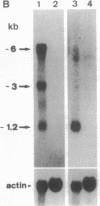
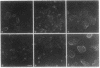
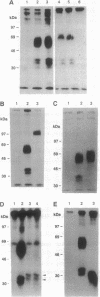
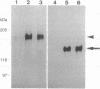
Abstract
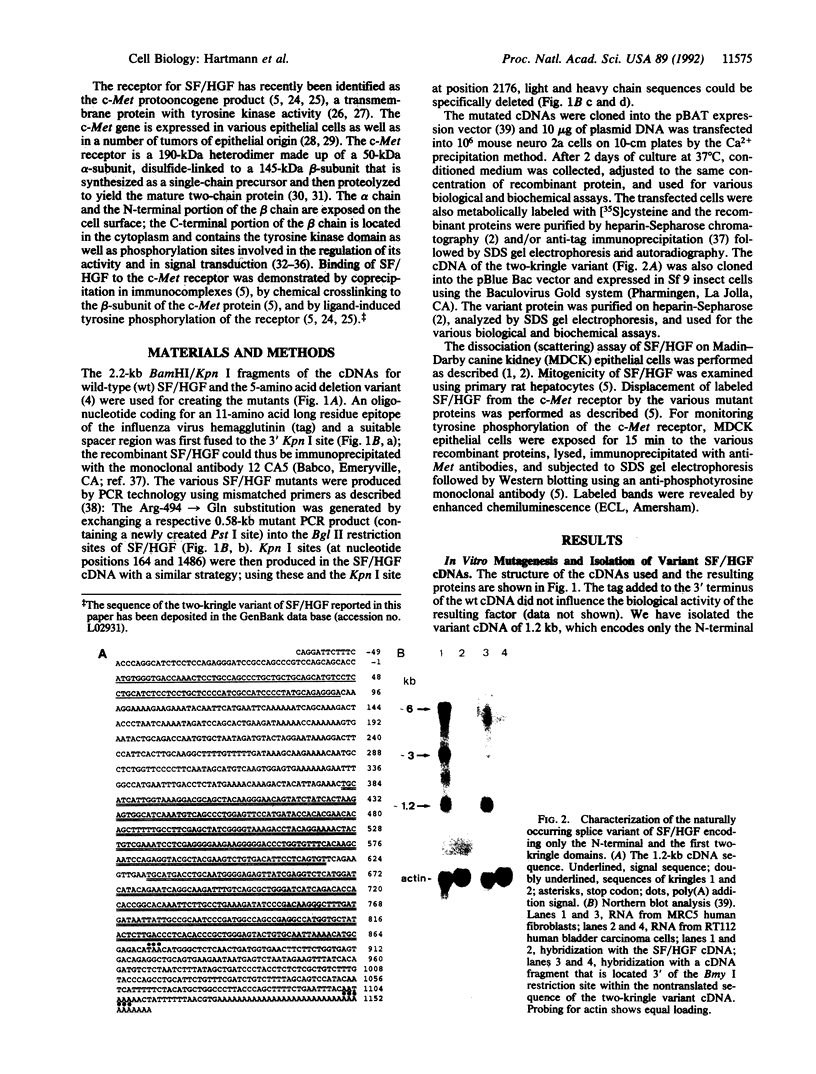
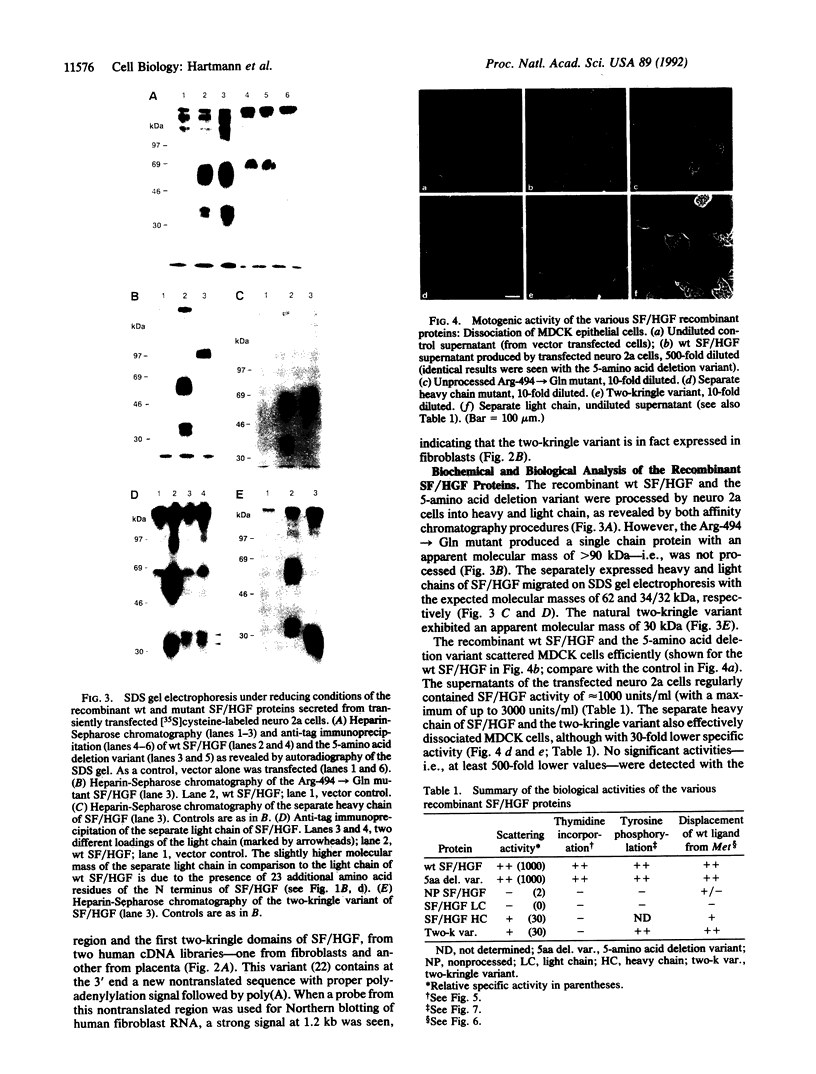
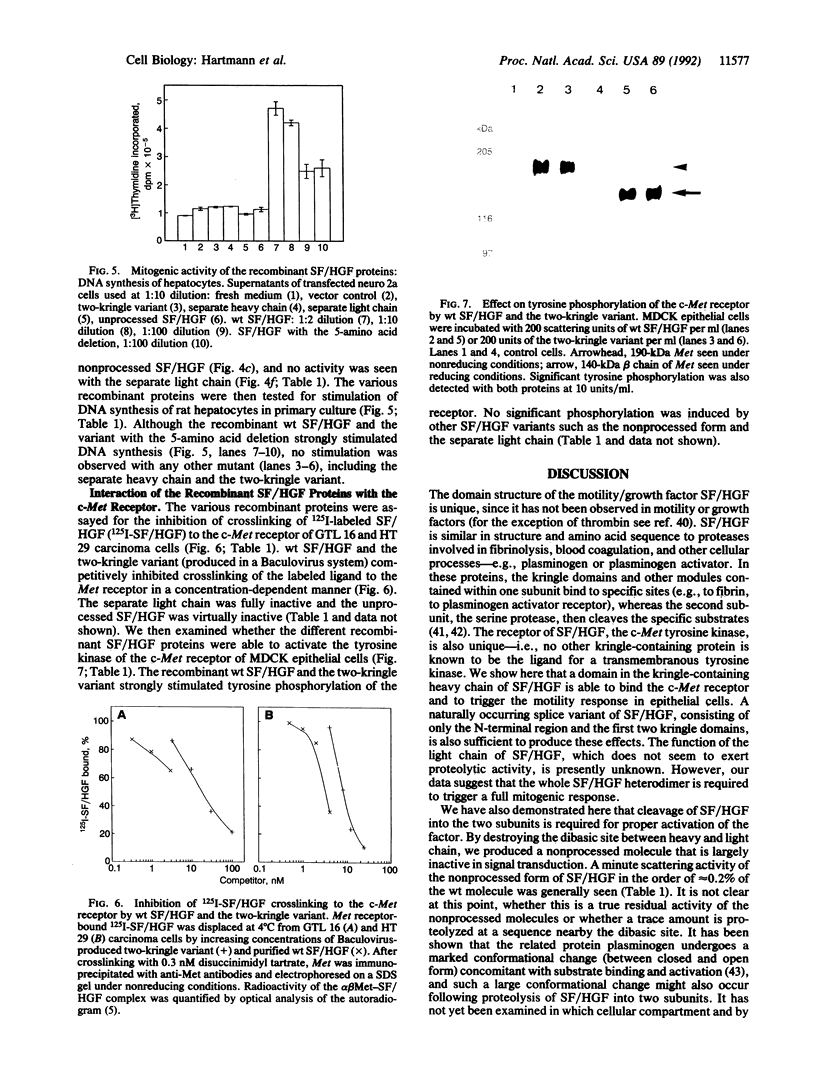
We recently found that scatter factor (SF), a cell motility factor with a multimodular structure, is identical to hepatocyte growth factor (HGF), a potent mitogen of various cell types. SF/HGF is the ligand of the c-Met receptor tyrosine kinase. Here we used transient expression of naturally occurring and in vitro mutagenized cDNAs of SF/HGF to delineate the protein domains necessary for biological activity and binding to the c-Met receptor. (i) A single-chain SF/HGF resulting from the destruction of the protease cleavage site between heavy and light chain (Arg-494--> Gln) was largely inactive, indicating that proteolytic cleavage is essential for acquisition of the biologically active conformation. (ii) A SF/HGF splice variant encoding a protein with a 5-amino acid deletion in the first kringle domain was as highly active as the wild-type molecule. (iii) The separately expressed light chain (with serine protease homology) was inactive in all assays tested. (iv) The separate heavy chain as well as a naturally occurring splice variant consisting of the N terminus and the first two kringle domains bound the c-Met receptor, stimulated tyrosine auto-phosphorylation, and induced scattering of epithelial cells but not mitogenesis. These data indicate that a functional domain in the N terminus/first two kringle regions of SF/HGF is sufficient for binding to the Met receptor and that this leads to the activation of the downstream signal cascade involved in the motility response. However, the complete SF/HGF protein seems to be required for mitogenic activity.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barr P. J. Mammalian subtilisins: the long-sought dibasic processing endoproteases. Cell. 1991 Jul 12;66(1):1–3. doi: 10.1016/0092-8674(91)90129-m. [DOI] [PubMed] [Google Scholar]
- Bottaro D. P., Rubin J. S., Faletto D. L., Chan A. M., Kmiecik T. E., Vande Woude G. F., Aaronson S. A. Identification of the hepatocyte growth factor receptor as the c-met proto-oncogene product. Science. 1991 Feb 15;251(4995):802–804. doi: 10.1126/science.1846706. [DOI] [PubMed] [Google Scholar]
- Chan A. M., Rubin J. S., Bottaro D. P., Hirschfield D. W., Chedid M., Aaronson S. A. Identification of a competitive HGF antagonist encoded by an alternative transcript. Science. 1991 Nov 29;254(5036):1382–1385. doi: 10.1126/science.1720571. [DOI] [PubMed] [Google Scholar]
- Di Renzo M. F., Narsimhan R. P., Olivero M., Bretti S., Giordano S., Medico E., Gaglia P., Zara P., Comoglio P. M. Expression of the Met/HGF receptor in normal and neoplastic human tissues. Oncogene. 1991 Nov;6(11):1997–2003. [PubMed] [Google Scholar]
- Ferracini R., Longati P., Naldini L., Vigna E., Comoglio P. M. Identification of the major autophosphorylation site of the Met/hepatocyte growth factor receptor tyrosine kinase. J Biol Chem. 1991 Oct 15;266(29):19558–19564. [PubMed] [Google Scholar]
- Field J., Nikawa J., Broek D., MacDonald B., Rodgers L., Wilson I. A., Lerner R. A., Wigler M. Purification of a RAS-responsive adenylyl cyclase complex from Saccharomyces cerevisiae by use of an epitope addition method. Mol Cell Biol. 1988 May;8(5):2159–2165. doi: 10.1128/mcb.8.5.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frixen U. H., Behrens J., Sachs M., Eberle G., Voss B., Warda A., Löchner D., Birchmeier W. E-cadherin-mediated cell-cell adhesion prevents invasiveness of human carcinoma cells. J Cell Biol. 1991 Apr;113(1):173–185. doi: 10.1083/jcb.113.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furie B., Furie B. C. The molecular basis of blood coagulation. Cell. 1988 May 20;53(4):505–518. doi: 10.1016/0092-8674(88)90567-3. [DOI] [PubMed] [Google Scholar]
- Gandino L., Di Renzo M. F., Giordano S., Bussolino F., Comoglio P. M. Protein kinase-c activation inhibits tyrosine phosphorylation of the c-met protein. Oncogene. 1990 May;5(5):721–725. [PubMed] [Google Scholar]
- Gandino L., Munaron L., Naldini L., Ferracini R., Magni M., Comoglio P. M. Intracellular calcium regulates the tyrosine kinase receptor encoded by the MET oncogene. J Biol Chem. 1991 Aug 25;266(24):16098–16104. [PubMed] [Google Scholar]
- Giordano S., Di Renzo M. F., Narsimhan R. P., Cooper C. S., Rosa C., Comoglio P. M. Biosynthesis of the protein encoded by the c-met proto-oncogene. Oncogene. 1989 Nov;4(11):1383–1388. [PubMed] [Google Scholar]
- Giordano S., Ponzetto C., Di Renzo M. F., Cooper C. S., Comoglio P. M. Tyrosine kinase receptor indistinguishable from the c-met protein. Nature. 1989 May 11;339(6220):155–156. doi: 10.1038/339155a0. [DOI] [PubMed] [Google Scholar]
- Gonzatti-Haces M., Seth A., Park M., Copeland T., Oroszlan S., Vande Woude G. F. Characterization of the TPR-MET oncogene p65 and the MET protooncogene p140 protein-tyrosine kinases. Proc Natl Acad Sci U S A. 1988 Jan;85(1):21–25. doi: 10.1073/pnas.85.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziani A., Gramaglia D., Cantley L. C., Comoglio P. M. The tyrosine-phosphorylated hepatocyte growth factor/scatter factor receptor associates with phosphatidylinositol 3-kinase. J Biol Chem. 1991 Nov 25;266(33):22087–22090. [PubMed] [Google Scholar]
- Higashio K., Shima N., Goto M., Itagaki Y., Nagao M., Yasuda H., Morinaga T. Identity of a tumor cytotoxic factor from human fibroblasts and hepatocyte growth factor. Biochem Biophys Res Commun. 1990 Jul 16;170(1):397–404. doi: 10.1016/0006-291x(90)91287-3. [DOI] [PubMed] [Google Scholar]
- Isacchi A., Statuto M., Chiesa R., Bergonzoni L., Rusnati M., Sarmientos P., Ragnotti G., Presta M. A six-amino acid deletion in basic fibroblast growth factor dissociates its mitogenic activity from its plasminogen activator-inducing capacity. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2628–2632. doi: 10.1073/pnas.88.7.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangel W. F., Lin B. H., Ramakrishnan V. Characterization of an extremely large, ligand-induced conformational change in plasminogen. Science. 1990 Apr 6;248(4951):69–73. doi: 10.1126/science.2108500. [DOI] [PubMed] [Google Scholar]
- Matsumoto K., Hashimoto K., Yoshikawa K., Nakamura T. Marked stimulation of growth and motility of human keratinocytes by hepatocyte growth factor. Exp Cell Res. 1991 Sep;196(1):114–120. doi: 10.1016/0014-4827(91)90462-4. [DOI] [PubMed] [Google Scholar]
- Matsumoto K., Takehara T., Inoue H., Hagiya M., Shimizu S., Nakamura T. Deletion of kringle domains or the N-terminal hairpin structure in hepatocyte growth factor results in marked decreases in related biological activities. Biochem Biophys Res Commun. 1991 Dec 16;181(2):691–699. doi: 10.1016/0006-291x(91)91246-9. [DOI] [PubMed] [Google Scholar]
- Miyazawa K., Kitamura A., Naka D., Kitamura N. An alternatively processed mRNA generated from human hepatocyte growth factor gene. Eur J Biochem. 1991 Apr 10;197(1):15–22. doi: 10.1111/j.1432-1033.1991.tb15876.x. [DOI] [PubMed] [Google Scholar]
- Miyazawa K., Tsubouchi H., Naka D., Takahashi K., Okigaki M., Arakaki N., Nakayama H., Hirono S., Sakiyama O., Takahashi K. Molecular cloning and sequence analysis of cDNA for human hepatocyte growth factor. Biochem Biophys Res Commun. 1989 Sep 15;163(2):967–973. doi: 10.1016/0006-291x(89)92316-4. [DOI] [PubMed] [Google Scholar]
- Montesano R., Matsumoto K., Nakamura T., Orci L. Identification of a fibroblast-derived epithelial morphogen as hepatocyte growth factor. Cell. 1991 Nov 29;67(5):901–908. doi: 10.1016/0092-8674(91)90363-4. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Nishizawa T., Hagiya M., Seki T., Shimonishi M., Sugimura A., Tashiro K., Shimizu S. Molecular cloning and expression of human hepatocyte growth factor. Nature. 1989 Nov 23;342(6248):440–443. doi: 10.1038/342440a0. [DOI] [PubMed] [Google Scholar]
- Naldini L., Vigna E., Ferracini R., Longati P., Gandino L., Prat M., Comoglio P. M. The tyrosine kinase encoded by the MET proto-oncogene is activated by autophosphorylation. Mol Cell Biol. 1991 Apr;11(4):1793–1803. doi: 10.1128/mcb.11.4.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naldini L., Vigna E., Narsimhan R. P., Gaudino G., Zarnegar R., Michalopoulos G. K., Comoglio P. M. Hepatocyte growth factor (HGF) stimulates the tyrosine kinase activity of the receptor encoded by the proto-oncogene c-MET. Oncogene. 1991 Apr;6(4):501–504. [PubMed] [Google Scholar]
- Naldini L., Weidner K. M., Vigna E., Gaudino G., Bardelli A., Ponzetto C., Narsimhan R. P., Hartmann G., Zarnegar R., Michalopoulos G. K. Scatter factor and hepatocyte growth factor are indistinguishable ligands for the MET receptor. EMBO J. 1991 Oct;10(10):2867–2878. doi: 10.1002/j.1460-2075.1991.tb07836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nistér M., Hammacher A., Mellström K., Siegbahn A., Rönnstrand L., Westermark B., Heldin C. H. A glioma-derived PDGF A chain homodimer has different functional activities from a PDGF AB heterodimer purified from human platelets. Cell. 1988 Mar 25;52(6):791–799. doi: 10.1016/0092-8674(88)90421-7. [DOI] [PubMed] [Google Scholar]
- Noji S., Tashiro K., Koyama E., Nohno T., Ohyama K., Taniguchi S., Nakamura T. Expression of hepatocyte growth factor gene in endothelial and Kupffer cells of damaged rat livers, as revealed by in situ hybridization. Biochem Biophys Res Commun. 1990 Nov 30;173(1):42–47. doi: 10.1016/s0006-291x(05)81018-6. [DOI] [PubMed] [Google Scholar]
- Park M., Dean M., Kaul K., Braun M. J., Gonda M. A., Vande Woude G. Sequence of MET protooncogene cDNA has features characteristic of the tyrosine kinase family of growth-factor receptors. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6379–6383. doi: 10.1073/pnas.84.18.6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prat M., Narsimhan R. P., Crepaldi T., Nicotra M. R., Natali P. G., Comoglio P. M. The receptor encoded by the human c-MET oncogene is expressed in hepatocytes, epithelial cells and solid tumors. Int J Cancer. 1991 Sep 30;49(3):323–328. doi: 10.1002/ijc.2910490302. [DOI] [PubMed] [Google Scholar]
- Rosen E. M., Goldberg I. D., Kacinski B. M., Buckholz T., Vinter D. W. Smooth muscle releases an epithelial cell scatter factor which binds to heparin. In Vitro Cell Dev Biol. 1989 Feb;25(2):163–173. doi: 10.1007/BF02626174. [DOI] [PubMed] [Google Scholar]
- Rubin J. S., Chan A. M., Bottaro D. P., Burgess W. H., Taylor W. G., Cech A. C., Hirschfield D. W., Wong J., Miki T., Finch P. W. A broad-spectrum human lung fibroblast-derived mitogen is a variant of hepatocyte growth factor. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):415–419. doi: 10.1073/pnas.88.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki T., Ihara I., Sugimura A., Shimonishi M., Nishizawa T., Asami O., Hagiya M., Nakamura T., Shimizu S. Isolation and expression of cDNA for different forms of hepatocyte growth factor from human leukocyte. Biochem Biophys Res Commun. 1990 Oct 15;172(1):321–327. doi: 10.1016/s0006-291x(05)80212-8. [DOI] [PubMed] [Google Scholar]
- Shiota G., Rhoads D. B., Wang T. C., Nakamura T., Schmidt E. V. Hepatocyte growth factor inhibits growth of hepatocellular carcinoma cells. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):373–377. doi: 10.1073/pnas.89.1.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern C. D., Ireland G. W., Herrick S. E., Gherardi E., Gray J., Perryman M., Stoker M. Epithelial scatter factor and development of the chick embryonic axis. Development. 1990 Dec;110(4):1271–1284. doi: 10.1242/dev.110.4.1271. [DOI] [PubMed] [Google Scholar]
- Tajima H., Matsumoto K., Nakamura T. Hepatocyte growth factor has potent anti-proliferative activity in various tumor cell lines. FEBS Lett. 1991 Oct 21;291(2):229–232. doi: 10.1016/0014-5793(91)81291-f. [DOI] [PubMed] [Google Scholar]
- Tashiro K., Hagiya M., Nishizawa T., Seki T., Shimonishi M., Shimizu S., Nakamura T. Deduced primary structure of rat hepatocyte growth factor and expression of the mRNA in rat tissues. Proc Natl Acad Sci U S A. 1990 Apr;87(8):3200–3204. doi: 10.1073/pnas.87.8.3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsubouchi H., Niitani Y., Hirono S., Nakayama H., Gohda E., Arakaki N., Sakiyama O., Takahashi K., Kimoto M., Kawakami S. Levels of the human hepatocyte growth factor in serum of patients with various liver diseases determined by an enzyme-linked immunosorbent assay. Hepatology. 1991 Jan;13(1):1–5. [PubMed] [Google Scholar]
- Vu T. K., Hung D. T., Wheaton V. I., Coughlin S. R. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991 Mar 22;64(6):1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- Weidner K. M., Arakaki N., Hartmann G., Vandekerckhove J., Weingart S., Rieder H., Fonatsch C., Tsubouchi H., Hishida T., Daikuhara Y. Evidence for the identity of human scatter factor and human hepatocyte growth factor. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7001–7005. doi: 10.1073/pnas.88.16.7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidner K. M., Behrens J., Vandekerckhove J., Birchmeier W. Scatter factor: molecular characteristics and effect on the invasiveness of epithelial cells. J Cell Biol. 1990 Nov;111(5 Pt 1):2097–2108. doi: 10.1083/jcb.111.5.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarden Y., Schreiber A. B., Schlessinger J. A nonmitogenic analogue of epidermal growth factor induces early responses mediated by epidermal growth factor. J Cell Biol. 1982 Mar;92(3):687–693. doi: 10.1083/jcb.92.3.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiyama Y., Arakaki N., Naka D., Takahashi K., Hirono S., Kondo J., Nakayama H., Gohda E., Kitamura N., Tsubouchi H. Identification of the N-terminal residue of the heavy chain of both native and recombinant human hepatocyte growth factor. Biochem Biophys Res Commun. 1991 Mar 15;175(2):660–667. doi: 10.1016/0006-291x(91)91616-k. [DOI] [PubMed] [Google Scholar]
- Zarnegar R., Michalopoulos G. Purification and biological characterization of human hepatopoietin A, a polypeptide growth factor for hepatocytes. Cancer Res. 1989 Jun 15;49(12):3314–3320. [PubMed] [Google Scholar]
- van Zonneveld A. J., Veerman H., Pannekoek H. Autonomous functions of structural domains on human tissue-type plasminogen activator. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4670–4674. doi: 10.1073/pnas.83.13.4670. [DOI] [PMC free article] [PubMed] [Google Scholar]