Abstract
Background
Over 1,000,000 cardiac catheterizations (CC) are performed annually in the United States. There is a small risk of complication that has persisted despite advances in technology. It is unknown whether daily CC procedural volume can influence this risk. In an effort to improve outcomes at our academic medical center, we investigated the relationship between daily CC volume and complication rates.
Methods
We obtained data from both the National Cardiovascular Data Registry (NCDR) Cath-PCI and Lumedx© databases reviewing the records of patients undergoing scheduled, non-emergent CC at our facility between January 2005 to June 2013. Daily CC volume was analyzed as were complications including death, post-procedure MI, cardiogenic shock, heart failure, stroke, tamponade, bleeding, hematoma and acute kidney injury (AKI).
Results
12,773 patients were identified who underwent 16,612 CCs on 2,118 days. The average age was 63 years (SD 12.4; range, 18–95). 61% were men. A total of 326 complications occurred in 243 patients on 233 separate days (2.0% CC complication rate). The average volume per day was 7.8 CCs. We found a low correlation between daily complications and CC volume (Spearman’s rho =0.11; P<0.01) though complication rates were lowest on days with 6–11 procedures; higher rates were found on slower and busier days.
Conclusions
We observed a U-shaped association between CC volume and rates of CC complications. The lowest complication rates were found on days with 6–11 procedures a day. The highest complication rate was seen with >11 procedures a day.
Keywords: cardiac catheterization (CC), ambulatory care, risk factors, health facility size
Introduction
Cardiac catheterization (CC) is a common procedure with more than a million performed annually in the United States (1). Prior studies have demonstrated an inverse relationship between annual CC procedure volume and the rate of complications. Higher volumes are associated with lower complication rates (2,3). Four hundred CCs a year has been suggested as a goal to define high volume centers (4,5). Facilities and physicians with high CC volumes consistently experience low rates of complications including bleeding, need for CABG and mortality (3,6-8). Daily volumes have not been analyzed and little is known about the effect of procedural volume on outcomes when trainees participate in CC.
Recognition of a possible relationship between trainee workload and patient outcomes led the AGME in 2003 to institute restrictions on trainee work hours (9). Some academic centers have reported an association between increased surgical volumes and increased complication rates (10). We hypothesized that an optimal daily volume for CCs may exist at our academic training center.
Methods
Data source and patient population
We reviewed records from a large academic training hospital. During the study period there was fixed daily staffing by two board certified invasive/interventional senior staff, two interventional fellows in training, post graduate year (PGY)-7, one first year PGY-4 and either one second year PGY-5 or third year PGY-6 cardiology Fellow. Three catheterization laboratories were available each staffed by two nurses or technologists. Staffing did not change over the study period. Vascular access is obtained by trainees of comparable experience under the supervision of senior staff that did not change significantly over the time period studied. Types of procedures performed include: left and right heart catheterizations, coronary angiograms, and percutaneous coronary interventions.
Data were obtained from the American College of Cardiology (ACC) National Cardiovascular Data Registry (NCDR) CathPCI Registry® and Lumedx© database of adult patients undergoing non-emergent CCs at a large tertiary care high CC volume hospital from January 2005 to June 2013. The patient identifiers were merged with the health care system electronic medical records to obtain additional demographic information.
Patients were included if they were adults with valid data on age and date of the CC procedure. We excluded patients undergoing CC on weekends or for emergent reasons. In the event of multiple CCs on a single patient, only the patient’s first procedure was utilized for patient-level analyses.
Measures
Demographic information collected included patient age at the time of CC, gender, and comorbidity burden in the year prior to their first CC procedure. The comorbidity burden of patients undergoing non-emergent CC was assessed using the Charlson comorbidity score (11,12). The Charlson sums weights for 19 conditions that are correlated with post-discharge mortality [including myocardial infarction (MI), dementia, diabetes, and diabetes with complications] as implemented in administrative data (13,14). The primary endpoint was complications as it relates to daily procedural volume. Procedural complications arising from CC were recorded as dichotomies in the NCDR CathPCI Registry® and included post-procedure MI, cardiogenic shock, heart failure, stroke, tamponade, bleeding, hematoma, acute kidney injury (AKI) defined as intraprocedural change in creatinine exceeding 0.3 μmol/L, and death. AKI was also determined by the use of ICD-9 code of 584.9 if occurring within 3 days of CC. In addition the status of the CC was obtained as recorded in the database as elective or urgent.
Analysis plan
Data were aggregated to the patient level to describe the sample, to the date-of-procedure level to obtain estimates of per-day risks, and to the procedure CC level to estimate CC-specific risks. The clinic, its patients, and the procedures were described by means (standard deviations), medians and frequencies (percentages). The relationship between daily procedural volume (as a continuous measure) and complications was assessed with Spearman’s rank correlation coefficient. This relationship was further explored with daily CC volume which was categorized into low, moderate or high after review of the finalized data. Due to the distribution of rates of complications we suggest that a low volume day is defined as a day with fewer than six CC procedures, a moderate day had 6–11 procedures, and a high volume day had 11 or more CC procedures. The patient demographics were compared across volume group classifications, employing Kruskal-Wallis tests for continuous measures and chi-square analyses for categorical measures. Multi-variable logistic regression was used to model complication occurring during the first CC procedure a patient had, assessing the influence of that day’s volume on complication and adjusting for age, gender, and comorbidities. A type I error of α=0.05 was assumed throughout. All analyses were performed using SAS v9.2 (Cary, NC, USA).
Results
Overall, 12,773 patients underwent 16,612 CCs on 2,118 days. About 59% of the procedures were considered elective with the rest categorized as urgent. Elective and urgent procedures were frequently performed together on the same day. An elective procedure was performed on 94% of the days studied with an urgent procedure occurring on 90% of the days studied. The average patient age was 63 years (SD =12.4; range, 18–95) and 61% were male (Table 1). Patients undergoing CC had an average Charlson comorbidity score of 2.0 (SD =2.1). MI and congestive heart failure were each represented in 28% of the sample. Nearly three-fourths had hypertension (73%), and 69% had dyslipidemia. Among the first recorded CCs, 243 (1.9%) patients had complications.
Table 1. Patient characteristics undergoing cardiac catheterization procedures (N=12,773).
| Patient characteristics | Mean ± SD or N (%) |
|---|---|
| Demographics | |
| Age [18–95] years | 63.5±12.4 |
| Female | 5,040 (39.5) |
| Charlson (0–15) | 2.0±2.1 |
| MI | 3,580 (28.0) |
| CHF | 3,524 (27.6) |
| PVD | 1,207 (9.5) |
| Stroke | 1,449 (11.3) |
| DM | 4,625 (36.2) |
| Procedures (N=16,612) | |
| # of complications | 326 (2.0) |
| Date-level measures (N=2,118) | |
| Procedures/day | 7.8±3.7 |
| Complications/day | 0.2±0.9 |
| Any complication | 233 (11.0) |
MI, myocardial infarction; Charlson, Charlson Comorbidity Index; CHF, congestive heart failure, PVD, peripheral vascular disease; DM, diabetes; SD, standard deviation.
On a procedural level, there were a total of 16,612 CCs performed with 326 complications occurring in 243 patients on 233 separate days (2.0% CC complication rate). The highest number of CCs completed on any given day during the study timeframe was 33 which occurred once, with the fewest being 1 CC which occurred on 72 days. The average volume per day was 7.8 CCs, and complications per day averaged 0.2. (Table 1) On days with a complication, the median procedural volume was 9 CCs compared to 8 CCs on days without a complication (P<0.01). Our population showed a low correlation between daily complications and CC volume (Spearman’s rho =0.11; P<0.01).
Complications rates by group
Table 2 contains patient demographics by CC daily volume category (low, moderate or high). Patients on low volume days had higher rates of MI, CHF, CKD and hypertension compared to patients undergoing a CC procedure on moderate or high volume days. The lowest complication rates occurred in the moderate volume group (1.4% vs. 2.18% for low volume days and 3.16% for high volume days; P<0.01). Additional data are presented in Figure 1 and Table 3.
Table 2. Patient characteristics by volume group classification (16,612 total procedures).
| Characteristic | Low-volume (1–5 events), N (%) | Moderate-volume (6–11 events), N (%) | High-volume (>11 events), N (%) | P values |
|---|---|---|---|---|
| Procedures (N=16,612) | 1,879 | 10,497 | 4,236 | |
| Age (mean ± SD) | 63.2±12.2 | 63.5±12.3 | 63.4±12.1 | 0.47 |
| Female | 734 (39.1) | 3,997 (38.1) | 1,563 (36.9) | 0.21 |
| Charlson (mean ± SD) | 2.3±2.2 | 2.1±2.2 | 2.1±2.1 | <0.01 |
| MI | 649 (34.5) | 3,094 (29.5) | 1,241 (29.3) | <0.01 |
| CHF | 573 (30.5) | 2,880 (27.4) | 1,091 (25.8) | <0.01 |
| PVD | 244 (13.0) | 1,028 (9.8) | 510 (12.0) | <0.01 |
| Stroke | 274 (14.6) | 1,287 (12.3) | 608 (14.4) | <0.01 |
| DM | 791 (42.1) | 3,994 (38.0) | 1,600(37.8 | <0.01 |
MI, myocardial infarction; Charlson, Charlson Comorbidity Index; CHF, congestive heart failure; PVD, peripheral vascular disease; DM, diabetes; SD, standard deviation.
Figure 1.
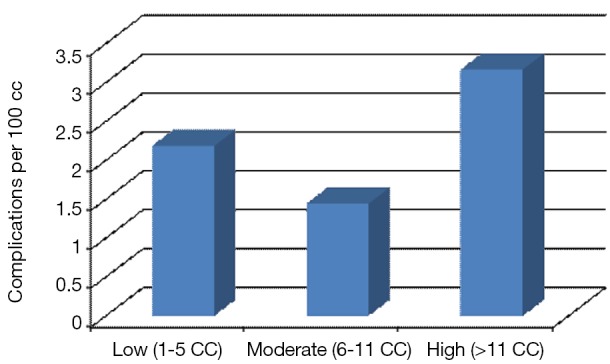
Complication rates among volume groups (N=2,118 weekdays).
Table 3. Complication rates by daily volume groups for the distribution of cardiac catheterization procedures (N=2,118 weekdays).
| Group | Days | Total complications | Total procedures | Complication (%) |
|---|---|---|---|---|
| Low (1–5 procedures) | 549 | 41 | 1,879 | 2.18 |
| Moderate (6–11 procedures) | 1,261 | 151 | 10,497 | 1.44 |
| High (>11 procedures) | 308 | 134 | 4,236 | 3.16 |
Multivariable logistic regression analysis
The complication rate was not affected by volume in a multivariable logistic regression model that adjusted for patient clinical and demographic factors. In this model, older age, female gender and greater comorbidity burden were each associated with greater odds of a complication. Specifically, female patients had nearly doubled odds of complications compared to males (OR =1.9; 95% CI: 1.3–2.8; Table 4). In the unadjusted model accounting for volume alone at the procedure level, high but not low volume was found to be a risk factor for complications [OR (high) =1.8; 95% CI: 1.4–2.2; Table 5].
Table 4. Multivariable logistic regression modeling complication during the first CC procedure, assessing the influence of that day’s volume on complication adjusted for patient factors (N=12,773 patients).
| Variable | OR (95% CI) | P value |
|---|---|---|
| Age (effect per decade) | 1.44 (1.21–1.73) | <0.01 |
| Female | 1.89 (1.26–2.84) | <0.01 |
| Charlson (range 0–15) | 1.20 (1.12–1.29) | <0.01 |
| Low vs. moderate volume day | 1.09 (0.56–2.15) | 0.80 |
| High vs. moderate volume day | 1.42 (0.90–2.23) | 0.13 |
Table 5. Multivariable logistic regression modeling complication (yes/no), assessing the influence of that day’s volume on complication at the CC procedure level (N=16,612 CCs; 326 complications).
| Variable | OR (95% CI) | P value |
|---|---|---|
| Moderate volume day (reference) | ||
| Low volume day | 1.24 (0.86–1.77) | 0.25 |
| High volume day | 1.77 (1.40–2.24) | <0.01 |
Discussion
Our data suggests an association between daily catheterization volume and complication rates at our academic institution. During the time period studied, we averaged around 2,850 procedures per year and based on current guidelines are considered a high volume facility. Cardiology trainees participate in all non-emergent cases.
A U-shaped association between daily procedural volume and complication rate calls into question whether or not there exists an optimal procedural volume for catheterization labs operating with trainee involvement in the majority of cases. Certainly, at our institution, we find evidence for an upper limit for daily procedural volume beyond which the safety of the procedure may decline (15). Previous research corroborates higher complication rates at centers with low volume, however, there have not been sufficient studies to evaluate patient safety with regard to increasing procedural volume amongst high volume centers. Similarly, there have been no studies comparing daily procedural volume and complication rates. Our findings illustrate the necessity of similar studies at both academic and non-academic high volume centers to better understand the relationship of procedural volume to patient safety. While higher annual procedural volumes, in general, are associated with lower complication rates, daily procedural volume may have an upper limit (4,5). By evaluating and adapting a schedule to accommodate optimal procedural volume, high volume centers may be able to decrease their annual complication rates further, specifically training facilities. Moreover, the current guideline statement of greater than 400 procedures per facility providing the lowest complication rate may underestimate the full risk continuum in high volume centers. There indeed may be an upper limit to annual procedural volume as well, at any given facility, past which complication rates begin to increase. Facilities wishing to improve their complication rates would benefit from a thorough analysis of the relationship of volume to complications on both a daily and an annual level.
A daily limit beyond which complication rates increase may be explained in part by fixed resources of staff and equipment. When a facility must accommodate increased daily volume time available for usual safety protocols is arbitrarily decreased. In addition, preparatory time in between patients is shortened and opportunity for staff breaks decreases. Increased demand and stress leading to hasty proceedings by staff may potentiate complications.
The slightly increased risk of complications found on low volume days is likely multifactorial. There may be some degree of selection bias whereby cases perceived to be particularly complex or challenging are scheduled on days with a lighter case volume to allow for more intensive monitoring or in anticipation of longer procedure times. Indeed, we observed that patients on lower volume days had more co-morbid conditions with higher rates of baseline CKD, MI and PVD. Another potential confounder may be found in the effects of the complications on daily volume. Diversion of resources due to complications could lead to an attenuation or cancellation of previously planned cases making case volume lower on these days than originally planned.
As with all retrospective analyses, there are some inherent limitations to our study. We used the NCDR cath PCI information system as well the Lumedex information system for data retrieval. Procedural information from our catheterization lab is stored in these systems and reported to the NCDR national registry. Information stored in this manor is subject to reporting bias; however, data from the NCDR registry has been validated nationwide with raw accuracy score rates above ninety percent (16,17). Another key limitation is the single center perspective from which the study was conducted. Given the rather stable practice during the study period along with trainees of consistent levels of experience participating in the majority of procedures, we believe our institution to be well suited in the evaluation of our hypothesis. Annual complication rates at our facility are similar to nationally reported averages at similar high volume centers (4,18). Hence, our results can be assumed to be generalizable to similar high volume centers. Nonetheless, we feel the optimal volume for any given facility is likely variable due to practitioner and patient variability, again highlighting the necessity for thorough evaluation within individual high volume centers.
Exclusion of patients undergoing emergency procedures also limits interpretation of the data. Though, our purpose was to evaluate risk associated with urgent and elective procedures which make up the vast majority of cases in our, and all catheterization labs. We feel it is important to consider emergent and non-emergent cases separately due to the nature of the patients and the situations. Emergent cases have expected higher rates of complications and may cloud the interpretation of the relationship of volume to complication rate (19). These cases were included, however, in daily procedural numbers. Complication rates pertaining to emergent cases remains to be examined in future research.
In conclusion, we find evidence for an upper limit on daily procedural volume beyond which CC complication rates appear to increase at our facility. We also found a less significant increase in complication rates on low volume days. The fewest complications occurred on days with 6–11 cases per day. Evaluating daily procedural volume may represent an opportunity to further reduce the risk associated with CC in high volume training facilities.
Acknowledgements
The authors would like to acknowledge Gregory J. Dehmer and D. Scott Gantt for their support of this project.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation 2015;131:e29-322. 10.1161/CIR.0000000000000152 [DOI] [PubMed] [Google Scholar]
- 2.McGrath PD, Wennberg DE, Dickens JD, Jr, et al. Relation between operator and hospital volume and outcomes following percutaneous coronary interventions in the era of the coronary stent. JAMA 2000;284:3139-44. 10.1001/jama.284.24.3139 [DOI] [PubMed] [Google Scholar]
- 3.Jolly SS, Cairns J, Yusuf S, et al. Procedural volume and outcomes with radial or femoral access for coronary angiography and intervention. J Am Coll Cardiol 2014;63:954-63. 10.1016/j.jacc.2013.10.052 [DOI] [PubMed] [Google Scholar]
- 4.Bashore TM, Balter S, Barac A, et al. 2012 American College of Cardiology Foundation/Society for Cardiovascular Angiography and Interventions expert consensus document on cardiac catheterization laboratory standards update: A report of the American College of Cardiology Foundation Task Force on Expert Consensus documents developed in collaboration with the Society of Thoracic Surgeons and Society for Vascular Medicine. J Am Coll Cardiol 2012;59:2221-305. 10.1016/j.jacc.2012.02.010 [DOI] [PubMed] [Google Scholar]
- 5.Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Catheter Cardiovasc Interv 2012;79:453-95. 10.1002/ccd.23438 [DOI] [PubMed] [Google Scholar]
- 6.Phillips KA, Luft HS, Ritchie JL. The association of hospital volumes of percutaneous transluminal coronary angioplasty with adverse outcomes, length of stay, and charges in California. Med Care 1995;33:502-14. 10.1097/00005650-199505000-00005 [DOI] [PubMed] [Google Scholar]
- 7.Hlatky MA, Dudley RA. Operator volume and clinical outcomes of primary coronary angioplasty for patients with acute myocardial infarction. Circulation 2001;104:2155-7. [PubMed] [Google Scholar]
- 8.Politi A, Galli M, Zerboni S, et al. Operator volume and outcomes of primary angioplasty for acute myocardial infarction in a single high-volume centre. J Cardiovasc Med (Hagerstown) 2006;7:761-7. 10.2459/01.JCM.0000247324.95653.ed [DOI] [PubMed] [Google Scholar]
- 9.Philibert I, Friedmann P, Williams WT, et al. New requirements for resident duty hours. JAMA 2002;288:1112-4. 10.1001/jama.288.9.1112 [DOI] [PubMed] [Google Scholar]
- 10.Salim A, Teixeira PG, Chan L, et al. Impact of the 80-hour workweek on patient care at a level I trauma center. Arch Surg 2007;142:708-12; discussion 712-4. 10.1001/archsurg.142.8.708 [DOI] [PubMed] [Google Scholar]
- 11.Charlson M, Peterson JC. Medical comorbidity and late life depression: what is known and what are the unmet needs? Biol Psychiatry 2002;52:226-35. 10.1016/S0006-3223(02)01422-1 [DOI] [PubMed] [Google Scholar]
- 12.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373-83. 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 13.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992;45:613-9. 10.1016/0895-4356(92)90133-8 [DOI] [PubMed] [Google Scholar]
- 14.Pugh MJ, Copeland LA, Zeber JE, et al. The impact of epilepsy on health status among younger and older adults. Epilepsia 2005;46:1820-7. 10.1111/j.1528-1167.2005.00291.x [DOI] [PubMed] [Google Scholar]
- 15.Kimmel SE, Berlin JA, Laskey WK. The relationship between coronary angioplasty procedure volume and major complications. JAMA 1995;274:1137-42. 10.1001/jama.1995.03530140049031 [DOI] [PubMed] [Google Scholar]
- 16.Messenger JC, Ho KK, Young CH, et al. The National Cardiovascular Data Registry (NCDR) Data Quality Brief: the NCDR Data Quality Program in 2012. J Am Coll Cardiol 2012;60:1484-8. 10.1016/j.jacc.2012.07.020 [DOI] [PubMed] [Google Scholar]
- 17.Moussa I, Hermann A, Messenger JC, et al. The NCDR CathPCI Registry: a US national perspective on care and outcomes for percutaneous coronary intervention. Heart 2013;99:297-303. 10.1136/heartjnl-2012-303379 [DOI] [PubMed] [Google Scholar]
- 18.Badheka AO, Patel NJ, Grover P, et al. Impact of annual operator and institutional volume on percutaneous coronary intervention outcomes: a 5-year United States experience (2005-2009). Circulation 2014;130:1392-406. 10.1161/CIRCULATIONAHA.114.009281 [DOI] [PubMed] [Google Scholar]
- 19.Zahn R, Gottwik M, Hochadel M, et al. Volume-outcome relation for contemporary percutaneous coronary interventions (PCI) in daily clinical practice: is it limited to high-risk patients? Results from the Registry of Percutaneous Coronary Interventions of the Arbeitsgemeinschaft Leitende Kardiologische Krankenhausarzte (ALKK). Heart 2008;94:329-35. 10.1136/hrt.2007.118737 [DOI] [PubMed] [Google Scholar]


