Abstract
Rationale: Early mobilization (EM) improves outcomes for mechanically ventilated patients. Variation in structure and organizational characteristics may affect implementation of EM practices.
Objectives: We queried intensive care unit (ICU) environment and standardized ICU practices to evaluate organizational characteristics that enable EM practice.
Methods: We recruited 151 ICUs in France, 150 in Germany, 150 in the United Kingdom, and 500 in the United States by telephone. Survey domains included respondent characteristics, hospital and ICU characteristics, and ICU practices and protocols.
Measurements and Main Results: We surveyed 1,484 ICU leaders and received a 64% response rate (951 ICUs). Eighty-eight percent of respondents were in nursing leadership roles; the remainder were physiotherapists. Surveyed ICUs were predominantly mixed medical-surgical units (67%), and 27% were medical ICUs. ICU staffing models differed significantly (P < 0.001 each) by country for high-intensity staffing, nurse/patient ratios, and dedicated physiotherapists. ICU practices differed by country, with EM practices present in 40% of French ICUs, 59% of German ICUs, 52% of U.K. ICUs, and 45% of U.S. ICUs. Formal written EM protocols were present in 24%, 30%, 20%, and 30%, respectively, of those countries’ ICUs. In multivariate analysis, EM practice was associated with multidisciplinary rounds (odds ratio [OR], 1.77; P = 0.001), setting daily goals for patients (OR, 1.62; P = 0.02), presence of a dedicated physiotherapist (OR, 2.48; P < 0.001), and the ICU’s being located in Germany (reference, United States; OR, 2.84; P < 0.001). EM practice was also associated with higher nurse staffing levels (1:1 nurse/patient ratio as a reference; 1:2 nurse/patient ratio OR, 0.59; P = 0.05; 1:3 nurse/patient ratio OR, 0.33; P = 0.005; 1:4 or less nurse/patient ratio OR, 0.37; P = 0.005). Those responding rarely cited ambulation of mechanically ventilated patients, use of a bedside cycle, or neuromuscular electrical stimulation as part of their EM practice. Physical therapy initiation, barriers to EM practice, and EM equipment were highly variable among respondents.
Conclusions: International ICU structure and practice is quite heterogeneous, and several factors (multidisciplinary rounds, setting daily goals for patients, presence of a dedicated physiotherapist, country, and nurse/patient staffing ratio) are significantly associated with the practice of EM. Practice and barriers may be far different based upon staffing structure. To achieve successful implementation, whether through trials or quality improvement, ICU staffing and practice patterns must be taken into account.
Keywords: critical care, physical therapy/physiotherapy, early mobilization, survey, intensive care unit
Clinical innovation often occurs through the testing of new treatments and techniques within a single country or region. Worldwide adoption occurs based on the broad appeal of improved patient outcomes. However, unrecognized environmental variation may yield unanticipated barriers to implementation (1). Knowledge of environmental and practice variations is important for appropriate study interpretation, implementation approach, and estimation of likelihood of local success (2).
One example of a universal practice in patient care is early exercise of the critically ill patient, often termed early mobilization (EM). Pioneering studies have demonstrated the feasibility and safety of early exercise, particularly in patients with respiratory failure undergoing mechanical ventilation (MV) (3–10). Prospective trials, most conducted in single centers in U.S. and Australian intensive care units (ICUs), have yielded mixed outcomes. Successful trials have measured improved patient functional outcome at hospital discharge, shorter duration of MV, shorter ICU and hospital lengths of stay, and shorter duration of delirium (11). Given the ability of EM to improve patient-centered outcomes, paired with the potential for greater ICU throughput, the practice has great appeal.
Despite this evidence, surveys and point prevalence studies of EM practice have shown limited penetration, particularly in patients undergoing MV (12–15). For those interested in implementing this practice in routine care, representation of regional variation may help to better contextualize the working environment and may aid in analysis of relevant barriers. Furthermore, the natural variation of ICU structure allows the study of hospital characteristics that may be most associated with (and hence most favorable for) the adoption of EM practice. Accordingly, we conducted a four-country survey of ICU leaders to characterize EM practices in relation to ICU and hospital structure, staffing, and related clinical practices. Preliminary results of this study were presented previously in abstract form (16, 17), and an in-depth analysis of some of the data was previously published (18).
Methods
Study Design
We designed a telephone survey (see Figure E1 in the online supplement) that was administered to randomly selected ICUs in France, Germany, the United Kingdom, and the United States. The survey was administered to the largest medical ICU, mixed medical-surgical ICU, or cardiac ICU in the hospital. The ICUs were stratified and selected randomly from publicly available lists of ICUs using a random number generator. Hospitals were stratified by country and size—small (50–199 beds), medium (200–399 beds), or large (≥400 beds). In the United States, hospitals were additionally stratified by American Hospital Association region (19).
In this study, we used survey procedures considered exempt from human subjects review under exemption 2 of the U.S. Code of Federal Regulations. In addition, each participant was read a verbal consent script to make sure he or she understood his or her rights as a survey participant and agreed to participate. The project was approved by the University of Pennsylvania Institutional Review Board (IRB number 818646).
Survey Development
We developed and administered a survey as previously described (18). In summary, the survey addressed the following domains (20, 21): respondent characteristics, hospital and ICU characteristics, and ICU practices and protocols. Hospital characteristics included number of beds, academic affiliation, and the total number of ICUs. ICU characteristics included size of the ICU; average ICU occupancy rate; and information about standard staffing models provided by physicians, nurses, and physical therapists (physiotherapists [PTs] in the European Union). We asked respondents about the following practices: daily multidisciplinary rounds, documentation of daily patient goals, setting a goal sedation target for MV patients, use of a standard sedation score for MV patients, and EM. Respondents were asked whether they had written protocols for the following: sedation, weaning from MV, and EM. Additionally, we asked respondents to identify the following details of EM: mechanism for initiation, types of patients included, eligibility criteria, team members and equipment used for mobilization, and respondents’ perceived barriers to implementation. To gather EM details, we asked participants to select information from predefined lists and gave them the option of providing other information or more detailed answers if needed (see Figure E1 in the online supplement).
We used standardized definitions in accordance with prior research. Daily multidisciplinary rounds were defined as rounds consisting of a physician, nurse, and other health care professionals, such as social workers, physical therapists, respiratory therapists, or a pharmacist (22). A protocol was defined as a written clinical pathway that provides a standard algorithm for caring for patients with a given condition (23). A high-intensity staffing model was defined as an ICU where either an intensivist has primary responsibility for all patients or an ICU where an intensivist provides a mandatory consult for all patients (24). We defined a dedicated therapist as one whose primary assignment is tending to patients in one or more ICUs. We did not define early. We defined mobility as “a planned series of exercise of a patient in a sequence that begins at a patient’s current mobility status and returns the patient to their baseline mobility status” (25).
The survey was reviewed by content experts and then piloted with a group of ICU directors and nurse managers. The survey was translated into French and German and then back into English to confirm proper translation. We targeted a goal response of approximately 30 ICUs with EM protocols from each of France, Germany, and the United Kingdom and about 100 ICUs with EM protocols from the United States. We estimated that 25% of ICUs would have an EM protocol. Given this, we elected to continue surveying until we reached a total of 150 ICUs in each of France, Germany, and the United Kingdom and a total of 500 in the United States. The test–retest reliability of this survey has been demonstrated previously (18).
Data Collection
The survey was administered over a 6-week period between September 2013 and November 2013. In France, Germany, and the United Kingdom, the telephone survey was administered to randomly chosen hospitals stratified by hospital size until a target sample size of 150 hospitals per country was reached. In the United States, the survey was administered to randomly chosen hospitals stratified by hospital density by region (as defined by the American Hospital Association [18, 19]) and hospital size until we reached the targeted sample of 500 hospitals. We sought to contact nurse leaders (nurse managers or directors, critical care nurse specialists) in the United States and nurse leaders or PTs in the European countries. We contacted potential respondents using publicly available information and then screened them to allow identification of eligible respondents. Respondents in the United States were eligible for a $25 incentive; no incentive was offered in the European countries. There was a single respondent per ICU and/or hospital.
Statistical Analysis
Descriptive statistics are presented as counts and percentages, with means (SD) or medians (interquartile range [IQR]) as appropriate. Univariable analyses of presence of EM practice (with or without a protocol) and EM practice with a protocol (termed EM protocol hereafter) were performed to evaluate associations with hospital- and ICU-level factors and ICU practices. Multivariable logistic regression was used to evaluate the association between either EM practice or EM protocol and hospital-level factors, ICU-level factors, ICU practices, and EM practices defined a priori. Variables were included in the analyses if their univariable association with individual protocol use met a significance level of a P value less than or equal to 0.2. Concerns about collinearity were reviewed by assessing correlation coefficients and by evaluating variance inflation factors for each independent variable. Stata version 12.1 software (StataCorp, College Station, TX) was used for all analyses. Given respondent discipline variation in the United Kingdom, a post hoc analysis was performed to compare respondent discipline with reported EM practices, EM activity levels, and/or barriers to EM. Significant differences between groups were reported at the α-level of 0.05. All P values were two-sided.
Results
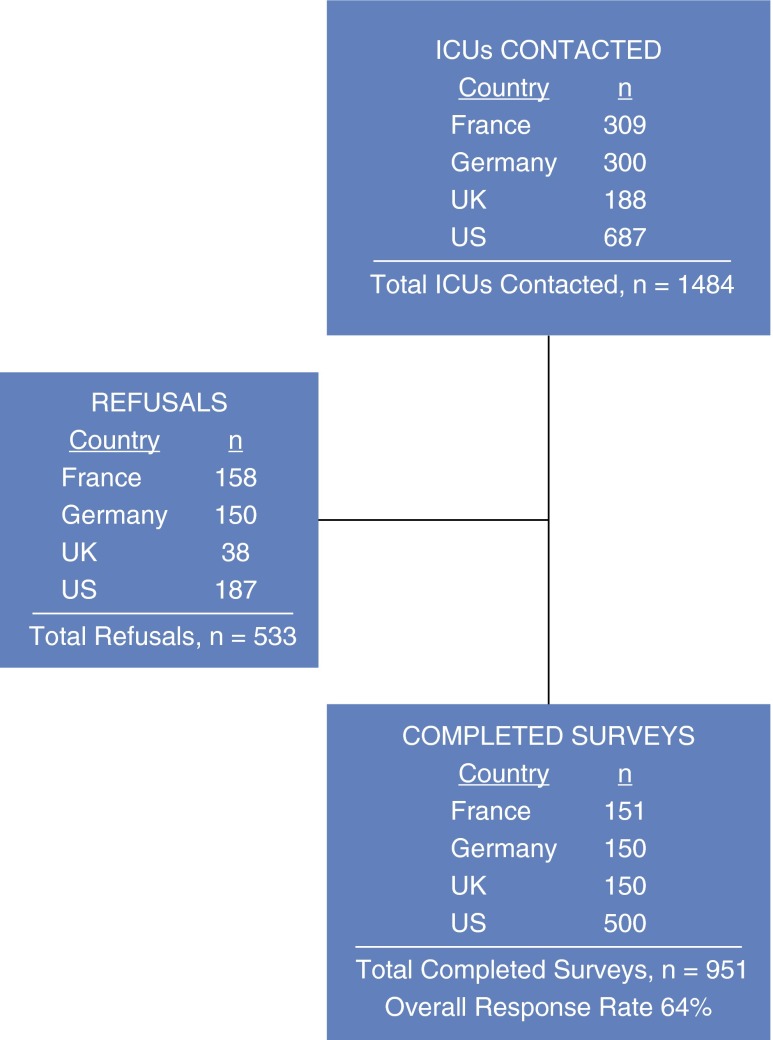
A total of 1,484 ICUs were contacted, and we received a total of 951 completed surveys (Figure 1). The overall response rate was 64%. Eighty-eight percent of respondents from all four surveyed countries were in nursing leadership roles in the ICUs (nurse managers or directors, critical care nurse specialists) (Table 1). Regional variation existed in respondent type; notably, 49% of respondents in the United Kingdom were PT leaders. Academic affiliation varied significantly by country (P < 0.001), with only 22% of French ICUs surveyed reporting an academic affiliation, in contrast to 65% of ICUs in Germany, 79% in the United Kingdom, and 51% in the United States. There was a median of one ICU (IQR, 1–3) present in the hospitals surveyed. There were a median of 13 ICU beds (IQR, 9–20). Most ICUs were mixed medical-surgical units (67% overall), and 27% were medical ICUs.
Figure 1.
Flow diagram of intensive care units (ICUs) contacted and survey completion.
Table 1.
Characteristics of respondents, hospitals, and intensive care units
| France (n = 151) | Germany (n = 150) | United Kingdom (n = 150) | United States (n = 500) | Total (n = 951) | |
|---|---|---|---|---|---|
| Respondent characteristics | |||||
| Nurse leader | 95% | 77% | 51% | >99% | 88% |
| Physician leader | 0% | 11% | 0% | 0% | 2% |
| Physiotherapist leader | 5% | 12% | 49% | <1% | 11% |
| Hospital characteristics | |||||
| Total beds | |||||
| <200 | 36% | 33% | 21% | 33% | 32% |
| 200–399 | 28% | 27% | 23% | 34% | 30% |
| ≥400 | 36% | 39% | 55% | 33% | 38% |
| Academic affiliation | 22% | 65% | 79% | 51% | 53% |
| Total number of adult ICUs, median (IQR) | 2 (1–4) | 1 (1–1) | 1 (1–1) | 1 (1–3) | 1 (1–3) |
| ICU characteristics | |||||
| Type of ICU | |||||
| Medical alone | 21% | 22% | 8% | 35% | 27% |
| Medical-surgical | 57% | 78% | 89% | 60% | 67% |
| Cardiac | 23% | 0% | 2% | 5% | 6% |
| ICU beds, median (IQR) | 9 (6–12) | 12 (8–16) | 10 (6–16) | 16 (12–24) | 13 (9–20) |
| 1–10 beds | 56% | 42% | 56% | 20% | 35% |
| 11–20 beds | 34% | 45% | 34% | 50% | 44% |
| >20 beds | 9% | 13% | 10% | 30% | 21% |
Definition of abbreviations: ICU = intensive care unit; IQR = interquartile range.
Data are presented as percentage or median (IQR). Percentages may not add to 100% due to rounding.
ICU staffing models were notably different between the four countries surveyed (Figure 2). In all countries, there was a high degree of intensivist availability. However, the presence of a high-intensity staffing model (24) was significantly different between the European ICUs and the U.S. ICUs (P < 0.001). The reported presence of dedicated PTs also varied significantly by country (P < 0.001). Nurse/patient ratios varied widely (P < 0.001), with U.K. ICUs almost uniformly reporting 1:1 nurse/patient ratios and French ICUs reporting 1:3 or 1:4 ratios.
Figure 2.
Physician, physical therapist or physiotherapist, and nurse staffing, by country. (A) Physician staffing model by country. Light blue and dark blue represent a high-intensity staffing model. Light red and dark red represent a low-intensity staffing model. (B) Dedicated physical therapist or physiotherapist staffing model by country. (C) Nurse staffing model by country. PT = physical therapist or physiotherapist.
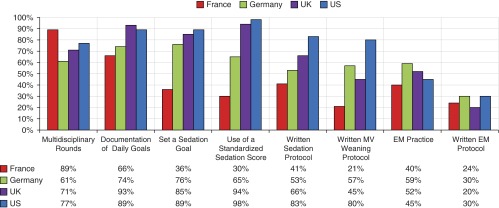
Reported practices and protocols also varied by country (Figure 3). Some practices were present in the majority of all four countries (e.g., documenting daily goals for patients; range, 66–93%), while others were quite disparate in prevalence (e.g., written MV weaning protocol; range, 21–80%). We found no significant change in between-country variability when we examined practices and protocols by respondent type and country. EM practice was reported in 48% of ICUs surveyed. Twenty-one percent of all ICUs (44% of all ICUs with an EM practice) noted that their EM practice was guided by a written protocol.
Figure 3.
Intensive care unit practices and protocols reported, by country. EM = early mobilization; MV = mechanical ventilation.
Multivariate analysis of EM practices and protocols across all countries was performed. EM practice was significantly associated with the following: lower nurse/patient staffing ratios, presence of a dedicated PT, multidisciplinary rounds, setting daily goals for patients, and country where the ICU was located (see Table 2). Factors not associated with EM practice included academic affiliation, hospital size, ICU type, a high-intensity staffing model for physicians, and written protocols for sedation administration or MV weaning. In diagnostic tests of the models, no independent variable had a variance inflation factor of 3 or greater, indicating that multicollinearity did not pose a problem.
Table 2.
Multivariate analyses for early mobilization practice and early mobilization protocol
| Variable | EM Practice | EM Protocol |
|---|---|---|
| Nurse/patient ratio | Overall P = 0.048 | Overall P = 0.002 |
| 1:1 or more | Reference | Reference |
| 1:2 | 0.59 (0.35–1.00), P = 0.05 | 0.63 (0.36–1.14), P = 0.13 |
| 1:3 | 0.33 (0.16–0.72), P = 0.005 | 0.20 (0.08–0.47), P < 0.001 |
| 1:4 or less | 0.37 (0.14–0.98), P = 0.005 | 0.18 (0.06–0.53), P = 0.002 |
| Dedicated PT | 2.48 (1.81–3.38), P < 0.001 | 2.97 (2.10–4.21), P < 0.001 |
| Multidisciplinary rounds | 1.77 (1.28–2.46), P = 0.001 | 1.76 (1.18–2.62), P = 0.005 |
| Daily goals | 1.62 (1.10–2.38), P = 0.02 | 2.18 (1.33–3.56), P = 0.002 |
| Country | Overall P < 0.001 | Overall P < 0.001 |
| United States | Reference | Reference |
| France | 1.17 (0.52–2.63), P = 0.70 | 2.00 (0.82–4.91), P = 0.13 |
| Germany | 2.84 (1.65–4.87), P < 0.001 | 2.10 (1.21–3.67), P = 0.008 |
| United Kingdom | 0.53 (0.29–0.99), P = 0.045 | 0.24 (0.12–0.48), P < 0.001 |
| Written protocol for sedation administration | NS | 1.82 (1.25–2.64), P = 0.002 |
Definition of abbreviations: EM = early mobilization; NS = not significant; PT = physical therapist or physiotherapist.
Factors associated with EM practice and EM protocol in multivariate analyses are listed. Analyses also included the following variables not found to be significantly associated with EM practice or EM protocol: academic affiliation, hospital size, intensive care unit type, a high-intensity staffing model for physicians, a written protocol for sedation administration, and a written protocol for mechanical ventilation weaning.
Data are presented as odds ratio (95% confidence interval), P value.
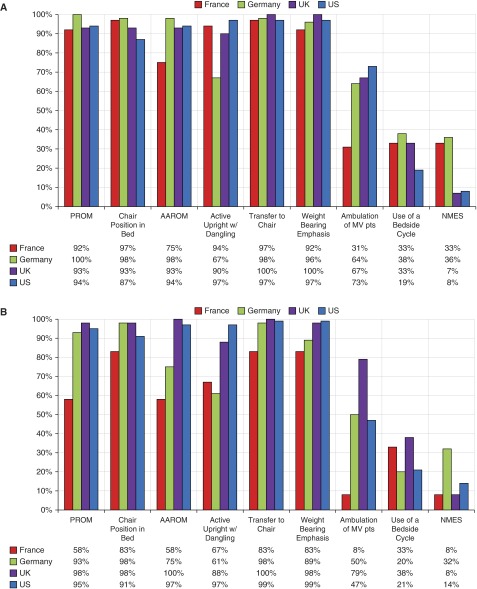
Stated activities included in EM practices and EM protocols are depicted in Figure 4. Passive range of motion, use of chair position in bed, active assisted range of motion, actively sitting upright with dangling, transferring to a chair, and weight bearing were all common. However, ambulation of mechanically ventilated patients, use of a bedside cycle, and neuromuscular electrical stimulation were uncommon.
Figure 4.
Activities included in early mobilization (EM) protocol (A) and EM practice (B), by country. AAROM = active assisted range of motion; MV = mechanical ventilation; NMES = neuromuscular electrical stimulation; PROM = passive range of motion; pts = patients.
To explore a potential relationship between respondent discipline and practice reporting, a post hoc analysis of U.K. results was conducted (total surveys, 150; nurse respondent, n = 76; PT respondent, n = 74). ICUs with PT respondents did not differ from nursing respondent ICUs with respect to academic affiliation, type of ICU, and number of ICU beds. When we stratified results by respondent type, we observed similarities in the proportion of ICUs reporting EM protocols (18% vs. 22%; P = 0.62) and the activities within those protocols (ambulation of MV patients, 64% vs. 69%; P = 0.99). However, units reporting EM practice (without protocol) differed by respondent (present in 43% of ICUs with nursing respondents vs. 61% of ICUs with PT respondents; P = 0.033). Furthermore, reported activities may have been different (68% of nursing respondents reported ambulation of MV patients vs. 86% of PT respondents; P = 0.16).
Other details of EM practice variability included mechanism of EM initiation and types of patients included (see Table E1). ICUs with EM protocol often were automatically triggered to start upon ICU entry. In contrast, ICUs with EM practice often required a physician’s order to start EM. Most commonly, all ICU patients, not just MV patients, were included in EM. Equipment used for mobilization (Figure 5) in both EM practice and EM protocol most commonly included a bed allowing the patient to assume the full chair position, mobile lifts, portable ventilators, and a patient rolling walker.
Figure 5.
Equipment used for mobilization for early mobilization (EM) protocol and EM practice, by country. (A) Equipment used as reported by country for intensive care units (ICUs) with EM protocols. (B) Equipment used as reported by country for ICUs with EM practice. NMES = neuromuscular electrical stimulation; pt = patient; reverse T = reverse Trendelenburg; side-side rot = side-to-side rotation.
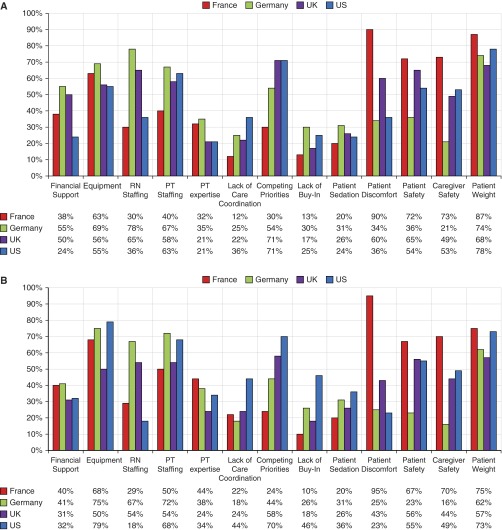
Respondents’ perceived barriers to EM (Figure 6, Table E2) were measured as well. Their commonly cited barriers included equipment, staffing, patient and caregiver safety, and competing priorities. In a post hoc analysis, we stratified barriers by respondent type as well as EM practice in the United Kingdom. In U.K. ICUs with EM protocols, PTs more commonly cited financial support (75% PT vs. 36% nursing; P = 0.063), equipment (75% PT vs. 36% nursing; P = 0.063), and PT staffing (88% PT vs. 43% nursing; P = 0.019) as barriers. In U.K. ICUs with an EM practice but no protocol, PTs more commonly cited PT staffing (66% PT vs. 46% nursing; P = 0.038), competing priorities (79% PT vs. 53% nursing; P = 0.063), and lack of buy-in (24% PTs vs. 0% nursing; P = 0.033). Also, nurses reported more concerns about patient discomfort (55% PT vs. 79% nursing; P = 0.13). Finally, in ICUs without an EM practice, barriers more commonly reported by nursing respondents included concerns about patient weight (45% PT vs. 65% nursing), patient safety (34% PT vs. 70% nursing), and patient discomfort (31% PT vs. 51% nursing). In these ICUs, PTs more commonly reported concerns about financial support (41% PT vs. 23% nursing) and equipment (66% PT vs. 40% nursing).
Figure 6.
Barriers to early mobilization by intensive care units (ICUs) with or without early mobilization (EM) practice. (A) Reported barriers, by country, for ICUs with EM practice. (B) Reported barriers, by country, for ICUs without EM practice. PT = physical therapist or physiotherapist; RN = registered nurse.
Discussion
In this large international survey, adoption of EM practices was reported by 48% of surveyed ICUs. These ICUs have highly variable approaches to intensive care delivery relevant to EM, including staffing structure, standardized practices, and the use of written protocols. On the basis of a simple average of each country’s reported implementation of individual practices, EM ranks as least common among the four processes of rounding, sedation, weaning, and mobilization. This finding is not surprising, given the relative age of the intervention (4–9, 11). Sedation and weaning interventions—particularly those driven by protocol—have undergone trial investigations and been the subject of guideline recommendations for years (26–28). Multidisciplinary rounds are suspected to be one of the primary interventions associated with the movement toward closed ICU models (22, 24). Although compelling randomized trial and quality improvement projects exist to support EM, lagging (self-reported) practice (12–15) likely reflects an implementation gap. Translating an intervention designed in one environment to a broad audience can be problematic.
For example, the international variability of staffing structure was substantial. Most striking is the variance in nurse/patient ratios. In this sample, the extremes are represented by ICUs in the United Kingdom, where 97% reported a 1:1 nurse/patient ratio, in contrast to French ICUs, with 90% reporting 1:3 or higher nurse/patient ratios (including 66% with a ratio of 1:4). Many studies have shown that heavier nursing workloads are associated with poor patient outcomes (29). Specifically, one meta-analysis of 90 studies found that increased registered nurse staffing was associated with lower mortality in intensive care, medical, and surgical units; reduced risk of hospital-acquired pneumonia, unplanned extubation, respiratory failure, cardiac arrest, and failure to rescue; and shorter lengths of stay for both surgical and ICU patients (30).
Similarly, in an observational study of 69 ICUs, a daily plan-of-care review and a lower bed/nurse ratio were both associated with a lower annual ICU mortality (31). It seems plausible to suspect that nurse/patient ratios may contribute to mobilization efficacy, and this is supported in part by our analyses demonstrating significant association between EM practice and nurse/patient ratios. Interestingly, even though nurse/patient ratios were highest in France, the French respondents did not disproportionately report nurse staffing as a barrier. This may demonstrate an inability of clinicians to recognize perceived norms as barriers to the successful implementation of a strategy tested in a distinct research environment.
The approach to physical therapy—or, alternatively, physiotherapy—is simply known (though not, perhaps, by all readers) to be practiced differently internationally (32, 33). In the United States, advanced ventilator care is the domain of the respiratory therapist, and physical therapy is a distinct discipline with certification focused on physical recovery. Advanced knowledge and comfort with MV support by physical (and occupational) therapists has been one of the substantial changes spurred on by EM practice. In contrast, the responsibilities of PTs in Europe commonly overlap both respiratory and physical therapy management.
The barriers to walking the MV patient may be very different, depending on this separation of professional duties. The team is more streamlined with physiotherapists; however, competing respiratory care demands across an ICU may prevent the delivery of such a time-intensive therapy. In the United Kingdom, for example, 78% of PTs noted competing priorities and 73% reported PT staffing as barriers to mobilization in ICUs with EM practice. Therefore, for the majority of patients without ventilator support, the physical therapy model may be most efficient. In multivariable analysis, units with dedicated physical therapy and/or physiotherapy services were 2.4 times more likely to have an EM practice.
The natural variation in ICU structure internationally yielded two other characteristics with a significant association with EM practice: conducting multidisciplinary rounds and setting explicit daily goals for patients. These two features reinforce the necessity of team communication to establish a culture receptive to EM. Managing sedation, delirium, procedures, and safety criteria for daily EM implementation requires deliberate dialogue across specialties. Observational and epidemiological investigations suggest that many potential ICU structural elements do not impact patient outcomes (e.g., nighttime intensivist staffing, daytime intensivist staffing, protocol volume) (34–38). In contrast, multidisciplinary rounds with daily goal communication may be one of the few durable interventions associated with success, spanning mortality and EM (18, 22, 24, 31, 39). This study also demonstrates that a team is necessary, including a dedicated PT and a nurse who are not so burdened by patient needs that daily exercise cannot be prioritized. In environments with lean nursing or therapist staffing, use of a team structure inclusive of an ICU mobility team, as studied previously (6), might be successful.
In general, multicenter collaboratives for research have been structured along geographic lines (e.g., the Canadian Critical Care Trials Group, the ARDS Network in the United States, the Australian and New Zealand Intensive Care Society). Our survey does increase understanding of the logical nature of this regarding ease of communication, feasibility of auditing practice, and, as emphasized here, uniformity of structure. Local dissemination of knowledge might strongly influence practice priorities. For example, German ICUs were associated with EM practice in multivariate analysis. Their success in reported EM implementation may contribute to uptake of EM locally. However, it may also mean that groups interpreting data for guidelines and advocating broad implementation must think constructively about communicating nuanced ICU structure and standardized practice.
Limitations
Our study has some limitations. First, we defined mobilization, but we did not define early. We chose not to define early on the basis of the lack of consensus in the EM literature. As a result, our respondents may have been describing general mobilization practices that may be more easily accomplished than truly early mobilization (within 72 h of presentation). Additionally, the survey included EM practices and protocols in four disparate countries. However, it is likely that even more varied practice is present in ICUs around the world. These differences may be examined in future studies. We did aim to minimize selection bias in the countries that we studied, however, by surveying a large number of randomly chosen and diverse ICUs.
A separate limitation is that the reported data in the survey may not reflect actual practice. This is important to note, given that in recent point prevalence studies done in the United States, Australia and New Zealand, and Germany, actual ambulation of ventilated patients occurred rarely (12–15), thus demonstrating a gap between reported perceived delivery and actual implementation. In addition, social desirability bias may play a role in respondents’ answers. We tried to prevent this by asking neutral questions rather than leading questions and by clearly stating that we were collecting data for research rather than for a different purpose, such as quality metrics. Moreover, respondent bias is possible. In the United Kingdom, about half of respondents were in nursing leadership roles, while the other half were PTs. The responses from the other three countries were almost entirely from individuals in nursing leadership roles. However, in a sensitivity analysis, we did not find significant differences based on respondent discipline in reported practices and protocols that included ambulation of MV patients. Finally, many prior studies have surveyed nursing staff (22, 34, 40).
Conclusions
The data derived from this survey shed light on the inherent tension between trial outcomes and broad implementation. EM, like all practices, probably suffers from an implementation lag. In this survey, we found substantial heterogeneity in reported EM practice. Additionally, some of the most significant factors associated with implementation may not be recognized as easily modifiable by the respondents, and thus may not be reported as barriers. These factors include nurse/patient ratios, physiotherapy staffing, communication practices, and the national culture of critical care. In future dissemination and implementation studies, researchers will need to pay specific attention to the environment in which interventions are being planned.
Footnotes
Supported by National Institutes of Health grant T32 HL007891 and Hill-Rom (Batesville, IN). Hill-Rom provided funding for the survey but did not participate in data analysis or interpretation or in the writing of the manuscript.
Author Contributions: R.N.B.: helped design the study, conducted the study, analyzed and interpreted the data, and drafted and critically revised the manuscript; D.J.W.: helped design the study, analyzed and interpreted the data, and critically revised the manuscript; D.J.M.: helped design the study, interpreted the data, and critically revised the manuscript; V.J.S.: helped design the study, interpreted the data, and critically revised the manuscript; W.D.S.: helped design the study, conducted the study, analyzed and interpreted the data, and drafted and critically revised the manuscript; and R.N.B.: is the guarantor of the content of the manuscript, including the data and analysis. All authors read and approved the final version of the manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Kitson A, Harvey G, McCormack B. Enabling the implementation of evidence based practice: a conceptual framework. Qual Health Care. 1998;7:149–158. doi: 10.1136/qshc.7.3.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Graham ID, Logan J. Innovations in knowledge transfer and continuity of care. Can J Nurs Res. 2004;36:89–103. [PubMed] [Google Scholar]
- 3.Stiller K, Phillips AC, Lambert P. The safety of mobilisation and its effect on haemodynamic and respiratory status of intensive care patients. Physiother Theory Pract. 2004;20:175–185. [Google Scholar]
- 4.Bailey P, Thomsen GE, Spuhler VJ, Blair R, Jewkes J, Bezdjian L, Veale K, Rodriquez L, Hopkins RO. Early activity is feasible and safe in respiratory failure patients. Crit Care Med. 2007;35:139–145. doi: 10.1097/01.CCM.0000251130.69568.87. [DOI] [PubMed] [Google Scholar]
- 5.Thomsen GE, Snow GL, Rodriguez L, Hopkins RO. Patients with respiratory failure increase ambulation after transfer to an intensive care unit where early activity is a priority. Crit Care Med. 2008;36:1119–1124. doi: 10.1097/CCM.0b013e318168f986. [DOI] [PubMed] [Google Scholar]
- 6.Morris PE, Goad A, Thompson C, Taylor K, Harry B, Passmore L, Ross A, Anderson L, Baker S, Sanchez M, et al. Early intensive care unit mobility therapy in the treatment of acute respiratory failure. Crit Care Med. 2008;36:2238–2243. doi: 10.1097/CCM.0b013e318180b90e. [DOI] [PubMed] [Google Scholar]
- 7.Schweickert WD, Pohlman MC, Pohlman AS, Nigos C, Pawlik AJ, Esbrook CL, Spears L, Miller M, Franczyk M, Deprizio D, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373:1874–1882. doi: 10.1016/S0140-6736(09)60658-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Needham DM, Korupolu R, Zanni JM, Pradhan P, Colantuoni E, Palmer JB, Brower RG, Fan E. Early physical medicine and rehabilitation for patients with acute respiratory failure: a quality improvement project. Arch Phys Med Rehabil. 2010;91:536–542. doi: 10.1016/j.apmr.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Burtin C, Clerckx B, Robbeets C, Ferdinande P, Langer D, Troosters T, Hermans G, Decramer M, Gosselink R. Early exercise in critically ill patients enhances short-term functional recovery. Crit Care Med. 2009;37:2499–2505. doi: 10.1097/CCM.0b013e3181a38937. [DOI] [PubMed] [Google Scholar]
- 10.McWilliams D, Weblin J, Atkins G, Bion J, Williams J, Elliott C, Whitehouse T, Snelson C. Enhancing rehabilitation of mechanically ventilated patients in the intensive care unit: a quality improvement project. J Crit Care. 2015;30:13–18. doi: 10.1016/j.jcrc.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 11.Stiller K. Physiotherapy in intensive care: an updated systematic review. Chest. 2013;144:825–847. doi: 10.1378/chest.12-2930. [DOI] [PubMed] [Google Scholar]
- 12.Hodgson C, Bellomo R, Berney S, Bailey M, Buhr H, Denehy L, Harrold M, Higgins A, Presneill J, Saxena M, et al. TEAM Study Investigators. Early mobilization and recovery in mechanically ventilated patients in the ICU: a bi-national, multi-centre, prospective cohort study. Crit Care. 2015;19:81. doi: 10.1186/s13054-015-0765-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berney SC, Harrold M, Webb SA, Seppelt I, Patman S, Thomas PJ, Denehy L. Intensive care unit mobility practices in Australia and New Zealand: a point prevalence study. Crit Care Resusc. 2013;15:260–265. [PubMed] [Google Scholar]
- 14.Nydahl P, Ruhl AP, Bartoszek G, Dubb R, Filipovic S, Flohr HJ, Kaltwasser A, Mende H, Rothaug O, Schuchhardt D, et al. Early mobilization of mechanically ventilated patients: a 1-day point-prevalence study in Germany. Crit Care Med. 2014;42:1178–1186. doi: 10.1097/CCM.0000000000000149. [DOI] [PubMed] [Google Scholar]
- 15.Jolley SE, Dale CR, Hough CL. Hospital-level factors associated with report of physical activity in patients on mechanical ventilation across Washington State. Ann Am Thorac Soc. 2015;12:209–215. doi: 10.1513/AnnalsATS.201410-480OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bakhru RN, McWilliams D, Spuhler V, Schweickert WD. Central nervous system and motor impairment in critical illness [abstract] Am J Respir Crit Care Med. 2014;189:A3933. [Google Scholar]
- 17.Bakhru RN, McWilliams DJ, Spuhler VJ, Wiebe DJ, Schweickert WD.A survey of international practices and infrastructure to support early mobilization [abstract 0066] Intensive Care Med 201440Suppl 1S26–S27. [Google Scholar]
- 18.Bakhru RN, Wiebe DJ, McWilliams DJ, Spuhler VJ, Schweickert WD. An environmental scan for early mobilization practices in U.S. ICUs. Crit Care Med. 2015;43:2360–2369. doi: 10.1097/CCM.0000000000001262. [DOI] [PubMed] [Google Scholar]
- 19.American Hospital Association American Hospital Association regions[accessed 2015 Oct]. Available from: http://www.aha.org/content/00-10/08regionalmap.pdf
- 20.Kelley K, Clark B, Brown V, Sitzia J. Good practice in the conduct and reporting of survey research. Int J Qual Health Care. 2003;15:261–266. doi: 10.1093/intqhc/mzg031. [DOI] [PubMed] [Google Scholar]
- 21.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Epidemiology. 2007;18:800–804. doi: 10.1097/EDE.0b013e3181577654. [DOI] [PubMed] [Google Scholar]
- 22.Kim MM, Barnato AE, Angus DC, Fleisher LA, Kahn JM. The effect of multidisciplinary care teams on intensive care unit mortality. Arch Intern Med. 2010;170:369–376. doi: 10.1001/archinternmed.2009.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prasad M, Christie JD, Bellamy SL, Rubenfeld GD, Kahn JM. The availability of clinical protocols in US teaching intensive care units. J Crit Care. 2010;25:610–619. doi: 10.1016/j.jcrc.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pronovost PJ, Angus DC, Dorman T, Robinson KA, Dremsizov TT, Young TL. Physician staffing patterns and clinical outcomes in critically ill patients: a systematic review. JAMA. 2002;288:2151–2162. doi: 10.1001/jama.288.17.2151. [DOI] [PubMed] [Google Scholar]
- 25.Bassett RD, Vollman KM, Brandwene L, Murray T. Integrating a multidisciplinary mobility programme into intensive care practice (IMMPTP): a multicentre collaborative. Intensive Crit Care Nurs. 2012;28:88–97. doi: 10.1016/j.iccn.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 26.Girard TD, Kress JP, Fuchs BD, Thomason JW, Schweickert WD, Pun BT, Taichman DB, Dunn JG, Pohlman AS, Kinniry PA, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet. 2008;371:126–134. doi: 10.1016/S0140-6736(08)60105-1. [DOI] [PubMed] [Google Scholar]
- 27.Kress JP, Pohlman AS, O’Connor MF, Hall JB. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000;342:1471–1477. doi: 10.1056/NEJM200005183422002. [DOI] [PubMed] [Google Scholar]
- 28.Ely EW, Baker AM, Dunagan DP, Burke HL, Smith AC, Kelly PT, Johnson MM, Browder RW, Bowton DL, Haponik EF. Effect on the duration of mechanical ventilation of identifying patients capable of breathing spontaneously. N Engl J Med. 1996;335:1864–1869. doi: 10.1056/NEJM199612193352502. [DOI] [PubMed] [Google Scholar]
- 29.Neuraz A, Guérin C, Payet C, Polazzi S, Aubrun F, Dailler F, Lehot JJ, Piriou V, Neidecker J, Rimmelé T, et al. Patient mortality is associated with staff resources and workload in the ICU: a multicenter observational study. Crit Care Med. 2015;43:1587–1594. doi: 10.1097/CCM.0000000000001015. [DOI] [PubMed] [Google Scholar]
- 30.Kane RL, Shamliyan TA, Mueller C, Duval S, Wilt TJ. The association of registered nurse staffing levels and patient outcomes: systematic review and meta-analysis. Med Care. 2007;45:1195–1204. doi: 10.1097/MLR.0b013e3181468ca3. [DOI] [PubMed] [Google Scholar]
- 31.Checkley W, Martin GS, Brown SM, Chang SY, Dabbagh O, Fremont RD, Girard TD, Rice TW, Howell MD, Johnson SB, et al. United States Critical Illness and Injury Trials Group Critical Illness Outcomes Study Investigators. Structure, process, and annual ICU mortality across 69 centers: United States Critical Illness and Injury Trials Group Critical Illness Outcomes Study. Crit Care Med. 2014;42:344–356. doi: 10.1097/CCM.0b013e3182a275d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Norrenberg M, Vincent JL European Society of Intensive Care Medicine. A profile of European intensive care unit physiotherapists. Intensive Care Med. 2000;26:988–994. doi: 10.1007/s001340051292. [DOI] [PubMed] [Google Scholar]
- 33.Skinner EH, Haines KJ, Berney S, Warrillow S, Harrold M, Denehy L. Usual care physiotherapy during acute hospitalization in subjects admitted to the ICU: an observational cohort study. Respir Care. 2015;60:1476–1485. doi: 10.4187/respcare.04064. [DOI] [PubMed] [Google Scholar]
- 34.Wallace DJ, Angus DC, Barnato AE, Kramer AA, Kahn JM. Nighttime intensivist staffing and mortality among critically ill patients. N Engl J Med. 2012;366:2093–2101. doi: 10.1056/NEJMsa1201918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kerlin MP, Small DS, Cooney E, Fuchs BD, Bellini LM, Mikkelsen ME, Schweickert WD, Bakhru RN, Gabler NB, Harhay MO, et al. A randomized trial of nighttime physician staffing in an intensive care unit. N Engl J Med. 2013;368:2201–2209. doi: 10.1056/NEJMoa1302854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kerlin MP, Harhay MO, Kahn JM, Halpern SD. Nighttime intensivist staffing, mortality, and limits on life support: a retrospective cohort study. Chest. 2015;147:951–958. doi: 10.1378/chest.14-0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Costa DK, Wallace DJ, Kahn JM. The association between daytime intensivist physician staffing and mortality in the context of other ICU organizational practices: a multicenter cohort study. Crit Care Med. 2015;43:2275–2282. doi: 10.1097/CCM.0000000000001259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sevransky JE, Checkley W, Herrera P, Pickering BW, Barr J, Brown SM, Chang SY, Chong D, Kaufman D, Fremont RD, et al. United States Critical Illness and Injury Trials Group-Critical Illness Outcomes Study Investigators. Protocols and hospital mortality in critically ill patients: the United States Critical Illness and Injury Trials Group Critical Illness Outcomes Study. Crit Care Med. 2015;43:2076–2084. doi: 10.1097/CCM.0000000000001157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pronovost P, Berenholtz S, Dorman T, Lipsett PA, Simmonds T, Haraden C. Improving communication in the ICU using daily goals. J Crit Care. 2003;18:71–75. doi: 10.1053/jcrc.2003.50008. [DOI] [PubMed] [Google Scholar]
- 40.Reineck LA, Wallace DJ, Barnato AE, Kahn JM. Nighttime intensivist staffing and the timing of death among ICU decedents: a retrospective cohort study. Crit Care. 2013;17:R216. doi: 10.1186/cc13033. [DOI] [PMC free article] [PubMed] [Google Scholar]