Abstract
Epigallocatechin gallate (EGCG) is the major polyphenolic compound of green tea. Polyphenolic compounds were extracted from the leaf of Camellia sinensis (Japanese green tea), and the minimum inhibitory concentration against canine oral bacteria was measured. Subsequently, we investigated the inhibitory effects of polyphenolic compounds and EGCG on the growth of canine oral bacteria. EGCG showed antimicrobial activity against a model bacterium, Streptococcus mutans. Our results indicate that EGCG can inhibit the growth and biofilm formation of S. mutans and that EGCG does not interact with streptococcal lipoteichoic acid (LTA). Furthermore, our findings suggest that EGCG interacts with other component(s) of the bacterial membrane aside from streptococcal LTA to inhibit biofilm formation and damage biofilms.
Keywords: biofilm, EGCG, polyphenolic compounds, streptococcal lipoteichoic acid
Tea is the most consumed beverage in the world, except for water [7]. Green tea, made from Camellia sinensis leaves, is a non-fermented tea and has more beneficial health effects than black tea or oolong tea [17]. Green tea contains several polyphenolic compounds, including flavins and polyphenols. Catechins are the most frequent and abundant polyphenolic compounds [12, 22]. The major green tea catechins include epigallocatechin gallate (EGCG) and epicatechin gallate (ECG), which are produced from the esterification of other catechins (C) and epicatechins (EC) with gallic acid. EGCG is the most abundant [9], accounting for 50 to 65% of total catechins, as reviewed by Zaveri [36] and Nagle et al. [24]. EGCG possesses a range of biological and medicinal properties, including antioxidant, anti-carcinogen, anti-obesity, antibacterial, antiviral and anti-enzymatic effects [28].
It has been reported that oral disorders, such as periodontitis, occur frequently in dogs [15, 16, 33]. Canine periodontitis occurs with plaque accumulation and subsequent gingivitis, and with bone resorption with aging. In the early stages, oxygen-resistant and other streptococci can adhere to the oral cavity. Plaque flora changes with increasing numbers of obligate anaerobic Gram-negative bacteria. The genus Streptococcus has been recognized as an initial colonizer [26].
Streptococcus mutans has various unique characteristics for survival in oral cavities of humans [1] and dogs [5, 6, 21]. The numbers of salivary S. mutans were different among various dog populations [21]. It has been reported that the quantity of caries-causing bacteria (S. mutans) is related to the environment in which dogs are kept [6, 21]. S. mutans is an important bacterium for biofilm formation [1, 5, 6], indicating that it would also be a model bacterium for testing antimicrobial substances in dogs [6]. One of the most documented characteristics of the virulence of S. mutans is its ability to produce glucosyltransferases, which synthesize intracellular polysaccharides and extracellular polysaccharides (EPS). The EPS, specifically the water-insoluble glucans, mediates the adherence of S. mutans and other oral bacterial species to tooth surfaces. This contributes to the formation of dental plaque biofilms [27] and allows the adhering bacteria to evade host defenses. The two major classes of these cell surface glycopolymers are teichoic acids (TA) and lipoteichoic acids (LTA), which are phosphate-rich molecules found in a wide range of Gram-positive bacteria [19, 29, 31]. They have been implicated in many persistent and chronic diseases, such as cystic fibrosis, endocarditis and infections, caused by biofilms growing on incorporated foreign materials, e.g. stents, indwelling catheters, bone implants and artificial valves [20]. Infections associated with implant surfaces or necrotic tissues like bone grafts can be fatal for the patient. Bacteria in biofilms are encased in a polysaccharide glycocalyx, which provides them with protection against the host defenses, antimicrobial drugs and biocides [3].
In this study, we investigated inhibition of growth of canine oral bacteria. Streptococci were highly sensitive to EGCG. Growth inhibition, anti-biofilm formation and anti-biofilm activity of catechins against S. mutans as a model bacterium were examined. Electron microscopic observations of S. mutans exposed to EGCG were also performed. Finally, the interaction between streptococcal LTA and EGCG was measured by a quartz crystal microbalance (QCM) binding assay.
MATERIALS AND METHODS
Bacterial strains and culture conditions: Various oral bacteria were isolated from gingival plaque taken from maxillary premolars of the dogs with periodontal disease (Table 1) as previously described [14]. The bacteria were grown in GAM broth (Nissui Co., Tokyo, Japan) for 24 to 48 hr at 37°C anaerobically. S. mutans was isolated by Hirose et al. as previously described [10] and was grown in Brain Heart Infusion (BHI) broth (Merck KGaA, Darmstadt, Germany) for 24 hr at 37°C.
Table 1. Minimum inhibitory concentration (MIC) of extracted polyphenolic compounds from Japanese green tea and EGCG against various isolates from oral cavity of dogs.
| Canine isolates | MIC (mg/ml) | |
|---|---|---|
| Polyphenolic compoundsa) from Japanese green tea |
EGCGb) | |
| Porphyromonas endodontalis INU-1 | 0.8 | 0.1 |
| Porphyromonas salivosa Festa-S | 0.4 | 0.1 |
| Porphyromonas gulae Festa-G | 0.4 | 0.05 |
| Prevotella intermedia INU-B4 | 0.1 | 0.025 |
| Prevotella melaninogenica INU-BL1 | 0.2 | 0.05 |
| Fusobacterium nucleatum INU-F2 | 0.2 | 0.05 |
| Lactobacillus acidophilus INU-L3 | 0.2 | 0.05 |
| Streptococcus spp. INU-7A3 | 0.1 | 0.0125 |
| Streptococcus spp. INU-8SO1 | 0.1 | 0.0125 |
| Streptococcus spp. INU-9SOA3 | 0.1 | 0.0125 |
| Peptostreptococcus spp. INU-PS | 0.4 | 0.025 |
a) Polyphenolic compounds were using 95% ethanol at 80°C for 4 hr to extract. It is a mixer that includes various components, such as tannins (EGCG and other catechins), minerals, nitrogenous components, caffeine and lipids, etc. b) EGCG is one of major polyphenolic compounds, which had been purified.
Catechins: Polyphenolic compounds were isolated from the leaf of C. sinensis by extraction using 95% ethanol (80°C for 4 hr) as previously described [12]. Five major catechins, epigallocatechin gallate (EGCG), epicatechin gallate (ECG), epigallocatechin (EGC), epicatechin (EC) and catechin (C), were detected at amounts of 17.8, 11.8, 4.2, 2.8 and 0.4%, respectively [12]. Purified forms of these five major catechins were also purchased (Nagara Science Co., Ltd., Gifu, Japan). The purity of the five major catechins was 98%.
Growth inhibition test: Canine oral bacteria were used for the growth inhibition test. The pre-cultured bacteria were diluted to 104 CFU (colony forming units) per ml, and then, a mixture of polyphenolic compounds [12] containing either the five major catechins and other components or only purified EGCG was added to 1 ml of bacterial suspension. S. mutans (model bacterium) was added to each of the catechin (EGCG, ECG, EGC, EC or C) solutions, which had final concentrations of 0.2, 0.1, 0.05, 0.025 or 0.0125 mg/ml, and were mixed and incubated at 37°C. One-hundred microliters of cell suspension, which had been treated with catechins for 3 or 5 hr, was used for a short-time killing assay and seeded on plates to incubate for 48 hr at 37°C. After incubation, the colonies were counted. Furthermore, cells were incubated with EGCG for 24 hr to measure the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC). Controls were prepared by mixing 1 ml of bacterial suspension, 0.9 ml of BHI broth and 0.1 ml of Hanks’ Balanced Salt Solution (HBSS, pH 7.4; Gibco, Grand Island, NY, U.S.A.). The MICs of the polyphenolic compound mix and EGCG are defined as the lowest concentrations that inhibited visible growth after overnight incubation. The MBC is defined as the lowest concentration of EGCG that killed 99.9% of the initial inoculum in a given time using a plate count assay of viable cells.
Biofilm formation test: The effect of EGCG on biofilm formation of S. mutans was measured by using the Minimum Biofilm Eradication Concentration-High Throughput Plate (MBECTM-HTP, Innovotech, Inc., Edmonton, AB, Canada). The pre-cultured S. mutans was diluted to a final concentration of 107 CFU/ml. One-hundred microliters of the bacteria dilution was mixed with 100 µl of 0.4 mg/ml EGCG and was added into the 96-well microtiter plate. The plate was covered with a lid equipped with 96 pegs, the surface of which was covered with hydroxyapatite, with each peg dipping into the bacterial suspension. Thus, biofilms could be formed on the surface of the pegs. The control was the bacterial suspension incubated under EGCG-free conditions. The plates were incubated at 37°C with shaking for 12, 24 and 36 hr. After different periods of incubation, the pegs were broken off, and the biofilms were disrupted from the surface of the pegs in 200 µl of physiological saline solution (PSS) with a sonicator. Twenty-microliter aliquots of the cell suspensions were then inoculated on BHI agar plates. The plates were incubated for 48 hr at 37°C, and colonies were counted. All measurements were done in triplicate.
Biofilm susceptibility assay: Biofilms of peg surfaces were formed from 24 hr cell suspensions of S. mutans containing 107 CFUs/ml. The biofilms formed on the pegs were inserted into 0.2 mg/ml of EGCG solution for 6 hr and 8 hr at 37°C. The survival of the bacteria was assessed as described above using the colony count method.
Observation of EGCG-treated bacteria surface using field emission-scanning electron microscope (FE-SEM): A 10 µl cell suspension of S. mutans was treated with EGCG at 37°C for 24 hr, mixed with 10 µl distilled water on a micro-glass and then dried. The dried cells were treated with saturated 70% ethanol for 5 min and saturated 100% ethanol for another 5 min. After air-drying, the sample was examined using a FE-SEM (SU8000; Hitachi High-Technologies Corporation, Tokyo, Japan).
The pegs treated with EGCG were removed from the plate, on which biofilms had formed, and were rinsed in 0.9% physiological saline for 1 min to remove planktonic culture. The samples were fixed with 2.5% glutaraldehyde (Kanto Chemical Co., Inc., Osaka, Tokyo, Japan) in 0.1 M cacodylic acid (Wako Pure Chemical Industries, Ltd., Osaka, Japan) at 4°C for 16 hr. The pegs were washed with 0.1 M cacodylic acid and distilled water for approximately 10 min each. Saturated 70% ethanol was applied for 15 to 20 min and then air-dried at least for 24 hr. The pegs were mounted on a stage and examined using an FE-SEM.
EGCG interaction with streptococcal LTA: The quartz crystal microbalance (QCM) is a very sensitive mass measuring device, which measures changes in resonant frequency upon a weight increase on the surface of sensor crystal oscillator. The use of QCM transducers offers sensitive, in situ detection of hybridization events, without the need for optical or redox indicators [34]. The QCM (Single-Q, AS ONE Co., Ltd., Osaka, Japan) has an automatic injection mechanism, mixer, sensor crystal oscillator and low capacity reaction vessel. The vibration frequency changes has 200 Hz was defined as substances have attached to sensor crystal oscillator. Firstly, 500 µl of HBSS was added to the low-capacity reaction vessel and mixed at 6,000 revolutions per minute (rpm) to stabilize at 37°C. Secondly, 5 µl of EGCG (16 mg/ml) was injected into the low capacity reaction vessel to stabilize. Subsequently, 5 µl of block agent that did not react with the substances was injected to fix the rest of sensor where it was not bound with EGCG. Finally, 5 µl of streptococcal LTA (1 mg/ml) from S. mutans (Sigma-Aldrich Japan Corporation, Tokyo, Japan) was injected to measure the frequency change.
Statistical analysis: The function program in Microsoft Excel (Microsoft Corporation) was used to conduct F-tests and t-tests for our results (estimation of bacterial cell numbers). The type of function used to analyze results of Figs. 1, 2 and 3. After statistical analysis, P-values of less than 0.05 were considered statistically significant.
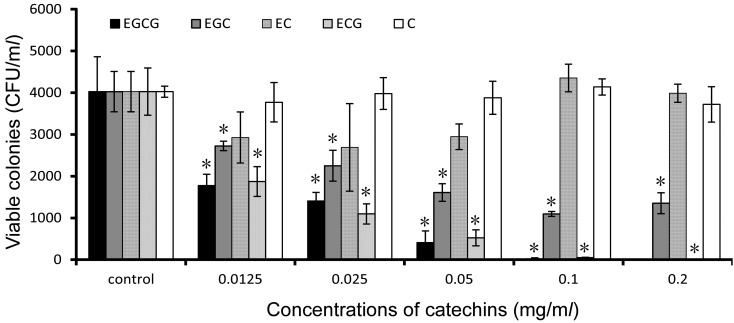
Fig. 1.
Antimicrobial activity of catechins against S. mutans. The bacteria cultures added different concentrations of catechins to incubate for 5 hr at 37°C. The viable colonies were calculated in colony forming units and analyzed by t-test. Standard deviations indicated by error bars were calculated from 3 independent experiments. * Significantly different from the untreated control (*P<0.05).
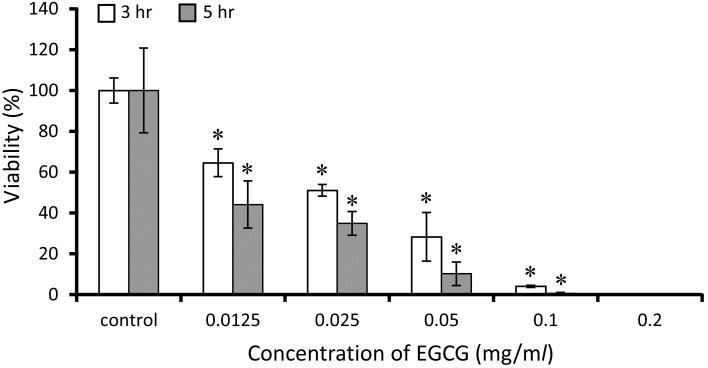
Fig. 2.
Antimicrobial activity of EGCG against S. mutans. The bacteria culture that added different concentrations of EGCG was incubated for 3 hr and 5 hr at 37°C. The viability (%) is represented as a percentage of the colonies that EGCG cell culture is compared with control. The result of viability analyzed by t-test. Standard deviations indicated by error bars were calculated from 3 independent experiments. * Significantly different from the untreated control (*P<0.05).
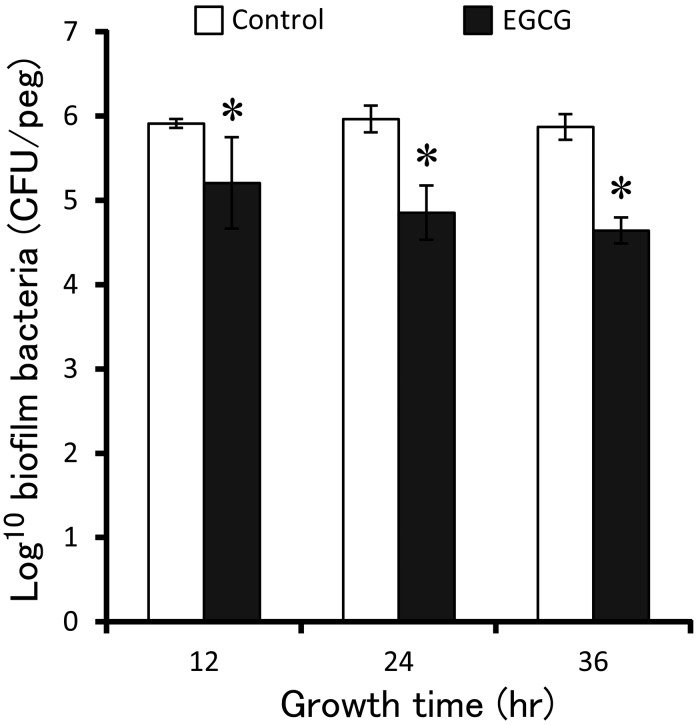
Fig. 3.
Inhibitory effect of EGCG on biofilm formation. The pegs inserted into 107 CFU/ml planktonic cultures that had added final concentration of 0.2 mg/ml of EGCG to incubate for 12, 24 and 36 hr to form biofilm, respectively. The bacteria were moved from biofilm of pegs and seeded on agar plate to count and analyzed by t-test. Standard deviations indicated by error bars were calculated from 3 independent experiments. * Significantly different from the untreated controls (*P<0.05).
RESULTS
Growth inhibition of canine oral bacteria: Various bacteria isolated from oral cavity were sensitive to the polyphenolic compounds mix and EGCG (Table 1). MIC ranges were 0.1–0.8 mg/ml for polyphenolic compounds and 0.0125–0.1 mg/ml for EGCG. Oral streptococci showed significant growth inhibition compared to controls which were in the absence of the polyphenolic compounds mix and EGCG.
Bactericidal effect of catechins: As shown in Fig. 1, the growth of S. mutans was inhibited by three kinds of catechins (EGCG, ECG and EGC), and the order of their inhibitory effect is EGCG >ECG >EGC. EC and C did not show inhibitory function against S. mutans. The MIC and MBC values of EGCG against S. mutans were 0.125 and 0.1 mg/ml, respectively.
The viabilities of S. mutans bacterial cells treated with 0.0125 to 0.1 mg/ml of EGCG clearly decreased in the short-time killing assay (Fig. 2). The percentage of viable bacterial cells, which were incubated at concentrations of 0.0125 to 0.1 mg/ml of EGCG for 3 hr and 5 hr comparing with controls, had decreased to 64.7–4.0% and 45.3–0.0%, respectively.
Inhibitory effect of EGCG on biofilm formation: The inhibitory effect of EGCG on biofilm formation of S. mutans was measured by counting colonies that were recovered from biofilms formed on the surface of pegs, as described in Materials and Methods. The number of CFUs obtained from biofilm, which had formed in the presence of EGCG, was less than that formed in the control culture (t-test, P<0.05) (Fig. 3), indicating that EGCG had an inhibitory effect on S. mutans biofilm formation. Thus, this result suggests that EGCG can interfere with some components of bacterial cells to inhibit biofilm formation.
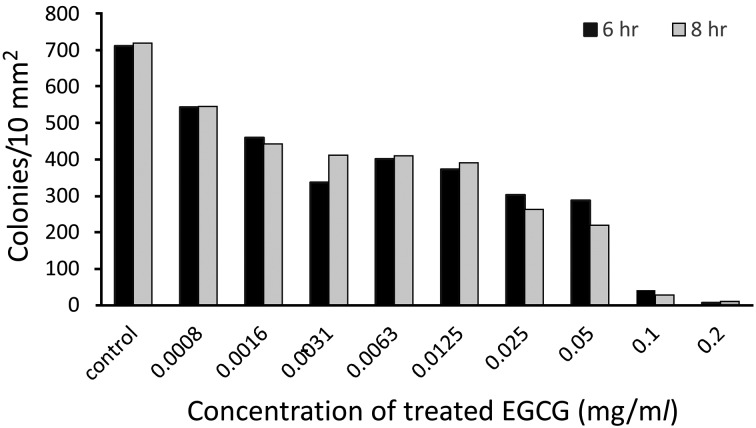
Biofilm susceptibility to EGCG: The effect of EGCG on the S. mutans biofilm was measured using a biofilm susceptibility assay as described above. For this purpose, biofilms were first established on pegs, and the biofilm bacteria were statistically significantly eradicated in 6 hr of incubation at a concentration of 0.2 mg/ml of EGCG, and bacteria from the treated biofilm were seeded on a plate to count colonies within 10 mm2 of agarose (Fig. 4). This anti-biofilm effect of EGCG was dose-dependent. The anti-biofilm effect of EGCG was further enhanced after 8 hr incubation, when no colonies were recovered from biofilms treated with 0.2 mg/ml EGCG.
Fig. 4.
Effect of EGCG on living microbes after biofilms formation. Biofilm pegs treated different concentrations of EGCG for 6 hr or 8 hr at 37°C, and then, bacteria were moved from biofilm of pegs to count living cell within per 10 square millimeter where seeded on ager plate.
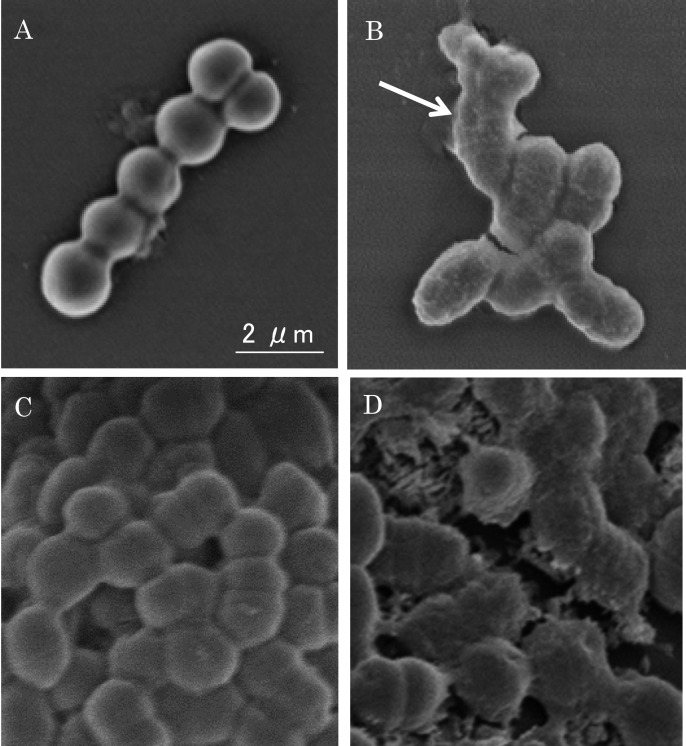
Bacterial cell surface damage after treatment with EGCG: The effect of EGCG on S. mutans cell morphology was observed using an FE-SEM. As shown in Fig. 5, untreated S. mutans cells had smooth surfaces. Cell surfaces treated with 0.2 mg/ml of EGCG became muddled, and some cell membranes broke, leading to leakage of the cytoplasm (Fig. 5B). These results demonstrate that the morphological changes to the cell surface were induced by EGCG.
Fig. 5.
The damage effect of EGCG on S. mutans cell morphology and biofilm. (A) Untreated control. (B) Treated with 0.2 mg/ml of EGCG for 24 hr. The arrow in Fig. 5B indicates “ring” phenomena around the damaged cells. (C) Untreated control of biofilm on MBECTM-HTP pegs. (D) Biofilm bacteria were treated with 0.2 mg/ml of EGCG for 24 hr.
A microscopy image of the S. mutans biofilm attached on the peg is shown in Fig. 5. In Fig. 5C, a regular form of the bacteria can be observed on the peg, showing the biofilm as a pellicle. The effect of EGCG on S. mutans biofilm damage was similar to that in cell suspensions (Fig. 5D). Cells of the biofilm became muddled, and some cell membranes broke, leading to leakage of cytoplasm. The results indicate that the bacteria in the biofilm were damaged by EGCG.
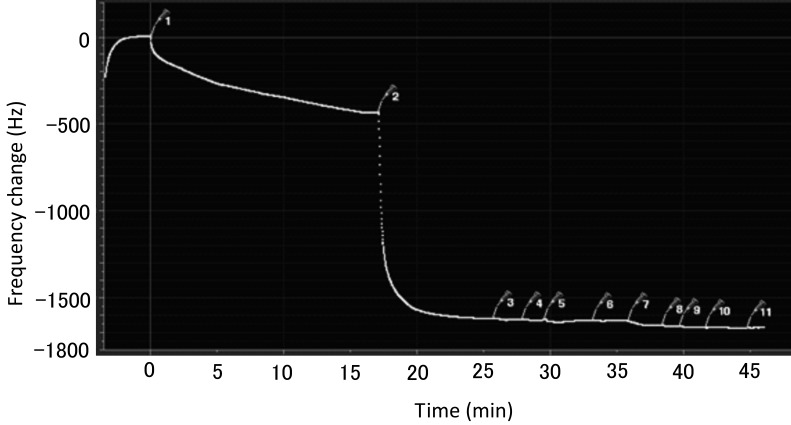
Intermolecular interaction between streptococcal LTA and EGCG: In the QCM test, when 5 µl of EGCG was injected, the vibration frequency decreased by 440 Hz, and the amount of streptococcal LTA solution injected each time was 5 µl, the vibration frequency only reduced by 50 Hz (Fig. 6). This experiment indicates that EGCG and streptococcal LTA have no intermolecular interaction.
Fig. 6.
Intermolecular interaction of EGCG and streptococcal LTA. This is one of the most typical data, in that the same experiment done three times. The numbers in the figure represent injecting times. 1, 5 µl of EGCG. 2, 5 µl of block agent. The numbers of 3 to 11 represent injected each 5 µl of streptococcal LTA.
DISCUSSION
In this study, oral bacteria isolated from dogs were found to be sensitive to polyphenolic compounds and EGCG, with streptococci being highly sensitive. In our previous study, we demonstrated that polyphenolic compounds inhibited plaque deposition, gingivitis and other porphyromonas in dogs when the compounds were supplemented in dog food (0.8 mg/g) [13]. We suspected that EGCG was the most effective catechin, because MIC increased when using purified EGCG.
Bacteria are the most numerous microbes in the mouth. One of the model bacteria of oral cavities in humans, S. mutans, adheres to the surface of the tooth indicating an area of demineralization of enamel [2, 27]. Besides, it has been reported that carotid injection of S. mutans and other related bacteria in a dog model leads to multifocal choroiditis with retinal detachment [23]. In recent reports, green tea has been used as an ingredient in feeds for calves [11]. Catechins of green tea have a broad spectrum of antimicrobial activity against both of gram-positive and gram-negative bacteria. In particular, galloylated derivatives, such as EGCG, have been documented to possess antimicrobial effects against oral streptococci [8]. Our study showed that one component of catechins, EGCG, demonstrates the highest antibacterial activity against the growth of S. mutans. The MBC value was higher than MIC. In the comparison of biofilm colonies formed in EGCG-treated and untreated culture, the CFUs of biofilm bacteria formed in the EGCG-treated culture were obviously less than that formed in the untreated culture. It was suggested that EGCG not only inhibits the growth of S. mutans, but also damages surface adsorption ability on the tooth in vitro.
The antimicrobial mechanism of EGCG is mainly attributable to irreversible damage of the microbial cytoplasmic membrane [30]. Electron microscopic analysis showed that EGCG induced cell membrane lysis and cytoplasm leakage as shown in Fig. 5. Microbial biofilms commonly exhibit increasing levels of resistance to most antibiotics or therapeutic agents [4]. Biofilm cells have shown to be more tolerant of antibiotics comparing with planktonic bacteria, and this makes it hard to treat S. mutans with modern medicine [32, 37]. Our data certified further that bacteria in biofilms display lower susceptibility to EGCG than those in suspension. As shown in Fig. 5D, EGCG has a striking effect on S. mutans cells, which establish the biofilm.
Gram-positive bacteria develop a profound cell-envelope structure; they lack the normal outer membrane, and the cell wall is usually much thicker than that of gram-negative species, with multiple peptidoglycan layers [35]. The LTA is anchored to the plasma membrane and extends from the cell surface to the peptidoglycan layer. LTA and wall-teichoic acid create what has been aptly been described as a “continuum of negative charge,” which extends from the bacterial cell surface beyond the outermost layers of peptidoglycan [16]. We performed a test on the intermolecular interaction with streptococcal LTA and EGCG, and the results indicated that EGCG did not bind to streptococcal LTA. Catechins are known to bind to various proteins (e.g., albumin, casein) to form macromolecular complexes in vitro [18, 25]. All results demonstrated that EGCG interacts with other component (s) of the bacterial membrane, which could be some proteins, to inhibit biofilm formation and damage bacterial cells and biofilms, not through streptococcal LTA. These findings highlight that the EGCG of green tea may be an attractive candidate for the prevention and treatment of oral caries. Further work to understand the relation between EGCG and components of the cell membrane is needed for the development of new means to fight the infections caused by canine oral bacteria in the future.
Acknowledgments
This work was supported by a part of grant-aid from Ministry of Education, Culture, Sports, Science and Technology of Japan.
REFERENCE
- 1.Bai L., Takagi S., Guo Y., Kuroda K., Ando T., Yoneyama H., Ito K., Isogai E.2013. Inhibition of Streptococcus mutans biofilm by LL-37. Int. J. Med. Sci. Biotechnol. 1: 56–64. [Google Scholar]
- 2.Berger M., Stich H., Hüster H., Roux P., Schawalder P.2006. Feline caries in two cats from a 13th century archeological excavation. J. Vet. Dent. 23: 13–17. doi: 10.1177/089875640602300102 [DOI] [PubMed] [Google Scholar]
- 3.Coraça-Hubér D. C., Fille M., Hausdorfer J., Pfaller K., Nogler M.2012. Evaluation of MBEC™-HTP biofilm model for studies of implant associated infections. J. Orthop. Res. 30: 1176–1180. doi: 10.1002/jor.22065 [DOI] [PubMed] [Google Scholar]
- 4.Costerton J. W., Stewart P. S., Greenberg E. P.1999. Bacterial biofilms: a common cause of persistent infections. Science 284: 1318–1322. doi: 10.1126/science.284.5418.1318 [DOI] [PubMed] [Google Scholar]
- 5.Zambori C., Cumpanasoiu C., Mladin B., Tirziu E.2013. Biofilms in oral cavity of dogs and implication in zoonotic infections. Anim. Sci. Biotechnol. 46: 156–158. [Google Scholar]
- 6.Zambori C., Tirziu E., Nichita I., Cumpanasoiu C., Gros R. V., Seres M., Mladin B., Mot D.2012. Biofilm implication in oral diseases of dogs and cats. . Anim. Sci. Biotechnol. 45: 208–212. [Google Scholar]
- 7.Fernando C. D., Soysa P.2015. Extraction Kinetics of phytochemicals and antioxidant activity during black tea (Camellia sinensis L.) brewing. Nutr. J. 14: 74. doi: 10.1186/s12937-015-0060-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamilton-Miller J. M. T.2001. Anti-cariogenic properties of tea (Camellia sinensis). J. Med. Microbiol. 50: 299–302. doi: 10.1099/0022-1317-50-4-299 [DOI] [PubMed] [Google Scholar]
- 9.Hayashi T., Ueda S., Tsuruta H., Kuwahara H., Osawa R.2012. Complexing of green tea catechins with food constituents and degradation of the complexes by Lactobacillus plantarum. Biosci. Microbiota Food Health 31: 27–36. doi: 10.12938/bmfh.31.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirose H., Hirose K., Isogai E., Miura H., Ueda I.1993. Close association between Streptococcus sobrinus in the saliva of young children and smooth-surface caries increment. Caries Res. 27: 292–297. doi: 10.1159/000261553 [DOI] [PubMed] [Google Scholar]
- 11.Ishihara N., Chu D. C., Akachi S., Juneja L. R.2001. Improvement of intestinal microflora balance and prevention of digestive and respiratory organ diseases in calves by green tea extracts. Livest. Prod. Sci. 68: 217–229. doi: 10.1016/S0301-6226(00)00233-5 [DOI] [Google Scholar]
- 12.Isogai E., Isogai H., Fujii N., Kimura K., Miura H., Hayashi M., Namioka S., Kawasaki M., Ikeda K.1992. Inhibitory effect of Japanese green tea extracts on growth of canine oral bacteria. Bifidobact. Microflora 11: 53–59. doi: 10.12938/bifidus1982.11.2_53 [DOI] [Google Scholar]
- 13.Isogai E., Isogai H., Kimura K., Nishikawa T., Fujii N., Benno Y.1995. Effects of Japanese green tea extract on canine periodontal diseases. Microb. Ecol. Health Dis. 8: 57–61. doi: 10.3109/08910609509141383 [DOI] [Google Scholar]
- 14.Isogai E., Isogai H., Miura H., Takano K., Aoi Y., Hayashi M., Namioka S.1989. Oral flora of mongrel and beagle dogs with periodontal disease. Jpn. J. Vet. Sci. 51: 110–118. doi: 10.1292/jvms1939.51.110 [DOI] [PubMed] [Google Scholar]
- 15.Isogai H., Isogai E., Okamoto H., Shirakawa H., Nakamura F., Matsumoto T., Watanabe T., Miura H., Aoi Y., Kagota W., et al. 1989. Epidemiological study on periodontal diseases and some other dental disorders in dogs. Jpn. J. Vet. Sci. 51: 1151–1162. doi: 10.1292/jvms1939.51.1151 [DOI] [PubMed] [Google Scholar]
- 16.Isogai H., Kosako Y., Benno Y., Isogai E.1999. Ecology of genus Porphyromonas in canine periodontal disease. Zentralbl. Veterinarmed. B. 46: 467–473. [DOI] [PubMed] [Google Scholar]
- 17.Jazani N. H., Zartoshti M., Shahabi S., Yekta Z., Nateghi S.2007. Evaluation of the synergetic effect of water soluble extracts of green tea (Camellia sinensis) on the activity of ciprofloxacin in urinary isolated E.coli. J. Biol. Sci. 7: 1500–1503. doi: 10.3923/jbs.2007.1500.1503 [DOI] [Google Scholar]
- 18.Keita Å. V., Söderholm J. D.2010. The intestinal barrier and its regulation by neuroimmune factors. Neurogastroenterol. Motil. 22: 718–733. doi: 10.1111/j.1365-2982.2010.01498.x [DOI] [PubMed] [Google Scholar]
- 19.Kristian S. A., Datta V., Weidenmaier C., Kansal R., Fedtke I., Peschel A., Gallo R. L., Nizet V.2005. D-alanylation of teichoic acids promotes group a streptococcus antimicrobial peptide resistance, neutrophil survival, and epithelial cell invasion. J. Bacteriol. 187: 6719–6725. doi: 10.1128/JB.187.19.6719-6725.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kunze B., Reck M., Dötsch A., Lemme A., Schummer D., Irschik H., Steinmetz H., Wagner-Döbler I.2010. Damage of Streptococcus mutans biofilms by carolacton, a secondary metabolite from the myxobacterium Sorangium cellulosum. BMC Microbiol. 10: 199. doi: 10.1186/1471-2180-10-199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lavy E., Golani Y., Friedamn M., Bdolah-Abram T., Steinberg D.2009. Comparison of the distribution of oral cavity bacteria in various dog populations. Isr. J. Vet. Med. 64: 78–83. [Google Scholar]
- 22.Łuczaj W., Skrzydlewska E.2005. Antioxidative properties of black tea. Prev. Med. 40: 910–918. doi: 10.1016/j.ypmed.2004.10.014 [DOI] [PubMed] [Google Scholar]
- 23.Meyers S. M., Vasil M. L., Yamamoto L.1982. Pathologic mechanisms of multifocal choroiditis with retinal detachment after carotid injection of Streptococcus mutans and other bacteria in dogs. Invest. Ophthalmol. Vis. Sci. 22: 165–173. [PubMed] [Google Scholar]
- 24.Nagle D. G., Ferreira D., Zhou Y. D.2006. Epigallocatechin-3-gallate (EGCG): chemical and biomedical perspectives. Phytochemistry 67: 1849–1855. doi: 10.1016/j.phytochem.2006.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Osawa R., Walsh T. P.1993. Visual reading method for detection of bacterial tannase. Appl. Environ. Microbiol. 59: 1251–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Periasamy S., Kolenbrander P. E.2009. Mutualistic biofilm communities develop with Porphyromonas gingivalis and initial, early, and late colonizers of enamel. J. Bacteriol. 191: 6804–6811. doi: 10.1128/JB.01006-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schilling K. M., Bowen W. H.1992. Glucans synthesized in situ in experimental salivary pellicle function as specific binding sites for Streptococcus mutans. Infect. Immun. 60: 284–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimamura T., Zhao W. H., Hu Z. Q.2007. Mechanism of action and potential for use of tea catechin as an antiinfective agent. Antiinfect. Agents Med. Chem. 6: 57–62. doi: 10.2174/187152107779314124 [DOI] [Google Scholar]
- 29.Swoboda J. G., Campbell J., Meredith T. C., Walker S.2010. Wall teichoic acid function, biosynthesis, and inhibition. ChemBioChem 11: 35–45. doi: 10.1002/cbic.200900557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor P. W., Hamilton-Miller J. M. T., Stapleton P. D.2005. Antimicrobial properties of green tea catechins. Food Sci. Technol. Bull. 2: 71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salton M. R.1967. Structure and function of bacterial cell membranes. Annu. Rev. Microbiol. 21: 417–442. doi: 10.1146/annurev.mi.21.100167.002221 [DOI] [PubMed] [Google Scholar]
- 32.Uppuluri P., Pierce C. G., López-Ribot J. L.2009. Candida albicans biofilm formation and its clinical consequences. Future Microbiol. 4: 1235–1237. doi: 10.2217/fmb.09.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wallis C., Marshall M., Colyer A., O’Flynn C., Deusch O., Harris S.2015. A longitudinal assessment of changes in bacterial community composition associated with the development of periodontal disease in dogs. Vet. Microbiol. 181: 271–282. doi: 10.1016/j.vetmic.2015.09.003 [DOI] [PubMed] [Google Scholar]
- 34.Wang J., Nielsen P. E., Jiang M., Cai X., Fernandes J. R., Grant D. H., Ozsoz M., Beglieter A., Mowat M.1997. Mismatch-sensitive hybridization detection by peptide nucleic acids immobilized on a quartz crystal microbalance. Anal. Chem. 69: 5200–5202. doi: 10.1021/ac9706077 [DOI] [PubMed] [Google Scholar]
- 35.Weidenmaier C., Peschel A.2008. Teichoic acids and related cell-wall glycopolymers in Gram-positive physiology and host interactions. Nat. Rev. Microbiol. 6: 276–287. doi: 10.1038/nrmicro1861 [DOI] [PubMed] [Google Scholar]
- 36.Zaveri N. T.2006. Green tea and its polyphenolic catechins: medicinal uses in cancer and noncancer applications. Life Sci. 78: 2073–2080. doi: 10.1016/j.lfs.2005.12.006 [DOI] [PubMed] [Google Scholar]
- 37.Zhang J., Biswas I.2009. A phenotypic microarray analysis of a Streptococcus mutans liaS mutant. Microbiology 155: 61–68. doi: 10.1099/mic.0.023077-0 [DOI] [PMC free article] [PubMed] [Google Scholar]