Abstract
Meeting the high demand for lanthanide-doped luminescent nanocrystals across a broad range of fields hinges upon the development of a robust synthetic protocol that provides rapid, just-in-time nanocrystal preparation. However, to date, almost all lanthanide-doped luminescent nanomaterials have relied on direct synthesis requiring stringent controls over crystal nucleation and growth at elevated temperatures. Here we demonstrate the use of a cation exchange strategy for expeditiously accessing large classes of such nanocrystals. By combining the process of cation exchange with energy migration, the luminescence properties of the nanocrystals can be easily tuned while preserving the size, morphology and crystal phase of the initial nanocrystal template. This post-synthesis strategy enables us to achieve upconversion luminescence in Ce3+ and Mn2+-activated hexagonal-phased nanocrystals, opening a gateway towards applications ranging from chemical sensing to anti-counterfeiting.
 De novo synthesis is the primary way to tune the emission colour of lanthanide-doped upconversion nanocrystals. Here, the authors introduce post-synthetic cation exchange as a strategy to access multiple colours of luminescent nanocrystals, preserving the size and morphology of the original template.
De novo synthesis is the primary way to tune the emission colour of lanthanide-doped upconversion nanocrystals. Here, the authors introduce post-synthetic cation exchange as a strategy to access multiple colours of luminescent nanocrystals, preserving the size and morphology of the original template.
With the rapid development of nanoscience and nanotechnology, lanthanide-doped upconversion nanocrystals1,2,3,4,5 have recently emerged as an important class of luminescent materials, owing to their potential applications ranging from biological imaging6,7,8 and multiplexing sensing9,10,11 to security encoding12,13,14 and volumetric display15. Despite significant progress made, the vast majority of approaches for making upconversion nanocrystals have involved de novo synthetic techniques such as hydrothermal reaction16,17, co-precipitation18,19,20 and thermal decomposition21,22,23. To access different colour emissions24,25,26, one has to perform a new set of reactions and require stringent control over a variety of experimental conditions, including the amount of dopant precursors and surfactants, solvent type, reaction time and temperature. This practice is clearly time-consuming and resource-intensive, and often leads to variation in particle size, phase and morphology16,20.
Cation exchange reactions at the nanoscale have recently emerged as a powerful tool for controlling composition and phase in colloidal semiconductor nanocrystals27,28,29,30,31. These reactions present an alternative solution for modulating emission colours in upconversion nanocrystals. However, different from the band-gap luminescence nature of quantum dots32,33,34,35,36, the emission from the upconversion nanocrystals stems directly from the lanthanides infused in the host lattice37,38,39,40,41. It is important to note that realizing efficient upconversion luminescence typically requires the homogeneous placement of sensitizer and activator ions in rather close proximity, as is the case for NaYF4 nanoparticles co-doped with Yb3+ and Er3+ (ref. 4). Although a high doping concentration of Yb3+ theoretically favours luminescence enhancement42,43,44, upconversion nanocrystals with a large Yb3+ content (for example, NaYbF4) are highly sensitive to the concentration quenching effect that depletes excitation energy and thus suppresses luminescence. This dilemma makes the cation exchange strategy practically unsuitable for emission colour modulation using conventional host materials (for example, NaYF4, NaLuF4 and NaYbF4; refs 45, 46, 47, 48).
It has been well-established that Gd3+-based host materials could effectively bridge the gap of energy transfer from sensitizers to activators through long-range energy migration in the sub-lattice24,41. Because of its large energy gap (∼4.0 eV) between the ground state (8S7/2) and the lowest excited state (6P7/2), the Gd3+ ion also serves as an ideal energy reservoir to suppress the concentration quenching of sensitized luminescence in crystalline nanophosphors.
Here we reason that utilization of a Gd3+-based host lattice may leverage multicolour synthesis in upconversion nanocrystals through cation exchange under mild conditions. By making use of myriad energy transfer pathways between dopant ions, our approach proves useful for accessing a plethora of optical nanomaterials of uniform size, shape and phase (Fig. 1). In particular, we achieve upconversion emission from Ce3+ or Mn2+ doped in hexagonal-phased nanocrystals. This allows us to generate a record long-lived luminescence of ∼600 ms for Mn2+-activated nanocrystals.
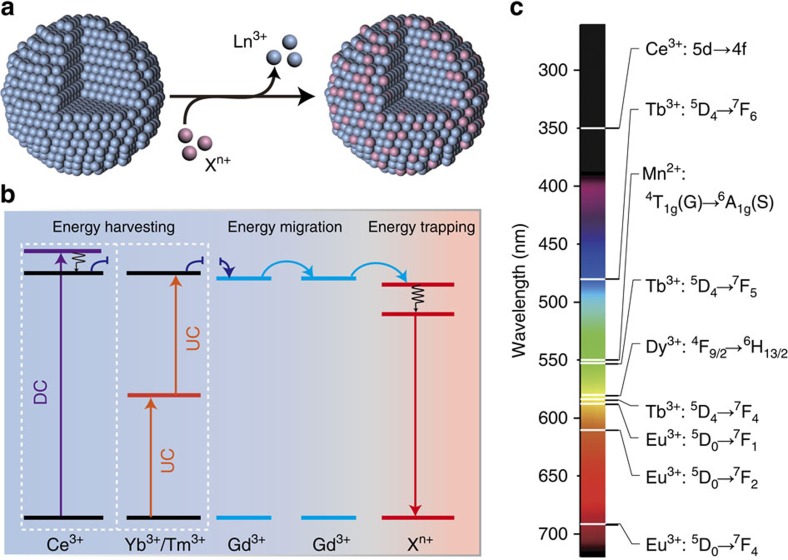
Figure 1. Rational design for emission tuning in lanthanide-doped nanocrystals through cation exchange.
(a) Schematic representation of a typical cation exchange process, occurring at the particle surface, between lanthanide (Ln3+) and exchange (Xn+) ions. (b) Proposed energy management process for cation exchange-mediated luminescence tuning in the nanocrystals. The energy transfer process mainly comprises energy harvesting, energy migration and energy trapping through different types of lanthanides. DC and UC represent downconversion and upconversion processes, respectively. (c) Typical luminescent ions used for cation exchange-mediated luminescence tuning and their main emitting transitions in the ultraviolet and visible part of the electromagnetic spectrum.
Results
Synthesis and characterization
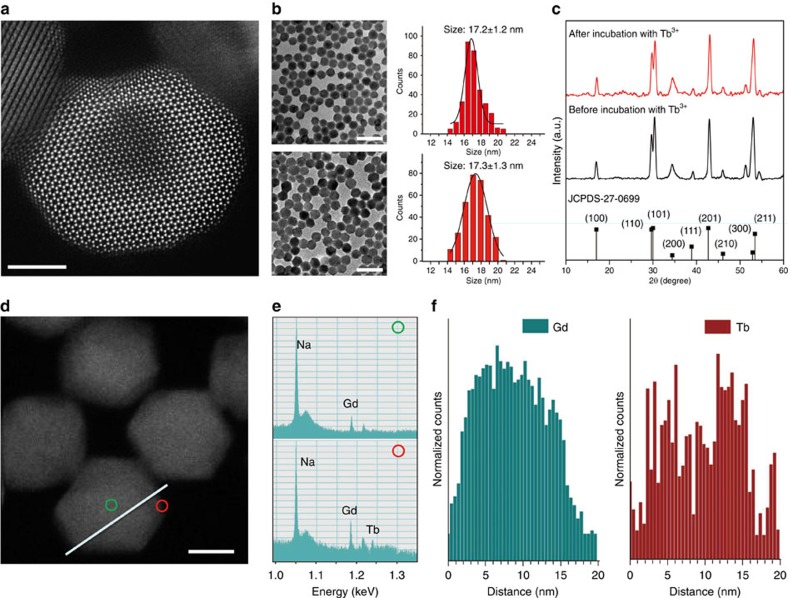
In a typical procedure, hexagonal phase NaGdF4:Yb/Tm@NaGdF4 core-shell nanocrystals were firstly synthesized as a model system by a co-precipitation procedure (Supplementary Fig. 1; ref. 24). Subsequently, surface-bound oleic acid molecules were removed by the treatment of HCl to generate ligand-free nanocrystals (Supplementary Figs 2 and 3). Cation exchange was then induced by mixing an aqueous solution containing a TbCl3 precursor with the as-prepared colloidal sample under ambient conditions for 1 h. High-resolution transmission electron microscopic (TEM) imaging reveals the single-crystalline hexagonal structure of the resulting nanocrystals after cation exchange (Fig. 2a and Supplementary Fig. 4). Low-resolution TEM imaging and the size distribution analysis of the nanocrystals before and after cation exchange show no obvious changes in the particle size and morphology (Fig. 2b and Supplementary Figs 5–7). In addition, X-ray diffraction of the samples confirms that the hexagonal phase is completely preserved after the post-synthetic treatment (Fig. 2c, Supplementary Figs 8 and 9 and Supplementary Note 1).
Figure 2. Structural characterization of NaGdF4:Yb/Tm@NaGdF4 nanoparticles before and after cation exchange.
(a) Typical high-resolution transmission electron microscopic (TEM) image of a nanoparticle treated with TbCl3 at room temperature. (b) Low-resolution TEM images and corresponding size distributions of the as-prepared NaGdF4:Yb/Tm@NaGdF4 nanoparticles, obtained before (top panel) and after (bottom panel) incubation with TbCl3. (c) Corresponding powder X-ray diffraction patterns of the core-shell nanoparticles. Note that all peaks can be well indexed in accordance with hexagonal-phase NaGdF4 structure (Joint Committee on Powder Diffraction Standards file number 27-0699). (d) Typical scanning transmission electron microscopic (STEM) image of the nanoparticles treated with TbCl3. (e) Electron energy loss spectroscopy (EELS) point analysis collected, respectively, from green and red circle marked in d. (f) EELS line profile recorded by scanning along the white line shown in d. Note that both elemental analyses in e,f reveal that more Tb content is present at particle outer layer, while more Gd content exists at particle inner layer. Scale bar, 5 nm for panel a, scale bar, 50 nm for panel b, scale bar, 10 nm for panel d.
Electron energy loss spectroscopy analysis on a single nanoparticle reveals that the Tb3+ ions are mainly located within the outmost layers of the core-shell nanocrystals (Fig. 2d–f and Supplementary Fig. 10). To further substantiate the occurrence of cation exchange, we carried out inductively coupled plasma mass spectroscopy analysis of the colloidal sample after cation exchange. For a series of experiments, we found that the amount of Gd3+ ions discharged from the core-shell nanocrystals increases with increasing Tb3+ concentration (Supplementary Figs 11–14 and Supplementary Note 2).
Spectroscopic study of cation-exchanged nanocrystals
Through Gd3+-mediated energy migration in the core-shell structure, the excitation energy could be efficiently transferred from the Yb/Tm pair in the core layer to the activator ions in the shell layers upon cation exchange (Fig. 3a and Supplementary Figs 15 and 16). The cation exchange process can be visualized by monitoring the emission colour change of the colloidal samples before and after addition of the Tb3+ or Eu3+ precursor (Fig. 3b and Supplementary Movies 1 and 2). The luminescence spectra of the samples measured at room temperature show an increase in Tb3+ or Eu3+ emission intensity but a decrease in Gd3+ intensity with increasing concentrations of the exchange ions (Supplementary Figs 17 and 18).
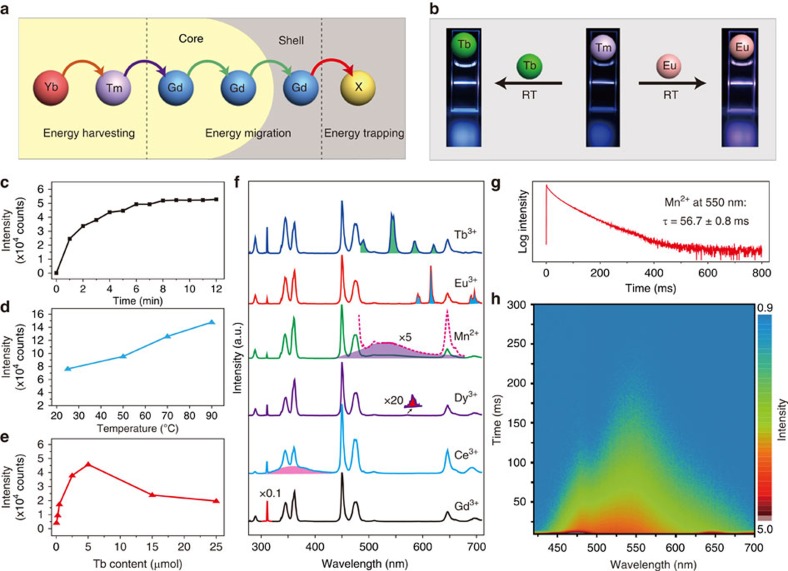
Figure 3. Optical investigation of Gd-based upconversion nanocrystals through cation exchange.
(a) Schematic representation of energy transfer process in NaGdF4:Yb/Tm@NaGdF4 nanocrystals after cation exchange with an activator ion (denoted as X). (b) Photoluminescence images showing the change in the emission colour of NaGdF4:Yb/Tm@NaGdF4 colloidal solutions upon addition of Tb3+ or Eu3+ ions at room temperature. (c–e) Emission intensity profiles of the nanocrystal solution measured as a function of cation exchange time, reaction temperature and Tb3+ concentration, respectively. Note that the emission of Tb3+ at 546 nm is used for intensity measurement. (f) Typical photoluminescence spectra of the nanocrystals treated with TbCl3, EuCl3, MnCl2, DyCl3 and CeCl3, respectively. Note that the activator emissions are highlighted with colour. All spectra were recorded under the irradiation of a 980 nm laser with a pump power of 1 W. (g) Lifetime decay curve of Mn2+ emission at 550 nm from the MnCl2-treated nanocrystals. (h) A transient photoluminescence decay image of the MnCl2-treated nanocrystals. The colour change from red to blue indicates the decrease in emission intensity.
The cation exchange process is generally controlled by three parameters: reaction time, temperature, and the ion concentration used for exchange in the solution (Supplementary Note 3). In our study, Tb3+-exchanged nanocrystals were taken as an example to study the influence of these three parameters on optical properties. We first investigated the time-dependent cation exchange at ambient conditions by monitoring the emission of a colloidal sample (NaGdF4:Yb/Tm@NaGdF4; 26.2 mg) after addition of TbCl3 (20 μmol). As shown in Fig. 3c, the intensity of Tb3+ emission gradually increased over time and reached a plateau after 8 min, at which time the cation exchange reached a dynamic equilibrium (Supplementary Fig. 19). We further performed the controls at different temperatures, and found that the emission can be greatly enhanced by increasing reaction temperature (Fig. 3d and Supplementary Fig. 20), indicating that high temperatures favour the cation exchange process. The emission intensity can also be boosted by slightly enriching the concentration of the exchange ions (Fig. 3e). The optimal concentration of Tb3+ for maximum particle emission was estimated to 5 mM (Supplementary Fig. 21).
Based on the above-mentioned optimization of reaction conditions, we successfully prepared Gd3+-based upconversion nanocrystals containing various types of activators (Eu3+, Dy3+, Ce3+, Mn2+) by the cation exchange approach (Fig. 3f, Supplementary Fig. 22, Supplementary Table 1 and Supplementary Note 4). It is worth noting that the upconversion emission of Ce3+ and Mn2+ is observed for the first time in hexagonal-phased NaGdF4 host materials (Supplementary Figs 23–27). Significantly, we obtained a record long-lived Mn2+ luminescence of ∼600 ms (lifetime: ∼56.7 ms) (Fig. 3g). The long decay time of Mn2+ emission can be used to resolve the spectral overlapping issue between Mn2+ and Tm3+ emissions by the time-gated spectroscopy (Fig. 3h). Interestingly, an emission colour change from cyan to green could be discerned by the naked eye on switching off of the 980-nm excitation source (Supplementary Movie 3).
Mechanistic investigation
The observation of optical emissions in cation-exchanged nanocrystals and inductively coupled plasma mass spectroscopy analysis reveal that the cation exchange process is dependent critically upon the nature of the exchanged ion; for example, ionic radius and valence charge (Supplementary Fig. 28). To facilitate the exchange of the Gd3+ host lattice with Mn2+, an elevated temperature of 90 °C is needed to overcome the charge imbalance and lattice strain due to the size mismatch. To understand the cation exchange process, we carried out first-principles calculations by estimating the formation energies of hexagonal-phased NaGdF4 nanocrystals doped with various ions (Supplementary Fig. 29 and Supplementary Table 2;refs 49,50). Our calculations indicate that lanthanides can readily replace Gd atoms at ambient conditions. By comparison, doping of Mn atoms into the NaGdF4 lattice at room temperature requires a large excess of energies (∼1.82 eV). We further investigated the charge transfer within the doped nanocrystals by employing charge distribution analysis. It was found that the amount of charge transfer from the doped lanthanides (Eu, Dy, Ce, Tb) to the surrounding F atoms is comparable to that between Gd and F atoms. However, the calculated amount of charge transfer from Mn to F decreased by about 0.5448e relative to that between Gd and F, giving rise to reduced dipole polarizability and increased formation energy in Mn-doped nanocrystals. In addition, the significant difference in ionic size between Mn2+ and Gd3+ (∼13.8 pm) is another major factor that hinders the cation-exchange process.
Apart from the enhancement of cation exchange at the particle surface, the thermal fluctuation at a high temperature is likely to accelerate the diffusion of the exchanged ions from a particle's surface to its inner region, so that an improved luminescence from the exchanged ions can be observed51,52. To confirm our hypothesis, we first mixed aqueous solutions of TbCl3 and NaGdF4:Yb/Tm@NaGdF4 at room temperature and then isolated the Tb3+-modified nanocrystals. Subsequently, we heated the nanocrystals at 90 °C for 1 h. As anticipated, we observed an increase in Tb3+ emission after heat treatment (Supplementary Fig. 30). Our calculations also suggest that the diffusion of doped ions (Tb3+, Eu3+, Dy3+, Ce3+ and Mn2+) in the NaGdF4 lattice can occur with ease (Supplementary Fig. 31). Taken together, these data indicate that the cation exchange and diffusion at elevated temperatures synergistically contribute to the increased emission of the exchanged cations (Supplementary Fig. 32 and Supplementary Note 5).
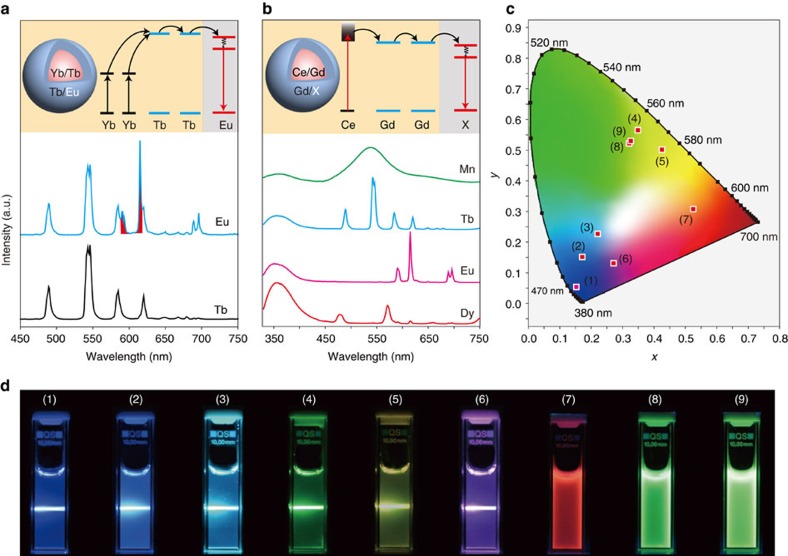
We next explored the applicability of this cation exchange approach to colour-tuning in other upconversion and downconversion systems. For instance, we observed an emission colour change for NaYbF4:Tb@NaTbF4 upconversion nanocrystals from green to yellow upon cation exchange of Tb3+ with Eu3+ at the particle's surface (Fig. 4a, Supplementary Figs 33–35 and Supplementary Note 6). In the cases where a downconversion process was demonstrated, the substitution of Gd3+ in NaGdF4:Ce@NaGdF4 nanocrystals by a series of activators (Tb3+, Eu3+, Dy3+, Mn2+) resulted in Ce3+-sensitized multicolour emissions (Fig. 4b, Supplementary Fig. 36 and Supplementary Note 7). Importantly, the capability of our approach to modulating emission colours in lanthanide-doped nanocrystals allowed rapid access to a myriad of different colour spaces with minimum sample processing time (Fig. 4c,d).
Figure 4. Multicolour synthesis in lanthanide-doped nanocrystals through cation exchange.
(a) Upconversion luminescence spectra of NaYbF4:Tb@NaTbF4 nanocrystals before and after treatment with EuCl3. Note that the Eu3+ emissions are highlighted in red. Inset shows the proposed energy transfer process in Eu3+-treated NaYbF4:Tb@NaTbF4 nanocrystals. Under the 980 nm excitation, a cooperative upconversion process firstly takes place in the Yb3+/Tb3+ pair, followed by an energy migration process in the Tb sub-lattice. The excited energy is subsequently trapped by Eu3+ ions. (b) Downconversion luminescence spectra of NaGdF4:Ce@NaGdF4 nanocrystals treated with TbCl3, EuCl3, MnCl2 and DyCl3. Inset shows the corresponding energy transfer mechanism in the nanocrystals. Under the ultraviolet excitation, the excited state of Ce3+ is firstly populated. Subsequently, an energy migration process in the Gd sub-lattice bridges energy transfer from Ce3+ to exchange ions (X=Tb3+, Eu3+, Mn2+ or Dy3+). (c) Commission Internationale de l'Eclairage (CIE) chromaticity coordinates of the emissions measured for the as-synthesized colloidal solutions. (d) Corresponding luminescence photographs of the colloidal solutions. Samples 1–9 are NaGdF4:Yb/Tm@NaGdF4, Mn2+-exchanged NaGdF4:Yb/Tm@NaGdF4, Tb3+-exchanged NaGdF4:Yb/Tm@NaGdF4, NaYbF4:Tb@NaTbF4, Eu3+-exchanged NaYbF4:Tb@NaTbF4, Eu3+-exchanged NaGdF4:Yb/Tm@NaGdF4, Eu3+-exchanged NaGdF4:Ce@NaGdF4, Mn2+-exchanged NaGdF4:Ce@NaGdF4, and Tb3+-exchanged NaGdF4:Ce@NaGdF4, respectively. Note that the luminescence photographs were taken under laser excitation at 980 nm for samples 1–6 and under ultraviolet lamp irradiation at 254 nm for samples 7–9.
Discussion
The combination of cation exchange and energy migration in lanthanide-doped nanocrystals enables us to precisely tailor the luminescence to the colours of interest, providing the possibility to achieve long-lived luminescence in unexplored regimes of lifetime and without concerning variation in particle size, phase and morphology. As with any methodology, there are seemingly obvious drawbacks. For example, it requires a subset of lanthanide ions capable of participating in the energy migration. However, our data suggest that the advantages of the presented approach far outweigh its limitations. In particular, our approach allows the development of a general, green protocol for preparing multicolour nanoprobes, combining efficient and rapid sample synthesis with significantly reduced solvent and reagent consumption. As such it is anticipated that this technique will greatly expand the repertoire of possible upconversion nanomaterials, with relevant applications for fields as diverse as chemical sensing, biological imaging, photodynamic therapy and anti-counterfeiting.
Methods
Nanocrystal synthesis
Cation-exchanged nanocrystals were prepared by incubating aqueous solution containing ligand-free template nanocrystals and activator ions at ambient conditions for 1 h. Additional experimental details are provided in the Supplementary Methods.
Characterization
Ultraviolet–visible spectra were measured on a SHIMADZU ultraviolet-3600 spectrophotometer. Fourier transform infrared spectroscopy spectra were recorded on a Varian 3100 fourier transform infrared spectrometer. Low-resolution TEM images were taken on a JEOL-1400 transmission electron microscope operating at an acceleration voltage of 100 kV. Scanning electron microscopy was carried out on a FEI NOVA NanoSEM 230 scanning electron microscope operated at 5 kV. Powder X-ray diffraction data was obtained on a Siemens D5005 X-ray diffractometer with Cu Kα radiation (λ=1.5406 Å). The upconversion luminescence spectra were recorded in an Edinburgh FSP920 equipped with a photomultiplier, in conjunction with 980 nm diode laser and a xenon arc lamp (Xe900). The measurement of luminescence lifetime was conducted using a lifetime spectrometer (FSP920, Edinburgh) equipped with a microsecond flash lamp as the excitation source. Upconversion luminescence microscopic images were obtained on an Olympus BX51 microscope with a Nikon DS-Ri1 imaging system adapted to a 980 nm diode laser. Digital photographs were taken with a Nikon D700 camera.
Data availability
The authors declare that the data that support the findings of this study are available within the article and its Supplementary Information files. All other relevant data are available from the corresponding author upon request.
Additional information
How to cite this article: Han, S. et al. Multicolour synthesis in lanthanide-doped nanocrystals through cation exchange in water. Nat. Commun. 7, 13059 doi: 10.1038/ncomms13059 (2016).
Supplementary Material
Supplementary Figures 1-36, Supplementary Tables 1-2, Supplementary Notes 1-7, Supplementary Methods and Supplementary References.
(Cation exchange with Tb3+). To visualize the cation exchange reaction under ambient conditions, we mixed ligand-free NaGdF4:Yb/Tm@NaGdF4 nanocrystals (2.5 mL; ~50 mg) with Tb3+ precursors (100 μL; 0.2 M). Upon addition of TbCl3 solution, we observed a gradual change in particle emission from blue to cyan, indicating the occurrence of cation exchange between the nanocrystals and Tb3+ ions. In addition, the green emission appeared brighter over time. After several minutes, the emission color remained intact, suggesting the establishment of an equilibrium in cation exchange process. The origin of green emission from Tb3+ was confirmed by covering a long-pass 500 nm filter on the front of the camera.
(Cation exchange with Eu3+). In a parallel experiment, we demonstrated the cation exchange between Eu3+ and Gd3+-based nanocrystals under ambient conditions. We added a 100- μL solution of EuCl3 (0.2 M) to a 2.5-mL water solution containing ligand-free NaGdF4:Yb/Tm@NaGdF4 nanocrystals (~50 mg). During the reaction, we observed a color change in particle emission from blue to red. The color change lasted for several minutes.
(Cation exchange with Mn2+). For illustration of long luminescence lifetime of Mn2+, we irradiated the Mn2+-exchanged NaGdF4:Yb/Tm@NaGdF4 nanocrystals dispersed in a cuvette with a 980 nm laser. The colloidal solution emitted a cyan color. After switching off of the 980 nm laser, we visualized a color change from white to green. This color change is due to the diminished Tm3+ emission and Mn2+ afterglow luminescence. Note that we repeated this process three times and the phenomenon was reproducible.
Acknowledgments
This work is supported by the Singapore Ministry of Education (Grant R143000627112, R143000642112), Agency for Science, Technology and Research (A*STAR) under the contracts of 122-PSE-0014 and 1231AFG028 (Singapore), National Research Foundation, Prime Minister's Office, Singapore under its Competitive Research Program (CRP Award No. NRF-CRP15-2015-03), National Basic Research Program of China (973 Program, Grant 2015CB932200), National Natural Science Foundation of China (61136003), and the CAS/SAFEA International Partnership Program for Creative Research Teams. Y.H. is grateful to KAUST Global Collaborative Research for the Academic Excellence Alliance (AEA) fund. We thank B. Zhou, Q. Sun, R. Deng, X. Xie, H. Xu and Q. Su for technical assistance and helpful discussion.
Footnotes
Author contributions S.H., W.H. and X.L. conceived the project and wrote the paper. S.H. performed the nanocrystal synthesis and luminescence measurements. X.Q. performed the DFT calculation. Y.Z. and Y.H. contributed to the high-resolution TEM imaging and analysis. S.H., Z.A., L.L., Y.H., W.H. and X.L. provided input into the design of the experiments and the preparation of the manuscript.
References
- Haase M. & Schäfer H. Upconverting nanoparticles. Angew. Chem. Int. Ed. 50, 5808–5829 (2011). [DOI] [PubMed] [Google Scholar]
- Auzel F. Upconversion and anti-Stokes processes with f and d ions in solids. Chem. Rev. 104, 139–173 (2004). [DOI] [PubMed] [Google Scholar]
- Bünzli J. C. G. & Piguet C. Taking advantage of luminescent lanthanide ions. Chem. Soc. Rev. 34, 1048–1077 (2005). [DOI] [PubMed] [Google Scholar]
- Zhou B., Shi B., Jin D. & Liu X. Controlling upconversion nanocrystals for emerging applications. Nat. Nanotechnol. 10, 924–936 (2015). [DOI] [PubMed] [Google Scholar]
- Liu X., Yan C. & Capobianco J. A. Photon upconversion nanomaterials. Chem. Soc. Rev. 44, 1299–1301 (2015). [DOI] [PubMed] [Google Scholar]
- Zhou J., Liu Z. & Li F. Upconversion nanophosphors for small-animal imaging. Chem. Soc. Rev. 41, 1323–1349 (2012). [DOI] [PubMed] [Google Scholar]
- Wu S. et al. Non-blinking and photostable upconverted luminescence from single lanthanide-doped nanocrystals. Proc. Natl Acad. Sci. USA 106, 10917–10921 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam S. H. et al. Long-term real-time tracking of lanthanide ion doped upconverting nanoparticles in living cells. Angew. Chem. Int. Ed. 50, 6093–6097 (2011). [DOI] [PubMed] [Google Scholar]
- Li L., Wu P., Hwang K. & Lu Y. An exceptionally simple strategy for DNA-functionalized up-conversion nanoparticles as biocompatible agents for nanoassembly, DNA delivery, and imaging. J. Am. Chem. Soc. 135, 2411–2414 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W. et al. Lanthanide-doped upconversion nano-bioprobes: electronic structures, optical properties, and biodetection. Chem. Soc. Rev. 44, 1379–1415 (2015). [DOI] [PubMed] [Google Scholar]
- Chen G., Qiu H., Prasad P. N. & Chen X. Upconversion nanoparticles: design, nanochemistry, and applications in theranostics. Chem. Rev. 114, 5161–5214 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorris H. H. & Wolfbeis O. S. Photon-upconverting nanoparticles for optical encoding and multiplexing of cells, biomolecules, and microspheres. Angew. Chem. Int. Ed. 52, 3584–3600 (2013). [DOI] [PubMed] [Google Scholar]
- Lee J. et al. Universal process-inert encoding architecture for polymer microparticles. Nat. Mater. 13, 524–529 (2014). [DOI] [PubMed] [Google Scholar]
- Lu Y. et al. On-the-fly decoding luminescence lifetimes in the microsecond region for lanthanide-encoded suspension arrays. Nat. Commun. 5, 3741 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng R. et al. Temporal full-colour tuning through non-steady-state upconversion. Nat. Nanotechnol. 10, 237–242 (2015). [DOI] [PubMed] [Google Scholar]
- Wang X., Zhuang J., Peng Q. & Li Y. A general strategy for nanocrystal synthesis. Nature 437, 121–124 (2005). [DOI] [PubMed] [Google Scholar]
- Wang F. et al. Simultaneous phase and size control of upconversion nanocrystals through lanthanide doping. Nature 463, 1061–1065 (2010). [DOI] [PubMed] [Google Scholar]
- Heer S., Kömpe K., Güdel H. U. & Haase M. Highly efficient multicolour upconversion emission in transparent colloids of lanthanide-doped NaYF4 nanocrystals. Adv. Mater. 16, 2102–2105 (2004). [Google Scholar]
- Schäfer H., Ptacek P., Kömpe K. & Haase M. Lanthanide-doped NaYF4 nanocrystals in aqueous solution displaying strong up-conversion emission. Chem. Mater. 19, 1396–1400 (2007). [Google Scholar]
- Gai S., Li C., Yang P. & Lin J. Recent progress in rare earth micro/nanocrystals: soft chemical synthesis, luminescent properties, and biomedical applications. Chem. Rev. 114, 2343–2389 (2014). [DOI] [PubMed] [Google Scholar]
- Ye X. et al. Competition of shape and interaction patchiness for self-assembling nanoplates. Nat. Chem. 5, 466–473 (2013). [DOI] [PubMed] [Google Scholar]
- Mai H. et al. High-quality sodium rare-earth fluoride nanocrystals: controlled synthesis and optical properties. J. Am. Chem. Soc. 128, 6426–6436 (2006). [DOI] [PubMed] [Google Scholar]
- Vetrone F., Naccache R., Mahalingam V., Morgan C. G. & Capobianco J. A. The active-core/active-shell approach: a strategy to enhance the upconversion luminescence in lanthanide-doped nanoparticles. Adv. Funct. Mater. 19, 2924–2929 (2009). [Google Scholar]
- Wang F. et al. Tuning upconversion through energy migration in core-shell nanoparticles. Nat. Mater. 10, 968–973 (2011). [DOI] [PubMed] [Google Scholar]
- Chan E. M. Combinatorial approaches for developing upconverting nanomaterials: high-throughput screening, modeling, and applications. Chem. Soc. Rev. 44, 1653–1679 (2015). [DOI] [PubMed] [Google Scholar]
- Dong H. et al. Lanthanide nanoparticles: from design toward bioimaging and therapy. Chem. Rev. 115, 10725–10815 (2015). [DOI] [PubMed] [Google Scholar]
- Son D. H., Hughes S. M., Yin Y. & Alivisatos A. P. Cation exchange reactions in ionic nanocrystals. Science 306, 1009–1012 (2004). [DOI] [PubMed] [Google Scholar]
- Robinson R. D. et al. Spontaneous superlattice formation in nanorods through partial cation exchange. Science 317, 355–358 (2007). [DOI] [PubMed] [Google Scholar]
- Li H. et al. Sequential cation exchange in nanocrystals: preservation of crystal phase and formation of metastable phases. Nano Lett. 11, 4964–4970 (2011). [DOI] [PubMed] [Google Scholar]
- Zhang J., Tang Y., Lee K. & Ouyang M. Nonepitaxial growth of hybrid core-shell nanostructures with large lattice mismatches. Science 327, 1634–1638 (2010). [DOI] [PubMed] [Google Scholar]
- Mocatta D. et al. Heavily doped semiconductor nanocrystal quantum dots. Science 332, 77–81 (2011). [DOI] [PubMed] [Google Scholar]
- Tabachnyk M. et al. Resonant energy transfer of triplet excitons from pentacene to PbSe nanocrystals. Nat. Mater. 13, 1033–1038 (2014). [DOI] [PubMed] [Google Scholar]
- Wu M. et al. Solid-state infrared-to-visible upconversion sensitized by colloidal nanocrystals. Nat. Photon. 10, 31–34 (2016). [Google Scholar]
- Smith A. M., Mohs A. M. & Nie S. Tuning the optical and electronic properties of colloidal nanocrystals by lattice strain. Nat. Nanotechnol. 4, 56–63 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T. et al. Self-assembled colloidal superparticles from nanorods. Science 338, 358–363 (2012). [DOI] [PubMed] [Google Scholar]
- Deutsch Z., Neeman L. & Oron D. Luminescence upconversion in colloidal double quantum dots. Nat. Nanotechnol. 8, 649–653 (2013). [DOI] [PubMed] [Google Scholar]
- Wang J. et al. Photon energy upconversion through thermal radiation with the power efficiency reaching 16%. Nat. Commun. 5, 5669 (2014). [DOI] [PubMed] [Google Scholar]
- Zhou J. et al. Ultrasensitive polarized up-conversion of Tm3+-Yb3+ doped β-NaYF4 single nanorod. Nano Lett. 13, 2241–2246 (2013). [DOI] [PubMed] [Google Scholar]
- Wachtler M. et al. Optical properties of rare-earth ions in lead germanate glasses. J. Am. Ceram. Soc. 81, 2045–2052 (1998). [Google Scholar]
- Speghini A., Piccinelli F. & Bettinelli M. Synthesis, characterization and luminescence spectroscopy of oxide nanopowders activated with trivalent lanthanide ions: The garnet family. Opt. Mater. 33, 247–257 (2011). [Google Scholar]
- Wegh R. T., Donker H., Oskam K. D. & Meijerink A. Visible quantum cutting in LiGdF4:Eu3+ through downconversion. Science 283, 663–666 (1999). [DOI] [PubMed] [Google Scholar]
- Gargas D. J. et al. Engineering bright sub-10-nm upconverting nanocrystals for single-molecule imaging. Nat. Nanotechnol. 9, 300–305 (2014). [DOI] [PubMed] [Google Scholar]
- Zhao J. et al. Single-nanocrystal sensitivity achieved by enhanced upconversion luminescence. Nat. Nanotechnol. 8, 729–734 (2013). [DOI] [PubMed] [Google Scholar]
- Wang J. et al. Enhancing multiphoton upconversion through energy clustering at sublattice level. Nat. Mater. 13, 157–162 (2013). [DOI] [PubMed] [Google Scholar]
- Dong C. & van Veggel F. C. J. M. Cation exchange in lanthanide fluoride nanoparticles. ACS Nano 3, 123–130 (2009). [DOI] [PubMed] [Google Scholar]
- Deng M. & Wang L. Unexpected luminescence enhancement of upconverting nanocrystals by cation exchange with well retained small particle size. Nano Res. 7, 782–793 (2014). [Google Scholar]
- Zhang F., Shi Y., Sun X., Zhao D. & Stucky G. D. Formation of hollow upconversion rare-earth fluoride nanospheres: nanoscale kirkendall effect during ion exchange. Chem. Mater. 21, 5237–5243 (2009). [Google Scholar]
- Dong C., Korinek A., Blasiak B., Tomanek B. & van Veggel F. C. J. M. Cation exchange: a facile method to make NaYF4:Yb,Tm-NaGdF4 core–shell nanoparticles with a thin, tunable, and uniform shell. Chem. Mater. 24, 1297–1305 (2012). [Google Scholar]
- Kresse G. & Hafner J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 47, 558–561 (1993). [DOI] [PubMed] [Google Scholar]
- Kresse G. & Furthmüller J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996). [DOI] [PubMed] [Google Scholar]
- Vlaskin V. A., Barrows C. J., Erickson C. S. & Gamelin D. R. Nanocrystal diffusion doping. J. Am. Chem. Soc. 135, 14380–14389 (2013). [DOI] [PubMed] [Google Scholar]
- Chen B. et al. Establishing the structural integrity of core-shell nanoparticles against elemental migration using luminescent lanthanide probes. Angew. Chem. Int. Ed. 54, 12788–12790 (2015). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures 1-36, Supplementary Tables 1-2, Supplementary Notes 1-7, Supplementary Methods and Supplementary References.
(Cation exchange with Tb3+). To visualize the cation exchange reaction under ambient conditions, we mixed ligand-free NaGdF4:Yb/Tm@NaGdF4 nanocrystals (2.5 mL; ~50 mg) with Tb3+ precursors (100 μL; 0.2 M). Upon addition of TbCl3 solution, we observed a gradual change in particle emission from blue to cyan, indicating the occurrence of cation exchange between the nanocrystals and Tb3+ ions. In addition, the green emission appeared brighter over time. After several minutes, the emission color remained intact, suggesting the establishment of an equilibrium in cation exchange process. The origin of green emission from Tb3+ was confirmed by covering a long-pass 500 nm filter on the front of the camera.
(Cation exchange with Eu3+). In a parallel experiment, we demonstrated the cation exchange between Eu3+ and Gd3+-based nanocrystals under ambient conditions. We added a 100- μL solution of EuCl3 (0.2 M) to a 2.5-mL water solution containing ligand-free NaGdF4:Yb/Tm@NaGdF4 nanocrystals (~50 mg). During the reaction, we observed a color change in particle emission from blue to red. The color change lasted for several minutes.
(Cation exchange with Mn2+). For illustration of long luminescence lifetime of Mn2+, we irradiated the Mn2+-exchanged NaGdF4:Yb/Tm@NaGdF4 nanocrystals dispersed in a cuvette with a 980 nm laser. The colloidal solution emitted a cyan color. After switching off of the 980 nm laser, we visualized a color change from white to green. This color change is due to the diminished Tm3+ emission and Mn2+ afterglow luminescence. Note that we repeated this process three times and the phenomenon was reproducible.
Data Availability Statement
The authors declare that the data that support the findings of this study are available within the article and its Supplementary Information files. All other relevant data are available from the corresponding author upon request.