Abstract
The striped stem borer, Chilo suppressalis (Walker), is an important insect pest of rice which shows substantial variation in developmental duration among individuals. This variation is currently poorly characterized but it is important from a control perspective because pesticides can only target early sensitive instars. It is unclear whether there are key stages that determine the length of developmental duration of individuals and/or whether variation in instar number contributes to this variation. In this study, a laboratory population and a population recently established from the field were used to test variation in development time across instar stages. The duration of developmental time of C. suppressalis started to diverge from the 5th instar onward. Individuals pupated at the 5th, 6th, 7th or even 8th instar stage. In both populations, both the instar at which the larva pupated and the duration of the last larval instar stage determined total developmental time of an individual. There was little impact of the developmental time of early instars on total developmental duration or on instar number prior to pupation. Sex influenced the number of instars but not development time within this number. The biological and applied significance of uneven development in C. suppressalis are discussed.
In insects, the number of larval moults and the duration of larval instars can be influenced by environmental factors such as temperature, photoperiod, nutrition, and carbon dioxide anaesthesia1,2,3,4,5,6. Instar number can also be changed by the presence of other individuals and by rearing density7,8. For instance in Diploptera punctata, there was a tendency for males reared in isolation to have an additional larval instar7, and the same phenomenon was found in the locust Schistocerca gregaria8.
It is generally thought that these responses reflect a compensatory scenario: adverse conditions lead to additional instars that allow insects to compensate and still reach a critical size for metamorphosis9. The notion that a threshold size for metamorphosis is required and linked to developmental duration emerged from research on the tobacco hornworm, Manduca sexta5,10. When 3rd or 4th instar larvae of this species were starved and molted at a subnormal weight, the resulting 5th instar larvae which usually develop into pupae continued to grow and molt, developing into 6th or 7th instar larvae before metamorphosis5. Instar transitions in themselves may depend on levels of oxygen available to developing larvae which will depend on tracheal development relative to larval size11.
As well as varying with environmental conditions, instar numbers can also vary among insects reared under the same constant conditions12, suggesting that there is inherent variation in instar development not necessarily connected to environmental responses. This instar variation may be partly due to sex: sexual size dimorphism is common in insects, and by having a higher number of instars females may be able to develop to a larger size on emergence9,13. In addition, instar number variation may reflect a genetic polymorphism in cases where variation in instars expressed under constant conditions occurs regardless of sex12. Variation in instars may also be unrelated to sex and genetic factors and represent a genetically fixed adaptive strategy; instar variability might serve to produce larvae with a range of emergence times to spread risk associated with emergence under unpredictable unfavorable periods, allowing organisms to hedge their bets9,13,14.
The striped stem borer, Chilo suppressalis (Walker), is an important insect pest of rice in China, India, south-east Asia, Iran and southern Europe. Field surveys of C. suppressalis populations show irregular ontogeny and overlapping generations in this pest, making it difficult to target pesticides at susceptible newly hatched larvae. In laboratory populations, some C. suppressalis exhibit prolonged developmental times, suggesting a high level of inter-individual variation15. It is unclear whether this variation is due to key instar stages that determine the length of individual developmental time and/or variation in instar numbers as well as sex.
The purpose of this study was to assess these sources of variability under constant conditions. Under a compensation model9, we might expect that shorter development at early instar stages could act as a trigger for an increase in instar number, particularly for females. We also tested for differences in developmental patterns between a population reared and selected in the laboratory for more than two years and a recently-established population. Larvae were separately fed with an artificial diet, and the developmental duration of each instar was scored. We suggest that instar number variability is only partly related to compensatory growth, given that instar number is linked to sex but not to the length of early developmental stages. Variability in larval development may also reflect an adaptive response to environmental unpredictability although this needs to be further tested. The applied significance of the results are discussed.
Results
Instar of pupation
The results showed C. suppressalis larvae underwent pupation after the 5th, 6th, and 7th larval instars (Table 1), with only one instance of pupation at the 8th instar (see Supplementary Table S1). The laboratory population (Ind-P) pupated mainly at the 5th and 6th instar stage, accounting for 44.5% and 53.6% of the total number tested for this population respectively. The recently collected population (Fie-P) pupated mainly at the 6th instar stage, followed by the 5th and 7th instar stages, accounting for 64.8%, 25.7% and 8.6% of the total respectively (Table 1). In both populations, individuals reaching pupae had a sex ratio close to 1:1, but ratios differed between instars. Overall, more males pupated at the 5th instar, while more females pupated at the 6th instar (Table 1). In both populations, the distribution across instars was heterogeneous across sex as assessed by contingency analysis (lab population, G1 = 20.44, P < 0.001, based on 5/6 instar only; field population, G2 = 8.07, P = 0.017, based on 5/6/7 instars). There was also a significant effect of population overall when combined across the sexes (G2 = 11.94, P = 0.002), reflecting a higher incidence of later instars in the field population.
Table 1. Number of Ind-P and Fie-P larvae pupating at different instar numbers*.
| 5 instars | 6 instars | 7 instars | Total pupating | |||||
|---|---|---|---|---|---|---|---|---|
| Ind-P | Fie-P | Ind-P | Fie-P | Ind-P | Fie-P | Ind-P | Fie-P | |
| Numbers pupating | 49 | 27 | 59 | 69 | 2 | 9 | 110 | 105 |
| % of total | 44.5% | 25.7% | 53.6% | 64.8% | 1.8% | 8.6% | — | — |
| Females | 13 | 10 | 41 | 36 | 1 | 8 | 55 | 54 |
| Males | 36 | 17 | 18 | 33 | 1 | 1 | 55 | 51 |
| Sex ratio (♀: ♂) | 0.36:1 | 0.59:1 | 2.28:1 | 1.09:1 | 1:1 | 8:1 | 1:1 | 1.06:1 |
*Data included are individuals with successful emergence. “−”: no data.
Some individuals developed into 7th or 8th instars, and even 9th instars, but most of these died without pupation. For Ind-P, two individual larvae died on day 10 and day 36 of the 7th instar stage. For Fie-P, two larvae died at the 7th instar stage on day 17 and day 32; one larva developed into an 8th instar and pupated on day 31; one larva developed into an 8th instar and died on day 26, and another individual developed into a 9th instar without pupation and died on day 20 (see Supplementary Table S1).
Developmental duration of Chilo suppressalis from hatching to emergence
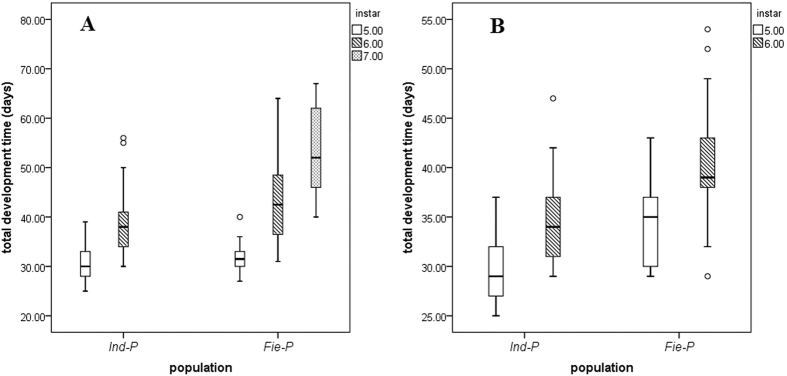
The developmental duration of each C. suppressalis individual is given in Dataset S1 (see Supplementary information). Using a generalized linear model and assuming the data followed a Poisson distribution, we showed that there was a significant effect of instar number (P < 0.001) and population (P < 0.001) on overall development time (Table 2). With increasing instar number, boxplots show that developmental duration increases, and this pattern is apparent for both sexes (Fig. 1). The population difference reflects a shorter development time overall for the laboratory population compared to Fie-P. Note that there were no significant sex effects or interactions among the variables. Therefore, within larvae having the same number of instars, the sexes had a similar developmental duration.
Table 2. Generalized linear model testing for the effects of variables on total development time.
| Effect | Wald Chi-Square | df | Significance |
|---|---|---|---|
| Population | 16.509 | 1 | <0.001 |
| Sex | 1.864 | 1 | 0.172 |
| Instar number | 84.268 | 2 | <0.001 |
| Population * Sex | 1.659 | 1 | 0.198 |
| Population * instar number | 0.558 | 2 | 0.455 |
| Sex * instar number | 3.560 | 2 | 0.059 |
| Population* sex* instar number | 0.557 | 2 | 0.455 |
Figure 1. Boxplots of total developmental duration of different instar numbers from the two populations, plotted separately for the two sexes.
(A) females; (B) males. Lines within boxes represent medians, boxes and whiskers represent quartiles.
For development time of the individual instar stages, the number of instars only had a significant effect on the development time of females at the 5th instar stage, but for males there was also an effect of instar number at the 1st and 2nd instar stages (Table 3: only females that had 5 or 6 instars were considered in these comparisons). Individuals that pupated after 5 instars had a markedly extended development time at the final instar stage when compared to larvae that had 6 and 7 instars. In addition, there was a tendency for males which pupated after 6 instars to have somewhat longer development times in the early instar developmental stages. There were also population differences at early instar stages (Table 3), reflecting the fact that for both sexes the laboratory population had a relatively shorter development period than Fie-P in the first two instars but a longer developmental period at the third instar stage. Females that pupated after 6 instars showed an extended development time at this final instar stage in contrast to those that had 7 instars. Moreover, the time spent at the final instar stage increased with the number of larval instars before pupation (Fig. 1).
Table 3. Median duration of development (days) for different instar stages when larvae underwent 5, 6 or 7 instars before pupation.
| Instar number | Population | Female | Male | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 4th | 5th | 6th | 7th | 1st | 2nd | 3rd | 4th | 5th | 6th | 7th | ||
| 5 instars | Ind-P | 2 (2,3) | 2 (2,5) | 3 (3,4) | 3 (3,4) | 11 (8,21) | — | — | 2 (2,3) | 2 (2,5) | 3 (3,4) | 3 (2,5) | 10 (7,18) | — | — |
| Fie-P | 3 (3,4) | 3 (3,4) | 3 (3,3) | 3 (3,4) | 12 (7,20) | — | — | 3 (2,4) | 3 (3,4) | 3 (3,4) | 4 (3,4) | 15 (9,24) | — | — | |
| 6 instars | Ind-P | 2 (2,3) | 3 (2,9) | 3 (1,6) | 3 (2,7) | 4 (2,8) | 14 (5,32) | — | 2 (2,3) | 3 (2,4) | 3 (2,4) | 3 (2,7) | 4 (3,5) | 10 (7,25) | — |
| Fie-P | 3 (2,5) | 3 (2,5) | 3 (2,5) | 3 (2,4) | 4 (4,10) | 18 (2,39) | — | 3 (2,4) | 3 (2,5) | 3 (2,4) | 3 (1,6) | 4 (3,9) | 16 (8,30) | — | |
| 7 instars | Ind-P | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| Fie-P | 3 (3,4) | 5 (2,6) | 3 (3,5) | 3 (2,10) | 4 (3,5) | 6 (4,12) | 20 (11,34) | — | — | — | — | — | — | — | |
| Probabilities (Kruskal-Wallis tests) | |||||||||||||||
| Instar number | 0.864 | 0.126 | 0.271 | 0.205 | <0.001 | 0.023 | <0.001 | 0.055 | 0.119 | <0.001 | |||||
| Population | <0.001 | <0.001 | 0.002 | 0.488 | 0.151 | 0.850 | <0.001 | <0.001 | 0.007 | 0.955 | 0.068 | 0.002 | |||
Numbers in brackets represent ranges. Probabilities for Kruskal -Wallis tests comparing instar number and population are given. “—”: no data. Bold numbers represent the last instar.
Variability in development within classes
We examined the association between total development time and the development time of each instar within sex, population and instar number, to see if the final instar largely controlled variation within as well as between the instar number groups. For females, the five correlations (Spearman) within groups between total development time and the final instar varied from 0.77 to 0.98 and all were highly significant (Table 4). For males, the four correlations involving the final development stage varied from 0.88 to 0.96 and were also all highly significant. In contrast, correlations for the other instar stages were weak and inconsistent in sign (Table 4). These results highlight the strong effect of the final instar stage on the total duration of development and much weaker effects of other stages within instar number groups, a trend that was consistent for the two populations.
Table 4. Non-parametric correlations between instar development and total development within different population and instar number groups.
| Females | Males | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 5 instars | 6 instars | 7 instars | 5 instars | 6 instars | |||||
| Larval stage | Ind-P | Fie-P | Ind-P | Fie-P | Fie-P | Ind-P | Fie-P | Ind-P | Fie-P |
| 1st | 0.287 | 0.292 | 0.306 | −0.023 | 0.000 | 0.235 | −0.142 | 0.130 | 0.298 |
| 2nd | 0.392 | −0.525 | 0.186 | 0.009 | 0.096 | 0.390* | 0.155 | −0.117 | 0.083 |
| 3rd | 0.382 | — | 0.207 | 0.043 | 0.187 | −0.038 | −0.103 | −0.332 | 0.103 |
| 4th | −0.149 | 0.070 | 0.198 | −0.157 | 0.082 | 0.370* | 0.073 | 0.541* | −0.001 |
| 5th | 0.927*** | 0.981*** | 0.097 | 0.149 | 0.228 | 0.891*** | 0.957*** | −0.297 | 0.093 |
| 6th | NA | NA | 0.912*** | 0.962*** | 0.418 | NA | NA | 0.878*** | 0.909*** |
| 7th | NA | NA | NA | NA | 0.766** | NA | NA | NA | NA |
| N | 13 | 10 | 41 | 36 | 8 | 36 | 17 | 18 | 33 |
*, **, ***Correlation is significant at the 0.05, 0.01, 0.001 level respectively. “NA”: not applicable.
This pattern may partly reflect a higher variability in development time at the final instar stage compared to the earlier stages. In order to characterize variability in development time, coefficients of variation (CV) were calculated within instar number groups. These indicate that the CVs of developmental duration from the first instar to the penultimate instar were far lower than those for the last instar, often differing by three fold or more (Table 5). Individuals that pupated after the same number of instars varied in their development at the last instar stage by as much as 20 days (see Supplementary Information Dataset S1).
Table 5. Coefficients of variation for developmental duration from first to penultimate instar, for last instar and for total development time.
| Number of instars | Population | Coefficient of Variation (CV) (%) | |||||
|---|---|---|---|---|---|---|---|
| Females | Males | ||||||
| 1st to penultimate | Last instar | Total | 1st to penultimate | Last instar | Total | ||
| 5 instars | Ind-P | 8.44 | 32.98 | 14.24 | 10.93 | 27.21 | 11.46 |
| Fie-P | 3.80 | 29.94 | 11.42 | 3.89 | 29.81 | 13.12 | |
| 6 instars | Ind-P | 10.20 | 35.10 | 15.56 | 10.77 | 40.76 | 13.51 |
| Fie-P | 10.65 | 43.52 | 19.56 | 12.87 | 31.60 | 14.20 | |
| 7 instars | Ind-P | — | — | — | — | — | — |
| Fie-P | 22.71 | 39.20 | 18.55 | — | — | — | |
“−”: no data.
Discussion
In this study, we found a high level of variability in developmental duration which was strongly associated with the number of instars at the larval stage as well as variation in the final instar stage before pupation regardless of instar number. Part of the difference in development time due to instar number reflected sex effects. As argued in Esperk et al. when females emerge larger they may achieve this by having more instars13. While we did not measure the size of emerging moths in this experiment, C. suppressalis females are larger than males and we have confirmed this in another colony of this species where we found that females were on average 30% larger than males regardless of the number of instars. Thus the tendency for females to have more instars may partly reflect the fact that they emerge at a larger size, and instar number likely contributes to the development of sexual dimorphism in this species. However, we also found no difference between the sexes in the duration of development once the number of instars had been taken into account. Given that females are larger than males, this points to females also having more efficient growth than males, allowing them to emerge at a larger size even when they have the same instar number.
Although instar number was associated with sex effects, we found it could also vary within sex. This type of variability has been noted for other species, although it is commonly related to external conditions. For instance, in D. punctata, all females had 4 larval instars, and males had 3 or 4 instars7. In this case, there is a tendency for males reared in isolation to have an additional larval instar and for instar number to vary with other rearing conditions7,16. In M. sexta, instar number is also linked to rearing conditions, and under suboptimal growing conditions growth is slower and larvae achieve final size through a greater number of smaller steps5,17. However in our work, all C. suppressalis individuals were reared in isolation with sufficient food, suggesting that factors other than nutrition are involved.
In other Lepidoptera, variation in larval instars that appears unrelated to sex or external conditions has also been found12. For instance, Barraclough et al. noted that growth in the geometrid moth, Pseudocoremia suavis, fell into two classes depending on instar number (5 or 6) and these classes were independent of sex14. In this case, the 6 instar larvae took longer to pupate but had shorter larval durations at the 3–5 instar stage, and changes in instar number likely represented a plastic response to different growth conditions and the need for larvae to reach a particular size for pupation. In contrast, in C. suppressalis the extra instars likely lead to an increase in the size of adults rather than involving the attainment of a critical pupation size; in a different colony we found that pupae emerging from larvae with 6 instars were around 12% larger than those emerging from larvae with 5 instars. The absence of substantive differences in development time of individual instar stages (apart from the final stage) between larvae with 5 or 6 instars also suggests that a critical size threshold is not involved in C. suppressalis. Instead it appears that variation in instar number (and overall development time) in C. suppressalis reflects inherent variation in development time rather than a plastic response to early development.
Insect growth from instar to instar is typically exponential so that most of growth occurs in the last larval instar18. Thus, the duration of the last larval instar is often longer than those in all other instars. In M. sexta, 90% of mass accumulation occurs in the 5th (last) instar, and the duration of last instar is the longest in all its larval instars18,19. This probably reflects the need for a final instar larva to feed and grow until it reaches a threshold critical weight, and then undergo changes linked to metamorphosis. In C. suppressalis, the average duration of final larval instar is longer than other stages, but the final larval instar duration of each C. suppressalis individual varied greatly, as reflected in the high CV values associated with this stage (Table 5). Furthermore, there were significant correlations between the total developmental duration and the last instar duration in individuals in all cases, in contrast to a lack of correlation between total development time and the period between the first instar to the penultimate instar (see Supplementary Table S2). We therefore suspect that mass accumulation rates in each larval instar are different in C. suppressalis individuals and that there is variability for critical weight in C. suppressalis.
Whether the variability in final instar development time and instar number has a genetic basis or represents a genetically fixed form of bet hedging strategy is unclear. We found instar number variability in both the Ind-P and Fie-P populations when cultured under the same conditions, despite inadvertent selection in the laboratory population against individuals that showed particularly rapid or slow development during the 15 generations that this population had been cultured; to facilitate maintenance of the Ind-P population, individuals that pupated too early or too late were routinely discarded each generation. If development time variation reflected genetic polymorphism, we might have therefore expected lower variability in the Ind-P population compared to the Fie-P population particularly as laboratory colonies of C. suppressalis can lose genetic variation quite rapidly20, but this was not reflected in the CVs (Table 5). On the other hand, a genetic shift may have occurred in this population because of the shorter larval duration and lower instar number in the Ind-P population.
Because of e variability in larval development, C. suppressalis will emerge from pupae across an extended period. In Jiangsu Province, there are two generations of this pest per year. Every year the first generation of C. suppressalis larva hatch from the beginning of May to the end of June. The newly hatched C. suppressalis larvae are most easily controlled but this extended period of asynchronous hatching makes it difficult to find the most suitable time for application of insecticides. This results in a large amount of insecticides being used to control C. suppressalis in the field, likely contributing to the development of resistance to different kinds of insecticides in this pest21,22,23,24.
In conclusion, we have shown that C. suppressalis has a high level of variability in development linked partly to the number of instars and sex. This variability is expressed under controlled conditions but its biological significance is unclear, apart from obvious effects on sexual dimorphism. One reason why larvae may develop at different rates relates to a tradeoff between size and development: larvae that develop more slowly (and thereby are exposed to an increased risk of parasitism) may nevertheless gain by being larger, leading to a higher reproductive output and mating success12. We suggest that two factors other than this tradeoff are also worth exploring in future studies. The first is that larvae show asynchronous development in order to reduce intraspecific competition, both among themselves and also among their offspring. Because female C. suppressalis lay eggs in batches, asynchronous development may allow larvae to utilize different resources. The second is that asynchronous development among offspring may represent a response to unpredictable conditions. When confronted by extreme environments, such as postponed planting time of rice due to low temperature, individuals that emergence early may not have access to feeding and breeding sites, whereas late emerging individuals may be at an advantage. However this advantage may be lost in warmer years when early emergence is favored.
Materials and Methods
Insects
Two groups of C. suppressalis larvae were used in this study: an indoor population (Ind-P), which has been cultured in the laboratory for 16 generations, and a field population (Fie-P), which was 1st generation larvae acquired newly from the field and propagated in the laboratory. To initiate both the Ind-P and Fie-P populations, C. suppressalis individuals were collected from rice fields in Ruichang County in China. The C. suppressalis individuals for the Ind-P populations were collected in 2012. The C. suppressalis individuals for Fie-P populations were collected in 2014. The laboratory line was maintained by turning over at least 500 pupae at the peak of emergence every generation, likely resulting in selection against larvae pupating early or late across multiple generations. Insects were grown at 28 ± 1 °C, with light-dark cycles of 16:8 h and relative humidity of 80%.
Methods
An artificial diet made in our laboratory was used for development. The diet was made according to Han et al.’s method25. Medium was cut into small pieces of 1 cm3, and placed into six-well plates. Neonate larvae that hatched within 2 hours were transferred into six-well plates with a brush, with one larva per well. A total of 25 plates (150 larvae) were set up. Paper towels were placed between the plate and cover to prevent larvae from escaping.
Data collection and statistical analysis
The development of each larva was observed every 24 hours, and the molting time, instar stage, pupation time and time of adult emergence were recorded. The total developmental duration refers to the time span from hatching of the egg to adult eclosion.
For analysis, we firstly compared the distribution of the sexes across different number of instar classes by undertaking contingency analysis and computing the G (likelihood ratio) statistic. We also compared the distribution of instars across the two populations with contingency analyses. We then ran a generalized linear model under a Poisson distribution to investigate the impact of population, instar number, and sex on total development time. This trait was not normally distributed even after log (or square root) transformation but did fit a Poisson distribution (Kolmogorov-Smirnov test, P = 0.109). We used boxplots to show the distribution of data in the different groups. We also explored the effects of sex and population on the development period of specific instars through non-parametric comparisons (Kruskal-Wallis tests). Finally, we used non-parametric correlations (Spearman) to investigate the association between instar development and total development within different population and instar number groups. All statistical analyses were performed with SPSS V13.0.
Additional Information
How to cite this article: Luo, G.-H. et al. Variability in development of the striped rice borer, Chilo suppressalis (Lepidoptera: Pyralidae), due to instar number and last instar duration. Sci. Rep. 6, 35231; doi: 10.1038/srep35231 (2016).
Supplementary Material
Acknowledgments
This work was supported by Special Fund for Agro-scientific Research in the Public Interest grant 201303017; Jiangsu Agriculture Science and Technology Innovation Fund grant CX(14)2025; Jiangsu Collaborative Innovation Center of Regional Modern Agriculture and Environmental Protection grant HSXT104.
Footnotes
Author Contributions G.-H.L. and J.Y. performed most of the experiments together. G.-H.L. and J.-C.F. conceived and designed the study together. G.-H.L. and Z.-C.Z. collected the insect samples together. G.-H.L., A.A.H., Q.Y., J.Y. and J.-C.F. wrote the paper together. All authors analyzed the data. All authors read and approved the final manuscript.
References
- Dmitry K., Aida S., Elena L. & Maria R. Stable and variable life-history responses to temperature and photoperiod in the beet webworm. Loxostege sticticalis. Entomol. Exp. Appl. 154, 228–241 (2015). [Google Scholar]
- Lopatina E., Balashov S. & Kipyatkov V. First demonstration of the influence of photoperiod on the thermal requirements for development in insects and in particular the linden-bug, Pyrrhocoris apterus (Heteroptera: Pyrrhocoridae). Eur. J. Entomol. 104, 23–31 (2007). [Google Scholar]
- Paul E. Lutz. Effects of temperature and photoperiod on larval development in Lestes Eurinus (Odonata: Lestidae). Ecology 49, 637–644 (1968). [Google Scholar]
- Beck S. D. Growth and retrogression in larvae of Trogoderma glabrum (Coleoptera: Dermestidae). 1. Characteristics under feeding and starvation conditions. Ann. Entomol. Soc. Am. 64, 149–155 (1971). [Google Scholar]
- Nijhout H. F. A threshold size for metamorphosis in the tobacco hornworm, Manduca sexta (L.). Biol. Bull. 149, 214–225 (1975). [DOI] [PubMed] [Google Scholar]
- Tanaka A. Regulation of body size during larval development in the German cockroach, Blattella germanica. J. Insect Physiol. 27, 587–592 (1981). [Google Scholar]
- Woodhead A. & Paulson C. Larval development of Diploptera punctata reared alone and in groups. J. Insect Physiol. 29, 665–668 (1983). [Google Scholar]
- Injeyan H. & Tobe S. Phase polymorphism in Schistocerca gregaria: reproductive parameters. J. Insect Physiol. 27, 97–102 (1981). [Google Scholar]
- Etile E. & Despland E. Developmental variation in the forest tent caterpillar: life history consequences of a threshold size for pupation. Oikos 117, 135–143 (2008). [Google Scholar]
- Nijhout H. F. & Williams C. Control of moulting and metamorphosis in the tobacco hornworm, Manduca sexta (L.): growth of the last-instar larva and the decision to pupate. J. Exp. Biol. 61, 481–491 (1974). [DOI] [PubMed] [Google Scholar]
- Kivela S. M., Friberg M., Wiklund C., Leimar O. & Gotthard K. Towards a mechanistic understanding of insect life history evolution: oxygen-dependent induction of moulting explains moulting sizes. Biol. J. Linn. Soc. 117, 586–600 (2016). [Google Scholar]
- Esperk T., Tammaru T. & Nylin S. Intraspecific variability in number of larval instars in insects. J. Econ. Entomol. 100, 627–645 (2007). [DOI] [PubMed] [Google Scholar]
- Esperk T., Tammaru T., Nylin S. & Teder T. Achieving high sexual size dimorphism in insects: females add instars. Ecol. Entomol. 32, 243–256 (2007). [Google Scholar]
- Barraclough E. I., Burgess E., Kean A. M. & Malone L. A. Growth and development in a lepidopteran with variable instar number, Pseudocoremia suavis (Geometridae), under standard rearing conditions and when parasitized by Meteorus pulchricornis (Hymenoptera: Braconidae). Eur. J. Entomol. 111, 501–511 (2014). [Google Scholar]
- Shang C. C., Wang Y. S. & Zon Y. H. Studies on successive mass rearing of the rice stem borer Chilo suppressalis Walker. Acta Entomologica Sinica (In Chinese with English abstract), 22, 164–167 (1979). [Google Scholar]
- Willis E. R., Riser G. R. & Roth L. M. Observations on reproduction and development in cockroaches. Ann. Entomol. Soc. Am. 51, 53–69 (1958). [Google Scholar]
- Davidowitz G., D’Amico L. J. & Nijhout H. F. Critical weight in the development of insect body size. Evol. Dev. 5, 188–197 (2003). [DOI] [PubMed] [Google Scholar]
- Davidowitz G. & Nijhout H. F. The physiological basis of reaction norms: the interaction among growth rate, the duration of growth and body size. Integr. Comp. Biol. 44, 443–449 (2004). [DOI] [PubMed] [Google Scholar]
- Davidowitz G., D’Amico L. J. & Nijhout H. F. The effects of environmental variation on a mechanism that controls insect body size. Evol. Ecol. Res. 6, 49–62 (2004). [Google Scholar]
- Liu Y. D., Han L. Z. & Hou M. L. Loss of genetic variation in laboratory colonies of Chilo suppressalis (Lepidoptera: Crambidae) revealed by mitochondrial and microsatellite DNA markers. Environ. Entomol. 44, 73–80 (2015). [DOI] [PubMed] [Google Scholar]
- He Y. P. et al. Differential susceptibilities to pyrethroids in field populations of Chilo suppressalis (Lepidoptera: Pyralidae). Pestic. Biochem. Phys. 89, 12–19 (2007). [Google Scholar]
- Li X., Huang Q., Yuan J. & Tang Z. Fipronil resistance mechanisms in the rice stem borer, Chilo suppressalis Walker. Pestic. Biochem. Phys. 89, 169–174 (2007). [Google Scholar]
- He Y. P. et al. Survey of susceptibilities to monosultap, triazophos, fipronil, and abamectin in Chilo suppressalis (Lepidoptera: Crambidae). J. Econ. Entomol. 100, 1854–1861 (2007). [DOI] [PubMed] [Google Scholar]
- Jiang X. et al. Mutation in acetylcholinesterase1 associated with triazophos resistance in rice stem borer, Chilo suppressalis (Lepidoptera: Pyralidae). Biochem. Bioph. Res. Co. 378, 269–272 (2009). [DOI] [PubMed] [Google Scholar]
- Han L., Li S., Liu P., Peng Y. & Hou M. New artificial diet for continuous rearing of Chilo suppressalis (Lepidoptera: Crambidae). Ann. Entomol. Soc. Am. 105, 253–258 (2012). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.