Abstract
Stillbirth and dystocia are major factors that negatively affect beef production. We sought to clarify serum selenium and liposoluble vitamin levels in Japanese Black cows that gave birth to stillborn calves (stillbirth cows). Blood samples were collected from 103 stillbirth cows and 95 cows that gave birth to healthy calves (control cows). Serum levels of selenium (45.8 ± 16.0 ng/ml) and vitamin A (73.0 ± 24.8 IU/dl) in stillbirth cows were lower (P<0.05) than those in control cows (52.2 ± 8.9 ng/ml and 93.3 ± 14.8 IU/dl, respectively). Our findings suggest that appropriate serum selenium and vitamin A levels are important for calving cows.
Keywords: cow, liposoluble vitamin, selenium, serum concentration, stillbirth
Recently, the incidence of bovine stillbirth has been increasing around world [5]. There are several risk factors associated with bovine stillbirth and dystocia including primiparity, long or short period of gestation, and season [21]. Herd owners should focus on the significant determinants that can be under their control, such as age at first calving and sire, in order to reduce the prevalence of perinatal mortality and improve perinatal welfare [13]. Smyth et al. [17] reported that abnormal thyroid glands of 365 calves, which were stillborn or had perinatal weak calf syndrome, contained significantly low levels of iodine. These cases were associated with a low concentration of selenium in the kidneys. The stillbirth rate of Holstein cows injected with a multimineral supplement (25 mg of selenium, copper, manganese and zinc) at 230 and 260 days of gestation (4.3%) was lower than that of control cows (6.1%) [12]. Pathological changes in the placenta have been reported in cows with hypovitaminosis A [1]. However, the blood levels of selenium and liposoluble vitamins remain unknown in cows that give birth to stillborn calves (hereafter stillbirth cows). The objective of our study was to elucidate whether the blood levels of selenium and liposoluble vitamins at calving are different between cows that gave birth to live calves and stillbirth cows.
We examined Japanese Black stillbirth cows (n=103) in 85 farms located in Miyazaki prefecture, Japan, between October 2009 and September 2012. All stillbirth cases were recorded by one of 27 veterinarians, with any parturition-related problems encountered by farm staff leading to the involvement of a veterinarian, and the same diagnostic criteria were applied to all of the cases. All animals were permanently housed in uninsulated open cubicles. Rice, Italian ryegrass or oat straw was individually fed to cows twice daily. Mixed feed was given to meet the requirements of the Japanese Feeding Standard for Beef Cattle. All animals were subject to artificial insemination (AI). A stillbirth was defined as fetus that had fully matured in the uterus for more than 240 days of pregnancy but was born dead.
Blood samples were collected from the 103 stillbirth cows by venipuncture and 105 stillborn fetuses (including two cases of twins) by cardiac puncture. Gestation length (mean ± SD) was 283.1 ± 13.1 days. Interval between time of calf’s death and blood sampling was 1.9 ± 2.8 hr. Age and parity of the cows were 5.2 ± 3.6 and 3.6 ± 3.1, respectively. The number of stillbirths was 32 in spring (March to May), 25 in summer (June to August), 19 in autumn (September to November) and 27 in winter (December to February). None of the cows showed abnormal clinical signs before parturition. Although placental separation in 17 cows and fetal abnormal position or posture in 6 cows were observed at parturition, no definitive causes could be assigned to stillbirth. No abnormal clinical signs were found in the stillborn fetuses, such as malformation and low-birthweight that meets the definition of weak calf syndrome. Hemolysis of fetal serum was recorded as no hemolysis, hemolysis (red) or severe hemolysis (dark red) as described previously [4, 19]. Because hemolysis and severe hemolysis were observed in 29.5 and 27.6% of fetal serum samples, respectively, the fetal samples were not used to analyze blood biochemistry but were used for antibody tests. As a control group, blood samples were collected from 95 cows (control cows) that gave birth to healthy calves at four farms in the same area (gestation length was 288.7 ± 2.8 days) at the expected date of calving between May 2011 and July 2011. All blood samples were centrifuged, and separated serum was stored at −20°C until analyzed.
Level of antibodies against viruses (Akabane, Aino, Chuzan, Peaton, D’Aguilar, Shamonda, BVDV, Parainfluenza 3 virus and Bovine-Herpesvirus-1) was determined using serum neutralization assays according to previously described procedure [20]. Selenium, vitamin A (retinol) and E (tocopherol) levels in sera were measured using high performance liquid chromatography (JASCO 800 Series HPLC, Japan Spectroscopic Co., Tokyo, Japan) and fluorescence detection (RF-550, Shimadzu, Kyoto, Japan) [10, 11]. Briefly, blood samples were mixed with nitric acid and perchloric acid and boiled, and then, selenium, retinol and tocopherol were extracted with cyclohexane. The Welch t-test was used to compare levels of selenium and liposoluble vitamins between stillbirth cows and control cows. Differences were considered significant, if P values were less than 0.05.
No antibodies against any of the viral pathogens were detected in the 105 fetuses. In the dams, the antibody titers against Aino, Akabane, Shamonda, BVDV and Parainfluenza 3 were 2–4,096, and those against Chuzan and Bovine-Herpesvirus-1 were between 2–2,048. The antibody titer against Peaton was 2–512, and that against D’Aguilar was 2–128.
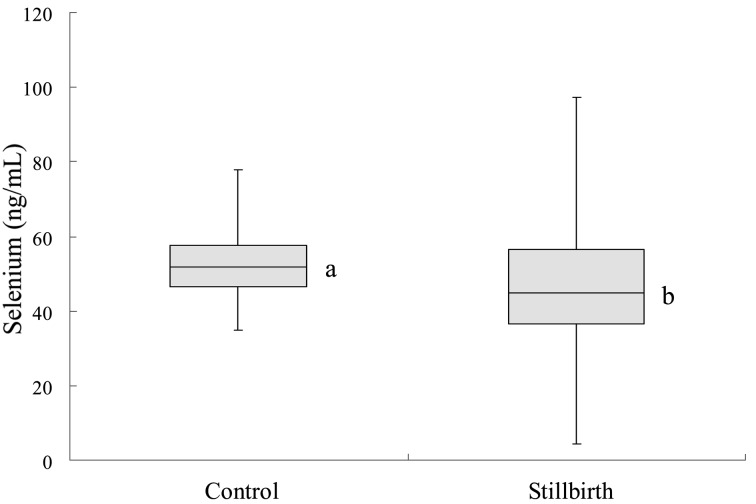
The mean serum concentration of selenium in stillbirth cows was 45.8 ± 16.0 ng/ml, which was lower (P<0.05) than that of control cows (52.2 ± 8.9 ng/ml; Fig. 1). Out of the 103 stillbirth cows, 23 animals (22.3%) had a mean serum selenium concentration of 34.4 ng/ml, which was more than two standard deviations lower than that of the control group and was considered deficient [6]. There was no significant difference in the percentage of selenium-deficient cows in different seasons. None of the control cows had a selenium deficiency, and no seasonal difference was found in the serum selenium concentration of the control cows.
Fig. 1.
Selenium in normally calving cows (control) and cows with stillbirth (stillbirth). a,b: Significant difference between the two groups (P<0.05). In the box plot, the horizontal line represents the median, and the whiskers indicate the lowest and highest data points that are within 1.5 the interquartile range.
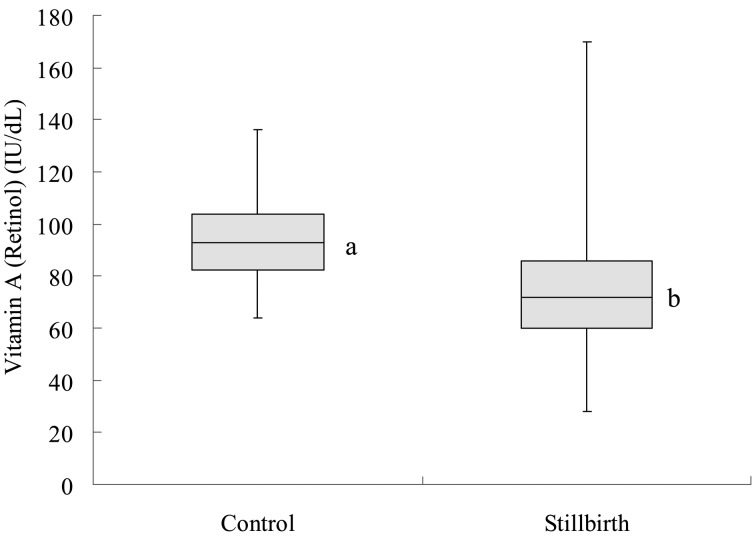
The average concentration of vitamin A (retinol) in the sera of stillbirth cows was 73.0 ± 24.8 IU/dl, which was lower (P<0.05) than that in control cows (93.3 ± 14.8 IU/dl; Fig. 2). A deficient level of serum vitamin A (80 IU/dl) was detected in 18.9% of the control cows (18/95). In contrast, a greater percentage (48.5%, 50/103) of the stillbirth cows had a deficient level of vitamin A (P<0.01). No significant seasonal difference was found in the percentage of vitamin A-deficient cows.
Fig. 2.
Vitamin A (retinol) in normally calving cows (control) and cows with stillbirth (stillbirth). a,b: Significant difference between the two groups (P<0.05). In the box plot, the horizontal line represents the median, and the whiskers indicate the lowest and highest data points that are within 1.5 the interquartile range.
While none of the control cows had deficient levels in serum concentrations of both selenium and vitamin A, 6 of the 103 stillbirth cows (5.8%) had deficient levels of serum selenium and vitamin A.
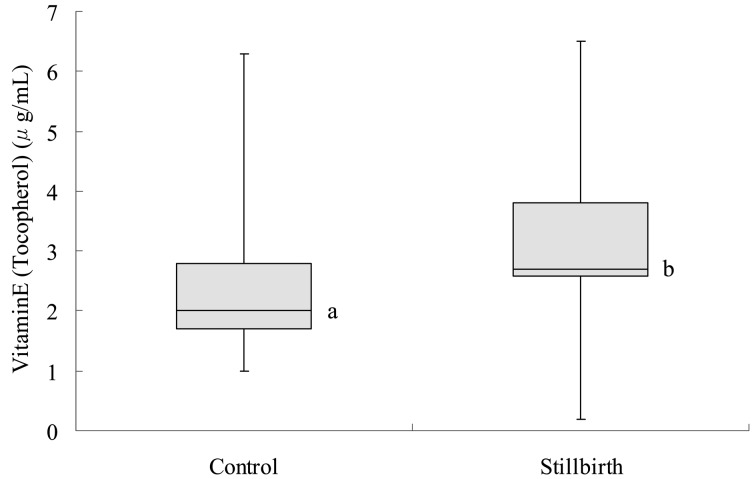
The average concentration of vitamin E (tocopherol) in the sera of stillbirth cows (2.9 ± 1.2 µg/ml) was higher (P<0.05) than that in the control group (2.3 ± 1.0 µg/ml; Fig. 3).
Fig. 3.
Vitamin E (tocopherol) in normally calving cows (control) and cows with stillbirth (stillbirth). a,b: Significant difference between the two groups (P<0.05). In the box plot, the horizontal line represents the median, and the whiskers indicate the lowest and highest data points that are within 1.5 the interquartile range.
Antibodies against viruses were not detected in sera from fetuses. In cows, however, there were some cases where virus antibody titers were high. When a cow is infected with a virus, such as an arbovirus, antibody titer can remain high for several years, thereby preventing infection of the fetus [11]. Therefore, we assumed that viral infections as the cause of stillbirth were unlikely in our study, despite the fact that stillbirth may be caused by viral and/or bacterial infection [18].
Deficiencies of selenium in cattle have been confirmed under natural grazing conditions in many countries in the world. Kamata et al. [9] reported that blood selenium concentrations during late pregnancy, at parturition and lactation in Holstein cows were 63.7 to 67.4, 52.6 to 56.1, and 106 ng/ml, respectively. In the present study, the serum selenium concentration in the control group of Japanese Black cows at parturition was similar to the reported data. However, selenium concentration in stillbirth cows was lower than that for control cows, and the average concentration of selenium in the sera of some of the stillbirth cows was considered deficient. Selenium is essential for thyroid hormone homeostasis [3] and for maintenance of pregnancy. In pregnant women with hypothyroidism, the fetus may die suddenly without clinical signs [16]. The death of the fetuses might have been associated with fetal myocardial degeneration and necrosis. These abnormalities might occur in cattle. In the present study, we examined only the blood samples taken soon after calving, because it was virtually impossible to predict stillbirth before calving. Further research on the profile of selenium concentrations during pregnancy to clarify its relationship with calving outcome is warranted.
Incidentally, the average concentration of selenium in the hay samples taken from nine out of the 85 farms that had stillbirth was very low (0.04 ± 0.02 ppm, data not shown). The supply of selenium-rich mineral salt may be recommended to decrease incidence of stillbirth in the cows that were given low selenium hay. In addition, the activities of the selenoenzyme glutathione peroxidase in the blood of Japanese Black cows should be examined as well as selenium concentrations in future studies.
Plasma vitamin A concentration of less than 80 IU/dl has been considered to be low in Japanese Black cows [15]. In the present study, serum vitamin A concentration in stillbirth cows was less than 80 IU/dl and lower than that in control cows. Angelov [1] reported that placentas and fetuses can be morphologically altered by hypovitaminosis A in the cow. Placentas can develop hyperplasia and metaplasia due to hypovitaminosis A. In the lung, tongue, intestines and vesical bladder of the fetus and in the endometrium of the cows that miscarried, hyperplasia and metaplasia of the epithelium were evident. It has also been reported that a higher incidence of retained placenta was correlated with lower levels of carotene intake in Jersey, Guernsey and Holstein cows and that vitamin A deficiency may result in an increase in stillborn or weak calves [7]. Fetuses in the present study, therefore, might have died because of the histopathological changes in the body and/or the placenta by vitamin A deficiency, although no abnormality was found macroscopically, and no microscopic verification was carried out. Quantification of plasma concentration of estrone sulfate during pregnancy should be valuable to monitor placental function and fetal growth [8].
In the present study, the vitamin E concentration in the stillbirth cows was higher than that in the control cows and previously reported normal level [14]. We have no explanation for this observation so far. However, it has been reported that cows that had high serum vitamin E level also had higher cholesterol level compared with cows that had low serum vitamin E level [2]. Although we had no opportunity to determine cholesterol level in our study, body conditions in the stillbirth cows were apparently not different from that in the normal cows (data not shown). It is inferred that stillbirth may still take place even though no matter how the nutritional status of the animal is.
In conclusion, serum levels of selenium and vitamin A in cows that had stillborn calves were significantly lower than those in cows that had normal calving. Adequate levels of selenium and vitamin A in the serum may be important for successful calving.
Acknowledgments
We are grateful to the Miyazaki Prefecture Livestock hygiene service centre for the measurement of virus antibody titers. We are grateful to the Nippon Zenyaku Kogyo Co., Ltd. for the measurement of selenium and liposoluble vitamin concentrations.
References
- 1.Angelov A. K.1983. [Morphological changes in the placenta and in fetuses in cows with hypovitaminosis A]. Vet. Med. Nauki 20: 54–60(in Bulgarian). [PubMed] [Google Scholar]
- 2.Erskine R. J., Bartlett P. C., Herdt T., Gaston P.1997. Effects of parenteral administration of vitamin E on health of periparturient dairy cows. J. Am. Vet. Med. Assoc. 211: 466–469. [PubMed] [Google Scholar]
- 3.Foroughi M. A., Dehghani H., Mahdavi-Shahri N., Bassami M. R.2013. Sodium selenite increases the transcript levels of iodothyronine deiodinases I and II in ovine and bovine fetal thyrocytes in vitro. J. Trace Elem. Med. Biol. 27: 213–220. doi: 10.1016/j.jtemb.2013.01.004 [DOI] [PubMed] [Google Scholar]
- 4.Gohary K., LeBlanc S. J., Lissemore K. D., Overton M. W., Von Massow M., Duffield T. F.2014. Effect of prepartum administration of recombinant bovine somatotropin on health and performance of lactating dairy cows. J. Dairy Sci. 97: 6231–6241. doi: 10.3168/jds.2014-8048 [DOI] [PubMed] [Google Scholar]
- 5.Hansen M., Misztal I., Lund M. S., Pedersen J., Christensen L. G.2004. Undesired phenotypic and genetic trend for stillbirth in Danish Holsteins. J. Dairy Sci. 87: 1477–1486. doi: 10.3168/jds.S0022-0302(04)73299-3 [DOI] [PubMed] [Google Scholar]
- 6.Harrison J. H., Hancock D. D., Conrad H. R.1984. Vitamin E and selenium for reproduction of the dairy cow. J. Dairy Sci. 67: 123–132. doi: 10.3168/jds.S0022-0302(84)81275-8 [DOI] [PubMed] [Google Scholar]
- 7.Hurley W. L., Doane R. M.1989. Recent developments in the roles of vitamins and minerals in reproduction. J. Dairy Sci. 72: 784–804. doi: 10.3168/jds.S0022-0302(89)79170-0 [DOI] [PubMed] [Google Scholar]
- 8.Isobe N., Nakao T., Uehara O., Yamashiro H., Kubota H.2003. Plasma concentration of estrone sulfate during pregnancy in different breeds of Japanese beef cattle. J. Reprod. Dev. 49: 369–374. doi: 10.1262/jrd.49.369 [DOI] [PubMed] [Google Scholar]
- 9.Kamata H., Terada F., Nishida T., Yoshida H., Hodate K., Shibata M.1998. Selenium balance in the pregnancy and lactation of dairy cattle, and blood selenium concentration of dam and its calf. Anim. Sci. Technol. 69: 1044–1049(JPN). [Google Scholar]
- 10.Kumada S.2011. Simultaneous quantification of Vitamin A, Vitamin E, and β-Carotene extracted with n-Hexane solution in bovine serum by high performance liquid chromatography. Bulletin 91: 22–25(Society of Beef Cattle Science). [Google Scholar]
- 11.Kumata S., Honda S.1993. Determination of selenium in bovine whole blood and serum by high performance liquid chromatography with fluorescence detection. J. Jpn. Vet. Med. Assoc. 46: 108–111(in Japanese). doi: 10.12935/jvma1951.46.108 [DOI] [Google Scholar]
- 12.Machado V. S., Bicalho M. L. S., Pereira R. V., Caixeta L. S., Knauer W. A., Oikonomou G., Gilbert R. O., Bicalho R. C.2013. Effect of an injectable trace mineral supplement containing selenium, copper, zinc, and manganese on the health and production of lactating Holstein cows. Vet. J. 197: 451–456. doi: 10.1016/j.tvjl.2013.02.022 [DOI] [PubMed] [Google Scholar]
- 13.Mee J. F., Berry D. P., Cromie A. R.2008. Prevalence of, and risk factors associated with, perinatal calf mortality in pasture-based Holstein-Friesian cows. Animal 2: 613–620. doi: 10.1017/S1751731108001699 [DOI] [PubMed] [Google Scholar]
- 14.Puls R.1994. Vitamin Levels in Animal Health: Diagnostic Data and Bibliographies, Sherpa International, Clearbrook. [Google Scholar]
- 15.Sekizawa F., Sawai K., Tanaka M., Okuda K.2012. Relationship between embryo collection results after superovulation treatment of Japanese Black cows and their plasma β-carotene and vitamin concentrations. J. Reprod. Dev. 58: 377–379. doi: 10.1262/jrd.11-075H [DOI] [PubMed] [Google Scholar]
- 16.Silver R. M.2007. Fetal death. Obstet. Gynecol. 109: 153–167. doi: 10.1097/01.AOG.0000248537.89739.96 [DOI] [PubMed] [Google Scholar]
- 17.Smyth J. A., Goodall E. A., McCoy M. A., Ellis W. A.1996. Stillbirth/perinatal weak calf syndrome: a study of calves with an abnormal thyroid gland. Vet. Rec. 139: 11–16. doi: 10.1136/vr.139.1.11 [DOI] [PubMed] [Google Scholar]
- 18.Smyth J. A., McNamee P. T., Kennedy D. G., McCullough S. J., Logan E. F., Ellis W. A.1992. Stillbirth/perinatal weak calf syndrome: preliminary pathological, microbiological and biochemical findings. Vet. Rec. 130: 237–240. doi: 10.1136/vr.130.12.237 [DOI] [PubMed] [Google Scholar]
- 19.Tatone E. H., Gordon J. L., LeBlanc S. J., Duffield T. F.2015. Evaluation of a handheld device for measurement of β-hydroxybutyrate concentration to identify prepartum dairy cattle at risk of developing postpartum hyperketonemia. J. Am. Vet. Med. Assoc. 246: 1112–1117. doi: 10.2460/javma.246.10.1112 [DOI] [PubMed] [Google Scholar]
- 20.Tsuda T., Yoshida K., Ohashi S., Yanase T., Sueyoshi M., Kamimura S., Misumi K., Hamana K., Sakamoto H., Yamakawa M.2004. Arthrogryposis, hydranencephaly and cerebellar hypoplasia syndrome in neonatal calves resulting from intrauterine infection with Aino virus. Vet. Res. 35: 531–538. doi: 10.1051/vetres:2004029 [DOI] [PubMed] [Google Scholar]
- 21.Uematsu M., Sasaki Y., Kitahara G., Sameshima H., Osawa T.2013. Risk factors for stillbirth and dystocia in Japanese Black cattle. Vet. J. 198: 212–216. doi: 10.1016/j.tvjl.2013.07.016 [DOI] [PubMed] [Google Scholar]