Abstract
Water shortage leads to a low quality of water, especially saline water in most parts of agricultural regions. This experiment was designed to determine the effects of saline irrigation on sorghum as a moderately salt-tolerant crop. To study salinity effects on photosynthetic pigment attributes including the chlorophyll content and chlorophyll fluorescence, an experiment was performed in a climate-controlled greenhouse at two vegetative and reproductive stages. The experimental design was factorial based on a completely randomized design with five NaCl concentrations (control, 50, 100, 150, and 200 mM), two grain and sweet-forage sorghum cultivars (Kimia and Pegah, respectively) and four replications. According to the experimental data, there were no significant differences between two grain and sweet-forage cultivars. Except for 100 and 150 mM NaCl, salinity significantly decreased the chlorophyll index and pigment contents of the leaf, while it increased the chlorophyll-a fluorescence characteristics. Although salinity reduced photosynthetic pigments and the crop yield, either grain or sweet-forage cultivars could significantly control the effect of salinity between 100 and 150 mM NaCl at both developmental stages, showing the possibility of using saline water in sorghum cultivation up to 150 mM NaCl.
Electronic supplementary material
The online version of this article (doi:10.1007/s10867-016-9428-1) contains supplementary material, which is available to authorized users.
Keywords: Fluorescence, Chlorophyll, Carotenoid, Pigment, Salinity, Sorghum
Introduction
Globally, water limitation has resulted in using poor-quality water resources including different kinds of saltwater (seawater, saline wells, drainage reuse, etc.). In arid and semi-arid regions, where there is a drastic water shortage, plants adaptable to low-quality water and lack of water must be cultivated. Sorghum, as a remarkable grain and industrial crop for inappropriate environments, is one of the most important crops among these plants.
Salinity influences many aspects of plant physiology including photosynthesis. Similar to most other environmental stresses, salinity has been known as an adverse factor for CO2 assimilation [1]. On the other hand, photosynthetic pigments are highly important among all parts of the photosynthesis system as their quantity and quality play vital roles in plant assimilation. Several studies have previously indicated that photosynthetic pigment contents decrease with salinity stress [2–5]. Moreover, the chlorophyll index (CI), as a non-destructive indicator for the total chlorophyll content, usually decreases under saline stress in various plants [6–8].
The function and structure of different photosynthetic ingredients seriously influence various photosynthetic pigment efficiencies, i.e., chlorophyll-a performance. Chlorophyll-a, as the antenna and the reaction center core in both photosystem I and photosystem II (PSI and PSII, respectively), plays a crucial role among the other photosynthetic pigments. Accordingly, the response of chlorophyll-a fluorescence to environmental factors has been principally considered in recent photosynthesis research [9–15]. Chlorophyll-a fluorescence is also a relatively rapid accessible attribute that can measure non-destructively under various circumstances. Indeed, photosynthesis and particularly photosystem II (PSII) behavior can be evaluated using the fast chlorophyll-a fluorescence transients induced by illuminating dark-adapted leaves [10]. This study was conducted to identify the chlorophyll-a fluorescence features of two grain and sweet-forage sorghum cultivars under different saline conditions.
Materials and methods
Experimental procedure
A pot study was performed at the College of Agriculture and Natural Resources, University of Tehran, Karaj, Iran. A factorial experiment based on a completely randomized design with four replications was conducted in a climate-controlled greenhouse. The day/night average of temperature, relative humidity and photoperiod were 30/25 °C, 40/50%, and 14/10 h, respectively. As well, the midday average of natural daylight intensity was 1500 μmol m−2 s−1. During the night, additional necessary illumination was supplied by tungsten (100 W m−2) and white-light fluorescent (23 W m−2) lamps while other artificial sources of light were eliminated.
Two sorghum [Sorghum bicolor (L.) Moench] genotypes, Kimia (a grain cultivar) and Pegah (a sweet-forage cultivar), were chosen for this research (Seed and Plant Improvement Institute, Karaj, Iran). Salinity was induced by adding sodium chloride (Merck KGaA, Darmstadt, Germany) to tap water in five concentrations including control (tap water as irrigation water), 50, 100, 150, and 200 mM, which corresponded to electrical conductivities (EC), as follows: control (tap water as irrigation water), 5, 10, 15, and 20 dS m−1. Saline treatments were conducted 2 weeks after sowing, continuing up to the end of the plant growth period. NaCl concentrations more than 50 mM were gradually treated by 50-mM steps every 3 days up to the final concentrations. The soil electrical conductivities of saline levels, estimated after the experiment, were 14, 47, 87, 132, and 181 mM, respectively. The details of soil and irrigation water are presented in Supplementary Information 1.
Seeds were sown in 30 × 20-cm polythene bags that were filled with 15 kg of soil and covered with 0.5 kg of gravel particles at the bottom. All pots were equipped with sub-holes to allow relatively mild drainage of excessive salts. To ensure seedling emergence, ten seeds were cultivated in every pot and the seedlings were thinned to two uniform-sizes per pot thereafter; 50 mg N kg−1 and 40 mg P kg−1 were utilized as the urea (at developmental stages 1 and 4: third and final visible, respectively) and triple superphosphate (before planting), respectively [16]. To avoid any moisture deficit, the pots were irrigated twice a week alternatively by saline and tap water up to field capacity (FC). Additionally, every 4 weeks, irrigation was done until the pots drained at the bottom and the drainage EC of each treatment was measured to be assured of its accuracy.
Leaf characteristics
All measurements were conducted on the youngest intact fully expanded leaf of the main stem in each plant. Three plants were measured in every replication at a vegetative (developmental stage 3: growing point differentiation) and a reproductive (developmental stage 7: soft dough) stage, described by Kansas State University [16].
Chlorophyll index
The relative chlorophyll content was estimated on the upper (adaxial) central surface of the leaf (except for the nervure) using a portable chlorophyllmeter (Chlorophyll Content Meter CL-01, Hansatech Instrument Ltd., King’s Lynn, Norfolk, UK) between 10:00 and 14:00 h. The relative chlorophyll content was determined based on dual wavelengths of the spectral absorbance: 620 nm (the red band) at which chlorophyll absorption is high and 940 nm as a reference wavelength. The results were expressed as a chlorophyll index [17].
Chlorophyll fluorescence
The chlorophyll-a fluorescence transients (the OJIP curve) were recorded by a portable plant efficiency analyzer (Pocket PEA, Hansatech Instrument Ltd., King’s Lynn, Norfolk, UK) between 10:00 to 14:00 h. The chlorophyll index measurements were made on the upper (adaxial) central surface of the leaf (except for the nervure). Before measuring, the leaves were fully dark-adapted for 15 min.
Chlorophyll fluorescence induction was prompted by a 3-s pulse of red light (peak wavelength of 627 nm) emitted from an LED lamp filtered by an NIR filter. This pulse was emitted at maximal saturation irradiance of 3500 μmol m−2 s−1 with 16-bit signal resolution. The acquisition rate of this analogue/digital signal resolution was (1) 10 μs for the first 300 μs, (2) 100 μs up to 3 ms afterward, (3) 1 ms up to 300 ms subsequently, and (4) 10 ms thereafter. The fluorescence signal was considered as FO at 50 μs [13, 18].
The JIP-test was used to analyze fast chlorophyll-a fluorescence transients providing structural and functional information about photosystem II (PSII) behavior [13, 18]. The original dark-adapted data and the equations, used for quantification of chlorophyll-a fluorescence characteristics, are represented in Supplementary Information 2.
Chlorophyll and carotenoid contents
After measuring non-destructive traits (chlorophyll index and chlorophyll fluorescence) on the same leaves, those leaves were gathered to measure their photosynthetic pigment concentrations as destructive traits; 0.1 g leaf samples were ground in liquid nitrogen and afterward the samples were extracted by 1.5 ml acetone (80%; v/v) via centrifuging at 10,000 rpm for 10 min. The spectral absorbances of supernatant solution were estimated at A663.2, A646.8, and A470 nm using a spectrophotometer (Unico SQ-2802S UV/VIS, United Products and Instrument Inc., Dayton, NJ, USA). Concentrations of total chlorophyll (Chla+b), chlorophyll-a (Chla), chlorophyll-b (Chlb), and total carotenoid (Carx+c: xanthophyll + carotene) were estimated by Wellburn [19] equations in 80% acetone. Based on these values, other traits were calculated according to Supplementary Information 3.
Statistical analysis
The results were analyzed by a general linear model (GLM) using the SAS 9.1 software package (SAS Institute Inc., Cary, NC, USA). Significant differences among individual means were determined based on the least significant difference (LSD) test at p ≤ 0.05. Since vegetative and reproductive stages behaved similarly in some parameters, they were pooled together. Correlation analysis was done according to significance of Pearson’s coefficients at p ≤ 0.05.
Results
Pigment contents and leaf ratios
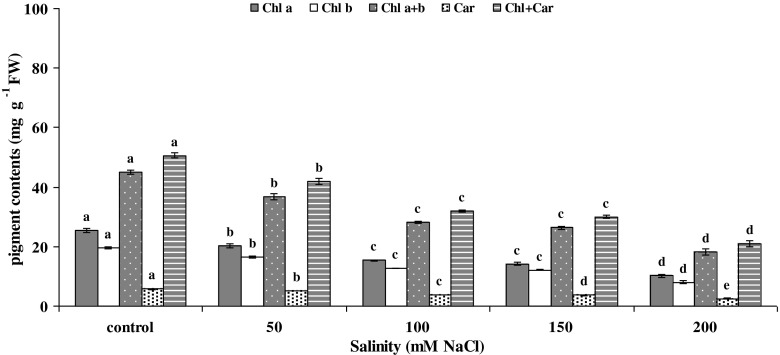
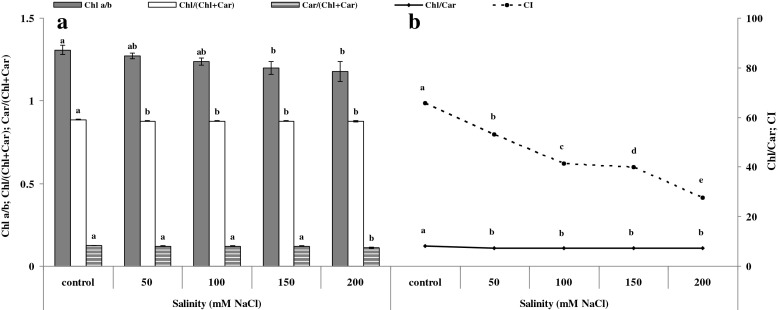
The grain and sweet-forage cultivars did not show any significant differences in any of the photosynthetic pigment contents and ratios (data not shown). All photosynthetic pigment contents and ratios significantly decreased by enhancing salinity at both the vegetative and reproductive stages (Figs. 1 and 2a, b). Furthermore, the chlorophyll index (CI) behaved the same (Fig. 2b). There were no significant differences among the interactions between salinity and cultivars (data not presented). Since vegetative and reproductive stages behaved similarly in some parameters, they were pooled together.
Fig. 1.
Changes of chlorophyll-a (Chl a), chlorophyll-b (Chl b), total chlorophyll (Chl a + b), total carotenoid (Car: xanthophyll + carotene) and total photosynthetic pigments (Chl + Car: total chlorophyll + total carotenoid) vs. salinity according to LSD (0.05) test (means ± SE; n = 4). Since vegetative and reproductive stages behaved similarly, they were pooled together
Fig. 2.
Changes of pigment ratios vs. salinity according to LSD (0.05) test (means ± SE; n = 4). a Chlorophyll a to b (Chl a/b), total chlorophyll to total chlorophyll and carotenoid (Chl/(Chl + Car)) and total carotenoid to total chlorophyll and carotenoid (Car/(Chl + Car)). b Total chlorophyll to total carotenoid (Chl/Car) and chlorophyll index (CI). Since vegetative and reproductive stages behaved similarly, they were pooled together
At both developmental stages, nearly all pigment contents and ratios responded identically to 100 and 150 mM NaCl. This result indicated that either grain or sweet-forage cultivars of sorghum could similarly tolerate these two levels of salinity (Figs. 1 and 2a, b).
Chlorophyll-a fluorescence of the leaf
Overall, chlorophyll-a fluorescence parameters were adversely influenced by salinity at the vegetative and reproductive stages (Tables 1, 2, 3, and 4). In some parameters, the vegetative and reproductive stages behaved similarly; therefore, they were pooled together. The two grain and sweet-forage sorghum cultivars (Kimia and Pegah, respectively) did not represent any significant differences in any of the chlorophyll-a fluorescence parameters (data not shown). Moreover, no significant differences were observed among the interactions between salinity and cultivars (data not shown).
Table 1.
Changes of extracted and technical fluorescence parameters vs. salinity
| Salinity (mM) | Control | 50 | 100 | 150 | 200 | |
|---|---|---|---|---|---|---|
| FO | Veg Rep |
292.00e ±2.15 512.25d ±2.16 |
319.25d ±3.75 539.62c ±3.75 |
382.62c ±5.57 621.37b ±10.63 |
403.75b ±4.37 619.37b ±4.06 |
527.37a ±2.35 746.37a ±2.51 |
| FM | Pooled stages | 3042.19d ±38.28 | 3197.63c ±22.84 | 3364.06b ±14.40 | 3402.00b ±10.06 | 3517.00a ±10.71 |
| FV | Pooled stages | 2640.06c ±39.15 | 2768.19b ±21.31 | 2862.06a ±12.68 | 2890.44a ±10.13 | 2880.13a ±11.75 |
| TM (ms) | Veg Rep |
860.75a ±5.80 713.87a ±5.32 |
799.62b ±3.61 673.87b ±4.55 |
742.50c ±6.56 639.75c ±6.08 |
730.50c ±6.29 621.50d ±6.46 |
666.87d ±4.73 588.25e ±4.17 |
| LD | Veg Rep |
0.10d ±0.01 0.16c ±0.01 |
0.10d ±0.01 0.16c ±0.01 |
0.12c ±0.01 0.17b ±0.01 |
0.13b ±0.01 0.17b ±0.01 |
0.16a ±0.01 0.20a ±0.01 |
| MO | Pooled stages | 0.63d ±0.01 | 0.82c ±0.01 | 1.00b ±0.01 | 1.01b ±0.01 | 1.48a ±0.03 |
| VJ | Veg Rep |
0.31d ±0.01 0.56c ±0.01 |
0.34c ±0.01 0.57b ±0.01 |
0.40b ±0.01 0.62a ±0.01 |
0.40b ±0.01 0.62a ±0.01 |
0.55a ±0.01 0.62a ±0.01 |
| VI | Pooled stages | 0.55d ±0.01 | 0.61c ±0.01 | 0.66b ±0.01 | 0.66b ±0.01 | 0.75a ±0.01 |
| SM | Veg Rep |
107.63a ±2.62 79.16a ±1.46 |
94.61b ±1.91 64.26b ±0.70 |
83.59c ±1.40 55.35c ±0.57 |
80.34c ±1.73 52.27d ±0.61 |
72.81d ±2.28 46.67e ±0.38 |
| N | Veg Rep |
185.29b ±4.98 104.29a ±0.93 |
201.55b ±5.56 100.84a ±1.54 |
194.08b ±4.35 95.03b ±1.70 |
186.89b ±4.33 91.48bc ±1.16 |
237.20a ±9.71 88.39c ±0.79 |
| RC/ABS | Pooled stages | 0.58a ±0.01 | 0.48b ±0.01 | 0.43c ±0.01 | 0.42c ±0.01 | 0.34d ±0.01 |
| RC/CS | Veg Rep |
1453.42a ±22.62 2107.31a ±18.45 |
1236.31b ±19.63 1854.32b ±22.91 |
1182.62b ±20.05 1745.47c ±21.79 |
1178.11b ±17.30 1732.91c ±16.33 |
848.96c ±40.25 1595.77d ±16.27 |
Within each row, means ± SE (n = 4) followed by at least one similar letter were not significantly different according to LSD (0.05) test. Since vegetative and reproductive stages behaved similarly in some parameters, they were pooled together. Except for TM, measured in ms, all the chlorophyll-a fluorescence parameters were quantified in an arbitrary unit (a.u.) or a relative unit (r.u.). FO: initial fluorescence intensity (at 50 μs); FM: peak or maximum fluorescence intensity; FV: variable fluorescence intensity; TM: time to reach FM; LD: non-photochemical loss in dark-adapted state of PSII; MO: net rate of closure in reaction centers; VJ: closed reaction centers at the J-step fluorescence intensity (at 2 ms); VI: closed reaction centers at the I-step fluorescence intensity (at 30 ms); SM: relative pool size of the plastoquinone; N: turnover number of quinone-a; RC/ABS: density of the reaction centers per absorption energy flux; RC/CS: density of the reaction centers per excited cross section of leaf area; Veg: vegetative stage; Rep: reproductive stage
Table 2.
Changes of flux ratios (quantum yields and quantum efficiencies) vs. salinity
| Salinity (mM) | Control | 50 | 100 | 150 | 200 | |
|---|---|---|---|---|---|---|
| Quantum yields | ||||||
| TRO/DIO | Pooled stages | 7.00a ±0.13 | 6.81a ±0.07 | 5.99b ±0.07 | 5.86b ±0.06 | 4.62c ±0.03 |
| ΦPo | Veg Rep |
0.89a ±0.01 0.84a ±0.01 |
0.89a ±0.01 0.84a ±0.01 |
0.88b ±0.01 0.83b ±0.01 |
0.87c ±0.01 0.83b ±0.01 |
0.84d ±0.01 0.80c ±0.01 |
| ΦEo | Veg Rep |
0.62a ±0.01 0.37a ±0.01 |
0.59b ±0.01 0.36b ±0.01 |
0.52c ±0.01 0.31c ±0.01 |
0.52c ±0.01 0.31c ±0.01 |
0.38d ±0.01 0.30c ±0.01 |
| ΦRo | Pooled stages | 0.42a ±0.01 | 0.36b ±0.01 | 0.31c ±0.01 | 0.31c ±0.01 | 0.23d ±0.01 |
| ΦDo | Veg Rep |
0.10d ±0.01 0.16c ±0.01 |
0.10d ±0.01 0.16c ±0.01 |
0.12c ±0.01 0.17b ±0.01 |
0.13b ±0.01 0.17b ±0.01 |
0.16a ±0.01 0.20a ±0.01 |
| Quantum efficiencies | ||||||
| ΨEo | Veg Rep |
0.69a ±0.01 0.44a ±0.01 |
0.66b ±0.01 0.42b ±0.01 |
0.60c ±0.01 0.38c ±0.01 |
0.60c ±0.01 0.38c ±0.01 |
0.45d ±0.01 0.38c ±0.01 |
| ΨRo | Pooled stages | 0.45a ±0.01 | 0.38b ±0.01 | 0.34c ±0.01 | 0.34c ±0.01 | 0.25d ±0.01 |
| δRo | Veg Rep |
0.81a ±0.01 0.77a ±0.01 |
0.75b ±0.01 0.65b ±0.01 |
0.74b ±0.01 0.63c ±0.01 |
0.74b ±0.01 0.63c ±0.01 |
0.66c ±0.01 0.54d ±0.01 |
| ETO/DIO | Veg Rep |
5.92a ±0.15 2.39a ±0.08 |
5.41b ±0.06 2.27a ±0.03 |
4.28c ±0.08 1.83b ±0.02 |
4.08c ±0.06 1.85b ±0.02 |
2.36d ±0.04 1.54c ±0.02 |
Within each row, means ± SE (n = 4) followed by at least one similar letter were not significantly different according to LSD (0.05) test. Since vegetative and reproductive stages behaved similarly in some parameters, they were pooled together. All the chlorophyll-a fluorescence parameters were quantified in an arbitrary unit (a.u.) or a relative unit (r.u.). TRO/DIO: maximum quantum yield of the primary PSII photochemistry relative to initial fluorescence (FO); ΦPo: maximum quantum yield of the primary PSII photochemistry relative to maximum fluorescence (FM); ΦEo: quantum yield of the electron transport flux from QA to QB of PSII; ΦRo: quantum yield consequent of the reduction in end electron acceptors of photosystem I (PSI); ΦDo: quantum yield of energy dissipation as heat; ΨEo: efficiency or probability with which a PSII trapped electron is transferred from QA to QB; ΨRo: efficiency or probability with which a PSII trapped electron is transferred from QA- to the end electron acceptors of PSI; δRo: efficiency or probability with which an electron from QB is transferred to the end electron acceptors of PSI; ETO/DIO: proportion of electron transport to energy dissipation as heat; Veg: vegetative stage; Rep: reproductive stage
Table 3.
Changes of specific energy fluxes and phenomenological energy fluxes vs. salinity
| Salinity (mM) | Control | 50 | 100 | 150 | 200 | |
|---|---|---|---|---|---|---|
| Specific Energy Fluxes | ||||||
| ABS/RC | Pooled stages | 1.74d ±0.04 | 2.12c ±0.02 | 2.36b ±0.03 | 2.39b ±0.03 | 3.13a ±0.09 |
| TRO/RC | Veg Rep |
1.72c ±0.04 1.32d ±0.02 |
2.13b ±0.03 1.57c ±0.01 |
2.32b ±0.04 1.72b ±0.02 |
2.33b ±0.03 1.75b ±0.02 |
3.27a ±0.14 1.89a ±0.02 |
| ETO/RC | Veg Rep |
1.19b ±0.04 0.58c ±0.02 |
1.40a ±0.02 0.66b ±0.01 |
1.39a ±0.03 0.65b ±0.01 |
1.39a ±0.03 0.66b ±0.01 |
1.49a ±0.08 0.72a ±0.01 |
| REO/RC | Veg Rep |
0.97a ±0.04 0.45a ±0.02 |
1.05a ±0.01 0.43ab ±0.01 |
1.03a ±0.02 0.41bc ±0.01 |
1.03a ±0.02 0.41bc ±0.01 |
0.97a ±0.04 0.39c ±0.01 |
| DIO/RC | Pooled stages | 0.22d ±0.01 | 0.27c ±0.01 | 0.34b ±0.01 | 0.35b ±0.01 | 0.55a ±0.01 |
| Phenomenological Energy Fluxes | ||||||
| ABS/CS | Pooled stages | 3042.19d ±38.28 | 3197.63c ±22.84 | 3364.06b ±14.40 | 3402.00b ±10.06 | 3517.00a ±10.71 |
| TRO/CS | Pooled stages | 2640.06c ±39.15 | 2768.19b ±21.31 | 2862.06a ±12.68 | 2890.44a ±10.13 | 2880.13a ±11.75 |
| ETO/CS | Veg Rep |
1727.00a ±38.70 1226.50a ±38.23 |
1727.63a ±21.79 1227.88a ±21.97 |
1635.63b ±14.22 1136.75b ±14.12 |
1644.38b ±13.27 1143.38b ±13.47 |
1242.50c ±21.53 1149.50b ±13.55 |
| REO/CS | Pooled stages | 1264.88a ±44.93 | 1127.29b ±25.15 | 1035.02c ±16.42 | 1042.56c ±14.68 | 798.27d ±21.42 |
| DIO/CS | Veg Rep |
292.00e ±2.15 512.25d ±2.16 |
319.25d ±3.75 539.62c ±3.75 |
382.62c ±5.57 621.37b ±10.64 |
403.75b ±4.37 619.37b ±4.06 |
527.37a ±2.35 746.37a ±2.51 |
Within each row, means ± SE (n = 4) followed by at least one similar letter were not significantly different according to LSD (0.05) test. Since vegetative and reproductive stages behaved similarly in some parameters, they were pooled together. All the chlorophyll-a fluorescence parameters were quantified in an arbitrary unit (a.u.) or a relative unit (r.u.). ABS/RC: absorbed energy flux per RC; TRO/RC: trapped energy flux per RC; ETO/RC: electron transport flux per RC; REO/RC: reduction energy flux per RC; DIO/RC: energy dissipation as heat per RC; ABS/CS: absorbed energy flux per CS; TRO/CS: trapped energy flux per CS; ETO/CS: electron transport flux per CS; REO/CS: reduction energy flux per CS; DIO/CS: energy dissipation as heat per CS; Veg: vegetative stage; Rep: reproductive stage
Table 4.
Changes of performance indices and driving forces parameters vs. salinity
| Salinity (mM) | Control | 50 | 100 | 150 | 200 | |
|---|---|---|---|---|---|---|
| Performance indices | ||||||
| PIABS,PSI | Veg Rep |
33.49a ±2.22 6.84a ±0.60 |
13.80b ±0.83 1.90b ±0.16 |
7.54c ±0.50 0.96c ±0.05 |
7.15c ±0.27 0.97c ±0.05 |
1.24d ±0.30 0.20c ±0.03 |
| PIABS,PSII | Pooled stages | 6.35a ±0.17 | 4.37b ±0.10 | 2.73c ±0.06 | 2.61c ±0.04 | 1.08d ±0.02 |
| PIABS,total | Pooled stages | 26.51a ±1.68 | 12.22b ±0.60 | 6.98c ±0.33 | 6.68c ±0.20 | 1.80d ±0.19 |
| PICS,PSI | Veg Rep |
94,072.00a ±7277.17 22,666.00a ±2193.95 |
40,807.00b ±2750.48 6585.00b ±580.05 |
23,498.00c ±1601.03 3487.00bc ±205.21 |
22,569.00c ±860.06 3560.00bc ±204.46 |
4047.00d ±990.80 761.00c ±113.16 |
| PICS,PSII | Pooled stages | 18,440.60a ±734.36 | 13,432.40b ±393.66 | 8869.20c ±221.50 | 8567.60c ±153.04 | 3804.20d ±77.10 |
| PICS,total | Pooled stages | 76,809.00a ±5818.89 | 37,128.00b ±2097.30 | 22,362.00c ±1116.40 | 21,632.00c ±676.20 | 6208.00d ±638.83 |
| Driving forces | ||||||
| DFABS,PSI | Veg Rep |
0.64a ±0.01 0.54a ±0.01 |
0.49b ±0.01 0.27b ±0.01 |
0.45b ±0.01 0.22c ±0.01 |
0.46b ±0.01 0.23c ±0.01 |
0.30c ±0.05 0.07d ±0.01 |
| DFABS,PSII | Pooled stages | 0.71a ±0.01 | 0.57b ±0.01 | 0.38c ±0.01 | 0.36c ±0.01 | 0.03d ±0.01 |
| DFABS,total | Pooled stages | 1.30a ±0.03 | 0.95b ±0.02 | 0.72c ±0.02 | 0.71c ±0.01 | 0.22d ±0.04 |
| DFCS,PSI | Veg Rep |
0.64a ±0.02 0.54a ±0.02 |
0.49b ±0.01 0.27b ±0.01 |
0.45b ±0.01 0.22c ±0.01 |
0.46b ±0.01 0.23c ±0.01 |
0.30c ±0.05 0.07d ±0.01 |
| DFCS,PSII | Pooled stages | 4.20a ±0.02 | 4.08b ±0.01 | 3.90c ±0.01 | 3.89c ±0.01 | 3.58d ±0.01 |
| DFCS,total | Pooled stages | 4.78a ±0.04 | 4.46b ±0.02 | 4.24c ±0.02 | 4.24c ±0.01 | 3.76d ±0.04 |
Within each row, means ± SE (n = 4) followed by at least one similar letter were not significantly different according to LSD (0.05) test. Since vegetative and reproductive stages behaved similarly in some parameters, they were pooled together. All the chlorophyll-a fluorescence parameters were quantified in an arbitrary unit (a.u.) or a relative unit (r.u.). PIABS,PSI: PSI performance index for energy conservation; PIABS,PSII: PSII performance index for energy conservation (from photons absorbed by PSII antenna chlorophyll to reduce QB); PIABS,total: total performance index for energy conservation (from photons absorbed by PSII antenna until the reduction of PSI acceptors); PICS,PSI: PSI performance index on CS basis; PICS,PSII: PSII performance index on CS basis; PICS,total: total performance index on CS basis; DFABS,PSI: PSI driving force on ABS basis; DFABS,PSII: PSII driving force on ABS basis; DFABS,total: total driving force on ABS basis; DFCS,PSI: PSI driving force on CS basis; DFCS,PSII: PSII driving force on CS basis; DFCS,total: total driving force on CS basis; Veg: vegetative stage; Rep: reproductive stage.
Extracted and technical parameters of chlorophyll-a fluorescence
At both developmental stages, salinity increased the initial fluorescence (FO), maximal fluorescence (FM), variable fluorescence (FV), and the proportion of initial to maximal fluorescence (LD). These results indicated that the stress increased solar energy dissipation as fluorescence (Table 1).
This condition was a result of increasing the net rate of closure in reaction centers of photosystems (MO) following the salinity. It meant all chlorophyll-a molecules in reaction centers were involved in the conversion of photons to electrons and there were no free molecules of chlorophyll-a to convert photons to electrons. Increasing MO resulted in further closed reaction centers of photosystems at the J-step (VJ) and I-step (VI) of chlorophyll-a fluorescence intensity (Table 1). This result was in agreement with the results of pigment contents because photosynthetic pigments including chlorophyll-a molecules decreased by salinity and a few number of these molecules could not absorb solar energy. Hence, the decrease in photosynthetic pigments, especially the chlorophyll-a content (Fig. 1), caused the decrease in the density of reaction centers per absorption energy flux in photosystems (RC/ABS) and a decrease in the density of reaction centers per cross section between photosystem I and photosystem II (RC/CS). The decrease in chlorophyll-a and reaction centers displayed low photon receptors. This situation led to finishing the excited electrons of the chlorophyll and closing the reaction centers. Consequently, the absorbed solar energy could not excite the chlorophyll-a electrons any more. Therefore, the fluorescence process increased to dissipate the excessive absorbed solar energy and inhibited the destruction of the photosynthetic apparatus.
In addition to fluorescence enhancement, the time to reach maximal fluorescence (TM) was shortened by salinity, which was a sign of accelerating fluorescence induction to dissipate the excessive absorbed solar energy. On the other hand, the enhancement of non-photochemical loss in a dark-adapted state of PSII (LD) showed that the stress affected initial fluorescence (FO) more than maximal fluorescence (FM) (Table 1; see Supplementary Information 2).
Similar to the content of pigments, the relative pool size of the plastoquinone (SM), as a kind of electron carrier per electron transport chain, was reduced by salinity at both developmental stages. It meant salinity reduced electron carriers as well as chlorophylls and carotenoids, which led to a decrease in electron transportation and a delay in converting photons to electrons followed by increasing chlorophyll-a fluorescence.
Nevertheless, the turnover number of quinone-a molecules (N) appeared differently at the vegetative and reproductive stages. During electron transporting, electrons are transferred by different electron carriers, i.e., the plastoquinones (PQ), the quinone-a molecules (QA), the quinone-b molecules (QB), etc. As QA receives the electron, it changes to QA-. By transferring the electron from QA- to QB, QA- turns to QA. The number of these turnovers is referred to as N. As QA decreases, the turnover of QA- to QA decreases and N declines.
It assumed that the amount of QA and QB molecules must be reduced by salinity as well as plastoquinones. However, the enhancement of N under 200 mM at the vegetative stage showed the opposite. The reduction of N at the reproductive stage might be due to the plant age. Indeed, the ability of electron transport in an old plant (reproductive stage) decreased more by salinity stress (Table 1). On the contrary, the vegetative stage indicated a significant enhancement of N under 200 mM salinity. It seemed that the turnover number of QA in a young plant (vegetative stage) could support electron transport under high salinity. However, the significantly few N at lower saline levels might be due to sufficient amounts of QA at the vegetative stage. As a matter of fact, it was not necessary to turnover QA- to QA quickly in order to receive new electrons under lower saline levels at the vegetative stage (Table 1). This result might be a sign of producing fewer excited electrons in older plants (reproductive stage) due to their incapable photosynthetic apparatus compared to younger plants (vegetative stage).
Flux ratios (quantum yields and quantum efficiencies) of chlorophyll-a fluorescence
Except for the quantum yield of dissipation (ΦDo), all the other quantum yields and quantum efficiencies decreased at the vegetative and reproductive stages. Approximately, all the quantum yields and quantum efficiencies behaved the same under 100 and 150 mM NaCl (Table 2).
Salinity reduced the quantum yield of the electron transport flux from QA to QB of photosystem II (ΦEo) and the quantum yield of reduction in the end electron acceptors of photosystem I (ΦRo). These results showed a decrease in electron transportation from QA to QB in photosystem II and afterward a decrease in electron transportation from QB to the end electron acceptors in photosystem I. Moreover, the quantum efficiencies showing that the electron transferred from QA to QB (ΨEo), the electron transferred from QB to the end electron acceptors of photosystem I (δRo) and the electron transferred to the end electron acceptors of photosystem I (ΨRo) were reduced by salinity (Table 2). Lower ΨEo and ΨRo demonstrated that there were a few open reaction centers at the J-step and I-step to produce excited electrons. Subsequently, a few excited electrons could transmit to the electron transport chain of the photochemical reactions of the photosynthesis. Such effects eventually led to the lower maximum quantum yield of the primary PSII photochemistry relative to the initial fluorescence (TRO/DIO) and also the lower maximum quantum yield of the primary PSII photochemistry relative to the maximal fluorescence (ΦPo). The reduction of these two parameters, as indicators of the activity of photosystem II, displayed that the leaf photosynthetic capacity was reduced by the salinity (Table 2).
Concerning the decrease in various pigment contents as a result of salinity (Figs. 1 and 2a, b), it appeared that the reduction of quantum yields and quantum efficiencies was a result of decreasing both the content of electron acceptors (PQ, QA, QB, etc.) and the potential of electron transferring.
The decrease in the density of reaction centers (RC/ABS and RC/CS), quantum yields and quantum efficiencies represented that the photon absorption and the electron transport were reduced by salinity; however, the electron transport decreased more. In other words, salinity decreased the reaction centers in photosystems and the electron acceptors of the electron transport chain, simultaneously. Nevertheless, the decrease was more in the electron acceptors of the electron transport chain because the quantum efficiencies of the electron transport were reduced (Table 2). These results revealed that there were so few electron acceptors in the electron transport chain that the electron transport chain could not even transfer a few exited electrons. This situation indicated that salinity damaged the electron transport chain of the photosynthesis system more than its reaction centers.
By decreasing the electron transport, the excessive absorbed solar energy that could not have been converted to electron transmission had to be dissipated as fluorescence and heat in order to not damage the photosynthetic structures. Hence, the proportion of electron transport to energy dissipation as heat (ETO/DIO) decreased while the quantum yield of dissipation as heat (ΦDo) increased (Table 2).
Specific energy fluxes and phenomenological energy fluxes of chlorophyll-a fluorescence
Except for the reduction of energy flux per reaction center (REO/RC), all specific fluxes and phenomenological fluxes responded similarly to the salinity stress at both the vegetative and reproductive stages (Table 3). The absorption energy flux per reaction centers (ABS/RC), the trapped energy flux per reaction centers (TRO/RC) and the electron transport flux per reaction centers (ETO/RC) were raised by increasing NaCl at both developmental stages. However, ETO/RC was only different under the control at the vegetative stage (Table 3). It was assumed that the reduction in the density of the reaction centers (Table 1) led to using all reaction centers and filling up the whole capacity of the reaction centers. This situation eventually intensified ABS/RC, TRO/RC, and ETO/RC. Nonetheless, the decreased slope of the density of the reaction centers was so harsh that the electron transport flux per reaction centers (ETO/RC) did not obviously change by the salinity at the reproductive stage. It demonstrated that the photosynthetic system could only transfer a few excited electrons released by a few reaction centers. This situation was regardless of the increase in the salinity and the decrease in the reaction centers and the electron acceptors. Therefore, the remaining excited electrons released by the reaction centers could not be transferred and their energy must be released as chlorophyll-a fluorescence.
On the other hand, the reduction energy flux per reaction center (REO/RC) behaved differently at the two developmental stages. Although REO/RC was insignificant at the vegetative stage, it significantly decreased under salinity at the reproductive stage (Table 3). The response of REO/RC was relatively in accordance with the turnover number of QA (N) at the vegetative stage. Since N could support the electron transport in young plants (Table 1), the electron transmission to the final PSI electron acceptors could continue (Table 3). Hence, the reduction of the end electron acceptors of PSI (REO/RC) did not significantly vary at the vegetative stage. However, N decreased due to the plant aging at the reproductive stage; therefore, REO/RC decreased.
In this situation, it was obvious that the energy dissipation as heat per reaction center (DIO/RC) increased to dissipate the excessive absorbed solar energy in order to not destroy the photosynthetic structures (Table 3) as well as the increase in chlorophyll-a fluorescence (Table 1). Nonetheless, 100 and 150 mM NaCl demonstrated similar specific fluxes.
On the other hand, salinity stress increased the absorption flux per cross section (ABS/CS) and the trapped energy flux per cross section (TRO/CS) while it decreased the electron transport flux per cross section (ETO/CS) and the reduction energy flux per cross section (REO/CS) simultaneously (Table 3). The enhancement of ABS/CS and TRO/CS indicated that the cross section reached its maximum potential for transferring electrons. Therefore, the electron acceptors were more involved in electron transferring due to few electron acceptors. This situation led to an increase in the proportion of absorbed energy flux to the cross section (ABS/CS) and the trapped energy flux per cross section (TRO/CS).
While the electron acceptors decreased, electron transferring per cross section (ETO/CS) declined. Consequently, it caused a decline of the reduction energy flux per cross section (REO/CS), implying a few electrons could be transferred to the end electron acceptors of photosystem I (Table 3). On the whole, it caused an increase in energy dissipation as heat per cross section (DIO/CS) in order to dissipate excessive absorbed energy (Table 3).
In the last three salinity levels, a similar TRO/CS might be a result of filling up the photon trapping capacity per cross section. As well, a similar ETO/CS under the first two salinity levels represented that 50 mM NaCl could not influence the electron transfer in the electron transport chain at any of the developmental stages. At the vegetative stage, ETO/CS was significantly different under 200 mM NaCl. However, it did not significantly vary at the reproductive stage. It might be related to the plant age as an older plant, having thicker leaves at the reproductive stage, contained more leaf tissues per leaf cross section. Therefore, the electron transfer measured by the fluorimeter might be recorded in a thicker tissue of the leaf compared to the leaf of a young plant at the vegetative stage. Consequently, more electron transfer might be detected at the same time, leading to ETO/CS without any significance among the last three salinity levels at the reproductive stage (Table 3).
Performance indices and driving force parameters of chlorophyll-a fluorescence
All performance indices and driving forces significantly decreased at both developmental stages (Table 4). The significant decline of all performance indices and driving forces obviously showed salinity stress deeply influenced the photochemical reactions of the photosynthetic apparatus. Nevertheless, non-significant differences between 100 and 150 mM NaCl demonstrated that there was a considerable tolerance up to 150 mM salinity in either the grain cultivar (Kimia) or the sweet-forage (Pegah) cultivar of sorghum (Table 4).
Crop yield and the relationship between chlorophyll-a fluorescence of the leaf
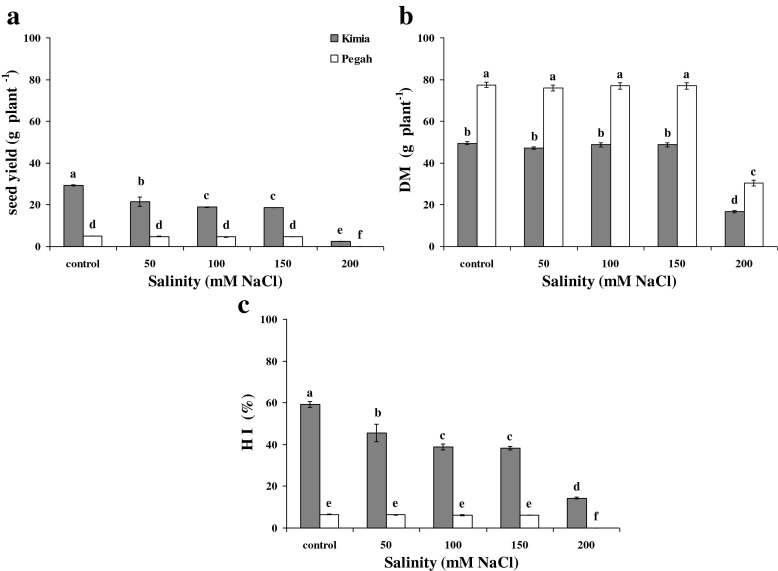
Seed yield per plant, total dry matter per plant and the harvest index (HI) were reduced by the interaction of salinity and cultivars (Fig. 3a-c). The grain cultivar (Kimia) showed higher seed yield per plant (Fig. 1a) while the sweet-forage cultivar (Pegah) displayed higher total dry matter per plant (Fig. 1b). The sweet-forage cultivar (Pegah) had only vegetative growth without showing any seed yields under 200 mM NaCl (Fig. 1a); therefore, it displayed no harvest index under this salinity level (Fig. 3c).
Fig. 3.
Interactions of salinity and sorghum cultivars on seed yield (a), total dry matter (b), and harvest index (HI) (c) according to LSD (0.05) test (means ± SE; n = 4)
On the other hand, the sweet-forage cultivar (Pegah) could control negative effects of salinity up to 150 mM NaCl in the seed yield per plant (Fig. 3a), total dry matter per plant (Fig. 3b) and the harvest index (Fig. 3c). As well, the grain cultivar (Kimia) could control negative effects of salinity on total dry matter per plant up to 150 mM NaCl (Fig. 3b). Although the grain cultivar (Kimia) displayed a decreasing trend in the seed yield per plant and the harvest index under salinity, it could control negative effects of salinity on these traits between 100 and 150 mM NaCl (Fig. 3a, c).
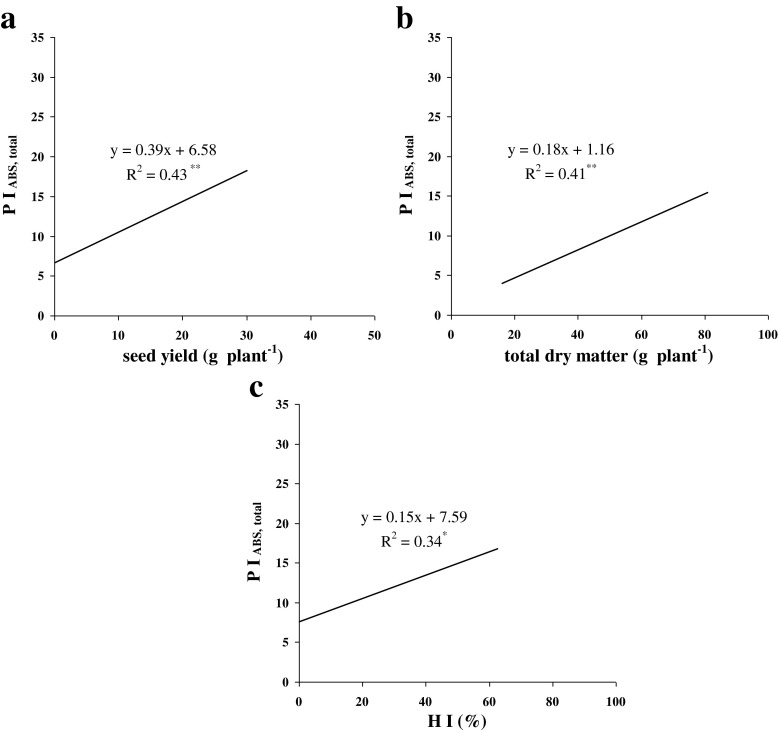
These results were in agreement with the chlorophyll-a fluorescence results. The positive correlations of total performance index (PIABS, total), as a key parameter showing the capability of the photochemical reactions of the photosynthesis, with the seed yield per plant (Fig. 4a), total dry matter per plant (Fig. 4b) and the harvest index (Fig. 4c) were compatible with present findings. By increasing PIABS, total, crop yields also significantly increased (Fig. 4a-c).
Fig. 4.
Correlation of total absorbed performance index (PIABS, total) with seed yield (a), total dry matter (b), and harvest index (c) of sorghum cultivars under salinity at p ≤ 0.05
Discussion
Pigment contents and ratios
The decrease in pigment contents and ratios in this study was in accordance with Netondo et al. [20] who reported a decline of chlorophyll-a, chlorophyll-b, carotenoid and chlorophyll a to b ratio in salt-tolerant cultivars of sorghum by increasing NaCl. Similar results were obtained in moderately salt-tolerant cultivars of soybean [3, 6, 21, 22] and okra [Abelmoschus esculentus (L.) Monech] [2]. On the other hand, chlorophyll-a, chlorophyll-b, total chlorophyll, and total carotenoid of a salt-sensitive cultivar of wheat decreased sharply by 250 mM NaCl in the study by Kang et al. [4]. In all these researches, lipid peroxidation and reactive oxygen species (ROS) played an essential role in damaging various photosynthetic membranes where photosynthetic pigments were bound. In addition, salinity decreased the chlorophyll index (CI) and total chlorophyll of salt-sensitive cultivars of soybean [6] and tomato [7].
Xing et al. [5] indicated that total chlorophyll content of a salt-tolerant cultivar of rice decreased by increasing salinity. Total chlorophyll was significantly higher than the control under a mild saline stress, however, it significantly decreased under a severe saline stress. The result was related to chloroplast membranes faced with moderate and severe saline stresses. Furthermore, the destruction of the thylakoid membrane structure was another reason for reducing the affinity between chlorophylls and proteins of the chloroplast. Subsequently, this situation lowered the activity of chlorophyll synthesis enzymes causing a decrease in chlorophyll stability and the breakdown of the chlorophyll without replacing it by enzymatic synthesis [5].
On the contrary, chlorophyll-a, chlorophyll-b, and total chlorophyll of salt-tolerant cultivars of wheat were increased under salinity levels more than 50 mM NaCl [23]. Nevertheless, no significant differences were seen among 100, 150, and 200 mM NaCl [23], which might be due to positive effects of salinity stress resulting in leaf thickness. This result was observed in a recent study exploring more chlorophyll contents per leaf area [23]. In addition, the ratio of chlorophyll-a to -b was significantly greater under the control rather than high saline levels. However, it was insignificant between control and low saline levels [23]. Raja Babu and Vijayalakshmi [24] reported more reduction in chlorophyll-b rather than chlorophyll-a in salt-sensitive cultivars of rice, indicating the susceptibility of chlorophyll-b to saline stress.
The pigment contents of pak choi [Brassica campestris ssp. Chinensis] decreased under 50 and 150 mM NaCl, however, pigment contents behaved diversely among salt-sensitive and salt-tolerant cultivars [25]. In a salt-sensitive cultivar, low sodium stress (50 mM NaCl) just decreased chlorophyll-a while it did not affect chlorophyll-b and total chlorophyll. However, high sodium stress (150 mM NaCl) reduced chlorophyll-a, chlorophyll-b and total chlorophyll of the salt-sensitive cultivar. Nonetheless, the ratio of chlorophyll a to b remained constant under both NaCl concentrations. In a moderately salt-tolerant cultivar, chlorophyll-a, chlorophyll-b, and total chlorophyll decreased under both NaCl concentrations. Nevertheless, the ratio of chlorophyll a to b remained almost unchanged. In a salt-tolerant cultivar, low sodium stress (50 mM NaCl) reduced chlorophyll-a while it raised chlorophyll-b and the ratio of chlorophyll a to b. However, total chlorophyll was not influenced by 50 mM NaCl. High sodium stress (150 mM NaCl) declined chlorophyll-a and total chlorophyll whereas chlorophyll-b was not varied. The ratio of chlorophyll a to b was rather greater under 150 mM NaCl [25].
Chlorophyll fluorescence
Extracted and technical parameters of chlorophyll-a fluorescence
The findings of this experiment were similar to the study of Yamane et al. [26], in which FO and FM decreased as salt-sensitive and salt-tolerant cultivars of rice were subjected to 75, 100, 150, and 200 mM NaCl. As well, Kafi [23] reported FM and TM were lowered by salinity in salt-tolerant cultivars of wheat [23]. It is known that photosynthesis including photochemical reactions is limited under saline conditions [1–7, 20, 22–26]. It is attributed to draught, degradation of chlorophyll molecules and also limitation of chlorophyll synthesis caused by salinity [1, 5, 7, 20, 23–26].The result of this situation is excessive energy that cannot be converted to electron flux and may damage the photosynthetic apparatus [13, 15, 18]. To prevent the damage, the excessive energy must dissipate rapidly and the most fast and easiest way to dissipate the excessive energy is fluorescence [13, 15, 18].
The increase in fluorescence parameters was observed in most of the stresses. FO, FM, and FV increased in different cultivars of maize and rice under zinc and cadmium stresses, respectively [27, 28]. Nevertheless, these parameters changed differently in chilling stress [29]. As FO rose under chilling, FM behaved vice versa [29]. In addition, an increase in closure of reaction centers at the J-step (VJ) and PSII non-photochemical loss (LD) and also a decrease in plastoquinone pool size were observed in different cultivars of potassium-deficient soybean [30]. Ripley et al. [12] observed a decrease in the density of the reaction centers per absorption energy flux (RC/ABS) in sorghum under low phosphorous. Similar responses were recorded in different cultivars of maize and rice under zinc and cadmium stresses, respectively [27, 28].
Flux ratios (quantum yields and quantum efficiencies) of chlorophyll-a fluorescence
Quantum yields and quantum efficiencies, referred to as flux ratios, are parameters to calculate the amount and the efficiency of absorbing quantums through photochemical reactions of the photosynthesis [10–15, 18]. These parameters can help us decipher the state of the quantum absorbing from solar energy in the leaf. It is understood that quantum yields and quantum efficiencies decrease under harsh situations including stresses [1–7, 10–15]. These parameters show the stress influences on absorbing and converting solar energy in photosynthesis [10–15].
Netondo et al. [20] found a particularly significant sharp drop of ΦPo as well as a mild fall of the electron transport in salt-tolerant cultivars of sorghum under 150 mM NaCl. These results had been related to the structural changes in PSII and the low effect of salinity on electron transportation, respectively. On the other hand, in Yamane et al. [26], ΦPo decreased under 75 and 100 mM NaCl although FO was unchanged. Yamane et al. [26] attributed these consequences to the swelling of thylakoid in chloroplasts of mesophyll cells exposed to the salinity. This situation destroyed thylakoid membranes and grana structures [26]. As the salinity increased, swollen thylakoids were enhanced in chloroplasts. The swelling of thylakoids under salinity was induced via lipid peroxidation, caused by reactive oxygen species (ROS) such as hydrogen peroxide and hydroxyl radical. In other words, PSII destruction was the direct effect of salinity. A lower decrease in ΦPo was possibly a result of the photoprotection [26].
Kafi [23] reported similar findings in salt-tolerant cultivars of wheat. Although ΦPo was significantly reduced with an increase under NaCl concentrations, it did not change up to 100 mM. A low value of ΦPo might be a sign of disturbance in RuBP (ribulose-1, 5-bisphosphate) regeneration under salt-stressed conditions, which seriously depends on the electron transmission from PSII to the electron acceptors [23].
A decline of quantum yields and quantum efficiencies was reported in other stresses. As reported by Ripley et al. [12], the proportion of electron transport to heat dissipation (ETO/DIO) of sorghum decreased due to low phosphorous application. This result was compatible with the present study. The decrease of phosphorus, as a key element of intermediates participating in the electron transport, led to reduced ETO/DIO. On the other hand, increasing arsenic decreased TRO/DIO via phosphorus deficiency in soybean [31]. It was related to the vital role of phosphorus in all energy cycles of biosystems [31]. Moreover, TRO/DIO was affected by a combination of zinc absence and drought, which drastically lowered TRO/DIO in maize [27].
Specific energy fluxes and phenomenological energy fluxes of chlorophyll-a fluorescence
Specific energy fluxes indicate the state of energy flux of photons in reaction centers of PSI and PSII whereas phenomenological energy fluxes describe the state of energy flux of photons in the cross section between PSI and PSII (Supplementary Information 2d, e) [10–15, 18, 31–33]. It is important to know what happens to photons inside the photosystems (the reaction centers) and between the photosystems (the cross- section) during photochemical reactions of the photosynthesis [10–15, 18, 32]. Therefore, measuring these parameters can help us to follow the state of the photon energy in the photosynthesis especially under stress conditions. However, these parameters have been rarely discussed in chlorophyll-a fluorescence research.
In agreement with the findings of this study, it has been reported that the absorbed energy flux per reaction centers (ABS/RC), the trapped energy flux per reaction centers (TRO/RC) and the energy dissipation as heat per reaction centers (DIO/RC) significantly increased in sorghum under low phosphorous stress [12]. This result was related to the decrease in the amount of phosphorus-containing intermediates participating in the electron transport [12]. However, the electron transport flux per reaction centers (ETO/RC) was not influenced by the stress [12].
On the other hand, taking into account that heat dissipation is the excessive photon absorption that cannot be trapped by the reaction centers, DIO/RC may be influenced by the ratio of active to inactive reaction centers in photosystems [12]. Accordingly, evaluation of DIO/RC can probably predict the proportional amount of inactive reaction centers in photosystems [32]. Similarly, the trapped excessive energy that cannot be transmitted by the cross section must be dissipated as heat per cross section. Hence, DIO/CS can probably be affected by the proportion of active to inactive parts of the cross section. Consequently, calculation of DIO/CS may estimate the relative rate of the inactive cross section.
Performance indices and driving force parameters of chlorophyll-a fluorescence
PIPSI, PIPSII and PItotal exhibit the performance of the electron flux in PSII, PSI and the whole photochemical reactions (total), respectively. However, DFPSI, DFPSII and DFtotal describe the redox potential in PSII, PSI and the whole photochemical reactions (total), respectively [15, 31]. Total performance indices (PIABS, total and PICS, total) and total driving forces (DFABS, total, DFCS, total) evaluate the whole of the photosynthetic apparatus from the first point in PSII toward the end point in PSI [14].
Performance indices are new important high-sensitive fluorescence parameters used as stress indicators that indicate the vitality of the photosynthetic apparatus. These indices are based on four factors including the density of the reaction centers per absorption energy flux (RC/ABS), the maximum quantum yield (ΦPo), the electron transport efficiency from QA to QB (ΨEo) and the electron transport efficiency from QB to the end electron acceptors of PSI (δRo) [14, 15, 30, 31]. In agreement with this finding, Ripley et al. [12] reported that the decrease in the PSII performance index (PIABS, PSII) of sorghum was in relation to significant decreases in ΦPo, ΦEo and ΨEo.
If a stressful condition influences one of these factors, performance indices will display variations clearly and distinctly [14, 15, 30, 31]. Due to this advantage, performance indices are used to screen genotypes in different researches [14, 15, 34]. Van Heerden et al. [11] noted that performance indices facilitated the screening of soybean genotypes much faster, easier, and more economical than other methods under dark chilling stress. Moreover, Tsimilli-Michael and Strasser [14] expressed that the photosynthetic performance indices were the most susceptible parameters for measuring chlorophyll-a fluorescence variations while the commonly used ΦPo was not susceptible enough. On the other hand, driving forces express the performance indices in chemistry language [15]. Actually, driving forces are related to the oxido-reduction reactions of the photochemical process [15].
PIABS, PSII is also dependent on the number of reaction centers [11]. Reaction centers consist of chlorophyll-a molecules containing nitrogen. Therefore, photosystems containing more reaction centers (RC/ABS) can trap more energy through their chlorophyll-a molecules (higher ΦPo = TRO/ABS). Therefore, they display greater electron transport yield (higher ΦEo = ETO/ABS) and lower dissipated energy as heat or fluorescence (lower ΦDo = DIO/ABS, DIO/RC, and DIO/CS) [12]. In accordance with these findings, van Heerden et al. [11] found a rapid increase in PIABS, PSII of different genotypes of soybean under nitrogen application while PIABS, PSII was reduced under nitrogen deficiency.
In addition to nitrogen, phosphorus is reported to be involved in performance indices. Ripley et al. [12] explained that the reduction in the amount of phosphorus-containing intermediates, taking part in the electron transport, led to lower performance indices in eight-week and twelve-week plants of sorghum. It was clear that the absorption of some elements including phosphorous had been limited by the stress.
On the other hand, the drought caused by some stresses such as salinity and chilling may influence the performance indices and the driving forces. Desotgiu et al. [35] reported that stomata closure following a decrease in the leaf water potential led to a decline of PIABS, total in a poplar [Oxford clone] subjected to water stress. Moreover, total driving forces of photosynthesis (DFABS,total and DFCS, total) decreased due to the drought caused by dark chilling stress in different cultivars of soybean [11]. It has been previously proved that water deficiency is one of the earliest negative effects of salinity. Therefore, performance indices and driving force parameters can display well early drought impacts of salinity stress before its late toxic impacts.
Crop yield and the relationship between chlorophyll-a fluorescence of the leaf
The decline of the seed yield, total dry matter and the harvest index has been reported in different cultivars of sorghum under salinity stress [36–38]. It was obvious that the decrease in photochemical reactions of photosynthesis resulted in the decline of crop yields especially the seed yield per plant and the harvest index of the grain cultivar [3, 10, 11, 20, 28, 30]. This result showed that seed yield per plant and the harvest index were more susceptible to salinity rather than biomass production. As well, the unchanged total dry matter up to 150 mM NaCl displayed that total dry matter was tolerant to salinity. Hence, it is possible to produce biomass by cultivating either the grain cultivar or the sweet-forage cultivar of sorghum under high salinity without decreasing the total dry matter. However, seed production of both cultivars decreases the high salinity.
The positive relations clearly presented dependency of crop yields on the capability of the photochemical reactions of the photosynthesis [10–12, 18, 30–34, 39, 40]. In other words, high PIABS, total leads to high crop yields and it may be possible to predict crop yields by measuring chlorophyll-a fluorescence parameters such as PIABS, total [9–13, 15, 17, 27].
Conclusions
Salinity decreased all photosynthetic pigments including chlorophyll-a. The chlorophyll-a fluorescence parameters as functional indicators of photochemical reactions showed drastic effects of increasing salinity on the photosynthesis. Despite the negative effects of NaCl on grain and sweet-forage cultivars of sorghum, both cultivars could control these effects between 100 and 150 mM NaCl. Considering the findings of the present study, it seems that sorghum could tolerate salinity up to 150 mM NaCl.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
(DOC 69 kb)
(DOC 122 kb)
(DOC 61 kb)
Contributor Information
Parvaneh Sayyad-Amin, Phone: +98 26 32227412, Email: p_sayyad_amin@ut.ac.ir.
Mohammad-Reza Jahansooz, Phone: +98 26 32227412, Email: jahansuz@ut.ac.ir.
Azam Borzouei, Phone: +98 26 34464060, Email: aborzouei@nrcam.org.
Fatemeh Ajili, Phone: +98 26 34464060, Email: ajili@nrcam.org.
References
- 1.Jiang Q, Roche D, Monaco TA, Durham S. Gas exchange, chlorophyll fluorescence parameters and carbon isotope discrimination of 14 barley genetic lines in response to salinity. Field Crop Res. 2006;96:269–278. doi: 10.1016/j.fcr.2005.07.010. [DOI] [Google Scholar]
- 2.Madhana Kumari P, Sekar K. Effect of plant growth regulators on chlorophyll and carotenoid content of salinity stressed okra seedlings. Asian J. Hortic. 2008;3:54–55. [Google Scholar]
- 3.Weisany W, Sohrabi Y, Heidari GR, Siosemardeh A, Ghassemi-Golezani K. Physiological responses of soybean [Glycine max L.] to zinc application under salinity stress. Aust. J. Crop. Sci. 2011;5:1441–1447. [Google Scholar]
- 4.Kang G, Li G, Zheng B, Han Q, Wang C, Zhu Y, Guo T. Proteomic analysis on salicylic acid-induced salt tolerance in common wheat seedlings [Triticum aestivum L.] Biochim. Biophys. Acta. 2012;1824:1324–1333. doi: 10.1016/j.bbapap.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 5.Xing W, Wang J, Liu H, Zou D, Zhao H. Influence of natural saline-alkalistress on chlorophyll content and chloroplast ultrastructure of two contrasting rice [Oryza sativa L. japonica] cultivars. Aust. J. Crop. Sci. 2013;7:289–292. [Google Scholar]
- 6.Miransari M, Smith DL. Overcoming the stressful effects of salinity and acidity on soybean nodulation and yields using signal molecule genistein under field conditions. J. Plant Nutr. 2007;30:1967–1992. doi: 10.1080/01904160701700384. [DOI] [Google Scholar]
- 7.Florina F, Giancarla V, Cerasela P, Sofia P. The effect of salt stress on chlorophyll content in several Romanian tomato varieties. J. Hortic. Forest Biotechnol. 2013;17:363–367. [Google Scholar]
- 8.Karimi H, Yusef-Zadeh H. The effect of salinity level on the morphological and physiological traits of two grape [Vitis vinifera L.] cultivars. Int. J. Agron. Plant Prod. 2013;4:1108–1117. [Google Scholar]
- 9.Maxwell K, Johnson GN. Chlorophyll fluorescence—a practical guide. J. Exp. Bot. 2000;51:659–668. doi: 10.1093/jexbot/51.345.659. [DOI] [PubMed] [Google Scholar]
- 10.van Heerden PDR, Tsimilli-Michael M, Kruger GHJ, Strasser RJ. Dark chilling effects on soybean genotypes during vegetative development: parallel studies of CO2 assimilation, chlorophyll a fluorescence kinetics O-J-I-P and nitrogen fixation. Physiol. Plant. 2003;117:476–491. doi: 10.1034/j.1399-3054.2003.00056.x. [DOI] [PubMed] [Google Scholar]
- 11.van Heerden PDR, Strasser RJ, Kruger GHJ. Reduction of dark chilling stress in N2-fixing soybean by nitrate as indicated by chlorophyll a fluorescence kinetics. Physiol. Plant. 2004;121:239–249. doi: 10.1111/j.0031-9317.2004.0312.x. [DOI] [PubMed] [Google Scholar]
- 12.Ripley BS, Redfern SP, Dames J. Quantification of the photosynthetic performance of phosphorus-deficient sorghum by means of chlorophyll-a fluorescence kinetics. S. Afr. J. Sci. 2004;100:615–618. [Google Scholar]
- 13.Strasser, R.J., Tsimilli-Michael, M., Srivastava, A.: Analysis of the fluorescence transient. In: Papageorgiou, G.C., Govindjee (eds.) Chlorophyll Fluorescence: a Signature of Photosynthesis, pp. 321–362. Springer, Dordrecht (2004)
- 14.Tsimilli-Michael, M., Strasser, R.: In vivo assessment of stress impact on plants’ vitality: applications in detecting and evaluating the beneficial role of mycorrhization on host plants. In: Varma, A. (ed.) Mycorrhiza: State of the Art, Genetics and Molecular Biology, Ecofunction,Biotechnology,Eco-physiology, Structure and Systematics, pp. 679–703. Springer, Dordrecht (2008)
- 15.Stirbet A, Govindjee On the relation between the Kautsky effect (chlorophyll a fluorescence induction) and photosystem II: basics and applications of the OJIP fluorescence transient. J. Photochem. Photobiol. B. 2011;104:236–257. doi: 10.1016/j.jphotobiol.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 16.Vanderlip RL. How a sorghum plant develops. Manhattan: Kansas State University; 1993. [Google Scholar]
- 17.Shrestha S, Brueck H, Asch HF. Chlorophyll index, photochemical reflectance index and chlorophyll fluorescence measurements of rice leaves supplied with different N levels. J. Photochem. Photobiol. B. 2012;113:7–13. doi: 10.1016/j.jphotobiol.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 18.Strasser, R.J., Srivastava, A., Tsimilli-Michael, M.: The fluorescence transient as a tool to characterize and screen photosynthetic samples. In: Yunus, M., Pathre, U., Mohanty, P. (eds.) Probing Photosynthesis: Mechanism, Regulation and Adaptation, pp. 445–485. Taylor and Francis, London (2000)
- 19.Wellburn AR. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. Aust. J. Plant Physiol. 1994;144:307–313. doi: 10.1016/S0176-1617(11)81192-2. [DOI] [Google Scholar]
- 20.Netondo GW, Onyango JC, Beck E. Sorghum and salinity: II. Gas exchange and chlorophyll fluorescence of sorghum under salt stress. Crop Sci. 2004;44:806–811. doi: 10.2135/cropsci2004.8060. [DOI] [Google Scholar]
- 21.Wang D, Wilson C, Shannon MC. Interpretation of salinity and irrigation effects on soybean canopy reflectance in visible and near-infrared spectrum domain. Int. J. Remote Sens. 2002;23:811–824. doi: 10.1080/01431160110070717. [DOI] [Google Scholar]
- 22.Wang D, Grieve CM, Suarez DL. Composition of irrigation water salinity affects growth characteristics and uptake of selenium and salt ions by soybean. J. Plant Nutr. 2005;28:1073–1088. doi: 10.1081/PLN-200058909. [DOI] [Google Scholar]
- 23.Kafi M. The effects of salinity and light on photosynthesis, respiration and chlorophyll fluorescence in salt-tolerant and salt-sensitive wheat [Triticum aestivum L.] cultivars. J. Agric. Sci. Technol. 2009;11:535–547. [Google Scholar]
- 24.Raja Babu C, Vijayalakshmi C. Impact of salt stress on chlorophyll fraction in rice [Oryza sativa L.] leaves. Plant Arch. 2008;8:969–971. [Google Scholar]
- 25.Memon SA, Wang LJ, Hou XL. Effect of 5-aminolevulinic acid (ALA) on antioxidative enzymes, chlorophyll content, and photosynthesis of Pakchoi [Brassica campestris ssp. Chinensis] under salt stress. Int. J. Agric. Sci. Technol. 2013;9:1333–1346. [Google Scholar]
- 26.Yamane K, Kawasaki M, Taniguchi M, Miyake H. Correlation between chloroplast ultrastructure and chlorophyll fluorescence characteristics in the leaves of rice [Oryza sativa L.] grown under salinity. Plant Prod. Sci. 2008;11:139–145. doi: 10.1626/pps.11.139. [DOI] [Google Scholar]
- 27.Wang H, Liu RL, Jin JY. Effects of zinc and soil moisture on photosynthetic rate and chlorophyll fluorescence parameters of maize. Biol. Plant. 2009;53:191–194. doi: 10.1007/s10535-009-0033-z. [DOI] [Google Scholar]
- 28.He JY, Ren YF, Zhu C, Yan YP, Jiang DJ. Effect of Cd on growth, photosynthetic gas exchange, and chlorophyll fluorescence of wild and Cd-sensitive mutant rice. Photosynthetica. 2008;46:466–470. doi: 10.1007/s11099-008-0080-2. [DOI] [Google Scholar]
- 29.Lin KH, Hwang WC, Lo HF. Chilling stress and chilling tolerance of sweet potato as sensed by chlorophyll fluorescence. Photosynthetica. 2007;45:628–632. doi: 10.1007/s11099-007-0108-z. [DOI] [Google Scholar]
- 30.Li XT, Cao P, Wang XG, Cao MJ, Yu HQ. Comparison of gas exchange and chlorophyll fluorescence of low-potassium-tolerant and -sensitive soybean [Glycine max (L.) Merr.] cultivars under low-potassium condition. Photosynthetica. 2011;49:633–636. doi: 10.1007/s11099-011-0073-4. [DOI] [Google Scholar]
- 31.Milivojevic DB, Nikolic BR, Drinik G. Effects of arsenic on phosphorus content in different organs and chlorophyll fluorescence in primary leaves of soybean. Biol. Plant. 2006;50:149–151. doi: 10.1007/s10535-005-0092-8. [DOI] [Google Scholar]
- 32.Falqueto AR, Silva FSP, Cassol D, Magalhaes Junior AM, Oliveira AC, Bacarin MA. Chlorophyll fluorescence in rice: probing of senescence driven changes of PSII activity on rice varieties differing in grain yield capacity. Braz. J. Plant Physiol. 2010;22:35–41. doi: 10.1590/S1677-04202010000100004. [DOI] [Google Scholar]
- 33.Cuchiara CC, Silva IMC, Martinazzo EG, Braga EJB, Bacarin MA, Peters JA. Chlorophyll fluorescence transient analysis in Alternanthera tenella Colla plants grown in nutrient solution with different concentrations of copper. J. Agric. Sci. 2013;5:8–16. [Google Scholar]
- 34.Kalaji, H.M., Guo, P.: Chlorophyll fluorescence: a useful tool in barley plant breeding programs. In: Sanchez, A., Gutierrez, S.J. (eds.) Photochemistry Research Progress, pp. 439–463. Nova Science Publishers, Hauppauge (2008)
- 35.Desotgiu R, Pollastrini M, Cascio C, Gerosa G, Marzuoli R, Bussotti F. Chlorophyll a fluorescence analysis along a vertical gradient of the crown in a poplar [Oxford clone] subjected to ozone and water stress. Tree Physiol. 2012;32:976–986. doi: 10.1093/treephys/tps062. [DOI] [PubMed] [Google Scholar]
- 36.Hussein MM, Abdel-Kader AA, Kady KA, Youssef RA, Alva AK. Sorghum response to foliar application of phosphorus and potassium with saline water irrigation. J. Crop Improv. 2010;24:324–336. doi: 10.1080/15427528.2010.499042. [DOI] [Google Scholar]
- 37.Begdullayeva T, Kienzler KM, Kan E, Ibragimov N, Lamers JPA. Response of Sorghum bicolor varieties to soil salinity for feed and food production in Karakalpakstan, Uzbekistan. Irrig. Drain. Syst. 2007;21:237–250. doi: 10.1007/s10795-007-9020-8. [DOI] [Google Scholar]
- 38.Amzallag GN. Perturbed reproductive development in salt-treated Sorghum bicolor: a consequence of modifications in regulation networks? J. Exp. Bot. 2005;56:2821–2829. doi: 10.1093/jxb/eri274. [DOI] [PubMed] [Google Scholar]
- 39.Srivastava A, Strasser RJ, Govindjee Greening of peas: parallel measurements of 77 K emission spectra, OJIP chlorophyll a fluorescence transient, period four oscillation of the initial fluorescence level, delayed light emission, and P700. Photosynthetica. 1999;3:365–392. doi: 10.1023/A:1007199408689. [DOI] [Google Scholar]
- 40.Stefanov D, Terashima I. Non-photochemical loss in PSII in high- and low-light-grown leaves of Vicia faba quantified by several fluorescence parameters including LNP, FO = F’M, a novel parameter. Physiol. Plant. 2008;133:327–338. doi: 10.1111/j.1399-3054.2008.01077.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 69 kb)
(DOC 122 kb)
(DOC 61 kb)