Abstract
The aim of this study was to investigate the effects of percutaneous transplanted autologous neurogenically-induced bone marrow-derived mesenchymal stem cells (NIBM-MSCs) in paraplegic dogs without deep pain perception (DPP) secondary to external spinal trauma. Thirteen client owned dogs that had failed in improvement neurologically at least 42 days after conservative management, decompression and decompression-stabilization were included in the study. Each dog received two doses of autologous 5.0 × 106 NIBM-MSCs suspension, which were positive to 2′,3′-Cyclic-nucleotide-3′-phosphodiesterase (CNPase) and Microtubule-associated protein 2 (MAP-2), as well as to Glial fibrillary acidic protein (GFAP) and beta III tubulin. The cells were injected into the spinal cord through the hemilaminectomy or laminectomy defects percutaneously with 21 days interval for 2 times. The results were evaluated using Texas Spinal Cord Injury Scale (TSCIS), somatosensory evoked potentials (SEP) and motor evoked potentials (MEP) at the admission time, cell transplantation procedures and during 2, 5, 7 and 12th months after the second cell transplantation. Improvement after cell transplantation in gait, nociception, proprioception, SEP and MEP results was observed in just 2 cases, and only gait score improvement was seen in 6 cases, and no improvement was recorded in 5 cases. All progresses were observed until 2nd month after the second cell transplantation, however, there was no improvement after this period. In conclusion, percutaneous transplantation of autologous NIBM-MSCs is a promising candidate modality for cases with spinal cord injury after spinal trauma and poor prognosis.
Keywords: autologous stem cells, external trauma, paraplegia, spinal cord injury, spinal stabilization
Spinal cord injury is commonly a consequence of spinal trauma in dogs and cats. They frequently involve vertebral fracture, luxation, fracture & luxation depending on the type and severity of the trauma. The strengths and weaknesses of the vertebral column are the underlying factors for the type of lesion and involved area. Spinal trauma may result from vehicular accidents, animal attacks or falling from height [11, 15, 37]. Spinal Cord Injury (SCI) is a debilitating and devastating condition for humans and also for companion animals. Even though the epidemiological data concerning the global prevalence of SCI are not available for companion animals, the estimated number for human sufferers reaches 250,000–300,000 in the U.S.A. alone [5]. Although substantial level of progress has been reported for medical and surgical treatment of spinal trauma, it is not yet possible to completely regain neuronal function after SCI [1, 14].
The primary focus of therapy is the preservation of function in surviving neural tissue, which often requires surgical decompression and stabilization of skeletal elements to prevent further trauma [15]. Recent advances in understanding pathophysiology, acute trauma care, interventional surgery, spinal instrumentation, rehabilitation and regenerative medicine have come to a certain level for spinal cord injury [21]. Recent focus in SCI therapy is to repair the injured spinal cord. The conclusion of the studies, carried out so far for this purpose, encourages the investigators to search regenerative possibilities [44, 45]. Experimental studies have shown that repair of the whole injured spinal cord area may not be necessary for functional recovery or to allow walking; restoring around 10–15% of connections in the spinal cord may be sufficient [9, 11, 20]. Replacing the lost cell types, and integrating newly transplanted or generated cells into the spinal cord circuitry is one of the most important areas for developing potential therapies of SCI [5]. Additionally, injected mesenchymal stem cells could produce trophic factors, cytokines and other neuroprotective factors in stroke or traumatic spinal cord injury [20, 30]. These factors contribute to survival by inducing the expression of differentiation factors for neural progenitor cells, hence playing a key role in the proliferation and differentiation of neural tissue by increasing the central nervous system plasticity [32, 34].
Stem cell therapy for SCI in canine practice is reported in limited studies with the use of different cell types and methods [14, 18, 25, 26, 32]. However, the absence of satisfactory clinical results for spinal cord injury cases, motivates us to investigate the effect of using percutaneous transplantation of autologous neurogenically-induced bone marrow-derived mesenchymal stem cells (NIBM-MSCs) in paraplegic dogs without deep pain perception (DPP), due to external trauma, and also not responded at least 42 days after the trauma to the conventional treatment methods.
MATERIALS AND METHODS
Animals: This prospective study includes client-owned companion dogs with spinal cord injury located between T3-L7 vertebrae secondary to external trauma, and paraplegia without DPP. The study protocol was approved by the Ankara University Animal Experimentations Local Ethics Committee (2011-15-395), and informed consents were obtained from each dog owner.
Inclusion criteria: Dogs with paraplegia, lacking DPP due to external trauma, weighing <20 kg, having physically intact spinal cord which is determined by magnetic resonance imaging (MRI) and confirmed in decompressive surgery (n=2), or just during decompressive surgery (n=11), the cases with no signs of functional integrity of spinal cord which was further confirmed by electrophysiology. Animals that showed no acceptable clinical improvement for at least 42 days (required time for the stem cell preparation) after the spinal cord injury, despite conventional surgical and physical treatments or only physical treatments (in chronic cases), were included in the study. Percutaneous intraspinal cell therapy applied for two times with 21 days intervals and followed up for at least 2 months after second cell transplantation.
Neurological examination: Texas Spinal Cord Injury Scale (TSCIS) which includes the evaluation of gait, proprioception and nociception [19] was performed to evaluate the neurologic score just after received spinal injury, as well as the outcomes (just before the 1st cell transplantation, 3 weeks after the 1st cell transplantation and once in 21 days after 2nd cell transplantation). Neurological improvement was assessed by comparing the difference between the neurological score at presentation and at the final examination.
Vertebral column radiography: Vertebral column instability was evaluated according to the three compartments theory. The dorsal compartment incorporates the articular processes, laminae, pedicles and spinous processes; the middle compartment includes the dorsal longitudinal ligament, the dorsal aspect of the vertebral body and the dorsal part of the annulus fibrosus; the ventral compartment contains the ventral longitudinal ligament, lateral and ventral parts of the annulus fibrosus, the nucleus pulposus and remaining parts of the vertebral body [38]. Damage to any two of the three compartments indicates instability, and spinal stabilization was carried out for those cases. However, involving one compartment was accepted as stabile, and just decompression was performed.
Magnetic resonance imaging: All MRI procedures were carried out under 40 µg/kg intravenous (IV) medetomidine hydrochloride (Domitor®, Pfizer AH, New York, NY, U.S.A.) and 5 mg/kg intramuscular (IM) ketamine hydrochloride (Ketasol®, Richter Pharma AG, Wels, Austria) anesthesia and under spontaneous-breathing. The MRI of two chronic cases (case nos: 1 and 11) was obtained by using a superconducting magnet 1.5 tesla MRI unit (Magnetom, Siemens AG, Munich, Germany) using spinal coil. T1 weighted images (TR: 370–700ms and TE: 15–20 ms) and T2 weighted images (TR: 2,000–4,000 ms, TE: 90–110 ms) were acquired at 2mm slice thickness, in three views as sagittal, transversal and axial planes. Gadolinium diethylene-triaminepentaacetic acid (Magnevist, Bayer Pharma AG, Berlin, Germany) was used as the paramagnetic contrast medium and was intravenously administered at a dose of 0.2 mM/kg IV.
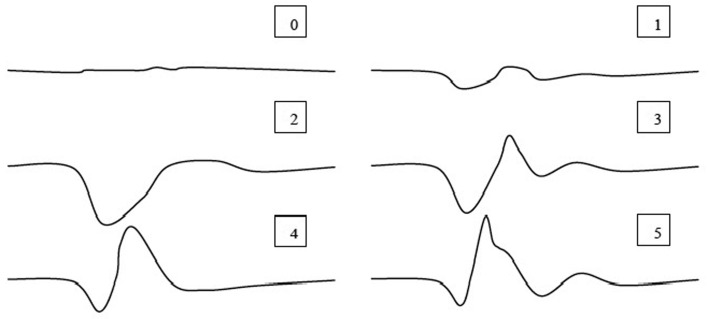
Electrophysiological examination: SEP were studied under the same general anesthesia protocol, which was provided for MRI. Briefly, tibial nerve was stimulated from the popliteal fossa because of easy accessibility to the nerve in this area, and the potentials were recorded from two segments cranial and two segments caudal of the lesion, by a hypodermic stainless steel needle. The recording needles were placed as active electrode inserted near to the arcuate ligament and the reference electrode inserted sub-fascially and about 2 cm laterally over the paraspinal muscles on the ipsilateral side. The ground electrode was inserted subcutaneously between the stimulating and recording electrodes. SEP were recorded from the scalp, and 250 responses were averaged. The injury potential ratings in posttraumatic SEP recordings were evaluated as; 0: isoelectric line, 1: major deformation, uncertain response, 2: complete injury potential, 3: incomplete injury potential, 4: morphological change and 5: normal response (Fig. 1) [40].
Fig. 1.
Grading of the injury potentials in posttraumatic SEP recordings.
Motor-evoked potentials were studied after magnetic stimulation two segments caudally and two segments cranially from the injury site using Double 70 mm Remote Control Coil (Magstim 200, The Magstim Co., Ltd., Whitland, U.K.). The recordings were carried out as active electrode inserted subcutaneously over the epimysium of gastrocnemius muscle and the reference electrode inserted subcutaneously over the Achilles tendon, and ground electrode was placed between stimulation and recording points [33]. The presence or absence of the potentials after the stimulation from two segments cranially from the injured area was taken into account in the evaluation..
Surgical techniques: According to the presence of compression and instability, hemilaminectomy or laminectomy was carried out for decompression within 24 hr of admission time in acute cases. Just decompression was performed in chronic cases within three days after admission to clinic. The cases with spinal instability were treated using screw+ poly-methyl methacrylate (PMMA) as usual. Sublaminar or transverse lamina wiring, or transarticular process pinning and tension wiring were also carried out, and combination of those techniques was demonstrated in Fig. 2. The details of the employed treatment method are presented in Table 1.
Fig. 2.
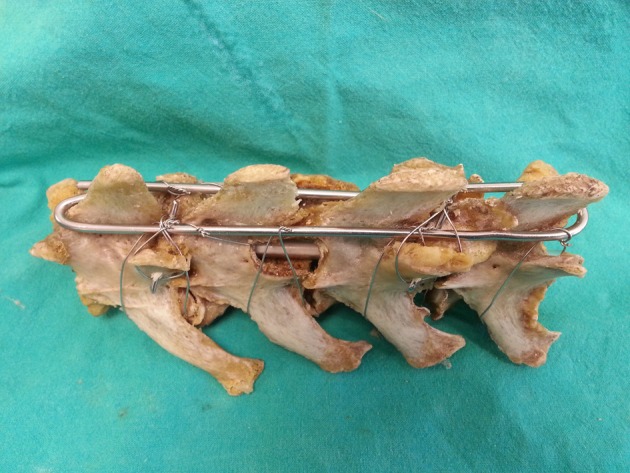
The employed spinal stabilization; transversal wiring, sublaminar wiring, transarticular process pinning and tension wiring.
Table 1. Clinical summary data for paraplegic dogs without nociception due to spinal trauma transplanted with autologous neurogenically-induced bone marrow-derived mesenchymal stem cells.
| Case no | Signalment of the dog | Neurologic examination at the admission time | X-ray/MRI | Surgical procedure | Neurologic examination | ||||
|---|---|---|---|---|---|---|---|---|---|
| At the time of first cell trans. (42.day) | At the time of 2nd cell trans. (63. day) | After the second cell transplantation | |||||||
| 2. months | 5. months | 7. months | |||||||
| 1 | 5m MI mix breed HBC AT: 1wk |
G0, P0, N0 Sca (4)Scr (0) Mcr (−)Mca (+) |
T13 proc. artc. and lamina fracture MRI: Hyperintense lesion in T2W images, and right side dorsolateral compression |
T13 right HL | G1, P0, N0 Sca (4), Scr (1) Mcr (−), Mca (+) |
G1, P0, N0 Sca (4), Scr (1) Mcr (−), Mca(+) |
G1, P0, N0 Sca (4), Scr (1) Mcr (−), Mca (+) |
G1, P0, N0 Sca (4), Scr (1) Mcr (−), Mca (+) |
G1, P0, N0 Sca (4), Scr (1) Mcr (−), Mca (+) There was no change at 12. Month |
| 2 | 2y FI husky HBC AT: 1d |
G0, P0, N0 Sca (4), Scr (1) Mcr (−), Mca (+) |
T12-13 fracture and luxation, 2/3 compression | T12-13 proc. artc. stabilized with pin and tension wire, and HL, LW between T11-L1 | G0, P0, N0 Sca (4), Scr (1) Mcr (−), Mca (+) |
G0, P0, N0 Sca (4), Scr (1) Mcr (−), Mca (+) |
G0, P0, N0 Sca (4), Scr (1) Mcr (−), Mca (+) |
||
| 3 | 6m FI mix breed HBC AT: 1d |
G0, P0, N0 Sca (4), Scr (1) Mcr (−), Mca (+) |
L1 burst fracture | HL in T13-L1 and LW including T12-13-L1-2-3 | G0, P0, N0 Sca (4), Scr (1) Mcr (−), Mca (+) |
G0, P0, N0 Sca (4), Scr (1) Mcr (−), Mca (+) |
G0, P0, N0 Sca (4), Scr (1) Mcr (−), Mca (+) |
||
| 4 | 7m MI mix breed HBC AT: 1d |
G0, P0, N0 Sca (4), Scr (1) Mcr (−), Mca (+) |
T12-13 luxation 1/3 compression, L5 (1/3 compression) vertebral body fracture |
T12 dorsal L T12-13 and L5-6 proc. artc. pin tension wire |
G0, P0, N0 Sca (4), Scr (1) Mcr (−), Mca (+) |
G0, P0, N0 Sca (4), Scr (1) Mcr (−), Mca (+) |
G0, P0, N0 Sca (4), Scr (1) Mcr (−), Mca (+) |
G0, P0, N0 Sca (4), Scr (1) Mcr (−), Mca (+) |
|
| 5 | 8m F mix breed HBC AT: 1d |
G0, P0, N0 Sca (4), Scr (1) Mcr (−), Mca (+) |
L2 (2/3 compression) and L7 (2/3) vertebral body fracture | L on L2-3, and stabilization with screw and PMMA, and
proc. artc. Lag screw fixation of L7-S1 |
G1, P0, N0 Sca (4), Scr (1) Mcr (−), Mca (+) |
G2, P0, N0 Sca (4), Scr (1) Mcr (−), Mca (+) |
G2, P0, N0 Sca (4), Scr (1) Mcr (−), Mca (+) |
G2, P0, N0 Sca (4), Scr (1) Mcr (−), Mca (+) |
|
| 6 | 2y FI mix breed Gunshot wound AT: 2d |
G0, P0, N0 Sca (2), Scr (0) Mcr (−), Mca (−) |
Pellet on T11 level at the left side | Pellet had taken off with HL | G0, P0, N0 Sca (2), Scr (0) Mcr (−), Mca (−) |
G1, P0, N0 Sca (2), Scr (0) Mcr (−), Mca (−) |
G1, P0, N0 Sca (2), Scr (0) Mcr (−), Mca (−) |
||
| 7 | 2.5m FI mix breed HBC AT: 2d |
G1, P0, N0 Sca (4), Scr (2) Mcr (−), Mca (+) |
L5 burst fracture | L4-5-6 sublaminar wiring, L4-5 left side HL |
G2, P0, N0 Sca (4), Scr (2) Mcr (−), Mca (+) |
G4, P1, N1 Sca (4), Scr (3) Mcr (+), Mca (+) |
G4, P1, N2 Sca (4), Scr (4) Mcr (+), Mca (+) |
G4, P1, N2 Sca (4), Scr (4) Mcr (+), Mca (+) |
G4, P1, N2 Sca (4), Scr (4) Mcr (+), Mca (+) |
| 8 | 8m FI mix breed HBC AT: 2d |
G0, P0, N0 Sca (4), Scr (0) Mcr (−), Mca (+) |
T12-13 luxation, 1/3 compression | T12-13 proc. artc. pin and tension wire stabilisation T12-13 Left side mini HL and disk fenestration |
G0, P0, N0 Sca (4), Scr (0) Mcr (−), Mca (+) |
G0, P0, N0 Sca (4), Scr (0) Mcr (−), Mca (+) |
G0, P0, N0 Sca (4), Scr (0) Mcr (−), Mca (+) |
G0, P0, N0 Sca (4), Scr (0) Mcr (−), Mca (+) |
G0, P0, N0 Sca (4), Scr (0) Mcr (−), Mca (+) |
| 9 | 1y MI pointer HBC AT: 2d |
G0, P0, N0 Sca (4), Scr (0) Mcr (−), Mca (+) |
T9-10 luxation 1/3 compression | T9-10 proc. artc. pin and tension wire and Left side HL, and T8 –T11 screw + PMMA | G0, P0, N0 Sca (4), Scr (0) Mcr (−), Mca (+) |
G1, P0, N0 Sca (4), Scr (0) Mcr (−), Mca (+) |
G1, P0, N0 Sca (4), Scr (0) Mcr (−), Mca(+) |
G1, P0, N0 Sca (4), Scr (0) Mcr (−), Mca (+) |
G1, P0, N0 Sca (4), Scr (0) Mcr (−), Mca (+) |
| 10 | 6m M mix breed HBC AT: 1d |
G0, P0, N0 Sca (1), Scr (0) Mcr (−), Mca (+) |
L5 vertebral body fracture 1/3 compression |
L4-5 right side H, vertebral body reconstruction plate
and L4-5 proc. artc. pin tension wire, |
G1, P0, N0 Sca (4), Scr (3) Mcr (−), Mca (+) |
G3, P0, N1 Sca (4), Scr (3) Mcr (−), Mca(+) |
G3, P1, N2 Sca (4), Scr (4) Mcr (+), Mca (+) |
G3, P1, N2 Sca (4), Scr (4) Mcr (+), Mca (+) |
G3, P1, N2 Sca (4), Scr (4) Mcr (+), Mca (+) |
| 11 | 8y F mix breed, HBC and Operated 1 year ago due to Scrinal trauma AT: 1 year |
G0, P0, N0 Sca (4), Scr (0) Mcr (−), Mca (+) |
MRI: Epidural fibrosis on T12 level Hyperintense lesion on T2 W 17 mm long at the Scrinal cord |
HL | G1, P0, N0 Sca (4), Scr (0) Mcr (−), Mca (+) |
G1, P0, N0 Sca (4), Scr (0) Mcr (−), Mca(+) |
G2, P0, N0 Sca (4), Scr (0) Mcr (−), Mca (+) |
G2, P0, N0 Sca (4), Scr (0) Mcr (−), Mca (+) |
G2, P0, N0 Sca (4), Scr (0) Mcr (−), Mca (+) |
| 12 | 4m FI mix breed HBC AT: 2d |
G0, P0, N0 Sca (4), Scr (0) Mcr (−), Mca (+) |
L3-4 luxation 2/3 compression |
L and Screw+PMMA in L2-5 | G0, P0, N0 Sca (4), Scr (0) Mcr (−), Mca (+) |
G1, P0, N0 Sca (4), Scr (0) Mcr (−), Mca (+) |
G1, P0, N0 Sca (4), Scr (0) Mcr (−), Mca (+) |
G1, P0, N0 Sca (4), Scr (0) Mcr (−), Mca (+) |
G1, P0, N0 Sca (4), Scr (0) Mcr (−), Mca (+) |
| 13 | 4y M mix breed HBC AT: 1 Month |
G0, P0, N0 Sca (4), Scr (0) Mcr (−), Mca (+) |
T12-13 fracture and luxation: 1/3 compression | L | G0, P0, N0 Sca (4), Scr (0) Mcr (−), Mca (+) |
G0, P0, N0 Sca (4), Scr (0) Mcr (−), Mca (+) |
G1, P0, N0 Sca (4), Scr (0) Mcr (−), Mca (+) |
G1, P0, N0 Sca (4), Scr (0) Mcr (−), Mca (+) |
G1, P0, N0 Sca (4), Scr (0) Mcr (−), Mca (+) |
artc.: articularis; AT: Admission Time; FI: Female intact; G: Gait score; HBC: Hit by car, HL: Hemilaminectomy; L: Laminectomy; Lt: Left; LW: Laminotransversal wiring; Mca: MEP caudal; MI: Male intact; Mcr: MEP cranial; N: Nociception; P: Proprioception, PMMA: polymethylmethacrylate; proc.: processus; Rt: Right; Sca: SEP caudal; Scr: SEP cranial; trans.: Transplantation; y: years.
In the cases that were admitted during acute-stage (<48 hr), surgical management (decompression or decompression + stabilization) and collection of the bone marrow were carried out during the same anesthesia. However, in the cases which were admitted in chronic stages (cases 1, 11 and 13), just the bone marrow was collected at the admission time for processing. In the former cases, decompression and first cell transplantation were carried out at the same session. All cases underwent physical therapy and rehabilitation at the interval between bone marrow collection and cell transplantation,
Intra-operative analgesia provided with a 0.02 mg/kg/hr IV constant-rate infusion of fentanyl (Fentanyl® 50 µg/ml, Johnson & Johnson, Diegem, Belgium) and administration of epidural morphine with 0.1 mg/kg (Morfin HCl® 0.01 g/ml, Osel, Istanbul, Turkey) at the decompression defect prior to closure of the incision. Analgesia at discharge was achieved with carprofen (Rimadyl®, Pfizer) with 2 mg/kg, PO, q 12 hr for 5–7 days.
Isolation and culture of BM-MSCs: Bone marrow was collected from the iliac crest and transported to the laboratory within an hour. BM-MSCs were cultured according to standard methods [6, 27]. Under sterile conditions, ~5–10 ml of bone marrow was suspended in α-MEM (HyClone®, GE Life Sciences, Logan, UT, U.S.A.) containing 1% penicillin–streptomycin (Pen/Strep) and 2 mM L-glutamine (Sigma-Aldrich, Munich, Germany) and was washed three times in 0.1 M phosphate buffer saline (PBS) with sequential centrifugation (at 25°C and 1,000 rpm). Subsequently, the cells were seeded in tissue culture flasks containing α-MEM supplemented with Pen/Strep, L-glutamine and 10% fetal bovine serum (BioWhittaker-Lonza, Basel, Switzerland) at 37°C with humidified 5% CO2. The non-adherent cells were removed by replacing the medium at the second day of subculture. The cells were cultured up to passage 2 under the same conditions, with medium changes every other day. At this point, ~5.0 × 106 BM-MSCs were separated and induced for differentiation into the neurogenic lineage. The remaining BM-MSCs were cultured likewise for the subsequent dose administration.
In vitro neurogenic differentiation of BM-MSCs: BM-MSCs were induced to differentiate into the neurogenic lineage by a two-step protocol [27]. Briefly, cells were placed in tissue culture flasks coated with poly hydroxyethyl methacrylate (BioWhittaker-Lonza) and cultured in the B-27 and N-2 (serum-free chemically-defined neurogenic supplements) containing neurobasal medium (MesenPro™ with 10% MesenPro™ supplement, Pen/Strep and L-Glutamine; Invitrogen), supplemented with 100 ng/ml human recombinant epidermal growth factor (EGF) and 10 ng/ml human recombinant basic fibroblast growth factor (bFGF) (both recombinant DNA expressed in yeast (S. cerevisiae); Merck-Millipore, Darmstadt, Germany). The resulting neurospheres were stained to confirm positivity to Nestin (Santa Cruz Biotechnology, Heidelberg, Germany).
At the second step, the acquired neurospheres were disaggregated by accutase (StemPro Accutase, Gibco, Grand Island, NY, U.S.A.), and the cell suspension was plated in tissue culture flasks which were coated with poly ornithine (Sigma-Aldrich). The neurosphere-derived cell suspension was then cultured for six days in the neurobasal medium supplemented with 100 ng/ml nerve growth factor (NGF) (from mouse submaxillary gland; Merck-Millipore, Darmstadt, Germany) and 10 ng/ml brain-derived neurotrophic factor (BDNF) (human recombinant; Merck-Millipore) for further induction into the neurogenic lineage. Immunohistochemistry revealed that these cells were positive to glial fibrillary acidic protein (GFAP) and anti-2’3′-cyclic nucleotide-3′-phosphodiesterase (CNPase), as well as to beta III tubulin and microtubule-associated protein-2 (MAP-2) (Abcam, Cambridge, MA, U.S.A.). At ~80% confluence, the cells were removed from the surface by 0.05% Trypsin/0.53 mM EDTA solution (Sigma-Aldrich) and washed three times with PBS. One ml of cell suspension was transferred into a syringe and was delivered to the surgery room within ~30 min.
Cell transplantation procedure: Three spinal needles (22G×90 mm quincke tip, Egemen, Izmir, Turkey) were inserted percutaneously through the hemilaminectomy or laminectomy defect intraspinally into three different points under general anesthesia with the same protocol which is used in MRI. The location of the needle tip was confirmed by observing cerebrospinal fluid leakage from the needle hub. Subsequently, the needle tip was directed into the spinal cord parenchyma percutaneously, and a slow injection was carried out. About 5.0 × 106 NIBM-MSCs suspended in 1.0 ml of PBS were injected by needles in equal volume. The cell transplantation procedure (~5.0 × 106 NIBM-MSCs) was repeated after 21 days. The neurological and electrophysiological examinations of the cases were performed at the 42nd day (time of the first cell transplantation), at the 63rd day (time of the second cell transplantation), and 2, 5, 7 and 12th months after the second cell transplantation.
RESULTS
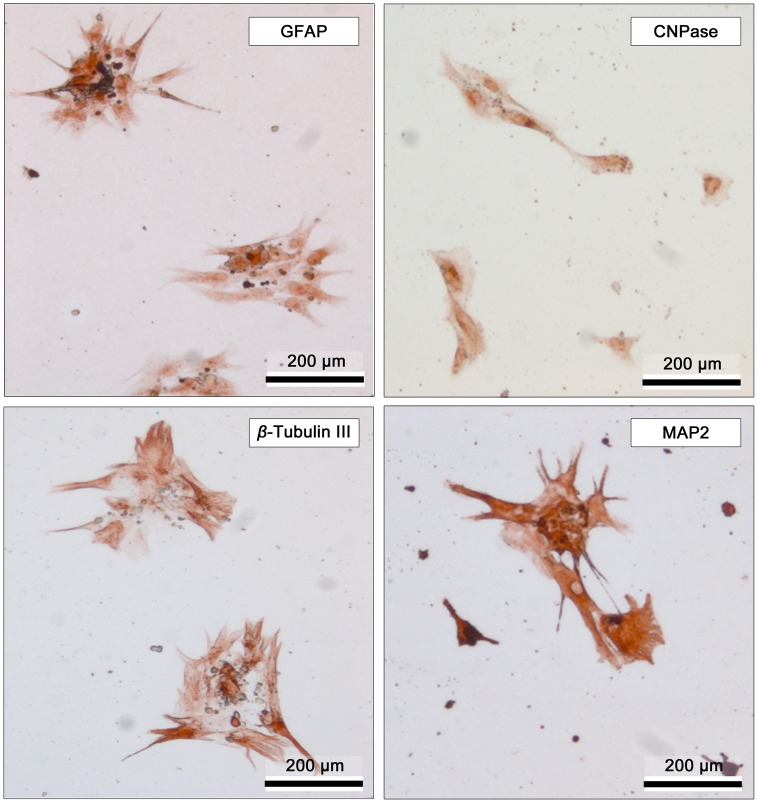
In most of the cases, BM-MSCs expanded quite well in-vitro demonstrating fusiform, fibroblastoid phenotype starting from the first passage (Fig. 3A). At the first neurogenic induction step, the cells formed neurospheres with a diameter of ~100–150 µm within 3 days (Fig. 3B). At this stage, >95% of the neurosphere cells were positive to Nestin. At the second step of neurogenic induction, the acquired Nestin-positive neurospheres were disaggregated, and the acquired cells were differentiated further into the subsequent stages forming neural tube-like structures, as well as multiple branching processes resembling early steps of development (Fig. 3C and 3D). The population of cells were positive to GFAP, CNPase, beta III tubulin and MAP-2 (Fig. 4). Immunohistochemistry revealed that differentiated cells were highly positive to MAP-2 (~90%) and partially positive to beta III tubulin (~37%), CNPase (~12%) and GFAP (~10%) markers, indicating the existence of a mixed population of mature and immature neurogenic cells, for the most part of neuronal cells.
Fig. 3.
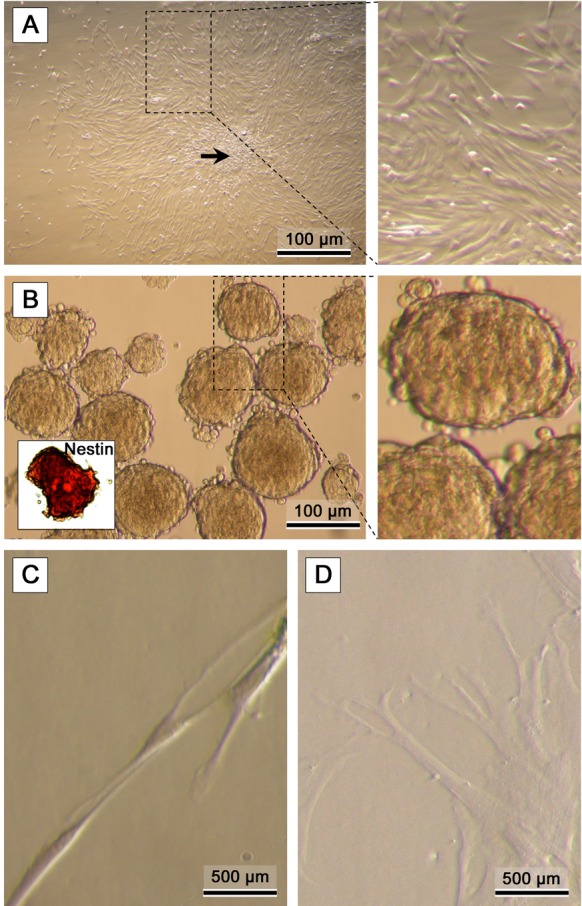
Representative micrographs of: (A) bone marrow MSCs. Formation of colonies (arrow) is typical in confluent MSC cultures (CFU-f). (B) Formation of Nestin-positive neurospheres (inner photo) after the first neurogenic induction step. (C, D) Disaggregated cells are directed into the second neurogenic induction stage yielding neurogenic phenotypes.
Fig. 4.
Representative immunohistochemistry images of BM-MSCs following neurogenic induction steps. GFAP (glial fibrillary acidic protein); CNPase (anti-2′3′-cyclic nucleotide-3′-phosphodiesterase), β-Tubulin III, MAP-2 (microtubule-associated protein-2.
Thirteen dogs (5 males and 8 females) fulfilled the case selection criteria. Neurological, radiological and electrophysiological findings are summarized in Table 1. Mean age during admission was 19.3 months (ranging from 2.5 months to 8 years), and the mean body weight of the dogs was 11 kg (range: 6–19 kg). Subjected breeds were mixed breed (n=12), Siberian husky (n=1) and Pointer (n=1). Ten dogs were presented within 48 hr of onset of clinical signs and operated at the same day. The rest of three dogs were presented one week, one month and one year after the trauma for each, and they were operated after stem cells preparation. At the admission time, all cases were paraplegic due to spinal trauma, and neuroanatomic localization was between T3-L7. Spinal trauma dispersed according to the radiographic images of the cases as; vertebral body fracture (n=3), fracture and luxation (n=2), laminar fracture (n=1), burst fracture (n=2), luxation (n=3), gunshot wound (n=1) and epidural fibrosis due to a former trauma (n=1). Spinal cord injury was further clarified by MRI at the admission time only in 2 chronic cases, in which the hyper-intense lesions (it may be myelomalacia or glial reaction) were seen at the spinal cord in T2 weighted imaging in the injured area of the spinal cord parenchyma. One case (case no: 1) had compression at the right dorsolateral side of T13 spinal cord segment in transversal images and a 24 mm-length hyper-intense lesion in sagittal and dorsal planes of MRI. The other case (case no: 11) had narrowing epidural space at T12 level, causing a 17 mm-long hyper-intense lesion in both dorsal and sagittal planes of MRI, and the lesion was interpreted as epidural fibrosis. In the remaining cases, traumatic lesions were obvious in survey radiography, and further advanced diagnostic imaging was declined. Spinal instability was determined according to the three compartments theory [38], and stabilization was carried out in 9 cases as follows: Sublaminar wire (n=1); pin-tension wire at the articular processes in both side (n=2); screw on vertebral bodies and PMMA (n=1); pin-tension wire at the articular processes+ screw and PMMA (n=1); pin-tension wire at the articular processes and laminotransversal wiring (n=1); laminotransversal wiring (n=1); screw on vertebral bodies+PMMA and lag screw on articular processes (n=1); vertebral body plate and pin-tension wire at the articular processes (n=1). Spinal trauma which did not cause instability was treated by laminectomy (n=1) and hemilaminectomy (n=3). In the case with the gunshot wound, the bullet was located dorsally at the vertebral body in epidural space. The color changes, which are reddish to brown, of dura mater were seen in all cases during the surgery. In 2 of the chronic cases (case nos.: 11 and 13), the spinal cord was covered by extensive fibrous tissue and was thinner in diameter.
The gait score for TSCIS at the time of admission was “0” in all cases except for 1 case with “1”, however, the proprioception and nociception scores were also “0” in all cases. Somatosensory-evoked potentials were flat-line at the cranial area of injury in 8 cases, “1” in 4 cases and “2” in 1 case. No motor-evoked potential was obtained from the gastrocnemius muscle after stimulating the spinal cord from the 2 segments cranial injured area.
At the 42nd day which is average time for preparing cells for each case after the operation or physical therapy: the gait score had improved one score for each in 5 cases. Neither proprioception nor nociception was changed after the conventional treatments for 42 days. The SEP were improved in one case at the cranial part of injured area with one score, and one case showed improvement in both caudal and cranial areas with 3 score. There was no improvement in MEP studies.
All cases received two doses of autologous 5.0 × 106 NIBM-MSCs suspension at the post-operative 42nd and 63rd days, through the decompression defect intraspinally, except for one case in which injection was performed subarachnoidally from the L5-6 intervertebral space by lumbar punction due to inaccessibility caused by PMMA (case no. 9). Technically injecting stem cells through the decompression defect was feasible. Injection was achieved by palpating the laminectomy or hemilaminectomy defect by the absence of spinous process or articular process, respectively, and insertion of spinal needles into the spinal cord. Appearance of the cerebrospinal fluid was accepted as landmark, the tip of needle was progressed into the spinal cord parenchyma, and injection was carried out.
Employed stabilization techniques did not fail in any of the cases during the observation period. Patients with PMMA had seroma formation at the operation site, however, seroma was drained, and the operation site was recovered uneventfully.
From the first to the second cell transplantation, 4 cases improved 1 gait score, and 2 cases improved 2 gait score. One of these cases which showed 2 gait score improvement, progressed 1 score for each in proprioception, nociception and proximal SEP, and also proximal MEP changed negative to positive. Two months after the second cell transplantation, 2 cases exhibited 1 score improvement in gait score. Two cases showed 1 score improvement in both nociception and proximal SEP, also one of these cases improved 1 score in proprioception, and proximal MEP changed negative to positive in this case. All progresses were observed until 2nd month after the second cell transplantation, however, there was no improvement after this period. In general, improvement of gait, nociception and proprioception after cell transplantation was observed in just 2 cases, and only gait score improvement was seen in 6 cases, and no improvement was recorded in 5 cases. SEP and MEP results had improved in both of the cases, which showed improvement nociception and proprioception. As a result, overall TSCIS score did not change after the cell transplantations in 5 cases, one score increased in 6 cases and five score in 2 cases.
DISCUSSION
To the authors’ knowledge, there is not an existing report on percutaneous NIBM-MSCs transplantation for the treatment of paraplegia due to external trauma in dogs. The present study reports promising results in 2 cases and also some improvement of the locomotion in 6 cases. Even though spontaneous improvement in this poorest neurologic grade has been reported, it is not so common [11, 29]. These hopeless cases have been selected for this study on purpose, and minus gains are acceptable [11]. According to the clinical and electrophysiological results, there was no remarkable difference between the time of admission and prior to cell transplantation (42nd day). When the results of data were recorded before cell transplantation with the end point of observation period, there are barely positive developments in clinical signs. This study does not exclude the possibility of spontaneous improvement entirely, yet presents acceptable positive results open to further investigation.
There is a wide range of studies regarding spinal stabilization techniques, involving the use of pins or screws and polymethymetacrylate, vertebral body plating, vertebral stapling, external skeletal fixation, veterinary string of pearls plates, pedicle screw–rod fixation, Lubra plates and tension bands [17, 22, 23, 39, 41, 43, 45]. The surgical stabilization applied in this case series was acceptable, and we report no implant failures in any case. Some modifications applied in the cases reported here, such as articular processes pining and tension wiring, and sublaminar wiring and/or subtransversal wiring, were also found versatile, cost effective and easy applicable techniques for providing spinal stabilization. For preventing further and repeated trauma due to spinal instability, spinal stabilization is crucial for the successful rehabilitation of paraplegic cases. These techniques are investigated in detail for both dogs and cats by the authors (OB, PC, unpublished data).
Bone marrow-derived stem cells can differentiate into glial cells or neurons which may improve the effects of regenerative therapy in spinal cord injury [24, 35]. The best therapeutic outcomes can be achieved with optimal dosage, timing and optimal administration route [42]. Bone marrow stromal cells do not differentiate into neural cells in the host tissue as determined by immunohistochemical analysis [47]. Thus, we carried out a serum-free MSC differentiation protocol which yielded a mixed population of partially mature neurogenic cells that were positive to GFAP, CNPase, beta III tubulin and MAP-2. MSCs may prove to be beneficial for treating SCI as they can be expanded and differentiated into the neurogenic lineage through neurospheres in vitro [13]. Additionally, the insufficient clinical results of spinal cord injury cases, motivates us to investigate autologous NIBM-MSCs in paraplegic dogs without DPP, due to external trauma. The percutaneous transplantation technique [18] preferred in this study is minimally invasive, reliable and easily applicable, and does not require advanced level of experience.
An experimental canine model utilizing neural-differentiated allogeneic MSC transplantation with Matrigel for the treatment of spinal cord injury, revealed functional improvement 1 week upon SCI. Findings were attributed to the neurotrophic effects, including increase in neurotrophin expression, decreased inflammation and astrogliosis, as well as increased neuronal extension and regeneration [31]. However, BM-MSCs in the spinal cord were not observable by the third week of injection. Multiple administration of BM-MSCs leads to improved cell grafting compared to a single application of BM-MSCs in spinal cord injury [26, 28, 35]. There was no chance to make histopathologic or immunohistochemical investigation of the injured spinal cord areas that may explain the mechanism of improvement in our cases, but increased neurotrophin expression or neuronal extension might be provided an improvement in the presented study.
In the present study, we preferred to transplant the cells 2 times with 21 days interval to sustain the positive effect for a longer period as it has been reported before [25]. We observed the beneficial effects of cell therapy in chronic spinal cord injury cases (more than 42 days) at the second month, but there was no improvement at the later periods. The use of BM-MSCs for SCI has provided re-myelination and neuroprotection from releasing cytokines in experimentally-induced spinal cord injuries in rat models [2, 12]. Even though autologous MSCs’ transplantation has shown more beneficial effects than allogeneic MSCs transplantation, allogeneic MSCs transplantation has also been found useful in improving functional recovery following SCI [16, 26, 46]. As the time required for preparing autologous NIBM-MSCs is relatively long, it is not possible to deliver these cells at the acute stage of spinal cord injury. There may be a possibility to shorten the time span between bone marrow collection and cell transplantation to around 28–30 days (i.e. 19–21 days of MSC expansion + 9 days of neurogenic induction), which will necessitate subtraction of the second dose. However, this was not the option in our study; and longer culture time was needed to obtain the required number of stem cells for the second transplantation. Use of banked allogenic stem cells can be a possibility to shorten the time span, however, potential drawbacks of using allogeneic source need to be evaluated in additional studies. Providing a suitable environment for cells assures better outcomes in chronic spinal cord injury. Researchers aim to solve the time limitation and support the therapy with additional modalities.
The results of cell injections into the CSF in dogs with intervertebral disc disease (IVDD) are as follows: 6 of 10 cases regained walking ability, but only a single case showed improvement in nociception [25]. The same group reports their results of intraspinal injection of bone marrow stromal cells to paraplegic dogs without DPP due to external trauma as: 2 out of 7 cases showed improvement in walking ability, but none had nociception [26]. This is consistent with the observation that BM-MSC transplantation in rats had no effect in sensory function. In a case series [29] about IVDD without DPP, walking ability was regained in 57.81% (37/64), and 9 dogs were euthanized within 3wk, and 18 of them survived but never regained nociception. However, 7 dogs out of 18 regained the ability to walk. The results of our study are in line with the previous studies [26], and improvement in gait score is higher than the nociception and proprioception scores presented by other groups. In another study conducted by our research group in dogs with IVDD and lacking DPP, in eight months-follow up, 4 cases were evaluated by the same parameter; gait score had improved in 3 cases, proprioception improved in 2 cases, and nociception improved in 3 cases [7]. Some recent studies [7, 26] show that the results of IVDD are better than external trauma even though the preferred cell types were different in the studies.
The success rate after external spinal trauma is lower (% 0) [4, 17] than that of IVDD (% 0–76) [3, 8, 10, 29, 36], as it involves the treatment of many structures (e.g. bone, ligaments), for overcoming instability, and also the possibility of iatrogenic trauma. Comparing recovery rates of different studies is complicated, due to many variables, such as case selection criteria, the duration of DPP absence or loss of motor function, and the treatment modality of choice. The rate of success and the results of the presented study are not dramatically different compared to the data in the literature [4, 29]. However, the improvement in nociception and proprioception can be accepted as a critical point for carrying out further investigations with higher number of cases and including new criteria.
Among previous reports with cases without DPP after external trauma, the success rate was also poor even with combined surgical or medical treatments [11, 17, 37]. Nevertheless, improvement in gait score in 6 of the cases, and improvement in proprioception and nociception in 2 cases can be accepted as encouraging results.
The main limitation of this study can be listed as; lack of histological and immuno-histochemical examinations, lack of a control group and being a single-center study design. Randomized multi-center clinical studies are required to clarify the contribution of NIBM-MSCs’ transplantation in dogs with SCI. However, it is possible to conclude that percutaneous intraspinal injection of autologous NIBM-MSCs provides some clinical benefits in dogs with paraplegia, lacking DPP after external spinal trauma.
Acknowledgments
Supported by The Scientific and Technological Research Council of Turkey (TÜBITAK) Research grant (no: 111O428). YME acknowledges the support of The Turkish Academy of Sciences (TÜBA, Ankara, Turkey) as associate member.
REFERENCES
- 1.Aghayan H. R., Arjmand B., Yaghoubi M., Moradi-Lakeh M., Kashani H., Shokraneh F.2014. Clinical outcome of autologous mononuclear cells transplantation for spinal cord injury: a systematic review and meta-analysis. Med. J. Islam. Repub. Iran 28: 112–120. [PMC free article] [PubMed] [Google Scholar]
- 2.Akiyama Y., Radtke C., Kocsis J. D.2002. Remyelination of the rat spinal cord by transplantation of identified bone marrow stromal cells. J. Neurosci. 22: 6623–6630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson S. M., Lippincott C. L., Gill P. J.1991. Hemilaminectomy in dogs without deep pain perception. Calif. Vet. 45: 24–28. [Google Scholar]
- 4.Bali M. S., Lang J., Jaggy A., Spreng D., Doherr M. G., Forterre F.2009. Comparative study of vertebral fractures and luxations in dogs and cats. Vet. Comp. Orthop. Traumatol. 22: 47–53. [PubMed] [Google Scholar]
- 5.Barreiro-Iglesias A.2010. Targeting ependymal stem cells in vivo as a non-invasive therapy for spinal cord injury. Dis. Model. Mech. 3: 667–668. doi: 10.1242/dmm.006643 [DOI] [PubMed] [Google Scholar]
- 6.Baykan E., Koc A., Elcin A. E,, Elcin Y. M.2014. Evaluation of a biomimetic poly(ε-caprolactone)/β-tricalcium phosphate multispiral scaffold for bone tissue engineering: in vitro and in vivo studies. Biointerphases 9: 029011. doi: 10.1116/1.4870781 [DOI] [PubMed] [Google Scholar]
- 7.Besalti O., Can P., Akpinar E., Aktas Z., Elcin A. E., Elcin Y. M.2015. Intraspinal transplantation of autologous neurogenically-induced bone marrow-derived mesenchymal stem cells in treatment of paraplegic dogs without deep pain perception secondary to intervertebral disk disease. Turk Neurosurg. 25: 625–632. [DOI] [PubMed] [Google Scholar]
- 8.Besalti O., Ozak A., Pekcan Z., Tong S., Eminaga S., Tacal T.2005. The role of extruded disk material in thoracolumbar intervertebral disk disease: a retrospective study in 40 dogs. Can. Vet. J. 46: 814–820. [PMC free article] [PubMed] [Google Scholar]
- 9.Blight A. R.1983. Cellular morphology of chronic spinal cord injury in the cat: analysis of myelinated axons by line-sampling. Neuroscience 10: 521–543. doi: 10.1016/0306-4522(83)90150-1 [DOI] [PubMed] [Google Scholar]
- 10.Brown N. O., Helphrey M. L., Prata R. G.1977. Thoracolumbar disk disease in the dog: A retrospective analysis of 187 cases. J. Am. Anim. Hosp. Assoc. 13: 665–672. [Google Scholar]
- 11.Bruce C. W., Brisson B. A., Gyselinck K.2008. Spinal fracture and luxation in dogs and cats: a retrospective evaluation of 95 cases. Vet. Comp. Orthop. Traumatol. 21: 280–284. [PubMed] [Google Scholar]
- 12.Chopp M., Zhang X. H., Li Y., Wang L., Chen J., Lu D., Lu M., Rosenblum M.2000. Spinal cord injury in rat: treatment with bone marrow stromal cell transplantation. Neuroreport 11: 3001–3005. doi: 10.1097/00001756-200009110-00035 [DOI] [PubMed] [Google Scholar]
- 13.Chung W. H., Park S. A., Lee J. H., Chung D. J., Yang W. J., Kang E. H., Choi C. B., Chang H. S., Kim D. H., Hwang S. H., Han H., Kim H. Y.2013. Percutaneous transplantation of human umbilical cord-derived mesenchymal stem cells in a dog suspected to have fibrocartilaginous embolic myelopathy. J. Vet. Sci. 14: 495–497. doi: 10.4142/jvs.2013.14.4.495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Granger N., Blamires H., Franklin R. J., Jeffery N. D.2012. Autologous olfactory mucosal cell transplants in clinical spinal cord injury: a randomized double-blinded trial in a canine translational model. Brain 135: 3227–3237. doi: 10.1093/brain/aws268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jeffery N. D.2010. Vertebral fracture and luxation in small animals. Vet. Clin. North Am. Small Anim. Pract. 40: 809–828. doi: 10.1016/j.cvsm.2010.05.004 [DOI] [PubMed] [Google Scholar]
- 16.Jung D. I., Ha J., Kang B. T., Kim J. W., Quan F. S., Lee J. H., Woo E. J., Park H. M.2009. A comparison of autologous and allogenic bone marrow-derived mesenchymal stem cell transplantation in canine spinal cord injury. J. Neurol. Sci. 285: 67–77. doi: 10.1016/j.jns.2009.05.027 [DOI] [PubMed] [Google Scholar]
- 17.Krauss M. W., Theyse L. F. H., Tryfonidou M. A., Hazewinkel H. A., Meij B. P.2012. Treatment of spinal fractures using Lubra plates. A retrospective clinical and radiological evaluation of 15 cases. Vet. Comp. Orthop. Traumatol. 25: 326–331. doi: 10.3415/VCOT-11-07-0096 [DOI] [PubMed] [Google Scholar]
- 18.Lee J. H., Chang H. S., Kang E. H., Chung D. J., Choi C. B., Lee J. H., Hwang S. H., Han H., Kim H. Y.2009. Percutaneous transplantation of human umbilical cord blood-derived multipotent stem cells in a canine model of spinal cord injury. J. Neurosurg. Spine 11: 749–757. doi: 10.3171/2009.6.SPINE08710 [DOI] [PubMed] [Google Scholar]
- 19.Levine G. J., Levine J. M., Budke C. M., Kerwin S. C., Au J., Vinayak A., Hettlich B. F., Slater M. R.2009. Description and repeatability of a newly developed spinal cord injury scale for dogs. Prev. Vet. Med. 89: 121–127. doi: 10.1016/j.prevetmed.2009.02.016 [DOI] [PubMed] [Google Scholar]
- 20.McDonald J. W.2004. Repairing the damaged spinal cord: from stem cells to activity-based restoration therapies. Clin. Neurosurg. 51: 207–227. [PubMed] [Google Scholar]
- 21.McDonald J. W., Liu X. Z., Qu Y., Liu S., Mickey S. K., Turetsky D., Gottlieb D. I., Choi D. W.1999. Transplanted embryonic stem cells survive, differentiate and promote recovery in injured rat spinal cord. Nat. Med. 5: 1410–1412. doi: 10.1038/70986 [DOI] [PubMed] [Google Scholar]
- 22.McKee W. M., Downes C. J.2008. Vertebral stabilisation and selective decompression for the management of triple thoracolumbar disc protrusions. J. Small Anim. Pract. 49: 536–539. doi: 10.1111/j.1748-5827.2008.00582.x [DOI] [PubMed] [Google Scholar]
- 23.Meij B. P., Suwankong N., Van der Veen A. J., Hazewinkel H. A.2007. Biomechanical flexion-extension forces in normal canine lumbosacral cadaver specimens before and after dorsal laminectomy-discectomy and pedicle screw-rod fixation. Vet. Surg. 36: 742–751. doi: 10.1111/j.1532-950X.2007.00331.x [DOI] [PubMed] [Google Scholar]
- 24.Muñoz-Elías G., Woodbury D., Black I. B.2003. Marrow stromal cells, mitosis, and neuronal differentiation: stem cell and precursor functions. Stem Cells 21: 437–448. doi: 10.1634/stemcells.21-4-437 [DOI] [PubMed] [Google Scholar]
- 25.Nishida H., Nakayama M., Tanaka H., Kitamura M., Hatoya S., Sugiura K., Suzuki Y., Ide C., Inaba T.2011. Evaluation of transplantation of autologous bone marrow stromal cells into the cerebrospinal fluid for treatment of chronic spinal cord injury in dogs. Am. J. Vet. Res. 72: 1118–1123. doi: 10.2460/ajvr.72.8.1118 [DOI] [PubMed] [Google Scholar]
- 26.Nishida H., Nakayama M., Tanaka H., Kitamura M., Hatoya S., Sugiura K., Harada Y., Suzuki Y., Ide C., Inaba T.2012. Safety of autologous bone marrow stromal cell transplantation in dogs with acute spinal cord injury. Vet. Surg. 41: 437–442. doi: 10.1111/j.1532-950X.2011.00959.x [DOI] [PubMed] [Google Scholar]
- 27.Odabas S., Elçin A. E., Elçin Y. M.2014. Isolation and characterization of mesenchymal stem cells. Methods Mol. Biol. 1109: 47–63. doi: 10.1007/978-1-4614-9437-9_3 [DOI] [PubMed] [Google Scholar]
- 28.Ohta M., Suzuki Y., Noda T., Ejiri Y., Dezawa M., Kataoka K., Chou H., Ishikawa N., Matsumoto N., Iwashita Y., Mizuta E., Kuno S., Ide C.2004. Bone marrow stromal cells infused into the cerebrospinal fluid promote functional recovery of the injured rat spinal cord with reduced cavity formation. Exp. Neurol. 187: 266–278. doi: 10.1016/j.expneurol.2004.01.021 [DOI] [PubMed] [Google Scholar]
- 29.Olby N., Levine J., Harris T., Muñana K., Skeen T., Sharp N.2003. Long-term functional outcome of dogs with severe injuries of the thoracolumbar spinal cord: 87 cases (1996-2001). J. Am. Vet. Med. Assoc. 222: 762–769. doi: 10.2460/javma.2003.222.762 [DOI] [PubMed] [Google Scholar]
- 30.Oudega M.2007. Schwann cell and olfactory ensheathing cell implantation for repair of the contused spinal cord. Acta Physiol. (Oxf.) 189: 181–189. doi: 10.1111/j.1748-1716.2006.01658.x [DOI] [PubMed] [Google Scholar]
- 31.Park S. S., Lee Y. J., Lee S. H., Lee D., Choi K., Kim W. H., Kweon O. K., Han H. J.2012. Functional recovery after spinal cord injury in dogs treated with a combination of Matrigel and neural-induced adipose-derived mesenchymal Stem cells. Cytotherapy 14: 584–597. doi: 10.3109/14653249.2012.658913 [DOI] [PubMed] [Google Scholar]
- 32.Penha E. M., Meira C. S., Guimarães E. T., Mendonça M. V., Gravely F. A., Pinheiro C. M., Pinheiro T. M., Barrouin-Melo S. M., Ribeiro-Dos-Santos R., Soares M. B.2014. Use of autologous mesenchymal stem cells derived from bone marrow for the treatment of naturally injured spinal cord in dogs. Stem Cells Int. 2014: 437521. doi: 10.1155/2014/437521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poma R., Parent J. M., Holmberg D. L., Partlow G. D., Monteith G., Sylvestre A. M.2002. Correlation between severity of clinical signs and motor evoked potentials after transcranial magnetic stimulation in large-breed dogs with cervical spinal cord disease. J. Am. Vet. Med. Assoc. 221: 60–64. doi: 10.2460/javma.2002.221.60 [DOI] [PubMed] [Google Scholar]
- 34.Ryu H. H., Kang B. J., Park S. S., Kim Y., Sung G. J., Woo H. M., Kim W. H., Kweon O. K.2012. Comparison of mesenchymal stem cells derived from fat, bone marrow, Wharton’s jelly, and umbilical cord blood for treating spinal cord injuries in dogs. J. Vet. Med. Sci. 74: 1617–1630. doi: 10.1292/jvms.12-0065 [DOI] [PubMed] [Google Scholar]
- 35.Sanchez-Ramos J., Song S., Cardozo-Pelaez F., Hazzi C., Stedeford T., Willing A., Freeman T. B., Saporta S., Janssen W., Patel N., Cooper D. R., Sanberg P. R.2000. Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp. Neurol. 164: 247–256. doi: 10.1006/exnr.2000.7389 [DOI] [PubMed] [Google Scholar]
- 36.Scott H. W., McKee W. M.1999. Laminectomy for 34 dogs with thoracolumbar intervertebral disc disease and loss of deep pain perception. J. Small Anim. Pract. 40: 417–422. doi: 10.1111/j.1748-5827.1999.tb03114.x [DOI] [PubMed] [Google Scholar]
- 37.Selcer R. R., Bubb W. J., Walker T. L.1991. Management of vertebral column fractures in dogs and cats: 211 cases (1977-1985). J. Am. Vet. Med. Assoc. 198: 1965–1968. [PubMed] [Google Scholar]
- 38.Shores A.1992. Spinal trauma. Pathophysiology and management of traumatic spinal injuries. Vet. Clin. North Am. Small Anim. Pract. 22: 859–888. doi: 10.1016/S0195-5616(92)50080-8 [DOI] [PubMed] [Google Scholar]
- 39.Shores A., Nichols C., Koelling H. A., Fox W. R.1988. Combined Kirschner-Ehmer apparatus and dorsal spinal plate fixation of caudal lumbar fractures in dogs: biomechanical properties. Am. J. Vet. Res. 49: 1979–1982. [PubMed] [Google Scholar]
- 40.Sirin Y. S., Keleş H., Besalti O., Vural A. S.2012. Comparison of ATP-MgCl2 and methylprednisolone in experimentally-induced spinal cord trauma. J. Clin. Anal. Med. 3: 442–447. doi: 10.4328/JCAM.846 [DOI] [Google Scholar]
- 41.Sturges B. K., Le Couteour R. A.2003. Vertebral fractures and luxations. pp. 1244–1262. In: Textbook of Small Animal Surgery, 3rd ed (Slatter, D. ed.), WB Saunders Co., Philadelphia. [Google Scholar]
- 42.Tohda C., Kuboyama T.2011. Current and future therapeutic strategies for functional repair of spinal cord injury. Pharmacol. Ther. 132: 57–71. doi: 10.1016/j.pharmthera.2011.05.006 [DOI] [PubMed] [Google Scholar]
- 43.Voss K., Montavon P. M.2004. Tension band stabilization of fractures and luxations of the thoracolumbar vertebrae in dogs and cats: 38 cases (1993-2002). J. Am. Vet. Med. Assoc. 225: 78–83. doi: 10.2460/javma.2004.225.78 [DOI] [PubMed] [Google Scholar]
- 44.Yılmaz T., Kaptanoğlu E.2015. Current and future medical therapeutic strategies for the functional repair of spinal cord injury. World J. Orthop. 6: 42–55. doi: 10.5312/wjo.v6.i1.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walker T. M., Pierce W. A., Welch R. D.2002. External fixation of the lumbar spine in a canine model. Vet. Surg. 31: 181–188. doi: 10.1053/jvet.2002.31045 [DOI] [PubMed] [Google Scholar]
- 46.Willing A. E., Lixian J., Milliken M., Poulos S., Zigova T., Song S., Hart C., Sanchez-Ramos J., Sanberg P. R.2003. Intravenous versus intrastriatal cord blood administration in a rodent model of stroke. J. Neurosci. Res. 73: 296–307. doi: 10.1002/jnr.10659 [DOI] [PubMed] [Google Scholar]
- 47.Wu S., Suzuki Y., Ejiri Y., Noda T., Bai H., Kitada M., Kataoka K., Ohta M., Chou H., Ide C.2003. Bone marrow stromal cells enhance differentiation of cocultured neurosphere cells and promote regeneration of injured spinal cord. J. Neurosci. Res. 72: 343–351. doi: 10.1002/jnr.10587 [DOI] [PubMed] [Google Scholar]