Abstract
Dental pulp is an accessible source of multipotent mesenchymal stromal cells (MSCs). The perspective role of dental pulp stem cells (DPSCs) in regenerative medicine demands an in vitro expansion and in vivo delivery which must deal with the safety issues about animal serum, usually required in cell culture practice. Human platelet lysate (PL) contains autologous growth factors and has been considered as valuable alternative to fetal bovine serum (FBS) in cell cultures. The optimum concentration to be added of such supplement is highly dependent on its preparation whose variability limits comparability of results. By in vitro experiments, we aimed to evaluate a standardised formulation of pooled PL. A low selected concentration of PL (1%) was able to support the growth and maintain the viability of the DPSCs. The use of PL in cell cultures did not impair cell surface signature typically expressed by MSCs and even upregulated the transcription of Sox2. Interestingly, DPSCs cultured in presence of PL exhibited a higher healing rate after injury and are less susceptible to toxicity mediated by exogenous H2O2 than those cultured with FBS. Moreover, PL addition was shown as a suitable option for protocols promoting osteogenic and chondrogenic differentiation of DPSCs. Taken together, our results indicated that PL is a valid substitute of FBS to culture and differentiate DPSCs for clinical-grade use.
1. Introduction
Dental pulp stem cells (DPSCs) are multipotent stromal/mesenchymal stem cells (MSCs) representing a source, free of ethical issues, of replacement cells worthwhile in human therapies. From a clinical point of view, it is necessary to avoid any immunological reaction to the xenogenic material; then the investigation on xeno-free ex vivo expansion of MSCs is highly encouraged. Many platelet derivates were tested so far in different forms for cell culture to replace animal serum supplementation. Currently, the use of human platelet lysate (PL) in clinical settings led to many therapeutic successes including orthopaedic, periodontal, oral, and maxillofacial surgeries [1]. PL is biocompatible and has no risk of transmissible viral disease or prionic contamination. Platelet concentrates can be prepared by buffy coat or platelet-rich plasma (PRP) method [2], even from expired blood derivates. Freeze-and-thaw cycles, sonication, and thrombin/CaCl2 activation have been used to obtain PL [2]. The variability in PL manufacturing influences the content of main growth factors (GFs) that will be available in cell culture, such as PDGF isoforms, TGF-β1, and also VEGF, HGF, and bFGF. The inconsistency of the results makes PL functionality in vitro still not exhaustively explored. Furthermore, specific and optimal culture conditions will be searched [3, 4] especially for MSCs [5].
DPSCs received great attention for their osteogenic potential; besides they demonstrated an angiogenic potential [6], a commitment to melanogenesis [7], differentiation in neurons [8], and islet-like aggregates formation [9]. The transcriptomes and cytogenetical stability of DPSCs expanded under PL and FBS were previously compared and revealed similar profiles [10]. Also CFU-potential, the immunophenotype, and trilineage differentiation according to MSC requisites were comparable [10]. 5% PL was defined as the concentration for increased cellular proliferation and for good mineralisation in vitro for DPSCs [10–12]. In addition, the same concentration was used to seed DPSCs on biomaterials, showing positive effects on regeneration in vivo [11]. Often 10% PL inhibited the proliferation of DPSCs. Interestingly, DPSC cultures in PL had a higher ALP activity compared to FBS [11, 12]. We initially combined different assays to select an optimal PL concentration for DPSCs growth, by evaluating different cell health indicators, such as membrane permeability, cell division, and metabolic activity. The comparison between FBS and the selected PL concentration for mesenchymal stem cell capacity was evaluated by surface marker expression and gene expression of relevant transcriptional factors.
We focused on PL effects on repairing properties of DPSCs by performing in vitro migration and survival assay. Finally, we introduced PL in osteogenic and chondrogenic inducer media in order to evaluate its effect on DPSCs differentiation.
We characterised a standardised commercially available human allogenic platelet lysate as an attractive candidate for in vitro culture of DPSCs [10]. The use of this safe, quality-controlled, and potentially advantageous supplement could establish a preparatory study for regenerative medicine applications.
2. Materials and Methods
2.1. DPSCs Source and Cell Culture
Human-impacted third molars were extracted from 20-year-old healthy patients from Calabrodental Clinic (Crotone). All donors signed a written informed consent according to the Ethical Committee policy. To isolate DPSCs, the obtained normal teeth were washed in saline solution containing antibiotic solutions and immediately transferred to the cell culture laboratory. Mesenchymal stem cells were isolated under sterile conditions according to a previously published method [13, 14]. Briefly, pulp tissues were isolated and washed several times with PBS and further cut with a scalpel into small pieces. Subsequently, the whole small pieces were dissociated to have single cell suspension by enzymatic digestion, using 3 mg/mL type I collagenase and 4 mg/mL dispase (Sigma-Aldrich, Saint Louis) in Hank's Balanced Salt Solution (Invitrogen, Carlsbad). Then, samples were incubated for 1 h at 37°C in agitation and the digest was diluted in alpha-minimal essential medium (α-MEM, Gibco, Grand Island) supplemented with 10% FBS (Gibco, Grand Island) and centrifuged at 300 ×g for 5 min. The pellet was finally resuspended in fresh medium, seeded in culture dishes, and incubated at 37°C 5% CO2. DPSCs were routinely expanded in growth medium consisting of α-MEM supplemented at 10% FBS. Trilineage differentiation of DPSCs was early assessed and cell stocks were cryopreserved. Two donors were used for the study. PL was purchased from Sclavo Diagnostics (Sovicille).
2.2. Viability Assays
For live/dead imaging, DPSCs were grown on sterile cover slides 24 × 24 mm and cultured for 5 days in FBS or PL media. After formalin fixation, the cells were stained with a solution of Calcein AM-EthD-III (Biotium, Hayward) according to the kit indications. Epifluorescent signal was detected and relative images were acquired through SP5 microscope (Leica, Wetzlar). Metabolic activity of DPSCs was assessed by PrestoBlue (Molecular Probes, Eugene) assay. Briefly, cryopreserved DPSCs were thawed and resuspended in α-MEM containing FBS at 10% or PL at 5%, 2%, and 1%. Cells were seeded at 2500 cell/well in one 96-well plate for each analysed time point; more than 6 wells were replicated for each condition. An aliquot of fresh medium was provided to the plates that were cultured for more than 4 days. The PrestoBlue reagent was incubated as supplier instruction to a final volume of 100 μL; after 2 hours the collected supernatant was measured for absorbance with Multiskan GO (Thermo Fisher, Waltham) spectrophotometer (570–600 nm). An additional control was set using human serum (HS) 10%. For viable cell counts, the samples were trypsinized after 3 days or 3 weeks and measured by ADAM-MC (AlphaMetrix, Rödermark) system to evaluate cell membrane permeability by dye exclusion.
2.3. Flow Cytometry
To investigate cellular proliferation, DPSCs were labelled with 5 μM of 5-chloromethylfluorescein diacetate (CMFDA) in α-MEM for 45 min at 37°C CO2. Cells were washed in medium and seeded at 105/well in a 12 well-plate, in duplicate, for different time points (2, 3, and 4 days). Cytoplasmic amount reduction of the dye was measured using NAVIOS flow cytometer (Beckman Coulter, Brea). The data were analysed with FlowJo software. For immunophenotype analysis, cells cultured for 1 week with 10% FBS or 1% PL were trypsinized and aliquoted in FACS tube. Cells were washed twice with PBS 0,1% BSA. To limit unspecific binding, a blocking step is performed by resuspension of the pellets with PBS 1% BSA for 15 min. Cells were stained on ice for 1 h with saturating concentrations of primary conjugated antibodies diluted 1 : 50 in PBS 0,1% BSA. CD13-PE (mouse IgG1), CD29-APC (mouse BALB/c IgG1), CD44-FITC (mouse IgG2b), CD45-APC-H7 (mouse IgG1), CD73-FITC (mouse IgG1), CD90-PE (mouse BALB/c IgG1), and CD105-APC (Mouse BALB/c IgG1) monoclonal antibodies purchased from BD (Franklin Lakes) and CD146-PE (mouse IgG1), CD34-FITC (mouse IgG2a), and HLA-DR-PE (recombinant human IgG1) monoclonal antibodies purchased from Miltenyi Biotec (Bergisch Gladbach) were used to define the MSC panel as previously described [15]. At least 10000 events were counted for each sample.
2.4. Real-Time PCR
To analyse cell differentiation state, gene expression was analysed as previously described [15, 16]. Briefly, RNA samples were obtained after extraction with PureLink RNA mini kit (Thermo Fisher, Waltham) following the manufacturer's instruction. Total RNA was quantified through spectrophotometry and 500 ng of RNA was subjected to reverse-transcription reaction using the High Capacity RNA-to-cDNA Kit (Applied Biosystem, Foster City). One microliter of cDNA was amplified by real-time PCR with the Power SYBR green PCR Master Mix (Applied Biosystem, Foster City). Real-time PCR reactions were carried out in a PikoReal 96 (Thermo Fisher, Waltham) apparatus with the following conditions: initial denaturation step at 95°C for 10 min, followed by 40 cycles of 10 s at 95°C and 1 min at 60°C. The specificity of PCR products was checked by analysis of melting curves. The expression of each gene was determined from the Ct value, and relative expression levels were calculated using the ΔΔCt method after normalisation to the expression of the HRPT housekeeping gene. All primer pairs sequences are listed in Table S1 in Supplementary Material available online at http://dx.doi.org/10.1155/2016/7230987.
2.5. Wound Scratch Assay
For scratch assay, DPSCs were seeded and grown until confluence in 35 mm dishes. An in vitro wound (600 μm average size) was created in the monolayer by scraping it over the total diameter with a sterile 10 μL pipette tip. Dishes were washed twice in α-MEM to smooth the scratch edges and remove any suspended cells. Cultures were refed with fresh complete medium containing 10% FBS or 1% PL. Reference markings were made on the external surface of the dishes to identify scraped zones to be photographed 2 hours and 24 hours after scratch. To quantify the wound healing capacity, images were manually analysed. A digital rectangle zone free of cells and centred on the wound breadth was set on 2-hour controls and thus was superimposed on the corresponding 24-hour images. Cell dense regions were drawn trough polygonal selection tool and measured by ImageJ software. Multiple selections were summed and healing was determined as percentage of the open (wound) area at 24 hours.
2.6. Chemotaxis Assay
An under-agarose method was adapted from Vogel et al. [17] Briefly, sterile melt 1% agarose in α-MEM was poured in 60 mm dishes. Three equally distant wells (≈5 mm) were made by pressing a sterile tip on the agarose gel surface. The gel was equilibrated overnight in α-MEM. DPSCs were harvested at exponential phase, washed in α-MEM, and seeded in the central well at concentration of 3.5 × 104 cells/70 μL. A volume of 70 μL medium containing 10% FBS or 1% PL was added to the right well in order to create a chemoattractant gradient in culture, while a same volume of α-MEM was added to the left well as negative control of chemotaxis. After 24 hours of incubation at 37°C 5% CO2, both left and right wells were refilled of their content. At 48 hours, the dishes were gently washed and fixed in formalin 5% overnight at 4°C. Four pictures (10x zoom) were taken at 24 hours for quantitative analysis at each interwell's zone. The images were tiled and cell number was manually counted in ImageJ software. Only cells completely outside the well and under the agarose were considered. The counted number of migrated cells is corrected subtracting the number of cells which moved towards the negative control well.
2.7. Cell Survival Assay
The damaging effect of millimolar concentrations of H2O2 on DPSCs was tested in dose-dependent manner after 1-hour exposition in basal medium (BM) which consisted of α-MEM. PrestoBlue assay was performed after 48 hours to detect the challenge on viability [18, 19]; therefore an EC50 of 500 μM was established for following experiments (Fig. S3). For cell survival test, DPSCs were seeded as 3.5 × 104 DPSCs/well of a 96-well plate and left to adhere in complete media, 10% FBS or 1% PL. On the next day, all the wells were washed with PBS and 500 μM H2O2 was added in basal medium (BM) or complete medium (CM) containing 10% FBS or 1% PL. Subsequently, cells were treated for 1 h in incubator. At the end of the treatment, H2O2 dilution was replaced with new complete medium. After 6 hours, at 37°C 5% CO2, PrestoBlue was incubated for 2 hours and its absorbance read. Alternatively, DPSCs were seeded in 12-well plates, treated as above, and trypsinized 6 hours later to perform viable counting by ADAM-MC.
2.8. In Vitro Osteogenic Differentiation
For osteogenic differentiation, growing cells were detached and seeded subconfluently in 60 mm Petri dishes. At 85–90% of confluence, cell medium was changed to osteogenic medium composed of α-MEM with glutamine (Gibco, Grand Island), 1% PL, 0,2 mM L-ascorbic acid-2-phosphate (Sigma-Aldrich, Saint Louis), 100 nM dexamethasone (Sigma-Aldrich, Saint Louis), 10 mM β-glycerophosphate (Sigma-Aldrich, Saint Louis), penicillin-streptomycin solution (Sigma-Aldrich, Saint Louis), and 0.25 mg/mL amphotericin B (Sigma-Aldrich, Saint Louis). The positive control group medium included 20% FBS in place of PL. Osteogenic induction was performed for 4 weeks, replacing media twice a week.
The differentiation was assessed by Alizarin red, quantified via spectrophotometry (405 nm) after dissolution in 10% acetic acid, and analysed by real-time PCR (primer listed in Table S1) for gene expression levels of osteogenic markers.
2.9. In Vitro Chondrogenic Differentiation
For chondrogenesis differentiation, DPSCs were initially detached and seeded subconfluently in 60 mm dishes. At 85–90% of confluence, growth medium was changed to chondrogenic medium composed of DMEM High Glucose (Gibco, Grand Island), 1% PL, ITS + 1 Supplement (Sigma-Aldrich, Saint Louis), 100 nM dexamethasone (Sigma-Aldrich, Saint Louis), 50 mg/mL L-ascorbic acid-2-phosphate (Sigma-Aldrich, Saint Louis), and freshly added 10 ng TGF-β1 (Miltenyi Biotec, Bergisch Gladbach). The positive control groups' medium did not include PL in the formula. Chondrogenic induction was performed for 3 weeks, replacing the media twice a week. Alcian blue staining was extracted in acetic acid and its relative quantification was performed by spectrophotometry (620 nm). For micromass culture 5 × 105 cells were pelleted at 300 g and washed in 1 mL of medium free of TGF-β1 and then left to aggregate in incubator in 1 mL of chondrogenic medium (with TGF-β1). Chondrogenic induction was performed for 3 weeks, replacing the media twice a week.
2.10. Histology
In several wound scratch assays, the scraped dishes were fixed and stained with eosin for qualitative late time points (3 days and 7 days) analysis and storage of samples. For osteogenesis confirmation, the dishes were fixed in 10% formalin for 15 min, washed in distilled water, and stained with Alizarin red (5 mg/mL) for 30 min. The samples were then washed several times with distilled water until clarity. For chondrogenesis confirmation, wells were fixed in 10% formalin for 15 min, washed in distilled water, and stained with Alcian blue pH 2.5 (Bio-Optica, Milan) following the supplier's indications. Finally, the samples were washed twice in abundant distilled water. Chondrogenic micromasses were formalin-fixed overnight, embedded into agarose block, and processed for classical histology. Sections of 3,5 μm thickness were prepared using microtome (Leica). The slides were finally stained with Alcian blue and counterstained in Azocarmine Red (Bio-Optica, Milan). All histological samples were photographed for qualitative analysis using optical microscopes and color cameras (Leica suite).
2.11. Statistics
All the plots were edited in GraphPad Prism software. The specific statistical method to interpret the graphs is described within the individual captions. Differences were considered statistically significant when P < 0.05. ∗ P < 0.05; ∗∗ P < 0.01; ∗∗∗ P < 0.001; ∗∗∗∗ P < 0.0001.
3. Results
3.1. Viability and Proliferation
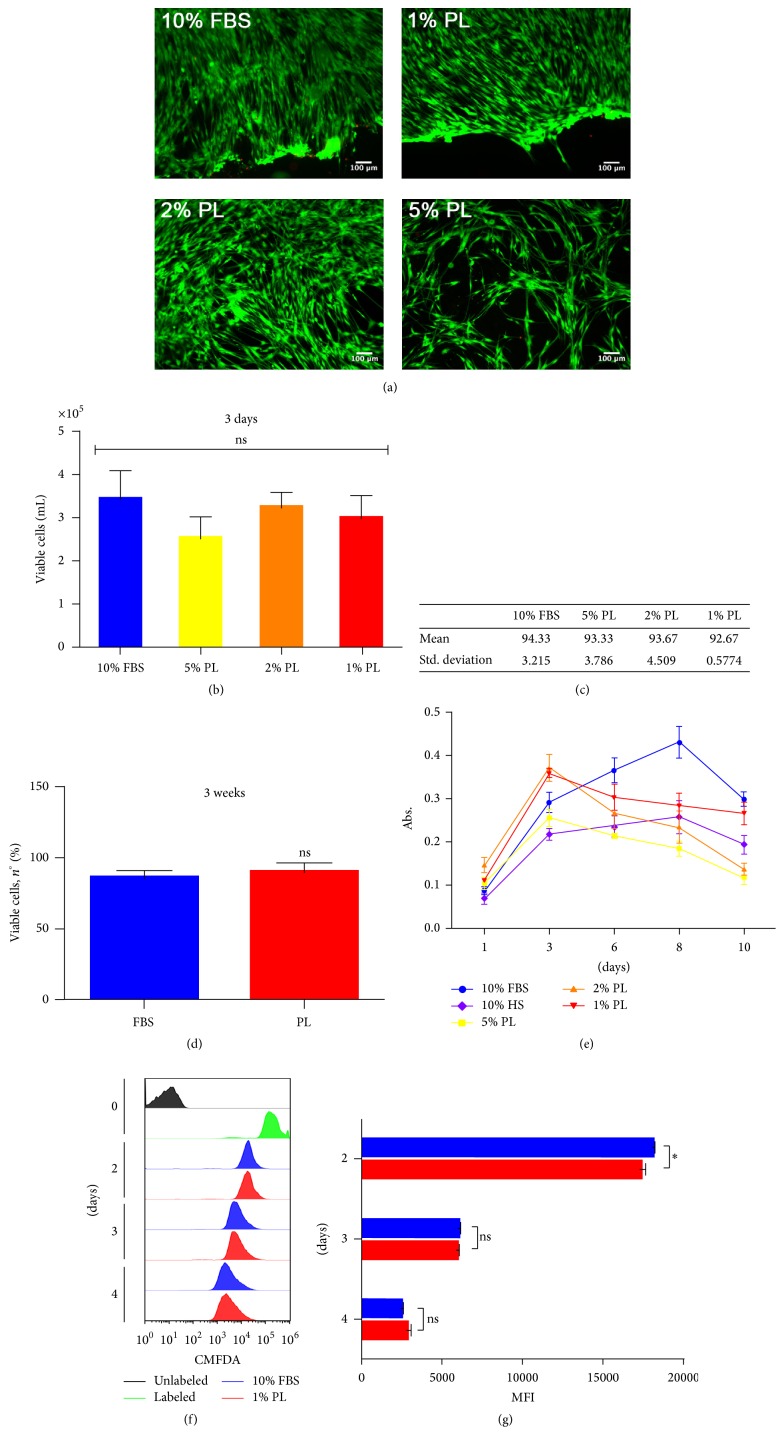
The effect of different concentrations of PL ranging from 5% (high) to 1% (low) was initially compared to 10% FBS by observing viability parameters. The imaging of DPSCs, grown at high density for 5 days and marked with fluorescent live/dead staining, reported a high viability rate in all compared conditions, with only rare detection of dead cells across the whole sample (Figure 1(a)). Slightly morphological changes appeared in 2% (mid) and 5% (high) concentrations of PL, where long intercellular processes and circular association were visible at minor density. After 3 days of culture, no statistically significant difference among the conditions was shown, despite the fact that the average number of cells grown with 5% PL seemed to be lower (Figure 1(b)). Through relative viability quantification of the counted samples, we confirmed the live/dead staining qualitative results (Figure 1(c)). Viable counts of long-term cultures showed that 1% PL is still competitive in viability with respect to 10% FBS (Figure 1(d)). Different trends of metabolic activity were emphasised instead by PrestoBlue assay (Figure 1(e)). In particular, compared to FBS, both 1% and 2% PL showed a higher metabolic rate during the first days of culture, while the 5% PL never outdid FBS. Despite the fact that 1% PL and 2% PL showed a similar trend, 1% concentration brought fewer changes during the last days and presented more similarity to FBS controls until the plateau growth phase (10 days). To further assess if this low PL concentration was sufficient to promote DPSCs proliferation, cells within first days of culture were analysed by flow cytometry for the CMFDA tracer (Figure 1(f)). CMFDA labelling of DPSCs highlighted a significant higher rate of proliferation in 1% PL compared to 10% FBS during 48 hours from seeding (Figure 1(g)).
Figure 1.
Effect of PL on the growth of DPSCs. (a) Representative images from live/dead fluorescence assay after 5 days of culture in different culture conditions. Green = live; red = dead. (b) Viable counts quantified by ADAM system after 3 days of culture. Data shown as mean + SD, n = 3, statistical significance according to 2-way ANOVA method (P < 0.05); ns: not significant. (c) Statistic values (average viability percentages) derived from samples in (b). (d) Viability after long-term culture of 3 weeks. Data shown as mean + SD, n = 2. ns: not significant according to unpaired t-test. (e) Cell growth activity measured by PrestoBlue assay. Data shown as mean + SD, n = 2. (f) CMFDA proliferation assay. Time-course comparing FBS and PL-selected condition. (g) Quantification of MFI derived from samples in (f). Statistics based on 2-way ANOVA method (∗ P < 0.05). ns: not significant.
3.2. Stem Cell Properties
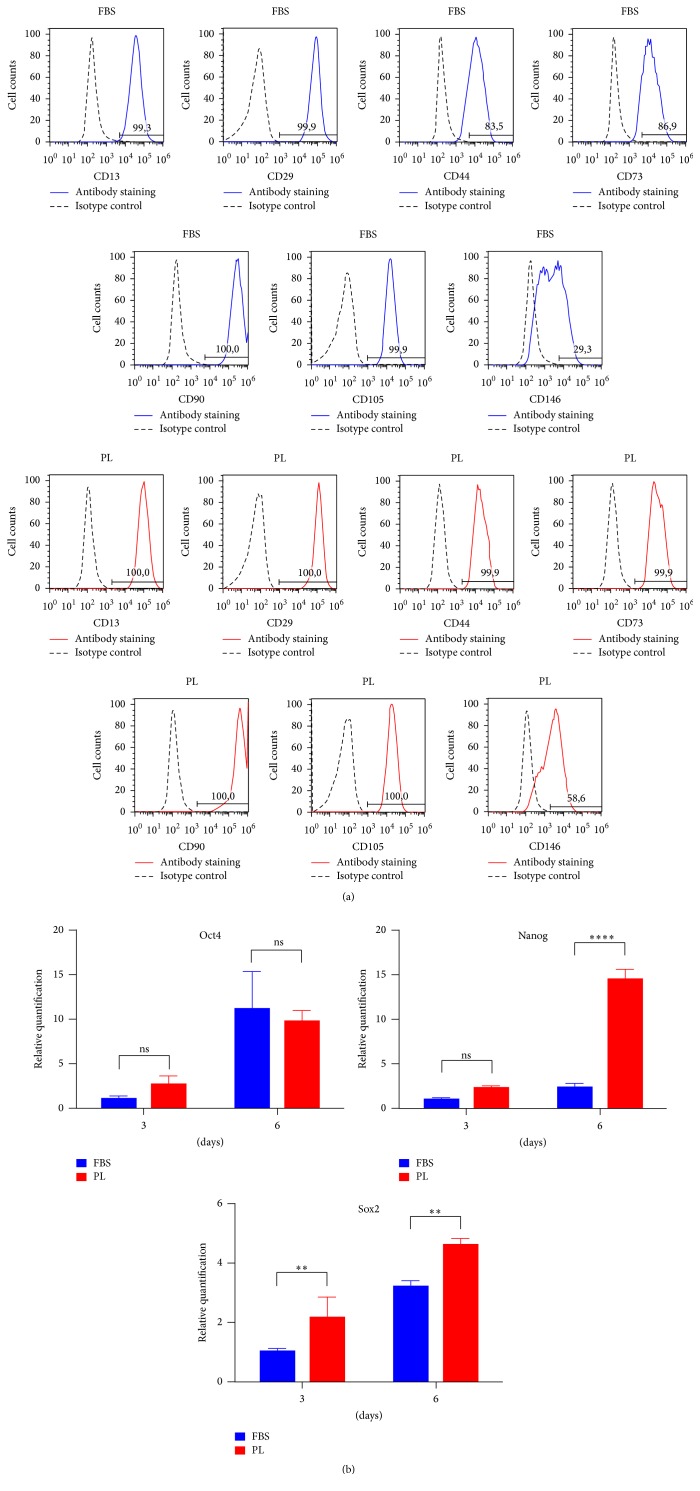
To verify the identity of MSCs after the switch to 1% PL as supplement in cell medium, the DPSCs were analysed for MSCs surface markers by flow cytometry. The immunophenotype of DPSCs cultured in 1% PL resulted in coexpression of the obligatory ISCT markers [20] CD73, CD90, and CD105 and also the positive signal by CD13, CD29, CD44, and CD146 (Figure 2(a)), while it did not express negative markers such as CD34, CD45, and HLA-DR (Fig. S1). Intriguingly, the half population of DPSCs increased their CD146 expression when cultured in 1% PL. Compared to their controls, fluorescence geometric mean of PL is higher for CD13 and CD73 (data not shown). OCT4, Nanog, and Sox2 pluripotency markers were evaluated by quantitative PCR (Figure 2(b)). Samples cultured with PL until 6 days maintained the level of transcription of OCT4 compared to those cultured with FBS. On the other hand, PL supplementation timely increased Sox2 transcripts levels, while Nanog levels were significantly higher only at the last time point.
Figure 2.
Stem cell markers comparison between FBS and PL cultures of DPSCs. (a) Immunophenotype for expressed MSC surface markers by flow cytometric analysis. The number specifies the percentage of gated cells positive for the indicated protein. Data representative of two experiments. (b) Relative levels of transcripts (fold change) for stemness markers derived from real-time PCR analysis. Statistical significance determined by 2-way ANOVA (∗ P < 0.05; ∗∗ P < 0.01; ∗∗∗ P < 0.001; ∗∗∗∗ P < 0.0001), mean + SD, n = 3.
3.3. Migration Capacity
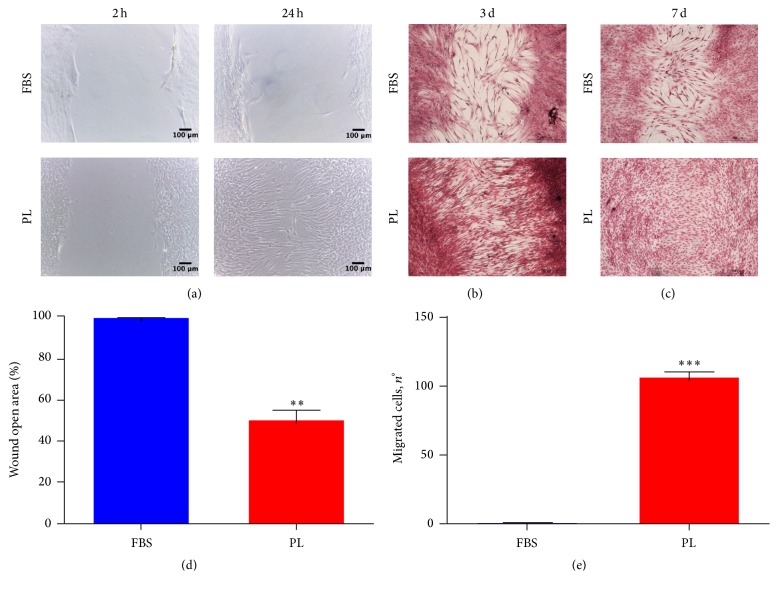
Platelet-derived GFs are involved in wound healing process [4]; thus we performed a scratch assay. Capturing images of different time points, we highlighted a greater level of wound closure for DPSCs scraped and then maintained in low-PL medium rather than in FBS medium. The enhanced cell migration in PL cultures from the wound edges is clearly evident 24 hours later (Figure 3(a)). Moreover, DPSCs cultured in low-PL medium continued to reduce the gap space after 3 and 7 days with a faster rate in comparison to FBS cultures (Figure 3(b)). One week after scratch, DPSCs in 1% PL were able to fully close the open wound area (Figure 3(c)). We also microscopically detected cell division in the wound space (Fig. S2), thus not restricting the repairing stimulation of PL to the migration of cells that were already present before the injury. Moreover, we counted the number of cells migrated at 24 h to have a quantitative result (Figure 3(d)). To address the question if this migrating ability can be associated with the soluble GFs contained in PL, we performed an under-agarose chemotaxis assay. After 24 hours, a conspicuous number of DPSCs positively responded to chemoattraction by PL, while very few cells migrated under the agarose gel to FBS containing compartment (Figure 3(e)).
Figure 3.
In vitro migration capacity of DPSCs is enhanced by PL. (a) Representative images of early time points during the scratch assay. Images were taken 2 hrs and 24 hrs after the initial scratch and are representative of 3 independent experiments. The cells were fixed and stained at different endpoints as shown in (b) for 3 days and (c) 7 days. (d) Quantitative analysis of wound healing at 24 hrs after scratch. 100% is set as open area 2 h time points. Data shown as mean + SD, n = 3. Statistical significance determined using unpaired t-test (∗∗ P < 0,1). (e) Quantification of chemotaxis derived from under-agarose assay. Data were collected using 24 h time points light microscope images. Data shown as mean + SD, n = 3. Statistical significance determined using unpaired t-test (∗∗∗ P < 0.001).
3.4. Resistance to Cellular Damage Induced by H2O2
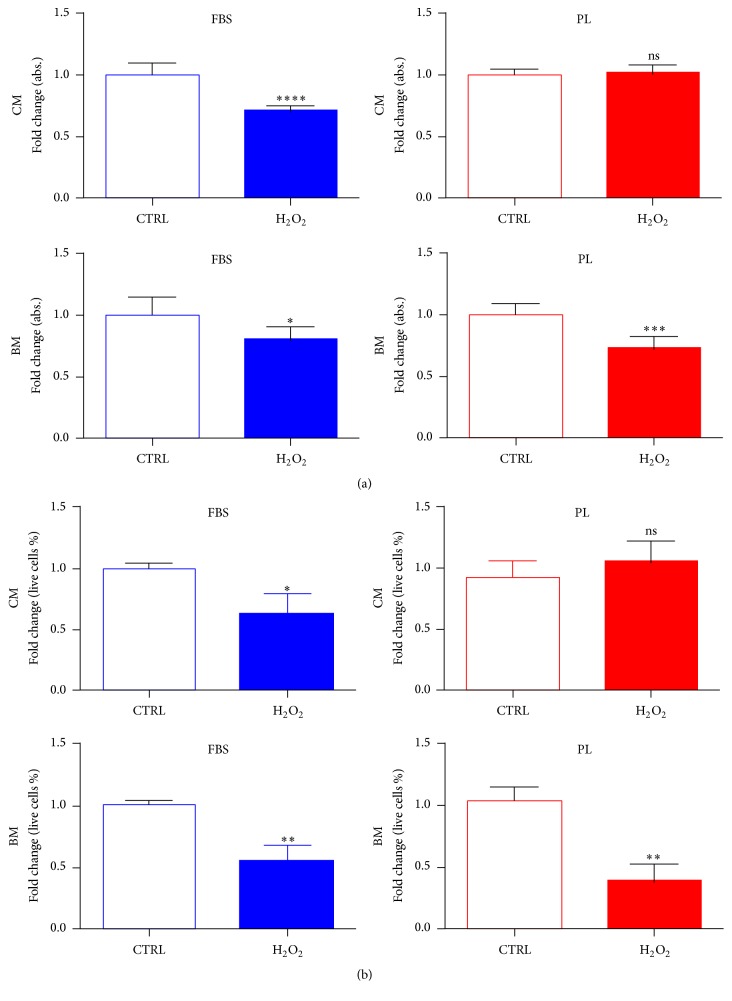
The early effect on survival/recovery of cells treated with 500 μM of H2O2 was analysed either by PrestoBlue assay (Figure 4(a)) or viable counting (Figure 4(b)) after 6 hours from the treatment. The presence in medium of PL before, during, and after treatment (complete media, CM) marked a nonsignificant difference output between untreated and treated samples, thus neutralising or rescuing the cells from the reactive oxygen species (ROS) action. On the contrary, the continuative presence of FBS in the wells (CM) was not sufficient to avoid cytotoxic effects of H2O2. Indeed, DPSCs conditioned in FBS or PL media but then exposed to H2O2 in basal media (BM) incubation reduced their vital activity measured by PrestoBlue reagent and were affected in own membrane integrity noticeable by dye exclusion cell counts.
Figure 4.
Impact of H2O2 on cellular viability is influenced by PL in culture. (a) Metabolic activity of cultures measured by PrestoBlue conversion 6 hrs after the treatment with H2O2. Data are expressed as mean + SD, n = 3, derived from 2 experiments. (b) Automatic viable cell counts after 6 hrs of treatment. Data were normalised to median value of control samples and shown as mean + SD, n = 3. To determine significant results, the unpaired t-test was performed (∗ P < 0.05; ∗∗ P < 0.01; ∗∗∗ P < 0.001; ∗∗∗∗ P < 0.0001). CM, treatment performed in complete medium; BM, treatment performed in basal medium.
3.5. Bony and Cartilaginous Differentiation
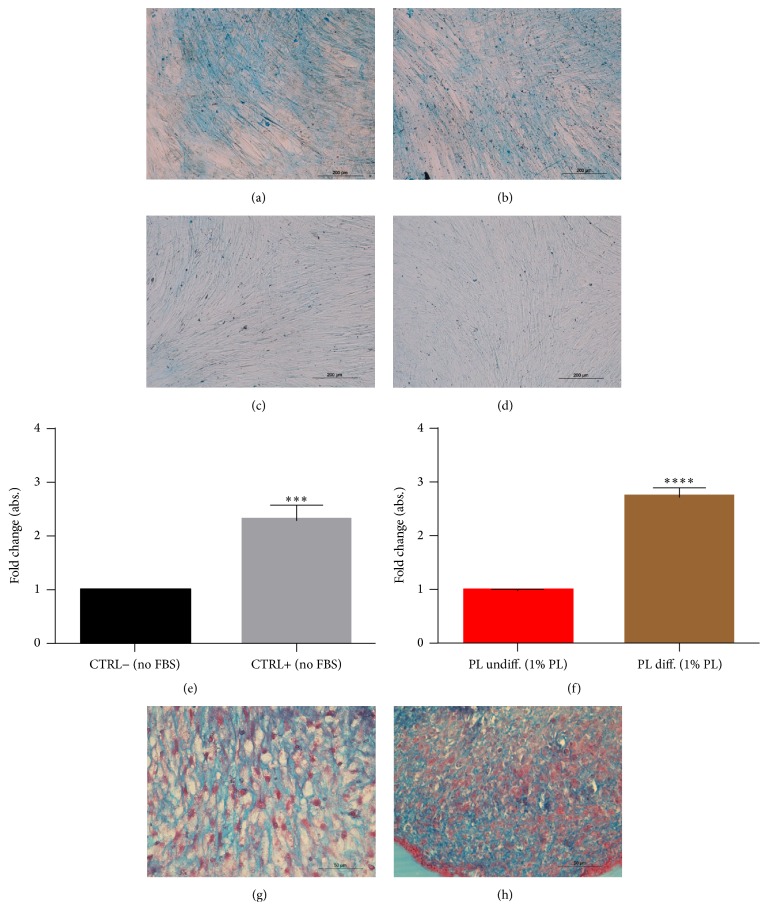
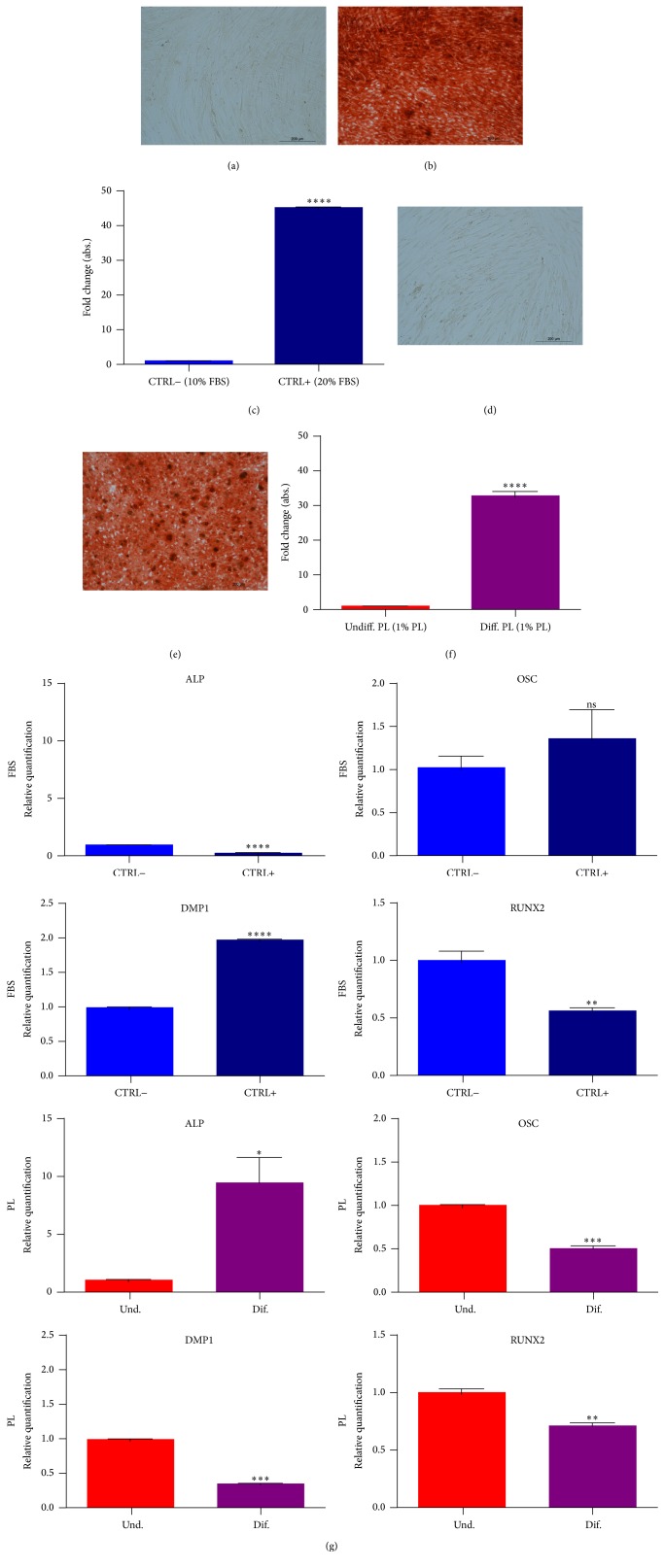
To test the suitability of PL for in vitro differentiation protocols, we basically substituted 20% FBS to 1% PL for osteogenesis of DPSCs; otherwise we added 1% PL to the serum-free formula for chondrogenesis. Results from Alcian blue staining showed the deposition of proteoglycans in both 2D (monolayers, Figures 5(a)–5(d)) and 3D conditions (micromasses, Figures 5(g) and 5(h)), confirming that PL did not impair chondrogenic differentiation of DPSCs. These results were confirmed by spectrophotometric measurement of Alcian blue complex formation (Figures 5(e) and 5(f)). Besides, FBS replacement with PL did not modify the formation of calcium deposits stainable by Alizarin red (Figures 6(a)–6(e)). Again, the differentiating condition in PL was statistically different compared to its respective undifferentiated control, as well as FBS condition (Figures 6(c) and 6(f)). Regarding genes analysis, PL samples showed at the end of differentiation a higher ALP expression compared to the respective basal state, a situation that did not correspond in FBS controls (Figure 6(g)). While FBS seemed to upregulate OSC and DMP-1 osteoblastic markers in 4 weeks in differentiating samples, we cannot affirm the same for PL differentiated cultures, in fact, showing lower relative levels at the same time of analysis (Figure 6(g)). RUNX2 transcription factor had a comparable fold increase in FBS samples as well as in PL samples (Figure 6(g)).
Figure 5.
Effect of PL on chondrogenic differentiation of DPSCs. (a–d) Representative images of DPSC cultured for 21 days in chondrogenic medium without (a) or with (b) PL. They were positively stained by Alcian blue, while cells maintained in 10% FBS (c) or 1% PL (d) growth media were not. (e, f) Quantification of Alcian blue by spectrophotometry for assay controls (e) and samples containing PL (f). Significant levels according to unpaired t-test (∗∗∗ P < 0.001 and ∗∗∗∗ P < 0.0001). Data shown as mean + SD, obtained from three experiments. (g, h) Micromass culture of DPSCs showing positive histological staining for chondrogenesis for control (g) as well as the micromasses cultured with differentiating medium with 1% PL (h).
Figure 6.
Effect of PL on osteogenic differentiation of DPSCs. Alizarin red staining on undifferentiated sample cultured in growth medium with 10% FBS (a) or 1% PL (d) and on samples differentiated with osteogenic medium containing 20% FBS (b) or 1% PL (e). Relative quantification of the same staining by spectrophotometry. Data shown as mean + SD, n = 3 samples. Two different experiments were performed. Significant levels according to unpaired t-test (∗ P < 0.05; ∗∗ P < 0.01; ∗∗∗ P < 0.001; ∗∗∗∗ P < 0.0001). (g) Relative quantification of osteogenic markers by real-time PCR on samples derived from the end (28 days) of the differentiating protocol. Data shown as mean + SD, n = 3. Significant levels according to unpaired t-test (∗ P < 0.05; ∗∗ P < 0.01; ∗∗∗ P < 0.001; ∗∗∗∗ P < 0.0001).
4. Discussion
So far, it has been observed that the proliferation rate of MSCs cultured in PL-supplemented medium was higher than that of those cultured in FBS-supplemented medium [21, 22]. The used concentrations of PL usually ranged from 10% to lesser than 1%, but the great heterogeneity of the results, mostly due to the preparation of PL, still delays the finding of the best concentrations to be used for specific cell type and application. The highest percentages, for example, 10% PL, often were not suitable to culture or expand the cells [11, 12], while 5% PL was the most tested and promising concentration for MSCs [23–26]. Here, we observed a good viability for 5% PL cultures but at the same time we noted resistance to trypsinization at this concentration. Partially, in accordance with other reports [27, 28], the DPSCs had easier and faster detaching from the plate compared to FBS when the concentrations were lower than 5% PL. At 1% PL, we could see that DPSCs attached quicker than FBS after cell seeding. Using the same low-PL concentration, DPSCs displayed a good viability and proliferation profile. Already Lee et al. showed the feasibility of low-percentage supplementation of PL for DPSCs growing and osteogenic differentiation [29]. On the base of our results, we choose 1% PL as selected concentration to adopt in culture for characterising DPSCs stemness, multipotency, and role in repair.
The immunophenotype of DPSCs cultured in PL was completely faithful to MSCs markers panel, confirming previous studies by colleagues [10]. Many stem cell lines express CD13; among the MSCs sources it is particularly present in oral tissues, while it is absent in bone marrow compartment [30]. The lack of this receptor was shown to impair in vitro adhesion to several ECM proteins, migration, and invasion [31]. Our PL cultures of DPSCs consistently expressed CD13. CD13 together with CD29 and CD73 [32] and CD44 [33] were associated with enhanced migrating phenotype of MSCs. CD146 is an endothelial and pericyte marker [34]. It is known to be downregulated in culture with FBS [35]. Moreover, our DPSCs expressed the CD146 receptor on their membrane when cultured in PL in comparison to FBS. This agrees with recent data about CD146 increased expression by BM-MSC in PL medium [24]. Both CD13 and CD146 are angiogenic markers; thus our study generated preliminary data to further include DPSCs angiogenesis protocols. Embryonic stem cell markers, in particular transcription factors such as Oct4, Nanog, and Sox2, were usually thought to have a similar role in maintenance of stemness also in DPSCs [36–38]. In general, they are indicative of a more immature phenotype for MSCs [39]. Nonetheless, it is very hard to understand the impact of their transcripts levels in DPSCs; an increase of their expression sustains a proliferating condition by cells. Our DPSCs cultures in PL sustained a higher gene expression of Sox2 [40], which also plays a role in migration of DPSCs [41].
MSCs can be exposed to various chemical or physical stresses, which may induce a cellular decay. MSCs [42] and HSCs [43] share with leucocytes the capacity of homing to damaged sites in several tissues. It is essential that DPSCs will move to the site of new skeletogenesis or during MSC-mediated tissue repair. Many migration assays are available tools for oral regeneration studies [44]. PL was also already described as efficient in promoting in vitro wound healing of dermal fibroblast [45], C2C12 mouse myoblasts [46], keratinocytes [47], and other human cells [48]. So far, an indirect enhanced migration and chemotaxis by BM-MSCs were induced by coating of platelet lysate on HA/b-TCP scaffolds [49]. In response to the injury, the DPSCs cultured in PL improved their ability to fix an induced mechanical damage such as a wound. In addition, we demonstrated that PL had a superior chemotactic activity than FBS, a reason which is likely correlated to the better migration of DPSCs towards the wounded region. Even though our experimental setting aimed to show how the healing of a scratched monolayer of DPSCs involved just the migration mechanism, surprisingly also cell genesis was active during the reparative process. Oxidative stress can lead to a wide range of cellular damage, such as damage to membrane lipids, proteins, and DNA. It is widely accepted that increasing concentration of free radicals augmented premature senescence even in vitro [50] or associated with bone disorders [51]. The platelets are very reactive in vivo against H2O2 which is a modulator and an efficient inducer of their aggregation [52]. SOD enzyme, also derived from the platelets, inhibited neutrophils excessive production in reactive oxygen species (ROS). Despite the role of platelets in releasing free radicals, their granules contained also the enzyme catalase. Our experiments supposed the rescue of DPSCs treated with H2O2, implying a neutralization action (supported by cell membrane permeability data) or high-level metabolic recovery (mostly mitochondrial). We speculate that the quite antioxidant activity detected with PL could mainly derive from catalase residues in the preparation and may be a beneficial protecting feature for MSCs, if this supplement is used. However, it is not possible to exclude that the less susceptibility to H2O2 is an intrinsic capacity that the cells acquire for the adding of PL. Nonetheless, a deeper comprehension would be desirable; this effect of PL we showed was not observable at the same level for FBS.
Moreover, PL is highly requested for cell-based therapies such as tissue engineering and bone and cartilage regenerative approaches [53]. Chondrogenic and osteogenic potentials were broadly studied and they were retained and influenced by PL added in cultures of MSCs [21, 54, 55]. MSCs cultured in 5% PL were seen to spontaneously activate osteoblastic gene expression [56]. Many authors documented that appropriate concentrations (i.e., 5% PL) enhanced, in particular, DPSCs mineralised differentiation and showed odontogenic potency [11, 12, 24]. The generation of protocols aimed at chondrogenesis taking advantage of PL is an expanding field [10, 21, 25, 57, 58], because PL is a rich source of natural TGF-β and the regenerative potential of primary chondroblasts is more restricted. PL was recently implemented in scaffolds construction [59] and scaffolds functionalization procedures [49]. We assessed the compatibility for the low-percentage concentration of commercial PL to be introduced in osteogenic and chondrogenic traditional protocols for DPSCs. Indeed successful colorimetric detection was measured either for calcium deposits by Alizarin or for proteoglycans by Alcian blue. We found an active gene transcription of the two osteoprogenitor markers ALP and RUNX2. Even if a total improvement in osteogenesis after the supplement comparison cannot be assumed at the selected concentration, the objective advantage of a xeno-free condition outlines an optimal reason to switch for our research-grade protocols in which GFs are delivered from PL. Actually, the positive controls conditions for differentiation were considerably different from testing conditions. Indeed, a 20% FBS and 1% PL, for the osteogenesis, were compared. Respectively, the presence of PL during chondrocytic development was used instead of absence of serum, suggested from the historical protocol [60].
Further studies will helpfully evaluate the feasibility of the use of PL in DPSCs isolation as already described for BM-MSC [23, 54, 61]. Other elucidations are required for the immunomodulation function in the presence of standardised PL preparations, like the one tested in this work. So far, the dual combination of PL with platelet-poor plasma (PPP) was shown to be particularly efficient to boost the number of clonogenic precursors from tissue biopsies, that is, primary cultures of MSCs from bone marrow, umbilical cord, and adipose tissue [62, 63].
5. Conclusion
Taken together, our results suggest that human allogenic PL is a suitable alternative to FBS for expansion and differentiation of DPSCs in vitro. Furthermore, up to our knowledge, this is the first study reporting the influence of PL on DPSCs migration and antioxidant effect during ex vivo maintenance.
Supplementary Material
The supplementary material provides the immunophenotype comparison for negative MSC markers, the images of cell growth occurring during in vitro wound closure, the dose response cytotoxic effect of H2O2, the primer list used for RT-qPCR experiments.
Acknowledgments
The present study was supported by ICARE Project-Infrastruttura Calabrese per la medicina Rigenerativa: “generazione di biobanche per la criopreservazione di cellule staminali umane e di tessuto osseo per uso clinico e design e sviluppo di bioscaffold innovativi” (PON03PE_00009_2.2).
Competing Interests
The authors declare that they have no competing interests.
References
- 1.Tatullo M., Marrelli M., Paduano F. The regenerative medicine in oral and maxillofacial surgery: the most important innovations in the clinical application of mesenchymal stem cells. International Journal of Medical Sciences. 2015;12(1):72–77. doi: 10.7150/ijms.10706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shih D. T.-B., Burnouf T. Preparation, quality criteria, and properties of human blood platelet lysate supplements for ex vivo stem cell expansion. New Biotechnology. 2015;32(1):199–211. doi: 10.1016/j.nbt.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rauch C., Feifel E., Amann E.-M., et al. Alternatives to the use of fetal bovine serum: human platelet lysates as a serum substitute in cell culture media. ALTEX. 2011;28(4):305–316. doi: 10.14573/altex.2011.4.305. [DOI] [PubMed] [Google Scholar]
- 4.Burnouf T., Strunk D., Koh M. B. C., Schallmoser K. Human platelet lysate: replacing fetal bovine serum as a gold standard for human cell propagation? Biomaterials. 2016;76:371–387. doi: 10.1016/j.biomaterials.2015.10.065. [DOI] [PubMed] [Google Scholar]
- 5.Hemeda H., Giebel B., Wagner W. Evaluation of human platelet lysate versus fetal bovine serum for culture of mesenchymal stromal cells. Cytotherapy. 2014;16(2):170–180. doi: 10.1016/j.jcyt.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 6.Marchionni C., Bonsi L., Alviano F., et al. Angiogenic potential of human dental pulp stromal (stem) cells. International Journal of Immunopathology and Pharmacology. 2009;22(3):699–706. doi: 10.1177/039463200902200315. [DOI] [PubMed] [Google Scholar]
- 7.Paino F., Ricci G., De Rosa A., et al. Ecto-mesenchymal stem cells from dental pulp are committed to differentiate into active melanocytes. European Cells and Materials. 2010;20:295–305. doi: 10.22203/ecm.v020a24. [DOI] [PubMed] [Google Scholar]
- 8.Arthur A., Rychkov G., Shi S., Koblar S. A., Gronthose S. Adult human dental pulp stem cells differentiate toward functionally active neurons under appropriate environmental cues. Stem Cells. 2008;26(7):1787–1795. doi: 10.1634/stemcells.2007-0979. [DOI] [PubMed] [Google Scholar]
- 9.Govindasamy V., Ronald V. S., Abdullah A. N., et al. Differentiation of dental pulp stem cells into islet-like aggregates. Journal of Dental Research. 2011;90(5):646–652. doi: 10.1177/0022034510396879. [DOI] [PubMed] [Google Scholar]
- 10.Govindasamy V., Ronald V. S., Abdullah A. N. B., et al. Human platelet lysate permits scale-up of dental pulp stromal cells for clinical applications. Cytotherapy. 2011;13(10):1221–1233. doi: 10.3109/14653249.2011.602337. [DOI] [PubMed] [Google Scholar]
- 11.Chen B., Sun H.-H., Wang H.-G., Kong H., Chen F.-M., Yu Q. The effects of human platelet lysate on dental pulp stem cells derived from impacted human third molars. Biomaterials. 2012;33(20):5023–5035. doi: 10.1016/j.biomaterials.2012.03.057. [DOI] [PubMed] [Google Scholar]
- 12.Abuarqoub D., Awidi A., Abuharfeil N. Comparison of osteo/odontogenic differentiation of human adult dental pulp stem cells and stem cells from apical papilla in the presence of platelet lysate. Archives of Oral Biology. 2015;60(10):1545–1553. doi: 10.1016/j.archoralbio.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 13.Gronthos S., Mankani M., Brahim J., Robey P. G., Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(25):13625–13630. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marrelli M., Paduano F., Tatullo M. Human periapical cyst-mesenchymal stem cells differentiate into neuronal cells. Journal of Dental Research. 2015;94(6):843–852. doi: 10.1177/0022034515570316. [DOI] [PubMed] [Google Scholar]
- 15.Paduano F., Marrelli M., White L. J., Shakesheff K. M., Tatullo M., Liu X. Odontogenic differentiation of human dental pulp stem cells on hydrogel scaffolds derived from decellularized bone extracellular matrix and collagen type I. PLoS ONE. 2016;11(2) doi: 10.1371/journal.pone.0148225.e0148225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marrelli M., Paduano F., Tatullo M. Cells isolated from human periapical cysts express mesenchymal stem cell-like properties. International Journal of Biological Sciences. 2013;9(10):1070–1078. doi: 10.7150/ijbs.6662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vogel S., Trapp T., Börger V., et al. Hepatocyte growth factor-mediated attraction of mesenchymal stem cells for apoptotic neuronal and cardiomyocytic cells. Cellular and Molecular Life Sciences. 2010;67(2):295–303. doi: 10.1007/s00018-009-0183-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baud O., Greene A. E., Li J., Wang H., Volpe J. J., Rosenberg P. A. Glutathione peroxidase-catalase cooperativity is required for resistance to hydrogen peroxide by mature rat oligodendrocytes. Journal of Neuroscience. 2004;24(7):1531–1540. doi: 10.1523/jneurosci.3989-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ido Y., Duranton A., Lan F., et al. Acute activation of AMP-activated protein kinase prevents H2O2-induced premature senescence in primary human keratinocytes. PLoS ONE. 2012;7(4) doi: 10.1371/journal.pone.0035092.e35092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dominici M., Le Blanc K., Mueller I., et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 21.Doucet C., Ernou I., Zhang Y., et al. Platelet lysates promote mesenchymal stem cell expansion: a safety substitute for animal serum in cell-based therapy applications. Journal of Cellular Physiology. 2005;205(2):228–236. doi: 10.1002/jcp.20391. [DOI] [PubMed] [Google Scholar]
- 22.Bieback K., Hecker A., Kocaömer A., et al. Human alternatives to fetal bovine serum for the expansion of mesenchymal stromal cells from bone marrow. STEM CELLS. 2009;27(9):2331–2341. doi: 10.1002/stem.139. [DOI] [PubMed] [Google Scholar]
- 23.Capelli C., Domenghini M., Borleri G., et al. Human platelet lysate allows expansion and clinical grade production of mesenchymal stromal cells from small samples of bone marrow aspirates or marrow filter washouts. Bone Marrow Transplantation. 2007;40(8):785–791. doi: 10.1038/sj.bmt.1705798. [DOI] [PubMed] [Google Scholar]
- 24.Brun J., Abruzzese T., Rolauffs B., Aicher W. K., Hart M. L. Choice of xenogenic-free expansion media significantly influences the myogenic differentiation potential of human bone marrow-derived mesenchymal stromal cells. Cytotherapy. 2016;18(3):344–359. doi: 10.1016/j.jcyt.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 25.Prins H.-J., Rozemuller H., Vonk-Griffioen S., et al. Bone-forming capacity of mesenchymal stromal cells when cultured in the presence of human platelet lysate as substitute for fetal bovine serum. Tissue Engineering—Part A. 2009;15(12):3741–3751. doi: 10.1089/ten.tea.2008.0666. [DOI] [PubMed] [Google Scholar]
- 26.Schallmoser K., Rohde E., Reinisch A., et al. Rapid large-scale expansion of functional mesenchymal stem cells from unmanipulated bone marrow without animal serum. Tissue Engineering—Part C: Methods. 2008;14(3):185–196. doi: 10.1089/ten.tec.2008.0060. [DOI] [PubMed] [Google Scholar]
- 27.Zaky S. H., Ottonello A., Strada P., Cancedda R., Mastrogiacomo M. Platelet lysate favours in vitro expansion of human bone marrow stromal cells for bone and cartilage engineering. Journal of Tissue Engineering and Regenerative Medicine. 2008;2(8):472–481. doi: 10.1002/term.119. [DOI] [PubMed] [Google Scholar]
- 28.Kocaoemer A., Kern S., Klüter H., Bieback K. Human AB serum and thrombin-activated platelet-rich plasma are suitable alternatives to fetal calf serum for the expansion of mesenchymal stem cells from adipose tissue. STEM CELLS. 2007;25(5):1270–1278. doi: 10.1634/stemcells.2006-0627. [DOI] [PubMed] [Google Scholar]
- 29.Lee U.-L., Jeon S. H., Park J.-Y., Choung P.-H. Effect of platelet-rich plasma on dental stem cells derived from human impacted third molars. Regenerative Medicine. 2011;6(1):67–79. doi: 10.2217/rme.10.96. [DOI] [PubMed] [Google Scholar]
- 30.Calloni R., Cordero E. A. A., Henriques J. A. P., Bonatto D. Reviewing and updating the major molecular markers for stem cells. Stem Cells and Development. 2013;22(9):1455–1476. doi: 10.1089/scd.2012.0637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rahman M. M., Subramani J., Ghosh M., et al. CD13 promotes mesenchymal stem cell-mediated regeneration of ischemic muscle. Frontiers in Physiology. 2014;4, article 402 doi: 10.3389/fphys.2013.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ode A., Kopf J., Kurtz A., et al. CD73 and CD29 concurrently mediate the mechanically induced decrease of migratory capacity of mesenchymal stromal cells. European Cells & Materials Journal. 2011;22:26–42. doi: 10.22203/ecm.v022a03. [DOI] [PubMed] [Google Scholar]
- 33.Zhu H., Mitsuhashi N., Klein A., et al. The role of the hyaluronan receptor CD44 in mesenchymal stem cell migration in the extracellular matrix. Stem Cells. 2006;24(4):928–935. doi: 10.1634/stemcells.2005-0186. [DOI] [PubMed] [Google Scholar]
- 34.Paduano F., Marrelli M., Palmieri F., Tatullo M. CD146 expression influences periapical cyst mesenchymal stem cell properties. Stem Cell Reviews. 2016 doi: 10.1007/s12015-016-9674-4. [DOI] [PubMed] [Google Scholar]
- 35.Müller I., Kordowich S., Holzwarth C., et al. Animal serum-free culture conditions for isolation and expansion of multipotent mesenchymal stromal cells from human BM. Cytotherapy. 2006;8(5):437–444. doi: 10.1080/14653240600920782. [DOI] [PubMed] [Google Scholar]
- 36.Huang C.-E., Hu F.-W., Yu C.-H., et al. Concurrent expression of Oct4 and Nanog maintains mesenchymal stem-like property of human dental pulp cells. International Journal of Molecular Sciences. 2014;15(10):18623–18639. doi: 10.3390/ijms151018623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.La Noce M., Paino F., Spina A., et al. Dental pulp stem cells: state of the art and suggestions for a true translation of research into therapy. Journal of Dentistry. 2014;42(7):761–768. doi: 10.1016/j.jdent.2014.02.018. [DOI] [PubMed] [Google Scholar]
- 38.Atari M., Barajas M., Hernández-Alfaro F., et al. Isolation of pluripotent stem cells from human third molar dental pulp. Histology and Histopathology. 2011;26(8):1057–1070. doi: 10.14670/HH-26.1057. [DOI] [PubMed] [Google Scholar]
- 39.Zhao W., Ji X., Zhang F., Li L., Ma L. Embryonic stem cell markers. Molecules. 2012;17(6):6196–6236. doi: 10.3390/molecules17066237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoon D. S., Kim Y. H., Jung H. S., Paik S., Lee J. W. Importance of Sox2 in maintenance of cell proliferation and multipotency of mesenchymal stem cells in low-density culture. Cell Proliferation. 2011;44(5):428–440. doi: 10.1111/j.1365-2184.2011.00770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu P., Cai J., Dong D., et al. Effects of SOX2 on proliferation, migration and adhesion of human dental pulp stem cells. PLoS ONE. 2015;10(10) doi: 10.1371/journal.pone.0141346.e0141346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maijenburg M. W., van der Schoot C. E., Voermans C. Mesenchymal stromal cell migration: possibilities to improve cellular therapy. Stem Cells and Development. 2012;21(1):19–29. doi: 10.1089/scd.2011.0270. [DOI] [PubMed] [Google Scholar]
- 43.Trotta T., Di Gioia S., Piro D., et al. Effect of acute lung injury on VLA-4 and CXCR4 expression in resident and circulating hematopoietic stem/progenitor cells. Respiration. 2013;85(3):252–264. doi: 10.1159/000341172. [DOI] [PubMed] [Google Scholar]
- 44.Weinreb M., Nemcovsky C. E. In vitro models for evaluation of periodontal wound healing/regeneration. Periodontology 2000. 2015;68(1):41–54. doi: 10.1111/prd.12079. [DOI] [PubMed] [Google Scholar]
- 45.Ranzato E., Mazzucco L., Patrone M., Burlando B. Platelet lysate promotes in vitro wound scratch closure of human dermal fibroblasts: different roles of cell calcium, P38, ERK and PI3K/AKT. Journal of Cellular and Molecular Medicine. 2009;13(8):2030–2038. doi: 10.1111/j.1582-4934.2008.00467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ranzato E., Balbo V., Boccafoschi F., Mazzucco L., Burlando B. Scratch wound closure of C2C12 mouse myoblasts is enhanced by human platelet lysate. Cell Biology International. 2009;33(9):911–917. doi: 10.1016/j.cellbi.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 47.Backly R. E., Ulivi V., Tonachini L., Cancedda R., Descalzi F., Mastrogiacomo M. Platelet lysate induces in vitro wound healing of human keratinocytes associated with a strong proinflammatory response. Tissue Engineering—Part A. 2011;17(13-14):1787–1800. doi: 10.1089/ten.tea.2010.0729. [DOI] [PubMed] [Google Scholar]
- 48.Barsotti M. C., Losi P., Briganti E., et al. Effect of platelet lysate on human cells involved in different phases of wound healing. PLoS ONE. 2013;8(12):1–11. doi: 10.1371/journal.pone.0084753.e84753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leotot J., Coquelin L., Bodivit G., et al. Platelet lysate coating on scaffolds directly and indirectly enhances cell migration, improving bone and blood vessel formation. Acta Biomaterialia. 2013;9(5):6630–6640. doi: 10.1016/j.actbio.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 50.Duan J., Duan J., Zhang Z., Tong T. Irreversible cellular senescence induced by prolonged exposure to H2O2 involves DNA-damage-and-repair genes and telomere shortening. The International Journal of Biochemistry & Cell Biology. 2005;37(7):1407–1420. doi: 10.1016/j.biocel.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 51.Li M., Zhao L., Liu J., et al. Hydrogen peroxide induces G2 cell cycle arrest and inhibits cell proliferation in osteoblasts. Anatomical Record. 2009;292(8):1107–1113. doi: 10.1002/ar.20925. [DOI] [PubMed] [Google Scholar]
- 52.Ikeda H., Koga Y., Oda T., et al. Free oxygen radicals contribute to platelet aggregation and cyclic flow variations in stenosed and endothelium-injured canine coronary arteries. Journal of the American College of Cardiology. 1994;24(7):1749–1756. doi: 10.1016/0735-1097(94)90183-x. [DOI] [PubMed] [Google Scholar]
- 53.Yamada Y., Nakamura S., Ito K., et al. A feasibility of useful cell-based therapy by bone regeneration with deciduous tooth stem cells, dental pulp stem cells, or bone-marrow-derived mesenchymal stem cells for clinical study using tissue engineering technology. Tissue Engineering—Part A. 2010;16(6):1891–1900. doi: 10.1089/ten.tea.2009.0732. [DOI] [PubMed] [Google Scholar]
- 54.Lucarelli E., Beccheroni A., Donati D., et al. Platelet-derived growth factors enhance proliferation of human stromal stem cells. Biomaterials. 2003;24(18):3095–3100. doi: 10.1016/S0142-9612(03)00114-5. [DOI] [PubMed] [Google Scholar]
- 55.Lange C., Cakiroglu F., Spiess A.-N., Cappallo-Obermann H., Dierlamm J., Zander A. R. Accelerated and safe expansion of human mesenchymal stromal cells in animal serum-free medium for transplantation and regenerative medicine. Journal of Cellular Physiology. 2007;213(1):18–26. doi: 10.1002/jcp.21081. [DOI] [PubMed] [Google Scholar]
- 56.Chevallier N., Anagnostou F., Zilber S., et al. Osteoblastic differentiation of human mesenchymal stem cells with platelet lysate. Biomaterials. 2010;31(2):270–278. doi: 10.1016/j.biomaterials.2009.09.043. [DOI] [PubMed] [Google Scholar]
- 57.Jonsdottir-Buch S. M., Lieder R., Sigurjonsson O. E. Platelet lysates produced from expired platelet concentrates support growth and osteogenic differentiation of mesenchymal stem cells. PLoS ONE. 2013;8(7, article e68984) doi: 10.1371/journal.pone.0068984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hildner F., Eder M. J., Hofer K., et al. Human platelet lysate successfully promotes proliferation and subsequent chondrogenic differentiation of adipose-derived stem cells: a comparison with articular chondrocytes. Journal of Tissue Engineering and Regenerative Medicine. 2015;9(7):808–818. doi: 10.1002/term.1649. [DOI] [PubMed] [Google Scholar]
- 59.Moroz A., Bittencourt R. A. C., Almeida R. P., Felisbino S. L., Deffune E. Platelet lysate 3D scaffold supports mesenchymal stem cell chondrogenesis: an improved approach in cartilage tissue engineering. Platelets. 2013;24(3):219–225. doi: 10.3109/09537104.2012.686255. [DOI] [PubMed] [Google Scholar]
- 60.Johnstone B., Hering T. M., Caplan A. I., Goldberg V. M., Yoo J. U. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Experimental Cell Research. 1998;238(1):265–272. doi: 10.1006/excr.1997.3858. [DOI] [PubMed] [Google Scholar]
- 61.Fekete N., Gadelorge M., Fürst D., et al. Platelet lysate from whole blood-derived pooled platelet concentrates and apheresis-derived platelet concentrates for the isolation and expansion of human bone marrow mesenchymal stromal cells: production process, content and identification of active components. Cytotherapy. 2012;14(5):540–554. doi: 10.3109/14653249.2012.655420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Muraglia A., Todeschi M. R., Papait A., et al. Combined platelet and plasma derivatives enhance proliferation of stem/progenitor cells maintaining their differentiation potential. Cytotherapy. 2015;17(12):1793–1806. doi: 10.1016/j.jcyt.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 63.Mori G., Brunetti G., Collucci S., et al. Osteoblast apoptosis in periodontal disease: role of TNF-related apoptosis-inducing ligand. International Journal of Immunopathology and Pharmacology. 2009;22(1):95–103. doi: 10.1177/039463200902200111. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The supplementary material provides the immunophenotype comparison for negative MSC markers, the images of cell growth occurring during in vitro wound closure, the dose response cytotoxic effect of H2O2, the primer list used for RT-qPCR experiments.