Abstract
A detailed investigation of nanostructured iron oxides/(oxy)hydroxides gathered after cultivation of bacteria from the genus Leptothrix as iron (II) oxidizers is presented. A specific type of medium is selected for the cultivation of the bacteria. Results for sediment powder and bio-film on glass substrate samples from the same media are discussed. XRD, Raman spectroscopy, SEM, and TEM images and PPMS measurements are used to prove the exact composition of the biogenic products and to interpret the oxidation process. Analysis of the data collected shows that around 80 % of the iron (II) from the growth medium has been transformed into iron (III) in the form of different (oxy)hydroxides, with the rest found to be in a mixed 2,5 valence in magnetite. Our investigation shows that the bio-film sample has a phase content different from that of the powdered biomass and that lepidocrocite (γ-FeOOH) is the predominant and the initial biogenic phase in both samples. Magnetite nanoparticles are a secondary product in the bio-film, part of which possesses a defective quasi-maghemite surface layer. In the powdered biomass, the oxidation steps are not fully completed. The initial products are non-stoichiometric and due to the mixed ferric and ferrous ions present, they develop into: (i) lepidocrocite (γ-FeOOH) as a basic sediment, (ii) magnetite (Fe3O4) and (iii) goethite (α-FeOOH) in small quantities. The average size of all iron-bearing particles is found to be below 30 nm. The magnetic measurements performed show a superparamagnetic behavior of the material at room temperature.
Keywords: Laboratory cultivated Leptothrix sp., Nanosized biogenic iron oxides/(oxy)hydroxides; Bio-coatings; Lepidocrocite (ɣ-FeOOH); Magnetite (Fe3O4); Goethite (α-FeOOH)
Introduction
A large part of Earth’s sedimentary iron deposits can be attributed directly or indirectly to microbial activity [1]. Microorganisms that are able to oxidize Fe (II) and form Fe (III) by-products are diverse in their morphology and overall physiology. Iron (II) oxidation at near neutral pH has long been thought to occur predominantly abiotically [2] and it still remains difficult to differentiate clearly between true biogenic iron-containing by-products and those formed as a result of abiotic processes [3]. One type of microorganism competing with the kinetically more favorable abiotic Fe (II) oxidation is the Sphaerotilus-Leptothrix group of bacteria. The abiotic rate of oxidation of Fe (II) in fully oxygenated water is rapid, which requires that most of the iron-oxidizing bacteria (FeOB) grow under microaerobic conditions [4, 5], although Leptothrix can grow under many different O2 conditions [6]. Under such conditions, the Beta-proteobacteria, capable of oxidizing not only Fe (II) but also Mn (II) at neutral pH, can accelerate the rate of iron oxidation by nearly a factor of three [5]. Numerous experiments have indicated that biological iron oxidation can contribute to more than 40% of the net rate of iron oxidation [5, 7, 8]. FeOB can be found in many different natural habitats, on the interface of oxic/anoxic zones where oxygen concentrations are optimal for their survival [9]. Microaerophilic FeOB belong to the group of sheathed bacteria and are distinguishable by their specific morphology. As a result of bacteria-mediated iron oxidation, red/orange microbial mats are formed, which comprise iron (oxy)hydroxides in a polysaccharide matrix in the specific form of tubular sheaths, with the exact composition of the sheaths yet to be fully determined. The question about the function of iron oxidation is still open for debate due to the lack of sufficient information and difficulties with the cultivation of isolates under laboratory conditions. Traditionally, it has been assumed that bacteria cannot utilize energy from the oxidation of iron (II) and the purpose of such a reaction is most probably connected with detoxification of harmful oxygen species, like peroxide [10, 11].
Considerable controversy also exists regarding their taxonomic affiliation, the kinetics of the abiotic polymerization and oxidation in the precipitated biomass after the bioprocess, the reason for the formation of sheaths, etc. The cultivation of FeOB that produces iron bioproducts under laboratory conditions would be a significant step in clarifying these processes. With regards to oxides, the development of conventional nanotechnologies is about to reach its limit. Our investigation is motivated by the fact that the possibilities of biological processes in this respect are yet to be fully comprehended, while the perfection that nature has achieved during billions of years of natural evolution is indisputable. On the other hand, the nanosized iron oxides/(oxy)hydroxides have been finding ever-wider applications in medicine. One of the basic problems in this respect is their biocompatibility and what, if not the natural product, could be more useful in this respect?
This paper is a continuation of previous works [12, 13] on obtaining and investigating biogenic powders containing nanostructured iron oxides/(oxy)hydroxides formed by Leptothrix bacteria. Our interest is with deepening the studies on the morphology and structure of iron oxides/(oxy)hydroxides that are products of the bacteria’s metabolism and, in particular, products that pass through nanosized fractions during the process of their formation.
Isolates from a natural source, which were identified as members of genus Leptothrix and Adler’s medium (AM) [14] as a growth medium, were used for cultivation of the bacteria under laboratory conditions. The current work’s focus is on acquiring new data for the biogenic material obtained from such a medium, based on investigations of powdered sediments and coatings on glass samples, further referred to as bio-film. XRD, Raman spectroscopy, SEM and TEM images, and PPMS (physical properties measurement system) measurements were used to prove the exact compositions of the bio sub-products and to interpret the oxidation process.
Materials and methods
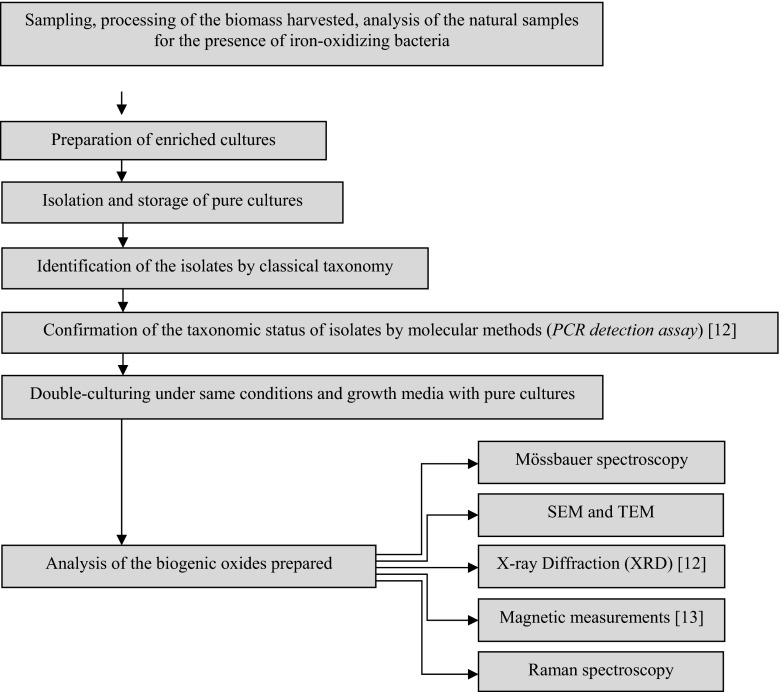
According to the specific environmental requirements of the targeted group of microorganisms, a habitat located in Vitosha Mountain (Bulgaria) was chosen as the main area of sampling. This is a large area with a well-defined sub-alpine zone, covered by shrubs, some conifers, and grass. The altitude above sea level is 1783 m with coordinates 42°35′15″N/23°14′55″E. The average temperatures at this altitude are relatively low (the average monthly temperature for January is −4 °C and in June, +13 °C). The region is characterized by a large number of small water sources, such as streams, springs, and swamps. The initial processing of the biomass harvested consisted in homogenization and filtration to remove various coarse particles (mud, plant matter, etc.). The procedure for sample preparation under laboratory conditions is outlined in Fig. 1.
Fig. 1.
Schematic illustration of the preparation and characterization procedures of the investigated material
The initial investigation, performed by light and scanning electron microscopy imaging of the biomass collected from the sampling area, showed the presence and morphology typical for FeOB with numerous tubular sheaths, as seen in Fig. 2.
Fig. 2.
Light microscopy image of tubular structures of a sample from the sampling area
Isolation of pure cultures was performed from enriched cultures obtained on solid media after dilutions according to classical procedures. The identification of isolates by classical taxonomy and the molecular methods used (PCR detection assay) have been described in detail previously [12].
To mimic the natural conditions for the development of the iron bacteria targeted, we used two types of cultivation vessels—Fernbach and Roux flasks. The specific shape of the selected vessels provides a high volume and surface for aeration of the culture medium in order to allow the bacteria to grow. The flasks with modified Adler medium [12, 15] were inoculated (10% v/v) with suspension of pure culture of Leptothrix sp. and cultivated at a temperature of 20 °C. The pH was maintained at 7.0. The growth medium used for propagation and selective cultivation of the iron bacteria was modified Adler’s medium (AM), with exact composition described in detail in [15]. The iron source was 1% of the volume of the growth medium. The iron source for AM was ammonium iron (II) sulfate ((NH4)2Fe(SO4)2·6H2O) and iron cuttings.
Two types of samples were studied. They were prepared from material belonging to flasks with pure culture of the genus Leptothrix, grown in identical media: (i) sedimented powder sample and (ii) bio-film deposited on glass substrate. The powder sample was obtained from a bacterial biomass, which after 40 days of cultivation was filtered by paper filters and dried at room temperature. The glass sample was prepared again after 40 days of cultivation of the targeted bacteria. The bio-film was prepared directly from the liquid medium by three drop-wise depositions of collected biomass and drying at room temperature afterwards. Apart from these procedures for sample preparation, no additional purification or chemical modification was performed. None of the initial biomass collected from nature was part of the samples under investigation and did not interfere with the measurements.
Powder X-ray diffraction patterns were collected within the range from 10 to 80° 2θ with a constant step of 0.02° 2θ on a Bruker D8 Advance diffractometer with Cu-Kα radiation and LynxEye detector. Phase identification was performed with the Diffracplus EVAusing ICDD-PDF2 Database. The electron microscope JEOL JSM-5510LV (JEOL, JAPAN) with a tungsten filament operated between 0.5 and 30 kV and commercial software provided by the producer were used for SEM image manipulations. The specimens were prepared by dropping the growth medium after cultivation onto cover glasses for light microscopy and drying. Transmission electron microscopy images were obtained with a TEM JEOL 2100 operated at 200-kV accelerating voltage. The specimens were dispersed in ethanol by ultrasonic treatment for 5 min and the suspensions were dripped on standard holey carbon/Cu grids. The glass slide sample was examined by Raman micro-spectroscopy using a LabRAM HR visible single spectrometer equipped with a microscope and a Peltier-cooled CCD detector. The 633-nm He-Ne laser line was used for excitation. The Raman measurements were performed at room temperature. The magnetic properties were measured on a physical properties measurement system (PPMS, Quantum Design).
Results and discussion
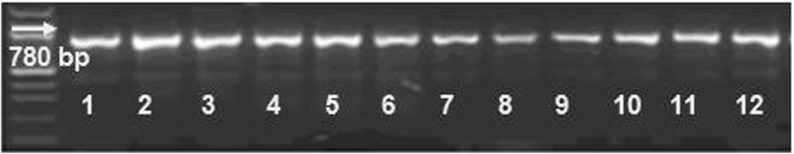
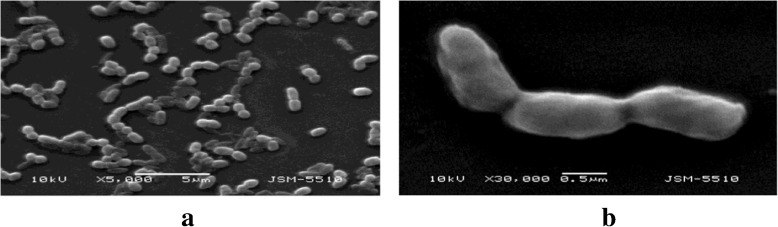
The methods of classical taxonomy applied confirmed that the pure cultures isolated belong to the genus Leptothrix. This was confirmed by a PCR-assay achieved with specific primers for genus Leptothrix (Fig. 3). The growth medium used is suitable for obtaining biogenic iron oxides, using neutrophilic iron bacteria belonging to the genus Leptothrix. Experiments with different growth media have also been described elsewhere [16]. Choosing the modified Adler medium was a decision based on assessing the results from the quantity of biomass obtained, in connection with the growth time in days. In order to achieve any type of application, the quantities of iron-bearing material obtained biotically have to compete with those obtained inorganically. Observing the quantities produced, it was also concluded that the Fernbach flasks provide better conditions for FeOB’s growth than the Roux flasks. Also, our previous investigation [12] showed that the use of Fernbach flasks is favorable from the point of view of biomass productivity. Thus, the physical characterization performed was concentrated on samples from Fernbach flasks. The typical morphology of the cells was confirmed by optical and SEM imaging. Figure 4 shows SEM images of FeOB cultured in Fernbach flasks on Adler medium. The typical cell’s morphology can be seen with a shape characteristic of the genus Leptothrix.
Fig. 3.
Amplification profile of the mofA gene – PCR. Visualization of the sizes of the amplified fragments on 3 % agarose gel
Fig. 4.
SEM of bacteria of the genus Leptothrix cultivated in Adler medium: a panoramic view; b typical cell shape with a rough surface
Under laboratory conditions, the growth of bacteria takes at least 7 days. However, none of the samples investigated contained well-formed, typical Leptothrix sp. sheaths, as revealed by SEM and light microscopy observations, even under longer periods of cultivation. This confirmed some previous investigations, which concluded that these bacteria, in most cases, lose their ability to form iron-containing sheaths when cultured in vitro [17, 18]. These bacteria bind Fe (III) oxides, such as ferric oxyhydroxide (FeOOH), through active enzymatic or passive sorption of dissolved Fe (II) to negatively charged cell walls or sheaths [19–23] through the representative oxidation reaction:
An electron is generated during biogenic oxidation of aqueous-phase Fe (II) to Fe (III) and has long been thought to be essential for autotrophic or mixotrophic metabolism in Leptothrix as an energy source [24–29]. The Fe-oxidizing protein of L. discophora SS-1has been reported to be excreted from bacterial cells in association with exopolymers [30–32]. A ∼150-kDa Fe-oxidizing protein was identified in concentrated spent cultures of L. discophora SS-1 [25]. This metal-oxidizing protein (corresponding to the enzyme) is considered to contribute to oxidation of Fe (II) and be associated with formation of the Leptothrix sheath and its metal encrustation [25, 26, 30, 31, 33].
The sheath material of Leptothrix contains organics such as polysaccharides, proteins, and lipids whose active groups are expected to play critical roles to bind aqueous-phase cations [34–36]. Chan et al. [24] showed a strong correlation between the presences of acidic polysaccharides with carboxyl functional groups to the distribution of iron oxyhydroxides in the Leptothrix sheath. The encrustation of inorganics in sheaths is most likely the result of biotic metal-oxidation and any associated reactions are presumably concerned with energy metabolism in Leptothrix cells [5]. Although careful approaches to study the interactions between bacterial exopolymers and metal ions in aqua-environments have been promoted the understanding of this matter i.e., how Fe-oxidizing factors are structurally associated with complex organic/inorganic composites such as Leptothrix sheaths, still remains to be elucidated [37]. The growth medium used did not lead to the formation of well-formed sheaths but we were able to prepare sufficient quantities of biomass with ochre coloring, obviously containing various types of iron oxides/(oxy) hydroxides.
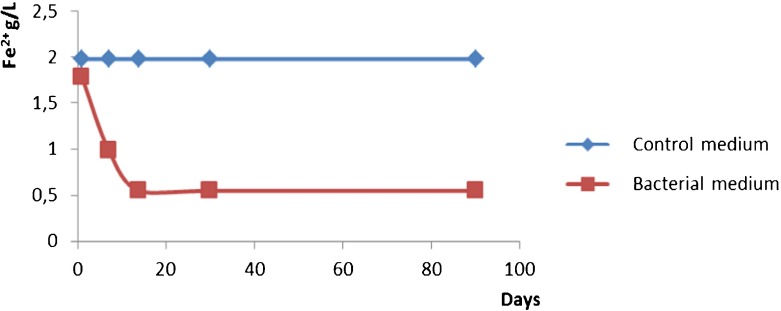
In previous work [12], the elemental composition especially for Fe content was analyzed by applying the neutron activation analysis (NAA) technique. The main objective was to explore the possibilities of the biotechnology approach for obtaining large quantities of iron by-products in laboratory conditions. The reported NAA results for Adler’s medium illustrate the changes in the content of iron in products from laboratory conditions and the natural environment. The data clearly indicated a strong increase in the iron content (both Fe (II) and Fe (III)) in laboratory conditions, which depends weakly on the time of cultivation. At the same time, probably due to better conditions when culturing in the laboratory, the Fe (II) oxidation is rapid in the first weeks, which leads to almost all the iron content to be in the form of Fe (III) compounds. The Fe (II) percentage in the bacterial sample is a good indicator for the bio-oxidation process and during the investigation the dynamics of iron oxidation was explored. Figure 5 confirms the decreases of the Fe (II) due to the bioprocess.
Fig. 5.
Dynamics of the oxidation of Fe (II) during cultivation of Leptothrix bacteria in Adler medium and the same dynamics in a control sample without bacteria
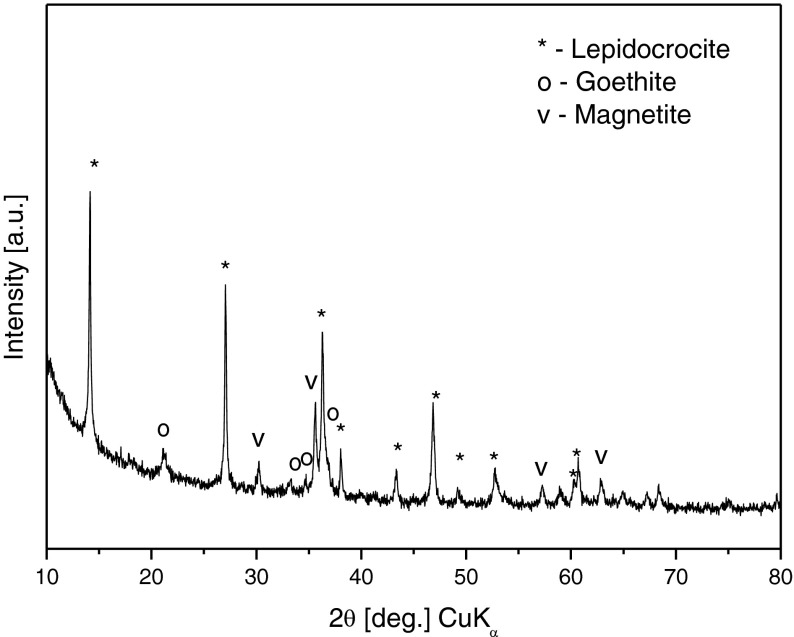
The material obtained after the cultivation of the bacteria, in the form of filtered and dried powder sample, was also characterized by XRD in our work [12] (Fig. 6) and the analysis of the spectra revealed that the phase composition was mixed: lepidocrocite (γ-FeOOH ) – 60%; magnetite (Fe3O4) – 22% and goethite (α-FeOOH) – 18%.
Fig. 6.
XRD phase analysis of AM powder sample
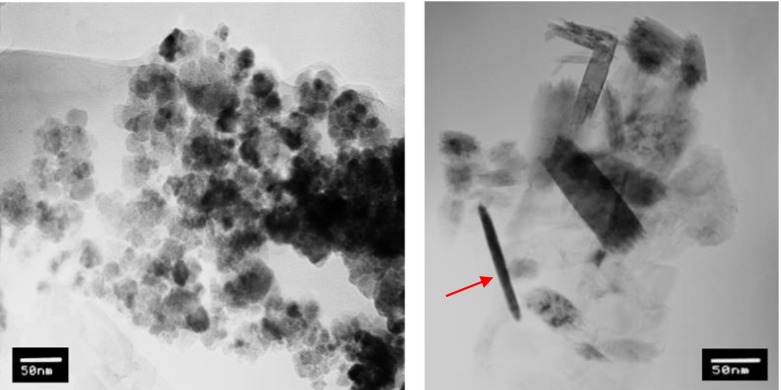
The TEM investigation of the sedimented and filtered powder sample from Adler medium showed spherical, nanosized particles with an average diameter of 10 to 30 nm, which were identified as magnetite (Fig. 7a). In Fig. 7b, one can see long lath-shaped particles typical for lepidocrocite with diameters ranging from 10 to 20 nm and lengths from 20 to 50 nm and also needle-like particles typical for goethite (indicated by an arrow). Thus, the TEM investigation confirmed the results for a three-phase system in the AM sample, as pointed out by XRD analysis.
Fig. 7.
TEM images of AM sample. a Particles with a spherical shape identified as magnetite and b lath-shaped particles of lepidocrocite and needle-like-shaped particles of (oxy)hydroxides, with the arrow indicating a typical goethite shape
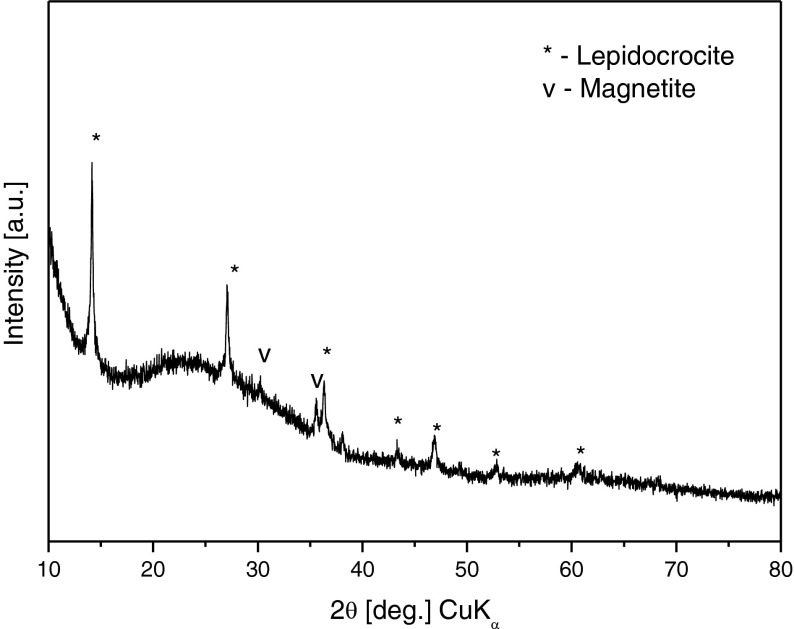
The XRD studies of the bio-film showed a phase content different from that of the powdered biomass. The predominant phase was again that of lepidocrocite, with negligible amounts of magnetite and traces of maghemite (Fig. 8). The phase content study of the bio-film deposited from Adler medium on the glass substrate revealed unambiguously the absence of goethite, in contrast with the presence of the latter in the powder sample.
Fig. 8.
XRD phase analysis of AM bio-film on a glass substrate
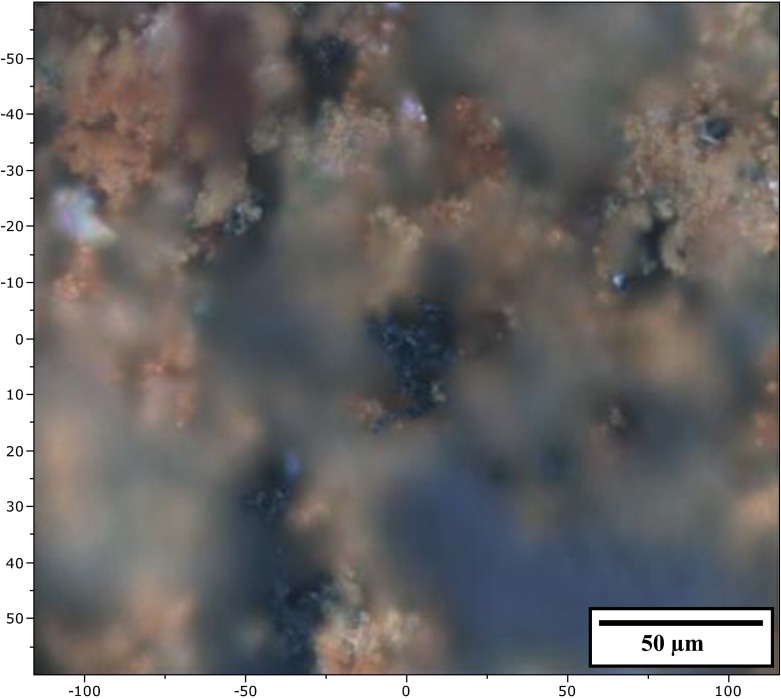
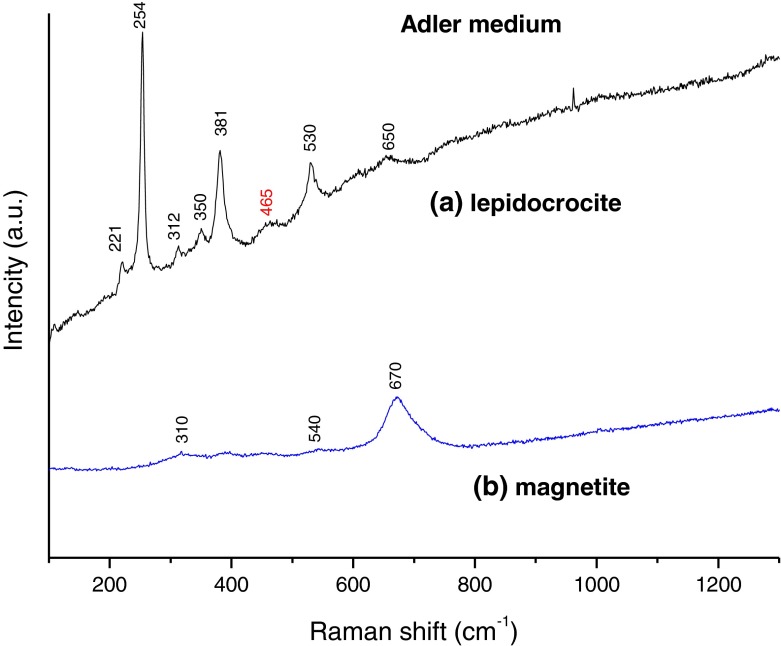
The bio-film was also examined by Raman spectroscopy at room temperature. Figure 9 presents an optical microscope photograph of spots, where the laser beam was focused (at x = 0 and y = 0) during the measurements.
Fig. 9.
Light microscope photograph of biogenic material deposited on a glass slide after cultivation of bacteria in Adler medium
The Raman spectra of the material are shown in Fig. 10. Table 1 summarizes the corresponding iron by-products detected and their positions in the Raman signal. We have to note that a strong luminescence signal was present during the measurements arising from all samples containing iron oxides/(oxy) hydroxides (other samples not shown), which greatly impeded our investigation. It stretched over the whole spectral range (100 to 3400 cm−1) of the spectra obtained. Such a luminescence was not observed in samples that did not contain iron compounds, or from the glass slide without a sample.
Fig. 10.
Raman spectra of Adler medium biogenic material deposited on a glass slide
Table 1.
Raman peak positions detected in bio-film sample
| Culture media (sample) | Raman peak positions detected in sample* (cm−1) | Assigned iron oxides/hydroxides** |
|---|---|---|
| Adler medium (AM) | 221, 254, 312, 350, 381,465, 530, 650 (Fig. 10a) |
lepidocrocite (γ-FeOOH) |
|
310, 540, 670
(Fig. 10b) |
magnetite (Fe3O4) |
*The prominent peak positions in the Raman signals detected are shown in bold
**The assignment of spectra is on the basis of literature data [38–44]. Since, to the best of our knowledge, Raman studies on biogenic material have not been performed before, the comparison was made with data for natural and synthetic minerals, corrosion products, etc
The spectral features found in the bio-film sample (Fig. 10a) match those of lepidocrocite (γ-FeO(OH)), with good crystallinity of the (oxy)hydroxide. All peaks in the spectra are narrow with a characteristic relative intensity. This confirms the XRD data for this sample. Examination of the photograph in Fig. 9 reveals areas of black color appearing in some places. These areas are surrounded by the reddish-brown color of the lepidocrocite. The results of analyzing this type of places are shown in Fig. 10b. The single broadband at 670 cm−1 is a well-known feature of magnetite (Fe3O4). Magnetite’s other two very weak bands at 310 and 530 cm−1 can also be found in the spectra shown. Magnetite is a ferrimagnetic material with iron in a mixed valence state. Its structural formula can be written as (Fe3+)A[Fe2+Fe3+]BO4, where ‘A’ and ‘B’ are magnetite’s tetrahedral and octahedral sub-lattices. Although this oxide is well known to be connected to different types of bacterial activity [45, 46], it is mainly a product of Fe (III) reduction by dissimilatory iron-reducing bacteria [47] and has never been found in FeOB by-products. The presence of traces of maghemite in the XRD spectrum can, therefore, be explained based on the results of our earlier studies [48, 49], where this was determined as being an oxidized surface layer with a maghemite-like structure characteristic for magnetite nanoparticles. The oxidation could be illustrated with the general formula (Fe3+)A[Fe3+ 5xFe2.5+ 2-6x □x]BO4 where □ is a ‘vacancy’ in the octahedral sub-lattice that increases up to the surface and x = 0 up to 0.3.
The appearance of magnetite in both AM samples to the amount of around 22% (as calculated from the XRD spectra) of the entire iron-bearing mass does not have a straightforward explanation. One possible reason for its formation can be deduced from a small very weak spectral feature found in Fig. 10a, which is not, by any means, characteristic for lepidocrocite. The band at around 460 cm−1 is a fingerprint for only one iron-bearing compound, namely, ferrous hydroxide (Fe(OH)2). It cannot be ruled out that these small traces of Fe(OH)2 are of biogenic origin, because the mechanism of the reactions involving the hydrogen peroxide (H2O2) derived from the Leptothrix sp. metabolism and the nutrition present are not fully understood, especially on the interface bacterial wall/iron source. Other possibilities include a reaction of the salts in the medium and water molecules, or abiotic adsorption of Fe (II) onto biogenic surfaces. In our opinion, hydroxide is most probably formed by a light-induced reduction of Fe (III) belonging to the lepidocrocite during the short period of preparing the glass slide.
A proof for this hypothesis can be found in the optical microscope photograph of the glass slide (Fig. 9). It can be seen that only some tips elevated above the biomass level are colored differently from reddish-brown. Also, the 460-cm−1 peak is only present in the lepidocrocite spectra.
Such small areas with concentrated Fe(OH)2 can act as centers for nucleation of magnetite. It is more thermodynamically stable than hydroxide and most probably it evolves via the scheme described by Herane et al. [50]. As they summarized, the formation of magnetite passes through an intermediate phase—green rust, which is unstable in air. We assume that the process Fe(OH)2 → Fe3O4 takes place in the upper layers of the bio-film and, as was mentioned before, the magnetite particles are fractionally oxidized to a maghemite-like structure afterwards.
No Raman peak positions of goethite were detected in the bio-film on glass substrate, although the XRD measurement showed that it was present in the powder sample, with nearly 19% of the volume. Lepidocrocite and goethite are both polymorphous forms of the iron oxy-hydroxide FeO(OH) and have the same chemical formula. Their different crystalline structures make them distinct minerals. Oxy-hydroxide formation and transformation kinetics are still poorly understood. Small changes in the conditions, such as pH and temperature, may lead to significant variability in the resulting products, because of the high dynamic surface state of the oxy-hydroxide in aqueous solutions [50]. Most probably, the by-products in the powder sample are non-stoichiometric and, due to the high reactivity and depending on the exposure conditions, intermediate products as well as magnetite and goethite can be formed.
The glass slide is coated by a mixture of iron-bearing compounds contained in a fluid, in which there are not only water molecules but also organics from the bacteria. Then, it is only in the short period of preparing the glass slide when a transformation of the iron-bearing compounds cut take place. After drying the bio-film, the iron oxides/(oxy)hydroxides are sealed in a type of matrix onto the glass, containing also biogenic leftovers of the bacteria and thus they retain their morphology and properties.
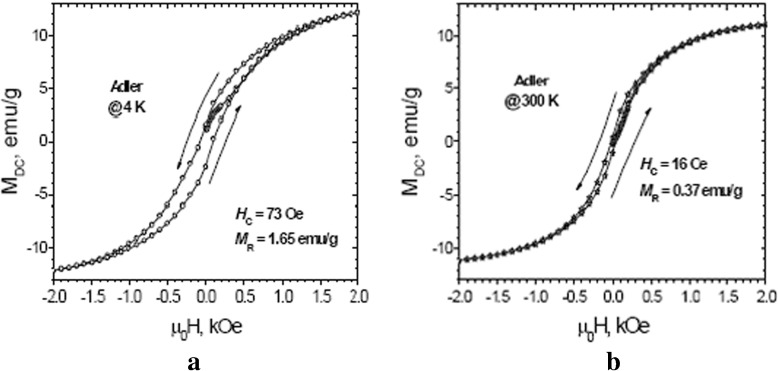
The powdered sample exhibited a strong response to a weak DC magnetic field, indicative of the magnetite-phase’s leading role regardless of its lower content. The magnetic properties were investigated by PPMS. We measured five-segment magnetic hysteresis up to 9 T at 4 and 300 K. They are presented in Fig. 11a and b. The coercive fields H c are 73 and 16 Oe at 4 and 300 K, respectively. Magnetization values are more typical for magnetite, rather than for any antiferromagnetic or paramagnetic material. The results indicate a superparamagnetic behavior of the sample at room temperature (300 K). The main contributor to the magnetic behavior of the biogenic product is the nanosized magnetite. The existence of a ‘none-zero’ low coercive field of 16 Oe could be due to the presence of a small amount of magnetite particles with a diameter larger than the superparamagnetic limit for this kind of particle, or a small contribution from the antiferromagnetic particles. The remanent magnetization M R is higher for the lower temperature.
Fig. 11.
Hysteresis loops M(H) of Adler medium sample at (a) 4 K and (b) 300 K
From all the data analyzed, it can be concluded that around 80 % of the Fe (II) from the growth media has been transformed into Fe (III) in the form of different (oxy)hydroxides, with the rest found to be in mixed 2,5 valance in magnetite. None of the initial precursors from the media were detected in the powdered material investigated. Lepidocrocite is the main phase in the material. There is a possibility that magnetite and goethite do appear after transformation from lepidocrocite, with lepidocrocite being the initial phase in the samples. In the presence of Fe (II) ions, lepidocrocite transforms into magnetite in alkaline medium at room temperature and the same conditions are needed to transform into goethite [51]. Although the pH in the medium was maintained at a constant neutral level, it cannot be ruled out that at certain places in the solution and at some points of time, conditions for such transformation do exist.
Conclusions
Bionanotechnology in laboratory conditions was employed to produce biogenic nanosized iron oxides/(oxy) hydroxides powders by cultivation of bacteria from the genus Leptothrix. The characterization of the biogenic iron oxides/(oxy) hydroxides obtained involved Raman spectroscopy, XRD, SEM, TEM, and magnetic measurements. A difference was observed in the phase content of the iron-containing nanosized particles in the powdered sample and that of bio-film coating deposited on a glass substrate. The film structure is a more refined product than the sedimented and filtered material and it is a result of the bacteria’s metabolism, performed directly in the solution. The results demonstrate that lepidocrocite (ɣ-FeOOH) is the main product of the bacteria’s metabolism, both in the bio-film and in the powdered sample. A secondary product in the bio-film consists of magnetite (Fe3O4) nanoparticles, part of which possess a defective quasi-maghemite surface layer with the general formula (Fe3+)A[Fe3+ 5xFe2.5+ 2-6x□x]BO4 where □ is a ‘vacancy’ in the octahedral sub-lattice. In the biomass filtered, the oxidation steps are not fully completed, the initial products are non-stoichiometric and due to the mixed ferric and ferrous ions present, it develops in two directions: (i) lepidocrocite (ɣ-FeOOH) as a basic sediment and (ii) magnetite (Fe3O4). The third phase of goethite (α-FeOOH) in small quantities appears, because of the highly dynamic surface state of the oxy-hydroxide in aqueous solutions. The bioproducts exhibit magnetic properties determined by the magnetite phase. The magnetic measurements showed a superparamagnetic behavior of the biogenic sample at room temperature, i.e., the nano-size of the particles is preserved upon the completion of the biotechnological process.
Acknowledgments
L. Slavov would like to acknowledge the important contribution of Prof. Eddy De Grave (Gent University, Belgium) for the discussions of phase transitions in different iron-bearing species and their authentication. This work is supported by the Bulgarian National Science Fund under project T 02-17/2014 and a FWO-BAS bilateral project.
References
- 1.Schieber J. Groundwater-Fed iron-rich microbial mats in a freshwater creek: growth cycles and fossilization potential of microbial feature. Lunar. Planet. Sci. Conf. 2004;35:1369–1370. [Google Scholar]
- 2.Kasama T, Murakami T. The effect of microorganisms on Fe precipitation rates at neutral pH. Chem. Geol. 2001;180:117–128. doi: 10.1016/S0009-2541(01)00309-6. [DOI] [Google Scholar]
- 3.Schieber J, Glamoclija M. Microbial mats built by iron bacteria: a modern example from southern Indiana. In: Schieber J, Bose PK, Eriksson PG, Banerjee S, Sarkar S, Altermann W, Catuneanu O, editors. Atlas of microbial mat features preserved within the clastic rock record. Holland: Elsevier; 2007. pp. 10–13. [Google Scholar]
- 4.Roden EE, Sobolev D, Glazer B, Luther GWI. Potential for microscale bacterial Fe redox cycling at the aerobic-anaerobic interface. Geomicrobiol. J. 2004;21:379–391. doi: 10.1080/01490450490485872. [DOI] [Google Scholar]
- 5.Emerson D, Fleming EJ, McBeth JM. Iron-oxidizing bacteria: an environmental and genomic perspective. Annu. Rev. Microbiol. 2010;64:561–583. doi: 10.1146/annurev.micro.112408.134208. [DOI] [PubMed] [Google Scholar]
- 6.Zhang JH, Lion LW, Nelson YM, Shuler ML, Ghiorse WC. Kinetics of Mn(II) oxidation by Leptothrix discophora SS1. Geochim. Cosmochim. Acta. 2002;66:773–781. doi: 10.1016/S0016-7037(01)00808-0. [DOI] [Google Scholar]
- 7.James RE, Ferris FG. Evidence for microbial-mediated iron oxidation at a neutrophilic groundwater spring. Chem. Geol. 2004;212:301–311. doi: 10.1016/j.chemgeo.2004.08.020. [DOI] [Google Scholar]
- 8.Neubauer SC, Emerson D, Megonigal JP. Life at the energetic edge: kinetics of circumneutral iron oxidation by lithotrophic iron-oxidizing bacteria isolated from the wetland-plant rhizosphere. Appl. Environ. Microbiol. 2002;68:3988–3995. doi: 10.1128/AEM.68.8.3988-3995.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rentz JA, Turner IP, Ullman JL. Removal of phosphorus from solution using biogenic iron oxides. Water Res. 2009;43:2029–2035. doi: 10.1016/j.watres.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 10.van Veen WL, Mulder EG, Deinema MH. The Sphaerotilus–Leptothrix group of bacteria. Microbiol. Mol. Biol. Rev. 1978;42:329–356. doi: 10.1128/mr.42.2.329-356.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spring S. The genera Leptothrix and Sphaerotilus. In: Dworkin M, Falkow S, Rosenberg E, Schleifer K-H, Stackebrandt E, editors. Prokaryotes. New York: Springer Verlag; 2006. pp. 758–777. [Google Scholar]
- 12.Angelova R, Groudeva V, Slavov L, Iliev M, Nedkov I, Sziklai- László I, Krezhov K. Investigation of iron-containing products from natural and laboratory cultivated Sphaerotilus–Leptothrix bacteria. J. Biol. Phys. 2015;41:367–375. doi: 10.1007/s10867-015-9384-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Angelova R, Blagoev B, Slavov L, Iliev M, Groudeva V, Nedkov I. Biogenic oxides from neutrophilic iron bacteria and possibilities for application in the nanotechnology. J. Phys. Conf. Ser. 2014;559:012019. doi: 10.1088/1742-6596/559/1/012019. [DOI] [Google Scholar]
- 14.Ellis, D.: Microbiology of the iron-depositing bacteria. Wexford College Press, Palm Springs (2003)
- 15.Angelova R, Iliev M, Mitova M, Voynova V, Tasheva L, Slavov L, Groudeva V, Antonova-Nikolova S. Optimization the cultivation of neutrophilic iron bacteria in laboratory conditions. J. BioSci. Biotech. 2014;SE/ONLINE:67–70. [Google Scholar]
- 16.Shopska M, Cherkezova-Zheleva Z, Paneva D, Iliev M, Kadinov G, Mitov I, Groudeva V. Biogenic iron compounds: XRD: Mossbauer and FTIR study. Cent. Eur. J. Chem. 2013;11:215–227. [Google Scholar]
- 17.Spring S, Kampfer P, Ludwig W, Schleifer KH. Polyphasic characterization of the Genus Leptothrix: new descriptions of Leptothrix mobilis sp. nov. and Leptothrix discophora sp. nov. nom. rev. and emended description of Leptothrix cholodnii emend. Syst. Appl. Microbiol. 1996;19:634–643. doi: 10.1016/S0723-2020(96)80036-1. [DOI] [Google Scholar]
- 18.Sawayama M, Suzuki T, Hashimoto H, Kasa T, Furutani M, Miyata N, Kunoh H, Takada J. Isolation of a Leptothrix Strain, OUMS1, from ocherous deposits in groundwater. Curr. Microbiol. 2011;63:173–180. doi: 10.1007/s00284-011-9957-6. [DOI] [PubMed] [Google Scholar]
- 19.Frankel RB, Bazylinski DA. Biologically induced mineralization by bacteria. Rev. Mineral. Geochem. 2003;54:95–114. doi: 10.2113/0540095. [DOI] [Google Scholar]
- 20.Northup DE, Lavoie KH. Geomicrobiology of caves: a review. Geomicrobiol J. 2001;18:199–222. doi: 10.1080/01490450152467741. [DOI] [Google Scholar]
- 21.Konhauser KO. Bacterial iron mineralization in nature. FEMS Microbiol. Rev. 1997;20:315–326. doi: 10.1111/j.1574-6976.1997.tb00317.x. [DOI] [Google Scholar]
- 22.Konhauser KO. Diversity of bacterial iron mineralization. Earth-Sci. Rev. 1998;43:91–121. doi: 10.1016/S0012-8252(97)00036-6. [DOI] [Google Scholar]
- 23.Lowenstam HA, Weiner S. On Biomineralization. New York: Oxford University Press; 1989. [Google Scholar]
- 24.Chan SC, Fakra CS, Edwards CD, Emerson D, Banfield FJ. Iron oxyhydroxide mineralization on microbial extracellular polysaccharides. Geochim. Cosmochim. Acta. 2009;73:3807–3818. doi: 10.1016/j.gca.2009.02.036. [DOI] [Google Scholar]
- 25.Corstjens PL, de Vrind JP, Westbroek P, de Vrind-de Jong EW. Enzymatic iron oxidation by Leptothrix discophora: identification of an iron-oxidizing protein. Appl. Environ. Microbiol. 1992;58:450–454. doi: 10.1128/aem.58.2.450-454.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Vrind-de Jong EW, Corstjens PL, Kempers ES, Westbroek P, de Vrind JP. Oxidation of manganese and iron by Leptothrix discophora: use of N, N, N1, N1-tetramethyl-p-phenylenediamine as an indicator of metal oxidation. Appl. Environ. Microbiol. 1990;56:3458–3462. doi: 10.1128/aem.56.11.3458-3462.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adams LF, Ghiorse WC. Physiology and ultrastructure of Leptothrix discophora SS-1. Arch. Microbiol. 1986;145:126–135. doi: 10.1007/BF00446769. [DOI] [Google Scholar]
- 28.Chan CS, de Stasio G, Welch SA, Girasole M, Frazer BH, Nesterova MV, Fakra S, Banfield JF. Microbial polysaccharides template assembly of nanocrystal fibers. Science. 2004;303:1656–1658. doi: 10.1126/science.1092098. [DOI] [PubMed] [Google Scholar]
- 29.Rentz JA, Kraiya C, Luther GW, III, Emerson D. Control of ferrous iron oxidation within circumneutral microbial iron mats by cellular activity and autocatalysis. Environ. Sci. Technol. 2007;41:6084–6089. doi: 10.1021/es062203e. [DOI] [PubMed] [Google Scholar]
- 30.Adams, L.F., Ghiorse, W.C.: Characterization of extracellular Mn2+−oxidizing activity and isolation of an Mn2+−oxidizing protein from Leptothrix discophora SS-1. J. Bacteriol. 169, 1279–1285 (1987) [DOI] [PMC free article] [PubMed]
- 31.Boogerd FC, de Vrind JP. Manganese oxidation by Leptothrix discophora. J. Bacteriol. 1987;169:489–494. doi: 10.1128/jb.169.2.489-494.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adams LF, Ghiorse WC. Oxidation state of Mn in the Mn oxide produced by Leptothrix discophora SS-1. Geochim. Cosmochim. Acta. 1988;52:2073–2076. doi: 10.1016/0016-7037(88)90186-X. [DOI] [Google Scholar]
- 33.Emerson D, Ghiorse WC. Isolation, cultural maintenance, and taxonomy of a sheath-forming strain of Leptothrix discophora and characterization of manganese-oxidizing activity associated with the sheath. Appl. Environ. Microbiol. 1992;58:4001–4010. doi: 10.1128/aem.58.12.4001-4010.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Furutani M, Suzuki T, Ishihara H, Hashimoto H, Kunoh H, Takada J. Initial assemblage of bacterial saccharic fibrils and element deposition to form an immature sheath in cultured Leptothrix sp. strain OUMS1. Minerals. 2011;1:157–166. doi: 10.3390/min1010157. [DOI] [Google Scholar]
- 35.Takeda M, Makita H, Ohno K, Nakahara Y, Koizumi J. Structural analysis of the sheath of a sheathed bacterium, Leptothrix cholodnii. Int. J. Biol. Macromol. 2005;37:92–98. doi: 10.1016/j.ijbiomac.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 36.Emerson D, Ghiorse WC. Ultrastructure and chemical composition of the sheath of Leptothrix discophora SP-6. J. Bacteriol. 1993;175:7808–7818. doi: 10.1128/jb.175.24.7808-7818.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kunoh, T., Hashimoto, H., Suzuki, T., Hayashi, N., Tamura, K., Takano, M., Kunoh, H., Takada, J.: Direct adherence of Fe(III) particles onto sheaths of Leptothrix sp. strain OUMS1 in culture. Minerals 6(4), (2016)
- 38.Das S, Hendry MJ. Application of Raman spectroscopy to identify iron minerals commonly found in mine wastes. Chem. Geol. 2011;290:101–108. doi: 10.1016/j.chemgeo.2011.09.001. [DOI] [Google Scholar]
- 39.Wang, A., Haskin, L.A., Jolliff, B.L.: Characterization of mineral products of oxidation and hydration by laser Raman spectroscopy: Implications for in situ petrologic investigation on the surface of Mars, Lunar Planet. Sci. XXVIII, abstract 1819, (1998)
- 40.Oh SJ, Cook DC, Townsend HE. Characterization of iron oxides commonly formed as corrosion products on steel. Hyperfine Interact. 1998;112:59–65. doi: 10.1023/A:1011076308501. [DOI] [Google Scholar]
- 41.Hanesch M. Raman spectroscopy of iron oxides and (oxy)hydroxides at low laser power and possible applications in environmental magnetic studies. Geophys. J. Int. 2009;177:941–948. doi: 10.1111/j.1365-246X.2009.04122.x. [DOI] [Google Scholar]
- 42.de Faria DLA, Silva SV, de Oliveira MT. Raman microspectroscopy of some iron oxides and oxyhydroxides. J. Raman Spectrosc. 1997;28:873–878. doi: 10.1002/(SICI)1097-4555(199711)28:11<873::AID-JRS177>3.0.CO;2-B. [DOI] [Google Scholar]
- 43.Bellot-Gurlet L, Neff D, Réguer S, Monnier J, Saheb M, Dillmann P. Raman studies of corrosion layers formed on archaeological irons in various media. J. Nanopart. Res. 2009;8:147–156. doi: 10.4028/www.scientific.net/JNanoR.8.147. [DOI] [Google Scholar]
- 44.Colomban, Ph.: Potential and drawbacks of Raman (Micro) spectrometry for the understanding of iron and steel corrosion. In: Chiaberge, M. (ed.) New Trends and Developments in Automotive System Engineering. pp. 567–584. In Tech. Pub. (2011)
- 45.Blakemore R. Magnetotactic bacteria. Science. 1975;190(4212):377–379. doi: 10.1126/science.170679. [DOI] [PubMed] [Google Scholar]
- 46.Matsunaga T, Sakaguchi T. Molecular mechanism of magnet formation in bacteria. J. Biosci. Bioeng. 2000;90:1–13. doi: 10.1016/S1389-1723(00)80001-8. [DOI] [PubMed] [Google Scholar]
- 47.Zegeye A, Abdelmoula M, Usman M, Hanna K, Ruby C. In situ monitoring of lepidocrocite bioreduction and magnetite formation by reflection Mössbauer spectroscopy. Am. Mineral. 2011;96:1410–1413. doi: 10.2138/am.2011.3794. [DOI] [Google Scholar]
- 48.Nedkov I. Nanosized magnetite for biomedical applications. J. Optoelectron. Adv. Mater. 2007;9:24–29. [Google Scholar]
- 49.Nedkov I, Slavov L, Blagoev B, Krezhov K. Surface effects in superparamagnetic magnetite particles. Bulg. J. Phys. 2013;40:348–360. [Google Scholar]
- 50.Herane, M., Garcia-Garcia, F., Sawtell, D., Montes-Atenas, G.: Study on lepidocrocite (γ − FeOOH) performance as adsorbent for heavy metals from aqueous aerated polluted solutions. Ciencia Abierta 31(5), (2007)
- 51.Cornell, R.M., Schwertmann, U.: The iron oxides: Structure, properties, reactions, occurrences and uses. Wiley-VCH, Weinheim (2003)