 cells/ml, P<0.001; and 100 mg/kg: 8.3 ± 2.8 × 105 cells/ml, P<0.001) in a dose-dependent manner (Fig. 1A).
Fig. 1.
Effect of Tyr on cellular infiltration (A) and protein concentration (B) in AqH 24 hr after LPS injection. Data represent the mean ± S.D. (n=8). *P<0.05, ***P<0.001 significantly different from LPS group.
The concentration of protein in AqH of the LPS group was 24.1 ± 2.7 mg/ml (n=8) compared with only 1.4 ± 0.3 mg/ml in the control group. Treatment with 10, 50 and 100 mg/kg of Tyr significantly reduced the protein concentration in AqH (10 mg/kg: 19.7 ± 4.2 mg/ml, P<0.05; 50 mg/kg: 16.1 ± 1.3 mg/ml, P<0.001; and 100 mg/kg: 14.3 ± 2.3 mg/ml, P<0.001) in a dose-dependent fashion (Fig. 1B).
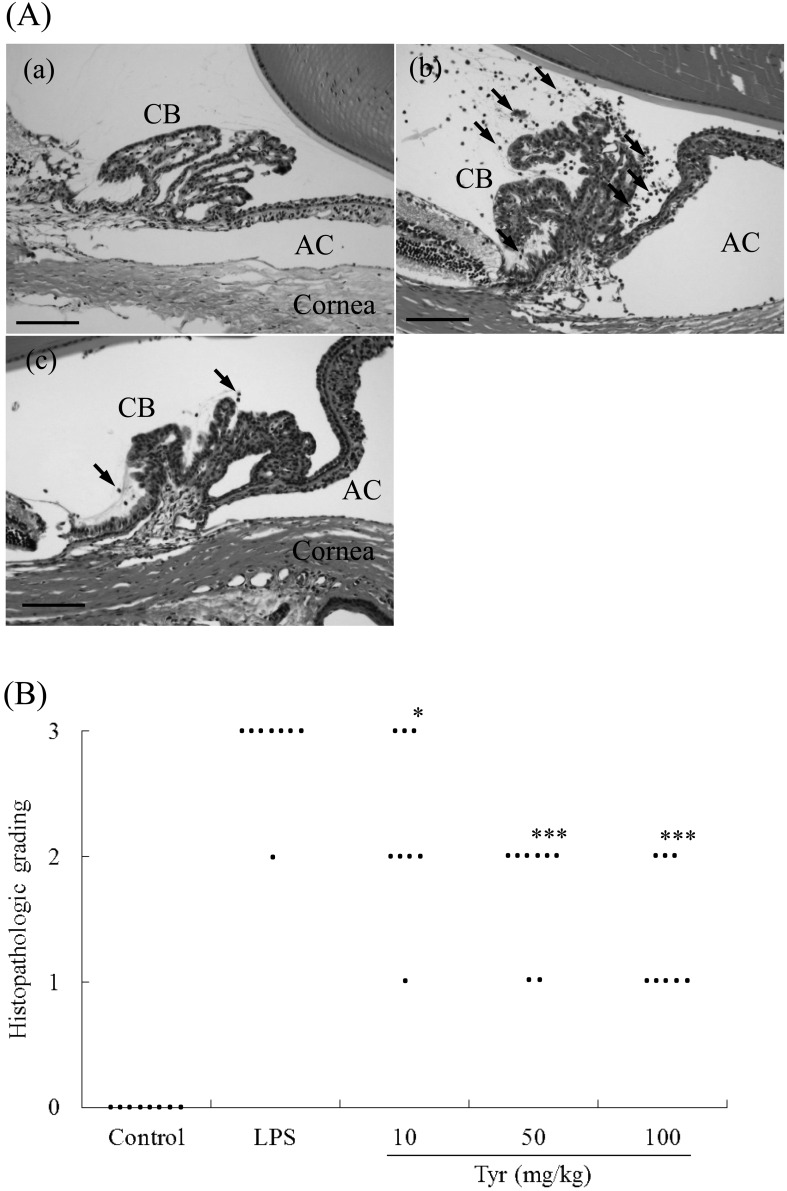
Histopathological findings: Tyr showed significant reduction of inflammation in the anterior segment compared with the LPS group (Fig. 2A). Cellular infiltration consisted of mainly macrophages and neutrophils. The mean histopathologic score in the LPS group was 2.875 (n=8). Conversely, no inflammation was detected in the control group. Treatment with 10, 50 and 100 mg/kg of Tyr significantly reduced inflammation in eyes in a dose-dependent manner (10 mg/kg: 2.429, P<0.05; 50 mg/kg: 1.75, P<0.001; and 100 mg/kg: 1.375, P<0.001) (Fig. 2B).
Fig. 2.
Histologic evaluation in the anterior segment 24 hr after LPS injection. A: histologic changes in the anterior segment 24 hr after LPS injection. Rats without LPS injection (a) showed no inflammation in the anterior segment. Untreated EIU rats (b) showed severe inflammatory cell infiltration. Rats injected with LPS and 100 mg/kg of Tyr showed a significant reduction in inflammation in the anterior segment (c). H&E staining; original magnification: ×200; Bars: 100 µm; AC: anterior chamber; CB: ciliary body; Arrows: inflammatory cells. B: effect of various doses of Tyr on the histopathologic score of EIU. The score represents the mean ± S.D. (n=8). *P<0.05, ***P<0.001 significantly different from LPS group.
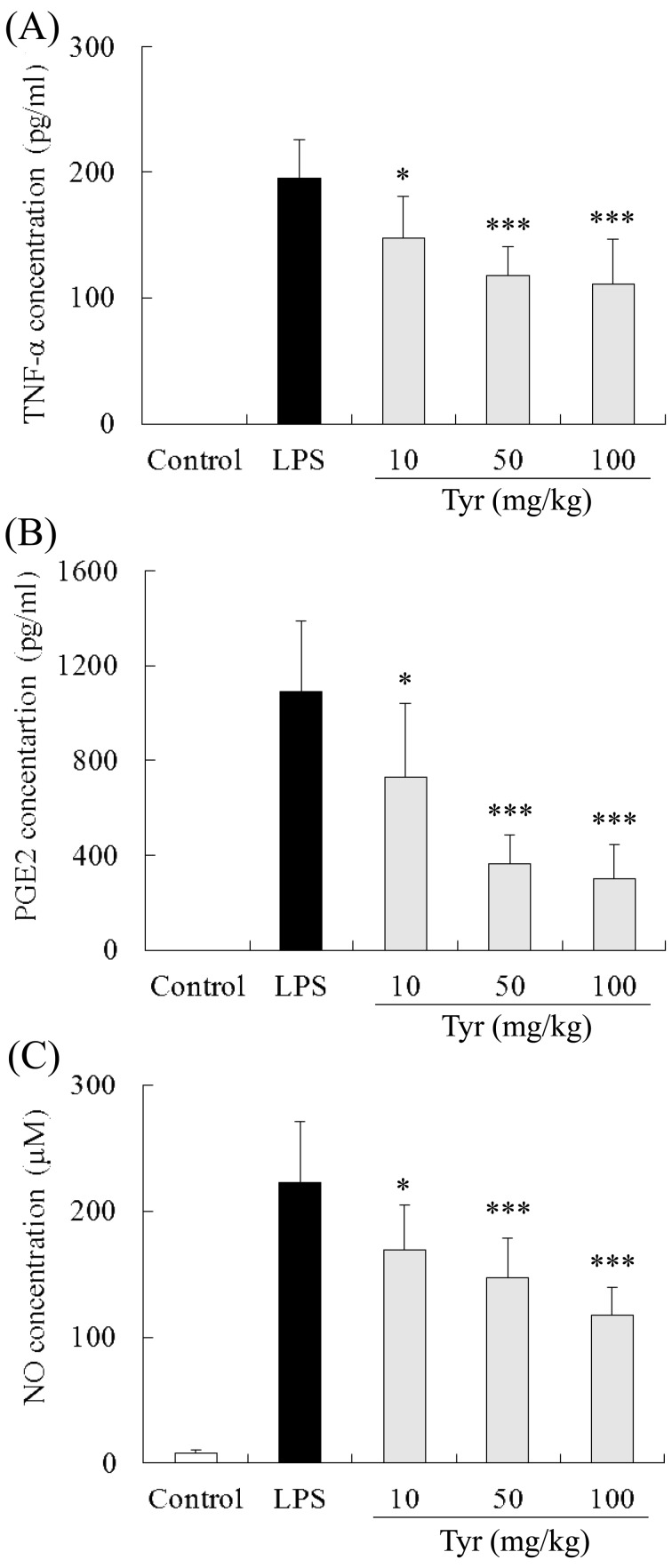
Effect of Tyr on TNF-α production in AqH: The level of TNF-α in AqH of untreated EIU rats (LPS group) was 195.2 ± 30.6 pg/ml (n=7). Treatment with 10, 50 and 100 mg/kg of Tyr significantly reduced the TNF-α concentration compared with the LPS group (10 mg/kg: 147.0 ± 33.5 pg/ml, P<0.05; 50 mg/kg: 117.5 ± 23.2 pg/ml, P<0.001; and 100 mg/kg: 111.2 ± 35.1 pg/ml, P<0.001) (Fig. 3A). In the control group, no TNF-α was detected in AqH.
Fig. 3.
Inhibitory effects of Tyr on TNF-α, PGE2 and NO production in EIU in rats. The aqueous humor of EIU rats collected 24 hr after LPS injection was used to measure the concentration of TNF-α (A), PGE2 (B) and NO (C). Data represent the mean ± S.D. (n=7). *P<0.05, ***P<0.001 significantly different from LPS group.
Effect of Tyr on PGE2 production in AqH: The PGE2 concentration in AqH of the LPS group was 1,088.4 ± 301.2 pg/ml (n=7). Treatment with 10, 50 and 100 mg/kg of Tyr significantly reduced the PGE2 concentration compared with the LPS group (10 mg/kg: 727.1 ± 311.3 pg/ml, P<0.05; 50 mg/kg: 365.5 ± 120.5 pg/ml, P<0.01; and 100 mg/kg: 298.8 ± 146.1 pg/ml, P<0.001) (Fig. 3B). In the control group, no PGE2 was detected in AqH.
Effect of Tyr on NO production in AqH: The level of NO in AqH of the LPS group was 222.6 ± 48.1 µM (n=7), while that in the control group was only 7.9 ± 2.3 µM. Treatment with 10, 50 and 100 mg/kg of Tyr significantly reduced the NO concentration compared with the LPS group (10 mg/kg: 169.4 ± 35.0 µM, P<0.05; 50 mg/kg: 147.2 ± 31.3 µM, P<0.001; and 100 mg/kg: 117.1 ± 22.7 µM, P<0.001) (Fig. 3C).
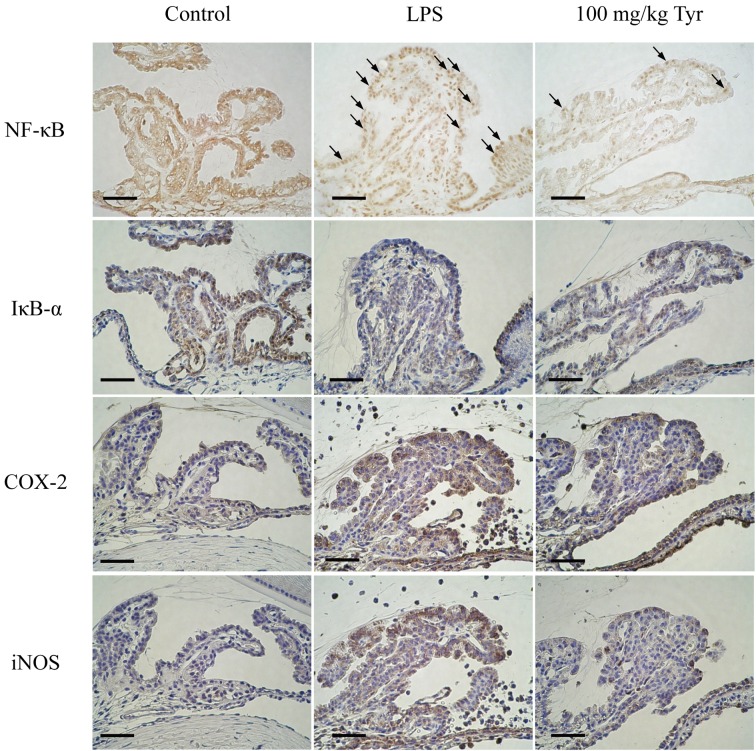
Immunohistochemistry of NF-κB, IκB-α, COX-2 and iNOS in ICB: At 3 hr after LPS injection, nuclear translocation of NF-κB and reduced IκB-α expression were observed in ICB of rats (LPS group). In the control group, both proteins were found in the cytoplasm of cells in ICB. Treatment with 100 mg/kg of Tyr remarkably suppressed both the nuclear translocation of NF-κB and reduction of IκB-α in ICB. At 24 hr after LPS injection, COX-2 and iNOS proteins were strongly expressed in ICB of rats (LPS group). Cells in ICB of the rats treated with 100 mg/kg of Tyr revealed markedly decreased both protein expression. In the control group, COX-2 and iNOS expression in ICB was not detected (Fig. 4).
Fig. 4.
Immunohistochemistry to determine the effects of Tyr on the nuclear translocation of NF-κB, IκB-α degradation, and COX-2 and iNOS expression in ICB of EIU rats. Sections from the eyes of rats with or without LPS treatment for 3 hr were incubated with polyclonal antibodies against NF-κB p65 and IκB-α, and for 24 hr against COX-2 and iNOS. Bars: 100 µm. Arrows: positive cells showing nuclear translocation of NF-κB.
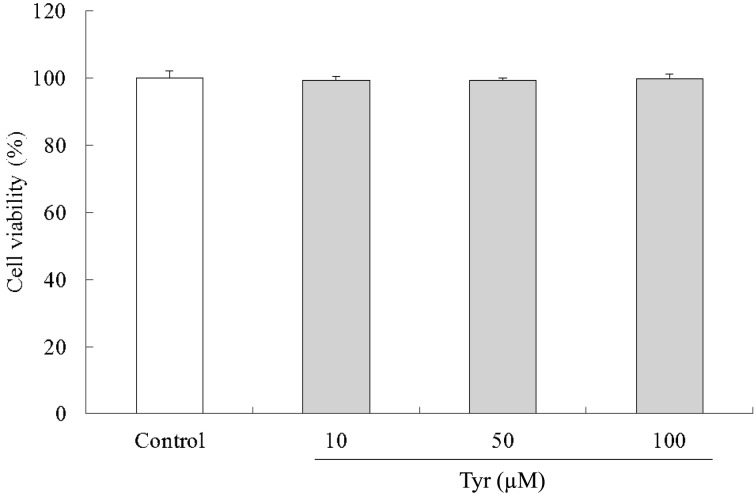
Effect of Tyr on cell viability: The survival curve showed that Tyr does not have any effect on the proliferation of cells at those concentrations (Fig. 5). As Tyr showed no effect on cell viability up to 100 µM in the RAW264.7 cells, Tyr was used at a concentration in the range 10 to 100 µM for in vitro studies.
Fig. 5.
Effect of Tyr on the viability of RAW264.7 cells. Cells (8 × 104 cells/ml) precultured for 24 hr were incubated with or without the indicated concentrations of Tyr for 48 hr. Cell viability was determined by Cell Counting Kit-8. Data represent the mean ± S.D. (n=3).
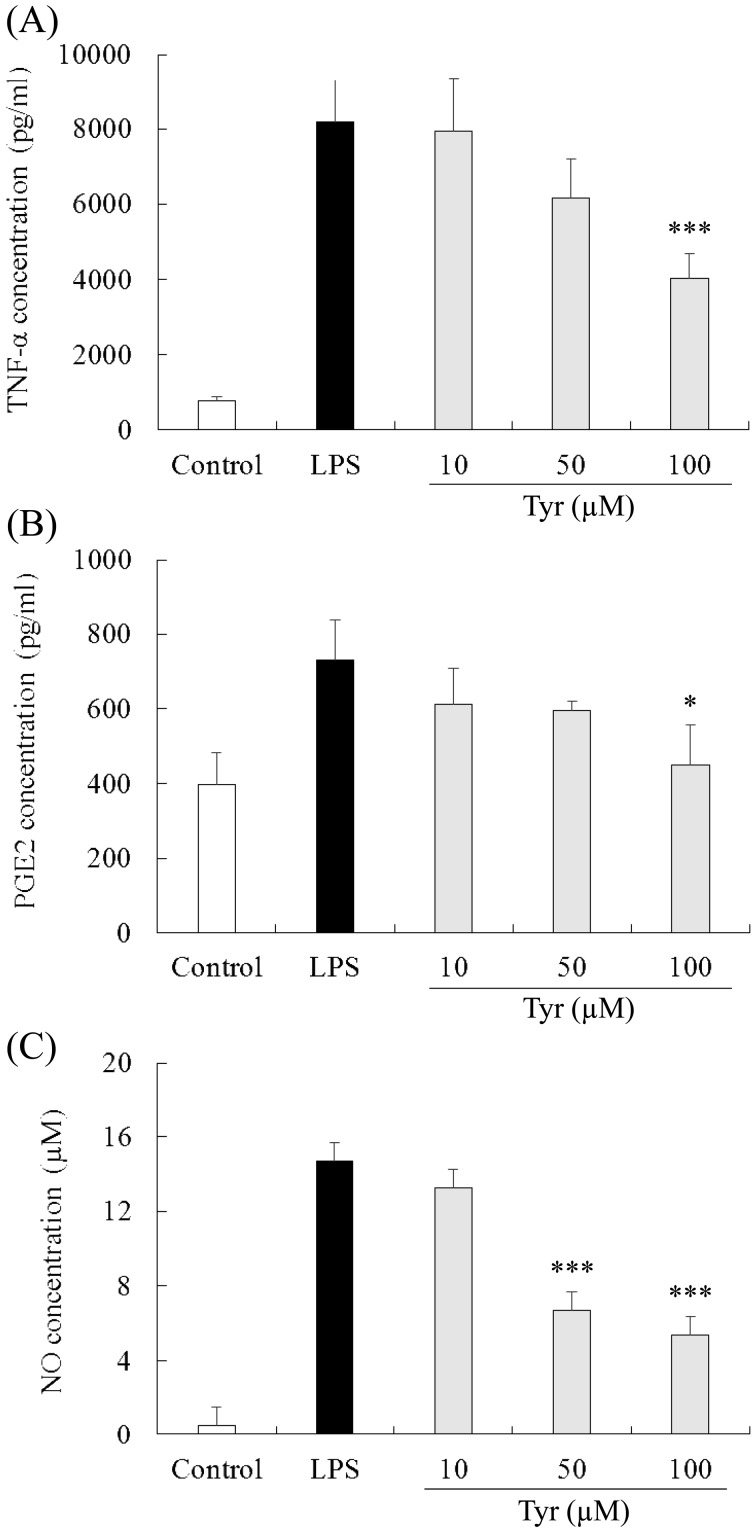
Effect of Tyr on TNF-α production in the culture medium: In the culture medium from the RAW264.7 cells stimulated with LPS for 24 hr, the TNF-α concentration was 8,172.0 ± 1,139.1 pg/ml (n=4). Treatment with 100 µM of Tyr significantly reduced the TNF-α concentration compared with the LPS group (4,027.3 ± 662.5, P<0.001), while 10 and 50 µM Tyr showed little and moderate effects on the reduction in TNF-α concentration, respectively (10 µM: 7,939.9 ± 1,408.1 pg/ml; and 50 µM: 6,163.5 ± 1,035.1 pg/ml) (Fig. 6A). In the control group, the TNF-α concentration in the medium was 761.0 ± 116.8 pg/ml.
Fig. 6.
Inhibitory effects of Tyr on TNF-α, PGE2 and NO production in LPS-stimulated RAW264.7 cells. Cells were incubated with the indicated concentrations of Tyr for 24 hr and subsequently with LPS for 24 hr. The treated culture media were used to measure the concentrations of TNF-α (A), PGE2 (B) and NO (C). Data represent the mean ± S.D. (n=4). *P<0.05, ***P<0.001 significantly different from LPS group.
Effect of Tyr on PGE2 production in the culture medium: In the control group, the PGE2 concentration in the medium was 398.4 ± 85.1 pg/ml (n=4). In the LPS group, the PGE2 concentration was 731.0 ± 106.4 pg/ml. Treatment with 100 µM of Tyr (449.5 ± 107.6 pg/ml, P<0.05) significantly reduced the PGE2 concentration, which was almost the same as that of the control group. Treatment with 10 and 50 µM Tyr showed equivalent effects on the decrease in the PGE2 concentration (10 µM: 611.9 ± 97.4 pg/ml; and 50 µM: 596.5 ± 22.5 pg/ml), and neither showed significant effects compared with the LPS group (Fig. 6B).
Effect of Tyr on NO production in the culture medium: In the LPS group, the NO concentration in the medium was 14.7 ± 0.7 µM (n=4), and in the control group, the NO concentration was 0.5 ± 0.4 µM. Treatment with 50 and 100 µM of Tyr significantly decreased the NO concentration (50 µM: 6.7 ± 0.8 pg/ml, P<0.001; and 100 mg/kg: 5.4 ± 1.2 pg/ml, P<0.001), while 10 µM of Tyr (13.3 ± 1.2 µM) showed a slight effect on decrease of NO concentration in the LPS group (Fig. 6C).
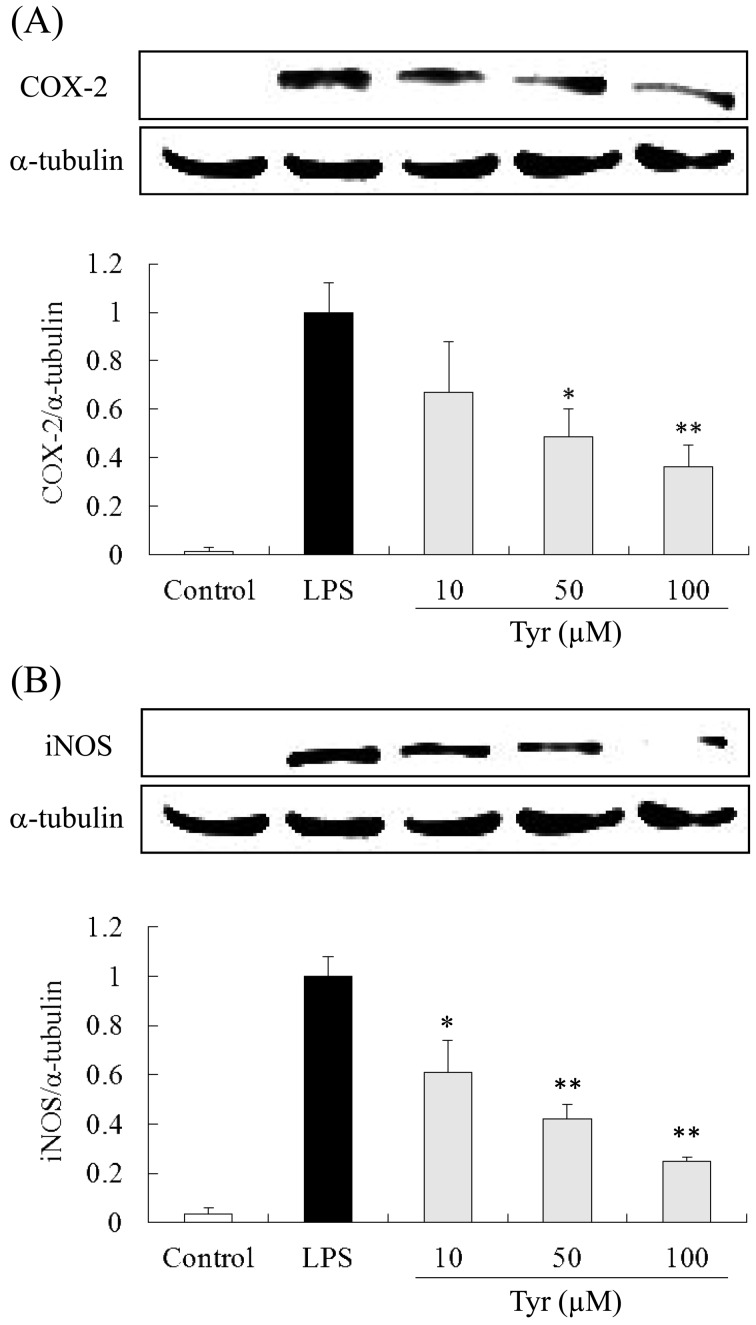
COX-2 and iNOS protein expression in RAW264.7 cells: COX-2 and iNOS expression in cells stimulated with LPS for 24 hr was strongly increased, while in the control group, little COX-2 and iNOS expression was detected. Tyr (10, 50 and 100 µM) reduced both protein expression in a dose-dependent manner. Particularly, 100 µM of Tyr significantly and most remarkably reduced both protein expression (Fig. 7A and 7B).
Fig. 7.
Inhibitory effects of Tyr on COX-2 (A) and iNOS (B) expression in LPS-stimulated RAW264.7 cells. Cells were incubated with the indicated concentrations of Tyr for 24 hr and subsequently with LPS for 24 hr. COX-2 and iNOS protein expression was detected by western blot analysis. Data represent the mean ± S.D. (n=3). *P<0.05, **P<0.01 significantly different from LPS group.
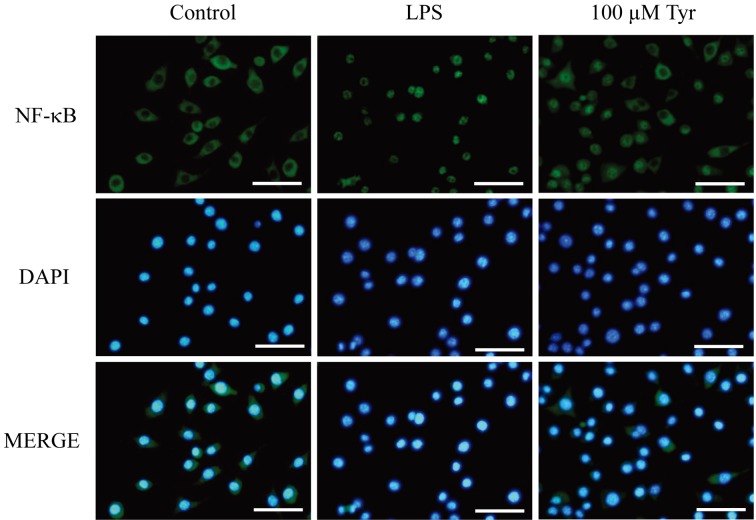
Immunocytochemistry of the p65 subunit of NF-κB in RAW264.7 cells: NF-κB is normally present in the cytoplasm (control group). Thirty min after LPS stimulation, NF-κB translocated into the nucleus in almost all of cells. Treatment with 100 µM of Tyr attenuated this nuclear translocation (Fig. 8).
Fig. 8.
Immunocytochemistry to determine the effect of Tyr on the nuclear translocation of NF-κB p65 in LPS-stimulated RAW264.7 cells. Cells were preincubated with 100 µM of Tyr for 24 hr and then stimulated with LPS for 30 min. Bars: 50 µm.
DISCUSSION
In the present study, Tyr significantly decreased LPS-induced cellular and protein infiltration in AqH of EIU rats and the histopathologic grade of EIU in a dose-dependent way. These results showed that Tyr significantly suppressed EIU development.
It is believed that EIU results from the release of numerous proinflammatory mediators, such as TNF-α, PGE2 and NO, by activated inflammatory cells, mainly in ICB [9, 13, 31]. TNF-α may play a major role in many inflammatory diseases, as it promotes and sustains inflammatory response by inducing adhesion molecules, particularly intercellular adhesion molecule (ICAM)-1, and stimulating the synthesis of PGE2, NO and other cytokines, such as IL-1β and IL-6 [1]. Local injection of TNF-α induced intraocular inflammation in rats [13]; therefore, inhibition of intraocular TNF-α production may suppress uveitis development. Anti-TNF-α antibody reportedly limited uveitis progression in patients with Behçet’s disease and remarkably improved the symptoms [28]. TNF-α expression is regulated by NF-κB, and conversely, TNF-α activates the NF-κB pathway as a stimulator; thus, this positive feedback loop further enhances the inflammatory response [10]. Our data showed that Tyr could suppress LPS-induced TNF-α elevation in AqH in a dose-dependent manner and nuclear translocation of activated NF-κB in ICB. Tyr could reduce the TNF-α level in the culture medium and inhibit activated NF-κB translocation in the LPS-stimulated RAW264.7 cells. These results indicate that Tyr consequently shows a suppressive effect on the positive loop between TNF-α and NF-κB in ICB. In addition to TNF-α, NF-κB also regulates COX-2 and iNOS expression, which are inducible enzymes synthesizing PGE2 and NO, respectively [5, 17, 40]. PGE2 and NO are also important inflammatory mediators involved in EIU pathophysiology [15, 17]. PGs represent a family of compounds with hormonal activity, but also have profound effects on inflammation. PGE2 has been detected in the anterior chamber in humans with acute anterior uveitis [43] and contributes to BAB breakdown and subsequent cellular infiltration and protein leakage into AqH in several animal models representing various forms of uveitis, including EIU [4, 33]. NO plays an important role in vascular homeostasis; however, it is an oxygen-free radical that may cause cellular and histologic damage [25]. Excessive NO production in the anterior segment also causes BAB breakdown in EIU by promoting vascular permeability [26]. It has been reported that treatment with diclofenac, a COX inhibitor, or NG-nitro-L-arginine methyl ester (L-NAME), a NOS inhibitor, reduced or delayed uveitis intensity induced by intravitreal injection of endotoxin in rabbits; these inhibitors were more effective on inflammation when combined than either alone [5]. Here, Tyr suppressed PGE2 and NO production in AqH, in a dose-dependent manner, and COX-2 and iNOS expression in ICB. Tyr reduced PGE2 and NO levels in the culture medium and suppressed COX-2 and iNOS expression in LPS-stimulated macrophage cells. TNF-α, PGE2 and NO may interact with each other in inflammatory response. PGs production may be involved in the regulation of NO synthesis by iNOS in RAW264.7 macrophages [42]. Conversely, NO activated COX enzymes and thereby led to a marked increase in PGE2 in the RAW264.7 cells [35]. NO may play a regulatory role in TNF production in EIU pathophysiology [29]. Thereby, the inhibition of all of these pathways would improve the therapeutic management of uveitis. The results of this study suggest that Tyr suppressed inflammatory mediator production, which might result in marked suppression of inflammatory manifestation in EIU rats.
NF-κB activity is enhanced in ICB of EIU rats, which leads to EIU development because of the excessive inflammatory mediator production, such as TNF-α, PGE2 and NO in the ocular tissue [19, 20]. Therefore, NF-κB is thought to play a critical role in ocular inflammation and be a therapeutic target in EIU. Pyrrolidine dithiocarbamate, an inhibitor of NF-κB, has been suggested to remarkably reduce inflammation in eyes with EIU by suppressing inflammation-related gene expression through inhibition of NF-κB activation in ICB [30]. Tyr can potentially downregulate NF-κB activity in a rat model of myocardial ischemic-reperfusion injury and several in vitro studies [11, 27, 40]; however, no previous study has addressed the effect of Tyr on blockade of the NF-κB pathway in ICB. Here, we showed for the first time that Tyr most likely exhibits a suppressive effect on LPS-induced NF-κB activation in ICB of EIU rats. A similar result was obtained from our in vitro study using LPS-stimulated RAW264.7 macrophages. These results suggested that inhibition of NF-κB activation in ICB by Tyr is responsible for the decreased inflammatory mediator production, such as TNF-α, PGE2 and NO in the ocular tissue. Kubota et al. showed that oral administration of resveratrol, which is the same dietary phenol as Tyr, prevented ocular inflammation in EIU in mouse by inhibiting NF-κB activation through suppression of IκB-α degradation and antioxidant activity [22]. Considering that Tyr suppressed IκB-α degradation, an endogenous inhibitor of NF-κB, in ICB of EIU rats, the inhibitory effect of Tyr on NF-κB activation might be attributed to the suppression of IκB-α degradation.
In summary, this study demonstrated that Tyr exhibits a dose-dependent anti-inflammatory effect both in a rat EIU model and LPS-stimulated RAW264.7 macrophages. The possible mechanisms for the anti-inflammatory effect of Tyr depend on the suppression of TNF-α, PGE2 and NO production in the ocular tissue through inhibition of NF-κB activation, in which the suppressing effect of IκB-α degradation might be involved.
REFERENCES
- 1.Akira S., Hirano T., Taga T., Kishimoto T.1990. Biology of multifunctional cytokines: IL 6 and related molecules (IL 1 and TNF). FASEB J. 4: 2860–2867. [PubMed] [Google Scholar]
- 2.Andrade P. B., Oliveira B. M., Seabra R. M., Ferreira M. A., Ferreres F., García-Viguera C.2001. Analysis of phenolic compounds in Spanish Albrariño and Portuguese Alvarinho and Loureiro wines by capillary zone electrophoresis and high-performance liquid chromatography. Electrophoresis 22: 1568–1572. doi: [DOI] [PubMed] [Google Scholar]
- 3.Baeuerle P. A., Henkel T.1994. Function and activation of NF-kappa B in the immune system. Annu. Rev. Immunol. 12: 141–179. doi: 10.1146/annurev.iy.12.040194.001041 [DOI] [PubMed] [Google Scholar]
- 4.Beitch B. R., Eakins K. E.1969. The effects of prostaglandins on the intraocular pressure of the rabbit. Br. J. Pharmacol. 37: 158–167. doi: 10.1111/j.1476-5381.1969.tb09533.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bellot J. L., Palmero M., García-Cabanes C., Espí R., Hariton C., Orts A.1996. Additive effect of nitric oxide and prostaglandin-E2 synthesis inhibitors in endotoxin-induced uveitis in the rabbit. Inflamm. Res. 45: 203–208. doi: 10.1007/BF02285162 [DOI] [PubMed] [Google Scholar]
- 6.Bertelli A. A., Migliori M., Panichi V., Longoni B., Origlia N., Ferretti A., Cuttano M. G., Giovannini L.2002. Oxidative stress and inflammatory reaction modulation by white wine. Ann. N. Y. Acad. Sci. 957: 295–301. doi: 10.1111/j.1749-6632.2002.tb02929.x [DOI] [PubMed] [Google Scholar]
- 7.Bhattacherjee P., Williams R. N., Eakins K. E.1983. An evaluation of ocular inflammation following the injection of bacterial endotoxin into the rat foot pad. Invest. Ophthalmol. Vis. Sci. 24: 196–202. [PubMed] [Google Scholar]
- 8.Bu Y., Rho S., Kim J., Kim M. Y., Lee D. H., Kim S. Y., Choi H., Kim H.2007. Neuroprotective effect of tyrosol on transient focal cerebral ischemia in rats. Neurosci. Lett. 414: 218–221. doi: 10.1016/j.neulet.2006.08.094 [DOI] [PubMed] [Google Scholar]
- 9.Bucolo C., Marrazzo G., Platania C. B., Romano G. L., Drago F., Salomone S.2014. Effects of topical indomethacin, bromfenac and nepafenac on lipopolysaccharide-induced ocular inflammation. J. Pharm. Pharmacol. 66: 954–960. doi: 10.1111/jphp.12224 [DOI] [PubMed] [Google Scholar]
- 10.Coward W. R., Okayama Y., Sagara H., Wilson S. J., Holgate S. T., Church M. K.2002. NF-κ B and TNF-α: a positive autocrine loop in human lung mast cells? J. Immunol. 169: 5287–5293. doi: 10.4049/jimmunol.169.9.5287 [DOI] [PubMed] [Google Scholar]
- 11.De Stefano D., Maiuri M. C., Simeon V., Grassia G., Soscia A., Cinelli M. P., Carnuccio R.2007. Lycopene, quercetin and tyrosol prevent macrophage activation induced by gliadin and IFN-γ. Eur. J. Pharmacol. 566: 192–199. doi: 10.1016/j.ejphar.2007.03.051 [DOI] [PubMed] [Google Scholar]
- 12.de Vos A. F., van Haren M. A., Verhagen C., Hoekzema R., Kijlstra A.1994. Kinetics of intraocular tumor necrosis factor and interleukin-6 in endotoxin-induced uveitis in the rat. Invest. Ophthalmol. Vis. Sci. 35: 1100–1106. [PubMed] [Google Scholar]
- 13.De Vos A. F., Van Haren M. A., Verhagen C., Hoekzema R., Kijlstra A.1995. Tumour necrosis factor-induced uveitis in the Lewis rat is associated with intraocular interleukin 6 production. Exp. Eye Res. 60: 199–207. doi: 10.1016/S0014-4835(95)80011-5 [DOI] [PubMed] [Google Scholar]
- 14.Ferrick M. R., Thurau S. R., Oppenheim M. H., Herbort C. P., Ni M., Zachariae C. O., Matsushima K., Chan C. C.1991. Ocular inflammation stimulated by intravitreal interleukin-8 and interleukin-1. Invest. Ophthalmol. Vis. Sci. 32: 1534–1539. [PubMed] [Google Scholar]
- 15.Helbig H., Kittredge K. L., Gurley R. C., Thurau S. R., Palestine A. G., Nussenblatt R. B.1990. Endotoxin-induced production of inflammatory mediators by cultured ciliary epithelial cells. Curr. Eye Res. 9: 501–505. doi: 10.3109/02713689008999616 [DOI] [PubMed] [Google Scholar]
- 16.Hoekzema R., Verhagen C., van Haren M., Kijlstra A.1992. Endotoxin-induced uveitis in the rat. The significance of intraocular interleukin-6. Invest. Ophthalmol. Vis. Sci. 33: 532–539. [PubMed] [Google Scholar]
- 17.Jacquemin E., de Kozak Y., Thillaye B., Courtois Y., Goureau O.1996. Expression of inducible nitric oxide synthase in the eye from endotoxin-induced uveitis rats. Invest. Ophthalmol. Vis. Sci. 37: 1187–1196. [PubMed] [Google Scholar]
- 18.Jin X. H., Ohgami K., Shiratori K., Suzuki Y., Hirano T., Koyama Y., Yoshida K., Ilieva I., Iseki K., Ohno S.2006. Inhibitory effects of lutein on endotoxin-induced uveitis in Lewis rats. Invest. Ophthalmol. Vis. Sci. 47: 2562–2568. doi: 10.1167/iovs.05-1429 [DOI] [PubMed] [Google Scholar]
- 19.Jin X. H., Ohgami K., Shiratori K., Suzuki Y., Koyama Y., Yoshida K., Ilieva I., Tanaka T., Onoe K., Ohno S.2006. Effects of blue honeysuckle (Lonicera caerulea L.) extract on lipopolysaccharide-induced inflammation in vitro and in vivo. Exp. Eye Res. 82: 860–867. doi: 10.1016/j.exer.2005.10.024 [DOI] [PubMed] [Google Scholar]
- 20.Kanai K., Ito Y., Nagai N., Itoh N., Hori Y., Chikazawa S., Hoshi F., Higuchi S.2012. Effects of instillation of eyedrops containing disulfiram and hydroxypropyl-β-cyclodextrin inclusion complex on endotoxin-induced uveitis in rats. Curr. Eye Res. 37: 124–131. doi: 10.3109/02713683.2011.622853 [DOI] [PubMed] [Google Scholar]
- 21.Kanai K., Itoh N., Ito Y., Nagai N., Hori Y., Chikazawa S., Hoshi F., Higuchi S.2011. Anti-inflammatory potency of oral disulfiram compared with dexamethasone on endotoxin-induced uveitis in rats. J. Vet. Med. Sci. 73: 517–520. doi: 10.1292/jvms.10-0239 [DOI] [PubMed] [Google Scholar]
- 22.Kubota S., Kurihara T., Mochimaru H., Satofuka S., Noda K., Ozawa Y., Oike Y., Ishida S., Tsubota K.2009. Prevention of ocular inflammation in endotoxin-induced uveitis with resveratrol by inhibiting oxidative damage and nuclear factor-kappaB activation. Invest. Ophthalmol. Vis. Sci. 50: 3512–3519. doi: 10.1167/iovs.08-2666 [DOI] [PubMed] [Google Scholar]
- 23.Lennikov A., Kitaichi N., Noda K., Mizuuchi K., Ando R., Dong Z., Fukuhara J., Kinoshita S., Namba K., Ohno S., Ishida S.2014. Amelioration of endotoxin-induced uveitis treated with the sea urchin pigment echinochrome in rats. Mol. Vis. 20: 171–177. [PMC free article] [PubMed] [Google Scholar]
- 24.Li Q., Verma I. M.2002. NF-kappaB regulation in the immune system. Nat. Rev. Immunol. 2: 725–734. doi: 10.1038/nri910 [DOI] [PubMed] [Google Scholar]
- 25.Lowenstein C. J., Dinerman J. L., Snyder S. H.1994. Nitric oxide: a physiologic messenger. Ann. Intern. Med. 120: 227–237. doi: 10.7326/0003-4819-120-3-199402010-00009 [DOI] [PubMed] [Google Scholar]
- 26.Mandai M., Yoshimura N., Yoshida M., Iwaki M., Honda Y.1994. The role of nitric oxide synthase in endotoxin-induced uveitis: effects of NG-nitro L-arginine. Invest. Ophthalmol. Vis. Sci. 35: 3673–3680. [PubMed] [Google Scholar]
- 27.Moreno J. J.2003. Effect of olive oil minor components on oxidative stress and arachidonic acid mobilization and metabolism by macrophages RAW 264.7. Free Radic. Biol. Med. 35: 1073–1081. doi: 10.1016/S0891-5849(03)00465-9 [DOI] [PubMed] [Google Scholar]
- 28.Nakamura S., Ohno S.2005. Anti-tumor necrosis factor alpha antibody in the treatment of Behçet’s disease. Int. Ophthalmol. Clin. 45: 179–189. doi: 10.1097/01.iio.0000155901.41938.3c [DOI] [PubMed] [Google Scholar]
- 29.Ohgami K., Shiratori K., Kotake S., Nishida T., Mizuki N., Yazawa K., Ohno S.2003. Effects of astaxanthin on lipopolysaccharide-induced inflammation in vitro and in vivo. Invest. Ophthalmol. Vis. Sci. 44: 2694–2701. doi: 10.1167/iovs.02-0822 [DOI] [PubMed] [Google Scholar]
- 30.Ohta K., Nakayama K., Kurokawa T., Kikuchi T., Yoshimura N.2002. Inhibitory effects of pyrrolidine dithiocarbamate on endotoxin-induced uveitis in Lewis rats. Invest. Ophthalmol. Vis. Sci. 43: 744–750. [PubMed] [Google Scholar]
- 31.Parks D. J., Cheung M. K., Chan C. C., Roberge F. G.1994. The role of nitric oxide in uveitis. Arch. Ophthalmol. 112: 544–546. doi: 10.1001/archopht.1994.01090160124032 [DOI] [PubMed] [Google Scholar]
- 32.Rafati D. S., Geissler K., Johnson K., Unabia G., Hulsebosch C., Nesic-Taylor O., Perez-Polo J. R.2008. Nuclear factor-kappaB decoy amelioration of spinal cord injury-induced inflammation and behavior outcomes. J. Neurosci. Res. 86: 566–580. doi: 10.1002/jnr.21508 [DOI] [PubMed] [Google Scholar]
- 33.Regnier A., Whitley R. D., Benard P., Bonnefoi M.1986. Effect of flunixin meglumine on the breakdown of the blood-aqueous barrier following paracentesis in the canine eye. J. Ocul. Pharmacol. 2: 165–170. doi: 10.1089/jop.1986.2.165 [DOI] [PubMed] [Google Scholar]
- 34.Rosenbaum J. T., McDevitt H. O., Guss R. B., Egbert P. R.1980. Endotoxin-induced uveitis in rats as a model for human disease. Nature 286: 611–613. doi: 10.1038/286611a0 [DOI] [PubMed] [Google Scholar]
- 35.Salvemini D., Misko T. P., Masferrer J. L., Seibert K., Currie M. G., Needleman P.1993. Nitric oxide activates cyclooxygenase enzymes. Proc. Natl. Acad. Sci. U.S.A. 90: 7240–7244. doi: 10.1073/pnas.90.15.7240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Samuel S. M., Thirunavukkarasu M., Penumathsa S. V., Paul D., Maulik N.2008. Akt/FOXO3a/SIRT1-mediated cardioprotection by n-tyrosol against ischemic stress in rat in vivo model of myocardial infarction: switching gears toward survival and longevity. J. Agric. Food Chem. 56: 9692–9698. doi: 10.1021/jf802050h [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sarker S. D., Bright C., Bartholomew B., Watson A. A., Nash R. J.2000. Calendin, tyrosol and two benzoic acid derivatives from Veronica persica (Scrophulariaceae). Biochem. Syst. Ecol. 28: 799–801. doi: 10.1016/S0305-1978(99)00122-2 [DOI] [PubMed] [Google Scholar]
- 38.Silverman N., Maniatis T.2001. NF-kappaB signaling pathways in mammalian and insect innate immunity. Genes Dev. 15: 2321–2342. doi: 10.1101/gad.909001 [DOI] [PubMed] [Google Scholar]
- 39.Srivastava S. K., Ramana K. V.2009. Focus on molecules: nuclear factor-kappaB. Exp. Eye Res. 88: 2–3. doi: 10.1016/j.exer.2008.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thirunavukkarasu M., Penumathsa S. V., Samuel S. M., Akita Y., Zhan L., Bertelli A. A., Maulik G., Maulik N.2008. White wine induced cardioprotection against ischemia-reperfusion injury is mediated by life extending Akt/FOXO3a/NFkappaB survival pathway. J. Agric. Food Chem. 56: 6733–6739. doi: 10.1021/jf801473v [DOI] [PubMed] [Google Scholar]
- 41.Tuck K. L., Hayball P. J.2002. Major phenolic compounds in olive oil: metabolism and health effects. J. Nutr. Biochem. 13: 636–644. doi: 10.1016/S0955-2863(02)00229-2 [DOI] [PubMed] [Google Scholar]
- 42.Vivancos M., Moreno J. J.2002. Role of Ca(2+)-independent phospholipase A(2) and cyclooxygenase/lipoxygenase pathways in the nitric oxide production by murine macrophages stimulated by lipopolysaccharides. Nitric Oxide 6: 255–262. doi: 10.1006/niox.2001.0410 [DOI] [PubMed] [Google Scholar]
- 43.Whitelocke R. A., Eakins K. E., Bennett A.1973. Acute anterior uveitis and prostaglandins. Proc. R. Soc. Med. 66: 429–434. [PMC free article] [PubMed] [Google Scholar]
- 44.Yokoseki O., Suzuki J., Kitabayashi H., Watanabe N., Wada Y., Aoki M., Morishita R., Kaneda Y., Ogihara T., Futamatsu H., Kobayashi Y., Isobe M.2001. cis Element decoy against nuclear factor-kappaB attenuates development of experimental autoimmune myocarditis in rats. Circ. Res. 89: 899–906. doi: 10.1161/hh2201.099373 [DOI] [PubMed] [Google Scholar]