Abstract
Increasing evidence shows that melatonin protected against age-related mitochondrial oxidative damage. However, the protective effects of melatonin against ovarian aging has not been explored. Young Kunming females (aged 2–3 months) were fed with melatonin added to drinking water for 6 or 12 months (mo). We found that long-term (12 mo) melatonin treatment significantly reduced ovarian aging, as indicated by substantial increases in litter size, pool of follicles, and telomere length as well as oocyte quantity and quality. Melatonin treatment suppressed ovarian mitochondrial oxidative damage by decreasing mitochondrial reactive oxygen species (mROS) generation, inhibiting apoptosis, repressing collapse of mitochondrial membrane potential and preserving respiratory chain complex activities. Female mice fed with melatonin had enhanced mitochondrial antioxidant activities, thus reducing the risk of mitochondrial oxidative damage cause by free radicals. Notably, melatonin treatment enhanced SIRT3 activity but not the protein expression level, and increased the binding affinity of FoxO3a to the promoters of both superoxide dismutase 2 (SOD2) and catalase (CAT). In conclusion, melatonin exerted protection against aging-induced fertility decline and maintenance of mitochondrial redox balance.
Female fertility peaks at approximately age 25 years and declines rapidly after age 35 years1. However, with current cultural and social trends, many women around the world delay childbearing and many face infertility when they attempt to conceive because of the ovarian aging, rather than changes in endometrial receptivity2. Ovarian aging is accompanied by a significant decline in telomere length3, ovarian follicle pool and oocyte reserve4, as well as an increased number of low-quality oocytes not competent for further development5,6. Thus, reproductive senescence is a growing public health problem.
Mitochondria are the primary energy generators in the body and are also the major source and target of free radicals7. In particular, mitochondrial oxidative stress is considered a major factor contributing to the aging process and leads to aberrant signaling pathways8,9. Aging increased mitochondrial reactive oxygen (mROS) and weakened antioxidative defense systems10. Therefore, it is necessary to attenuate oxidative stress and strengthen the antioxidant system to modulate altered signaling pathway molecules to constrain the aging process.
Melatonin (N-acetyl-5-methoxytryptamine) is an endogenously produced indoleamine that can modulate many important physiological reactions in the body11. Long-term administration of melatonin, whose pineal secretion decreases with age, was highly effectively at preventing age-dependent impairment of redox status in various tissues and cells12. Melatonin modulates mitochondria-related functions and strengthens its antioxidant defense systems, effects facilitated by its amphiphilic nature, enabling it to penetrate all morphophysiological barriers and enter all subcellular compartments. Furthermore, melatonin and its metabolites were powerful antioxidants and free radical scavengers13,14. This property enabled them to protect cellular membranes, the electron transport chain and mitochondria from oxidative injury. Melatonin would be readily bioavailable to the ovary and could directly affect ovarian function after oral ingestion15. Thus, the use of melatonin to improve age-related mitochondrial oxidative stress in the ovaries seems feasible.
The aims of this study were to determine whether mice receiving long-term treatment with melatonin could counteract age-associated infertility and ovarian mitochondrial oxidative stress in a mouse model of ovarian aging in vivo and in vitro.
Materials and Methods
Ethics Statement
This study was carried out in strict accordance with the guidelines for the care and use of animals of Northwest A&F University. All animal experimental procedures were approved by the Animal Care Commission of the College of Veterinary Medicine, Northwest A&F University. Every effort was made to minimize animal pain, suffering, and distress and to reduce the number of animals used.
Reagents
Unless otherwise stated, all chemicals and reagents used in this study were from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA).
Animals and treatments
Female Kunming strain mice at 2–3 mo old (outbreed albino mice originated from the ICR strain) were obtained from the Experimental Animal Center of the Fourth Military Medical University (Xi’an, China) and housed in a temperature-controlled animal facility, kept on a cycle of 12 h light and 12 h dark with free access to food and water. Mice were randomly divided into two groups: half received vehicle as a control and the other half received melatonin at 10 mg/kg of body weight during the hours of darkness (18:00–06:00). The dose of melatonin was selected based on that used in previous aging studies in mice12,16. Melatonin was dissolved in a minimum volume of absolute ethanol plus tap water to yield a dose of 10 mg/kg of body weight. The concentration of ethanol in the final solution was 0.066%. A fresh vehicle and melatonin solutions were prepared twice per week. Water bottles were covered with aluminum foil to protect solutions from light. Following treatment with melatonin or vehicle for 6–12 months. Female mice of different age group were paired with young adult Kunming males of proven fertility at 22:00 h. Successfully mated were confirmed by the presence of a vaginal plug before 10:00 h the following morning (day 1 of pregnancy) and were bred individually. Pregnant time of each mouse, number of pups in one litter and development of pups were recorded.
Determination of melatonin levels
Melatonin levels were determined from plasma samples that were collected from each groups. The mice were decapitated after 20% urethane solution. Blood was collected by cardiac puncture, five hundred microliters of were collected in a small-heparinized and kept on ice. The blood was centrifuged at 1,500 rpm for 20 min at 4 °C. Plasma was kept at −80 °C until analysis. Melatonin levels were determined taking all the necessary care to prevent light degradation by using the melatonin enzyme immunoassay kit (IBL Hamburg, Germany).
Ovary serial sectioning and follicle counting
Ovaries were collected and fixed in 4% paraformaldehyde for 24 h, then paraffin embedded. Serial sections (5-μm thickness) were cut and placed onto glass microscope slides. After staining with hematoxylin and eosin Y, the stained sections were analyzed for the number of follicles at four different developmental stages under an optical microscope (Nikon U-III Multipoint Sensor System, Japan). The follicles were classified as previously described17.
Estimation of mitochondrial DNA (mtDNA) copy numbers
Oocyte manipulation techniques are described in more detail in Supplemental materials. A single GV or MII oocyte was added to a PCR tube with 20 μL of lysis buffer (50 mM Tris, 0.1 mM EDTA, 100 μg/mL Proteinase K and 0.5% Tween-20) and incubated at 55 °C for 30 min, then 95 °C for 10 min. To obtain purified plasmid standard DNA, PCR products were amplified with a mouse mtDNA-specific primer as previously described18, and were ligated into PMD-19T vector (Takara).
Immunofluorescence
For chromosome and spindle analysis, oocytes from each group were washed twice in PBS containing 0.1% polyvinylpyrrolidone (PBS-PVP) and then fixed with freshly prepared 4% paraformaldehyde on ice for 30 min, permeabilized with 0.1% Triton X-100 for 30 min and washed three times. After blocking in 1% Bull Serum Albumin (BSA)-supplemented PBS for 1 h, oocytes were incubated overnight at 4 °C with fluorescein isothiocyanate-conjugated α-tubulin antibody to visualize microtubules in the spindle. Chromosomes were stained with DAPI for 5 min. Embryos were harvested for the detection of Oct4-positive cells with an anti-Oct4 mouse monoclonal antibody (Santa Cruz, CA, USA). DNA fragmentation was assessed in blastocysts using a DeadEnd Fluorometric TUNEL System (Promega, Madison, WI), according to the manuscript’s instructions.
Telomerase activity assay
The telomerase activity in ovaries of was measured using the telomerase (TE) ELISA kit (CUSABIO, Wuhan, China).
Measurement of average telomere length
DNA was extracted from ovaries using TIAN amp Genomic DNA Kit (Tian Gen Biotech CO., Ltd., Beijing, China) as described by the manufacturer and absorbance at 260 nm measured with a spectrophotometric plate reader (BioTek, Winooski, Vermont, USA). Average telomere length was measured from genomic DNA using a quantitative real-time PCR (qRT-PCR) assay. The telomere signal was normalized to that from the reference control gene to generate a T/S ratio indicative of relative telomere length.
Mitochondrial ROS production assay
Mitochondrial ROS production was determined using the CM-H2DCFDA indicator dye (Molecular Probes; Invitrogen, Eugene, OR, USA)19.
Mitochondrial DNA damage
Samples of mitochondrial DNA (1 mg/mL) were isolated using Mitochondrial DNA Isolation Kit (BioVision, Milpitas, CA, USA) and incubated for 5 min at 95 °C, then rapidly chilled on ice. DNA sample were digested to nucleosides by incubating the denatured DNA with 3 μL sodium acetate (20 mM, pH 5.2) and 2 μL of nuclease P1 (6.25 U/μL) for 2 h at 37 °C. Each sample was then incubated with 2 μL alkaline phosphatase (0.31 U/μL) and 6 μL Tris (100 mM, pH 7.5) for 1 h at 37 °C. The reaction mixtures were then centrifuged for 5 min at 6000 × g and the supernatants assayed for DNA oxidation with an 8-hydroxy-2′-deoxyguanosine (8-OHdG) ELISA assay (Cell Biolabs, CA, USA).
Measurement of mitochondrial antioxidant function
The mitochondrial fractions were assayed for the superoxide dismutase 2 (SOD2), catalase (CAT) and glutathione (GSH/GSSG) activities. All activities were measured using the kit, following the manufacturer’s instructions (Beyotime Institute of Biotechnology, Jiangsu, China).
Isolation of cytosolic, mitochondria and nuclear fractions
Mitochondria or nuclear fractions were immediately extracted using the Mitochondrial or Nuclear Isolation Kit (Beyotime), respectively. All manipulations were performed on ice. Protein concentrations were determined with the BCA Protein Assay Kit (Pierce Biotech, Rockford, IL, USA).
Western blotting
Protein samples were separated by gradient electrophoresis on 8–15% SDS/PAGE gels. The following antibodies were used to detect the proteins of interest: Glyceraldehyde-3-phosphate dehydrogenase (GAPDH), LAMIN A, β-TUBULIN, SIRT3, Cytochrome c, Bax, Bcl-2, and Cytochrome c oxidase (COX) IV from Cell Signaling Technology (Danvers, MA, USA) and FoxO3a, SOD2, CAT from Abcam (Cambridge, UK). Western blot analysis was performed as described previously20 using the protocols provided by the primary antibody suppliers.
Determination of adenosine triphosphate (ATP) content
The levels of ATP were measured with a luminometer (Bioluminat Junior, Berthold, Germany) with an ATP Bioluminescence Assay Kit (Beyotime).
Ovarian mitochondrial membrane potential (MMP) and respiratory chain complex activity (I-IV) measurements
Before analysis, mitochondria samples were subjected to three freeze-thaw cycles to disrupt membranes and expose the enzymes. All enzymatic activities were measured at 37 °C. MMP was determined using JC-1 as previously described21. Complex I (NADH-ubiquinone oxidoreductase) activity was assayed by measuring decreased absorbance at 340 nm, indicating reduction of NADH oxidation. Complex II (succinate-ubiquinone oxidoreductase) activity was assayed by monitoring reduction of 2,6-dichlorophenolindophenol (DCPIP) at 600 nm. Complex III (CoQ-cytochrome c oxidoreductase) was assayed by monitoring reduction of cytochrome c at 550 nm. Complex IV (cytochrome c oxidase) was assayed by monitoring the oxidation of reduced cytochrome at 550 nm16.
Mouse primary granulosa cell collection and culture
Mouse granulosa cells were collected from the ovaries of immature (3 weeks) Kunming mice using the follicle puncture method, as described previously22. The granulosa cells were cultured in DMEM/F12 supplemented with 10% FBS, 100 IU/mL penicillin, and 100 μg/mL streptomycin at 37 °C and 5% CO2. Cells were incubated with 2 mM H2O2 in the presence or absence of melatonin (100 μg/mL) for 2 h. After incubation, cells were used for further analysis.
Cell viability assay
Cell viability was evaluated by the Cell Counting Kit-8 (CCK-8, Beyotime). After treatment, 10 μL CCK-8 solution was added to each well and incubated at 37 °C for 4 h.
Apoptosis analysis by flow cytometry
Cell suspension was treated with 5 μL of Annexin V and 2.5 μL of propidium iodide (Beyotime) in the dark for 15 min, and analyzed by flow cytometry using a FACSCalibur flow cytometer (Becton-Dickinson, San Jose, CA, USA).
SIRT3 activity
Protein was extracted from cells and tissue using a mild lysis buffer (50 mM Tris-HCl pH 8, 125 mM NaCl, 1 mM DTT, 5 mM MgCl2, 1 mM EDTA, 10% glycerol, and 0.1% NP-40). SIRT3 activity was determined using the CycLex SIRT3 Deacetylase Fluorometric Assay Kit according to the manufacturer’s instructions (MBL International Corp. Japan).
RNA interference of SIRT3
The siRNA for SIRT3 was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Granulosa cells were transfected with 100 nM SIRT3 small interfering and non-targeted control siRNA according to the manufacturer’s protocol. Twenty-four hours after transfection, cells were harvested for further experiments.
Chromatin immunoprecipitation (ChIP)
A ChIP assay was performed using the Pierce Agarose ChIP kit as described previously23. The relative binding of FoxO3a to the SOD2 and CAT promoters was determined using an ABI StepOnePlus PCR system (Applied Biosystems), SYBR Premix ExTaq II (TaKaRa) and the following primer sequences, SOD2: 5′-TTATGGAAACATTTGATAGCCACTGCTTCTTAGAC-3′ (Forward) and 5′-CGCGTGCTTGCTACAGCCACGC-3′ (Reverse); CAT: 5′-GCCAACAAGATTGCCTTCTC-3′ (Forward) and 5′-ACTGTCCGACATGGTGTAGGATT-3′ (Reverse). Techniques are described in more detail in Supplemental materials.
Statistical analysis
Data (means ± SD) were analyzed using SPSS software (SPSS Inc., Chicago, IL, USA). Differences were analyzed using one-way analysis of variance (ANOVA) or t-test. For statistical analysis of the percentage values, the χ2 test were performed. Differences at P < 0.05 were considered to be statistically significant.
Results
Melatonin counteracted age-related fertility decline
Initially, we first determined plasma melatonin levels in female mice treated with melatonin and in the mice of the same age without treatment. Plasma melatonin levels in treated-mice significantly increased at both times tested in compared to the respective control group (Table 1). The values showed a peak of melatonin in the middle of the dark period, and the endogenous melatonin levels significantly decreased at the end of the dark period. However, in melatonin-treated mice, the plasma levels remained constantly higher compared with untreated mice (Supplementary Table S1).
Table 1. Melatonin levels in plasma of treated and untreated female mice.
| Length of treatment (mo) | Group | Melatonin Levels (pg/mL) |
|---|---|---|
| 6 | Vehicle | 26.2 ± 3.7 |
| Melatonin | 43.0 ± 2.1** | |
| 12 | Vehicle | 21.8 ± 5.3 |
| Melatonin | 75.5 ± 6.8** |
Serum melatonin levels at different times were determined. Un-paired Student’s t-test was used to compare two groups. **p < 0.01.
Next, to determine whether supplementation with melatonin was able to rescue the reduced fertility associated with aging, mice treated with melatonin, which showed only a slightly increased litter size. In mice at the age of 14–16 mo, the average number of pups in the melatonin treated groups was significantly higher than from age-matched mice not receiving melatonin. It is notable that there was an increased pregnancy failure in the aged female mice following successful mating regardless of melatonin treatment, as indicated by the presence of mating plugs and mice bearing pups. These data indicate that the melatonin delays age-associated infertility, but could not completely prevent eventual reproductive aging (Table 2 and Supplementary Table S2).
Table 2. Effect of melatonin on female fertility.
| Length of treatment (mo) | Groups | No. of mice examined | No. of mice with vaginal plug | No. of mice bearing pups | Total number | Average litter size of pregnant mice |
|---|---|---|---|---|---|---|
| 6 | Vehicle | 23 | 19 | 17 | 109 | 6.5 ± 2.3 |
| Melatonin | 21 | 20 | 20 | 162 | 7.1 ± 1.8 | |
| 12 | Vehicle | 29 | 22 | 11 | 32 | 2.9 ± 1.6 |
| Melatonin | 25 | 21 | 16 | 90 | 5.7 ± 2.2** |
*P < 0.05, **P < 0.05 versus vehicle; values are mean ± SD.
Melatonin attenuates the loss of follicles and follicle atresia
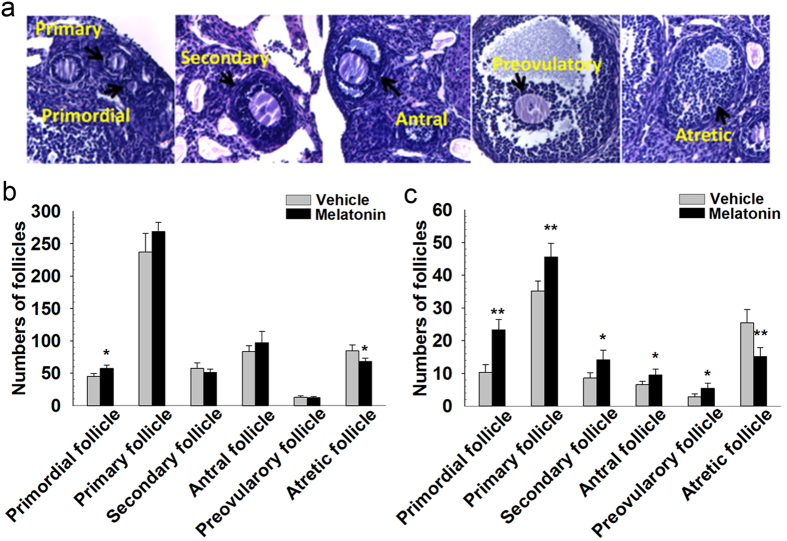
Follicles at different developmental stages in the ovaries were shown in Fig. 1a. Mice treated with melatonin for 6 mo had more primordial follicles compared with untreated age-matched mice. The number of atretic follicles was significantly decreased in aged mice with melatonin treatment. Melatonin administration for 6 mo did not, however, improve the number of growing and mature follicles (Fig. 1b). The numbers of primordial and primary follicles, and few growing and mature follicles continued to decline with age, but were higher in mice treated with melatonin for 12 mo than in age-matched untreated mice, the number of atretic follicles was significantly lower in mice treated with melatonin at this age (Fig. 1c).
Figure 1. Follicle counts in mice treated with melatonin for 6 or 12 mo.
(a) Follicles at different developmental stages in the ovaries. Numbers of different types of follicles in the ovaries of female mice exposed orally to melatonin for 6 (b) or 12 mo (c) Bars represent means ± SD (n = 5/group). *P < 0.05; **P < 0.01 versus the Vehicle group.
Melatonin increases the quantity and quality of oocytes
Next, we observed the oocytes retrieved from ovaries and oviducts of mice. As shown in Table 3 and Supplementary Table S3, the maternal age had a striking effect on the mean number of oocytes retrieved from ovaries and oviducts per mouse. The mean numbers of both ovarian GV oocytes and ovulated MII oocytes were higher in mice treated with melatonin for 12 mo than in age-matched untreated mice.
Table 3. Effect of melatonin on number of oocytes retrieved from ovaries and oviducts.
| Length of treatment (months) | Groups | No. of mice examined | Ovarian GV oocytes |
No. of mice examined | Ovulated MII oocytes |
||
|---|---|---|---|---|---|---|---|
| Total number | Number of oocytes recovered/mouse Mean ± SD (n) | Total number | Number of oocytes recovered/mouse Mean ± SD (n) | ||||
| 6 | Vehicle | 13 | 402 | 30.5 ± 5.7 | 12 | 227 | 18.2 ± 4.4 |
| Melatonin | 12 | 386 | 31.9 ± 4.5 | 12 | 239 | 19.7. ± 5.6 | |
| 12 | Vehicle | 19 | 313 | 16.3 ± 4.1 | 26 | 118 | 4.5 ± 3.8 |
| Melatonin | 19 | 447 | 23.2 ± 3.2* | 19 | 186 | 9.6 ± 3.4* | |
*P < 0.05, **P < 0.01 versus vehicle; values are mean ± SD.
In vitro maturation of GV oocytes were shown in Table 4 and Supplementary Table S4. In mice at the age of 14–16 mo, maturation rate of oocytes from mice treated with melatonin was significantly lower than those of oocytes from the age-matched untreated mice.
Table 4. Effect of melatonin on in vitro maturation of GV oocytes.
| Length of treatment (mo) | Groups | No. of mice examined | No. of oocytes cultured | Maturation stage examined in 16–18 h of culture (%) |
||
|---|---|---|---|---|---|---|
| GV | MI | MII | ||||
| 6 | Vehicle | 13 | 395 | 34 (8.6) | 21 (5.3) | 340 (86.1) |
| Melatonin | 13 | 404 | 32 (7.9) | 24 (6.1) | 350 (86.6) | |
| 12 | Vehicle | 21 | 366 | 41 (11.2) | 33 (9.0) | 301 (79.8) |
| Melatonin | 17 | 390 | 36 (9.7) | 27 (6.9)* | 339 (83.4)* | |
*P < 0.05, **P < 0.01 versus vehicle; χ2 test was used.
Pronuclear formation of oocytes from mice of different ages in 4–7 h of IVF were shown in Table 5 and Supplementary Table S5. Pronuclear formation was accomplished in 7 h of IVF and the rate of oocytes forming pronuclei has no significant difference in each group. In mice at the age of 14–16 mo, the pronuclei formed in oocytes from aged mice without treatment later than that from mice treated with melatonin. Early embryonic development of oocytes after IVF is also shown in Table 5. Mice treated with melatonin for 12 mo increased the rates of oocytes developing to 2-cell and blastocyst than that of age-matched mice without treated.
Table 5. Effect of melatonin on pronuclear formation and development of MII oocytes after in vitro fertilization.
| Length of treatment (mo) | Groups | No. of mice examined | No. of oocytes cultured | No. of oocytes forming pronuclei (%) |
No. of embryo (%) |
||||
|---|---|---|---|---|---|---|---|---|---|
| 4 h | 5 h | 6 h | 7 h | 2-cell | Blastocysts | ||||
| 6 | Vehicle | 12 | 227 | 15(6.6)[7.4] | 76(33.5)[37.6] | 187(82.4)[92.6] | 202(89.0)[100] | 181(79.8) | 139(61.2) |
| Melatonin | 12 | 235 | 16(6.8)[7.7] | 89(37.9)[42.6] | 197(83.8)[94.3] | 209(88.9)[100] | 190(80.9) | 149(63.4) | |
| 12 | Vehicle | 26 | 144 | 6(4.2)[5.1] | 51(35.4)[43.2] | 105(72.9)[89.0] | 118(81.9)[100] | 107(74.3) | 80(55.6) |
| Melatonin | 19 | 186 | 21(11.3)*[13.5] | 94(50.5)**[60.3] | 150(80.6)*[96.2] | 156(83.9)[100] | 146(78.5)* | 117 (62.9)* | |
Values in [] are represented the percentages of oocytes forming pronuclei at certain time of IVF/the total oocytes forming pronuclei.
*P < 0.05, **P < 0.01 versus vehicle; values are mean ± SD, by χ2 test.
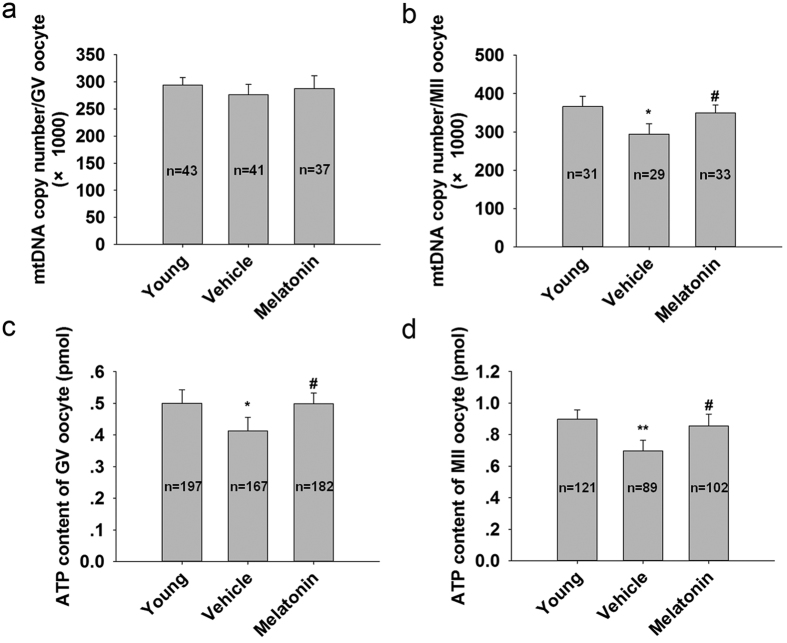
Reports indicate that insufficient mtDNA levels and ATP contents could be directly associated with diminished oocyte competence24. The data showed that mtDNA copy numbers in GV oocytes in the vehicle group showed no difference from that of mice treated with melatonin for 12 mo (Fig. 2a). However, the average mtDNA copy numbers in MII oocytes from melatonin-treated mice were significantly higher compared with the age-matched mice without treatment (Fig. 2b). Mice treated with melatonin for 12 mo significantly increased the ATP levels both in GV (Fig. 2c) and MII oocytes (Fig. 2d).
Figure 2. MtDNA copy numbers and ATP levels after ovulation induction following melatonin treatment for 12 mo.
(a) MtDNA copy number per GV oocyte. (b): MtDNA copy number per MII oocyte. (c): ATP content per GV oocyte. (d) ATP content per MII oocyte. Data are means ± SD, n shows number of oocytes. *P < 0.05; **P < 0.01 versus the Young group. #p < 0.05 versus the Vehicle group.
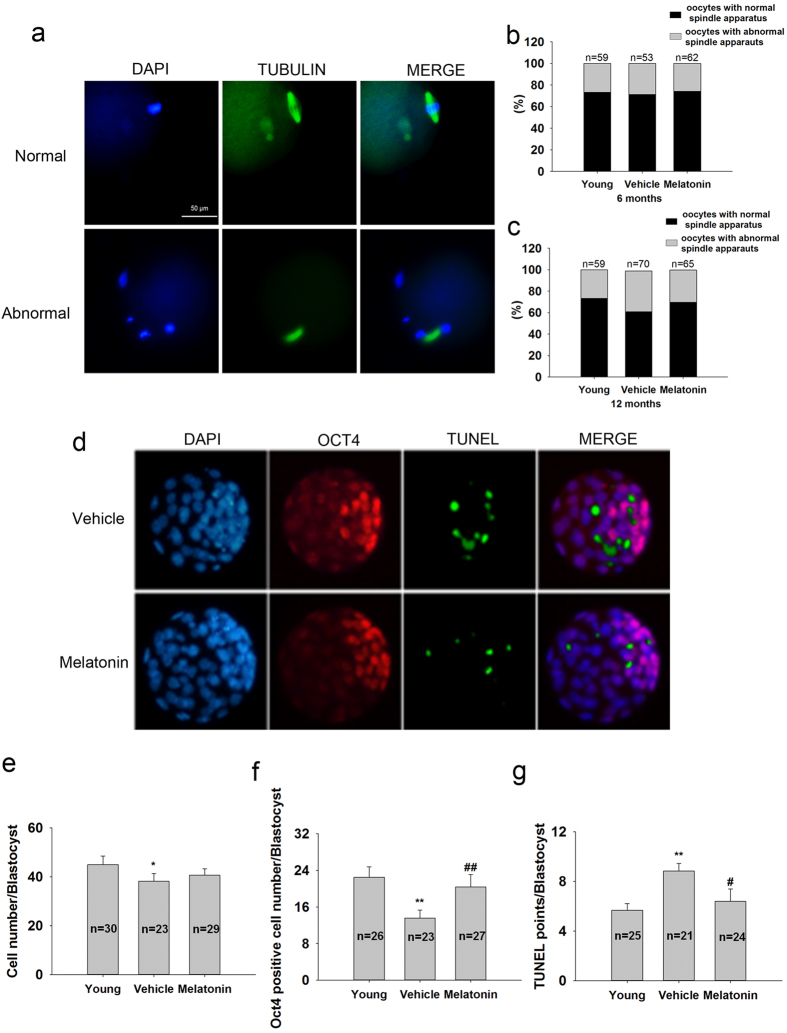
Oocyte quality was further determined by spindle morphology and chromosome alignment (Fig. 3a). The frequency of normal oocytes in the untreated group showed no difference from that of mice treated with melatonin for 6 mo (Fig. 3b). Mice treated with melatonin for 12 mo exhibited a higher frequency of normal oocytes than age-matched untreated control (Fig. 3c). Oct4 immunostaining not only marks pluripotent cells but also indicates the inner cell mass of blastocysts25. Mice treated with melatonin for 12 mo did not influence the total number of cells in blastocysts (Fig. 3d,e), but significantly increased the number of Oct4-positive cells (Fig. 3d,f) and reduced the frequency of apoptosis (Fig. 3d,g) in blastocysts.
Figure 3. Oocyte quality and embryo development potential after ovulation induction following melatonin treatment.
(a) Representative images of normal or abnormal chromosomes and spindle apparatus. A normal spindle apparatus was characterized as having chromosomes located exclusively on the equatorial plate and a barrel-shaped structure. Percentage of oocytes with normal or abnormal spindle apparatus following melatonin treatment for 6 (b) or 12 mo (c). (d) Representative immunofluorescence images for Oct4 (red) and TUNEL (green) staining in embryos cultured for 108 h. Nuclei stained with DAPI (blue). (e) Cell number in each embryo estimated by counting nuclei. (f) Number of Oct4-positive cells, indicative of inner cell mass cell number of each embryo. (g) Number of TUNEL-positive cells of each embryo. n = number of oocytes or embryos counted. The bars show means ± SD. *P < 0.05; **P < 0.01 versus the Young group. #p < 0.05; ##p < 0.01 versus the Vehicle group.
Melatonin suppressed ovarian mitochondrial oxidative stress and apoptosis
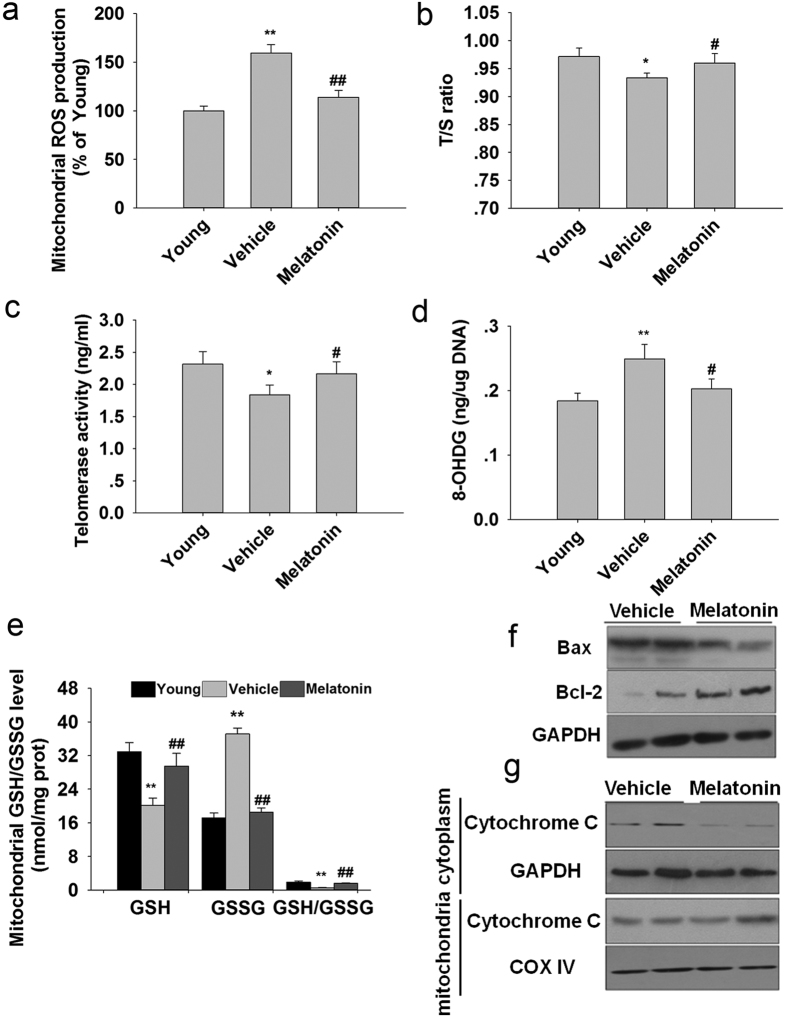
Mice treated with melatonin significantly decreased mitochondrial ROS production (Fig. 4a). Telomeres erode rapidly in response to oxidative stress. Ovaries from mice treated with melatonin for 12 mo had longer telomeres than those from age-matched mice without treatment (Fig. 4b). Telomerase activity in mice treated with melatonin for 12 mo was higher than in age-matched untreated mice (Fig. 4c). Aged mice treated with melatonin for 12 mo had markedly reduced 8-OHdG level in mitochondria compared with those of age-matched untreated mice (Fig. 4d). Long-term treatment with melatonin prevented aging-associated oxidative stress in the ovarian mitochondria, as assessed by the recovery of GSH levels and GSH/GSSG ratios (Fig. 4e).
Figure 4. Mitochondrial oxidative stress and apoptosis in the ovaries of mice following melatonin treatment for 12 mo.
(a): Mitochondrial ROS production. (b) The average telomere lengths of ovaries, shown as the T/S ratio, were determined by qRT-PCR. (c) Telomerase activities in the ovaries were analyzed by ELISA. (d) Indicators of mitochondrial DNA oxidation (8-OHdG). (e) GSSG/GSH ratio. Mitochondrial-related apoptosis were analyzed by western blotting. (f) Bax/Bcl-2 ratio. (g) Mitochondrial and cytosolic cytochrome c. Data are means ± SD (n = 7/group). *P < 0.05; **P < 0.01 versus the Young group. #p < 0.05; ##p < 0.01 versus the Vehicle group.
Melatonin treatment significantly suppressed Bax protein expression and decreased the Bax/Bcl-2 ratio (Fig. 4f) and decreased cytoplasmic cytochrome c levels (Fig. 4g) compared with age-matched mice without treatment.
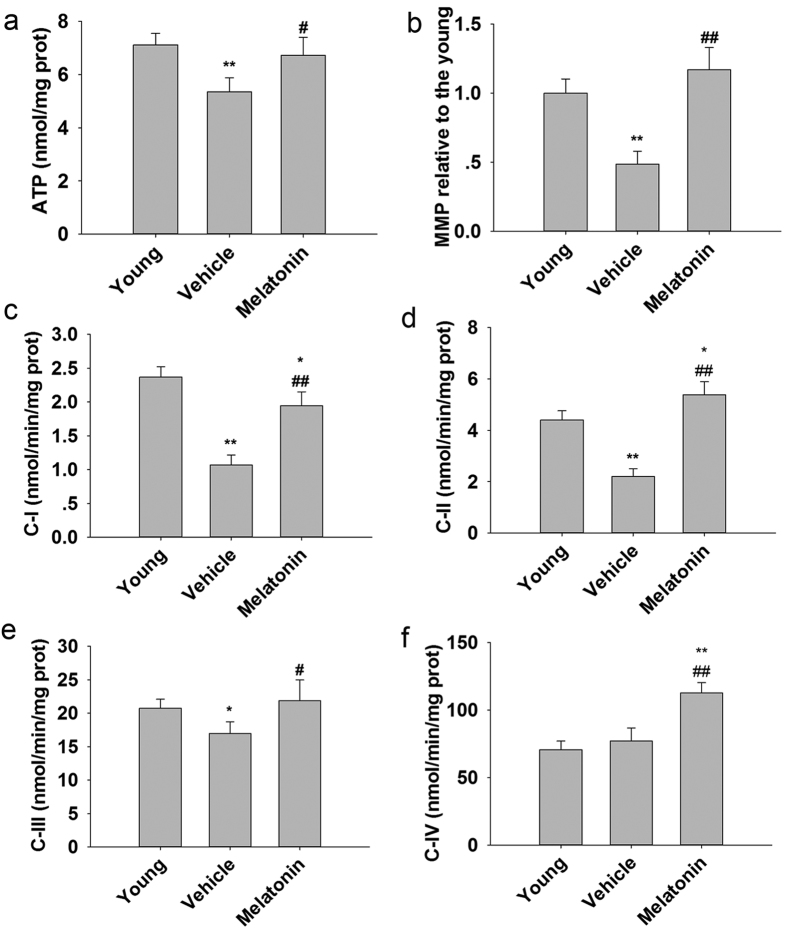
Melatonin attenuated ovarian mitochondrial dysfunction
In the ovarian mitochondria from female mice treated with melatonin for 12 mo, ATP content (Fig. 5a) and MMP (Fig. 5b) were significantly higher than in age-matched untreated females. Mitochondria, especially mitochondrial respiratory chain, has been shown to be major sources of ROS26. We measured the activities of complexes I-IV of mitochondrial electron transport chain (ETC). Aging did not affect activity of complex IV (Fig. 5f), but there was a significant decrease in activities of complex I (Fig. 5c), II (Fig. 5d) and III (Fig. 5e) in untreated mice at 14–16 mo of age compared with in young mice. Administration of melatonin resulted in a remarkable augmentation of the ETC activities, as compared with age-matched mice without treatment. In agreement with the reduced mitochondrial ROS production, long-term treatment with melatonin significantly elevated the ETC activities.
Figure 5. Mitochondrial function in the ovaries of mice following administration of melatonin for 12 mo.
(a) ATP level. (b) MMP value. (c) Mitochondrial complex I activity. (d) Mitochondrial complex II activity. (e) Mitochondrial complex III activity. (f) Mitochondrial complex IV activity. Data are means ± SD (n = 10/group). *P < 0.05; **P < 0.01 versus the Young group. #p < 0.05; ##p < 0.01 versus the Vehicle group.
Melatonin protects granulosa cells from oxidative stress in vitro
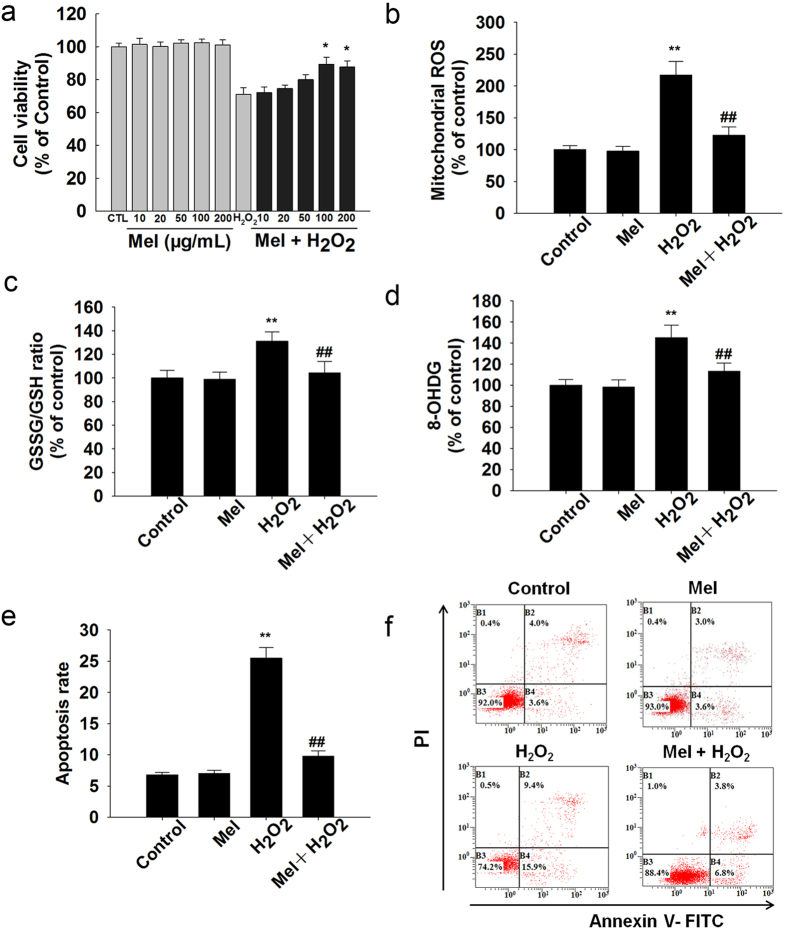
Granulosa cell is one of the main components of ovary and it is a appropriate object for in vitro experiments. According to lots of previous studies, granulosa cells were examined to indicate ovarian aging in the in vitro experiments. The H2O2-induced decrease in cell viability was significantly attenuated after treatment with melatonin (Fig. 6a). In vitro analysis showed that melatonin blocked mitochondrial ROS elevation induced by H2O2 in granulosa cells (Fig. 6b). Moreover, co-incubation with melatonin prevented H2O2-induced oxidative stress (Fig. 6c), DNA damage (Fig. 6d) and apoptosis (Fig. 6e,f).
Figure 6. Effect of melatonin on the H2O2-induced oxidative damage and apoptosis in granulosa cells in vitro.
(a) Cell viability was measured by CCK-8. (b) Mitochondrial reactive oxygen species (mROS). (c) GSSG/GSH ratio. (d) The elevation of mitochondrial oxidative stress was further confirmed by 8-OHdG. (e,f): Apoptosis rate. All values are the mean ± SD of the results from three independent experiments. **p < 0.01 versus the Control group. #p < 0.05; ##p < 0.01 versus the H2O2 group.
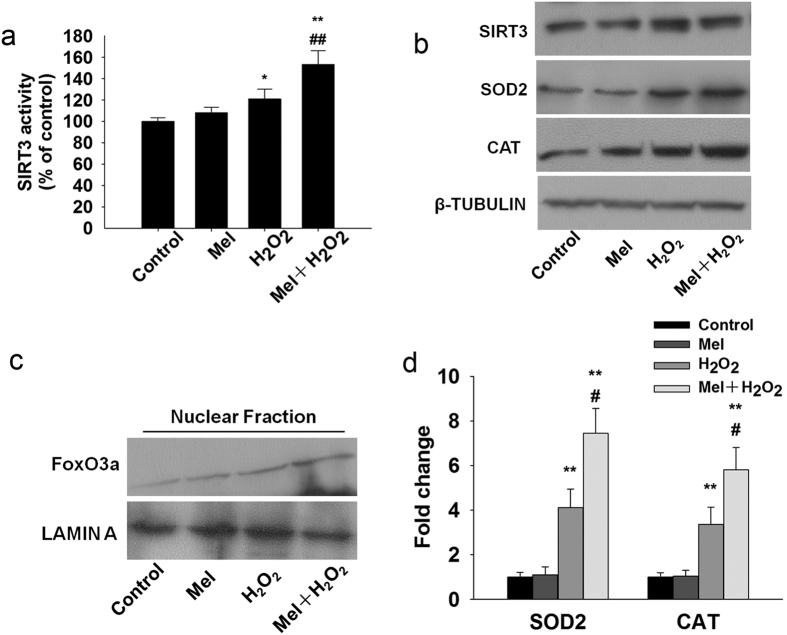
Melatonin upregulates SOD2 and CAT through the interaction of SIRT3 with FoxO3a in vitro
We firstly examined whether the expression of aging-related SIRT3 is modulated by oxidative stress in granulosa cells. Interestingly, the results revealed that granulosa cells stressed with H2O2 induced a significant increase in SIRT3 expression (Supplementary Fig. S1a) and activity (Supplementary Fig. S1b), however, it dropped significantly after two hours. Cell viability was further determined using the CCK-8 assay (Supplementary Fig. S1c). Overexpression of SIRT3 restore the cell viability (Supplementary Fig. S1e), while SIRT3-siRNA exhibited a marked reduction in cell viability (Supplementary Fig. S1d) in response to H2O2, indicating that SIRT3 is necessary to prevent granulosa cells against senescence-related oxidative stress.
Our data showed that melatonin enhanced the SIRT3 activity (Fig. 7a) without significantly affecting SIRT3 protein levels (Fig. 7b) in response to oxidative stress. FoxO3a protein expression significantly increased in the nuclear fraction of granulosa cells treated with melatonin compared to control (Fig. 7c) under oxidative stress. In ChIP assay, we found that SIRT3 promoted the binding of FoxO3a to the promoter of SOD2 and CAT (Fig. 7d). The increased protein expression of SOD2 and CAT were further confirmed with western blot analysis.
Figure 7. Melatonin increase the SIRT3 activity, nuclear translocation of FoxO3a and binding of FoxO3a to the promoters of SOD2 and CAT in granulosa cells following H2O2 treatment.
(a) Graph shows a significant increase in the SIRT3 activity. (b) Western blots showed the expression of SIRT3, SOD2 and CAT in granulosa cells. (c) Western blot analysis shows the nuclear translocation of FoxO3a in granulosa cells. (d) FoxO3a has a strong binding affinity to the promoters of mitochondrial antioxidants SOD2 and CAT in granulosa cells. *p < 0.05; **p < 0.01 versus the Control group. #p < 0.05; ##p < 0.01 versus the H2O2 group. All values are the mean ± SD of the results from three independent experiments.
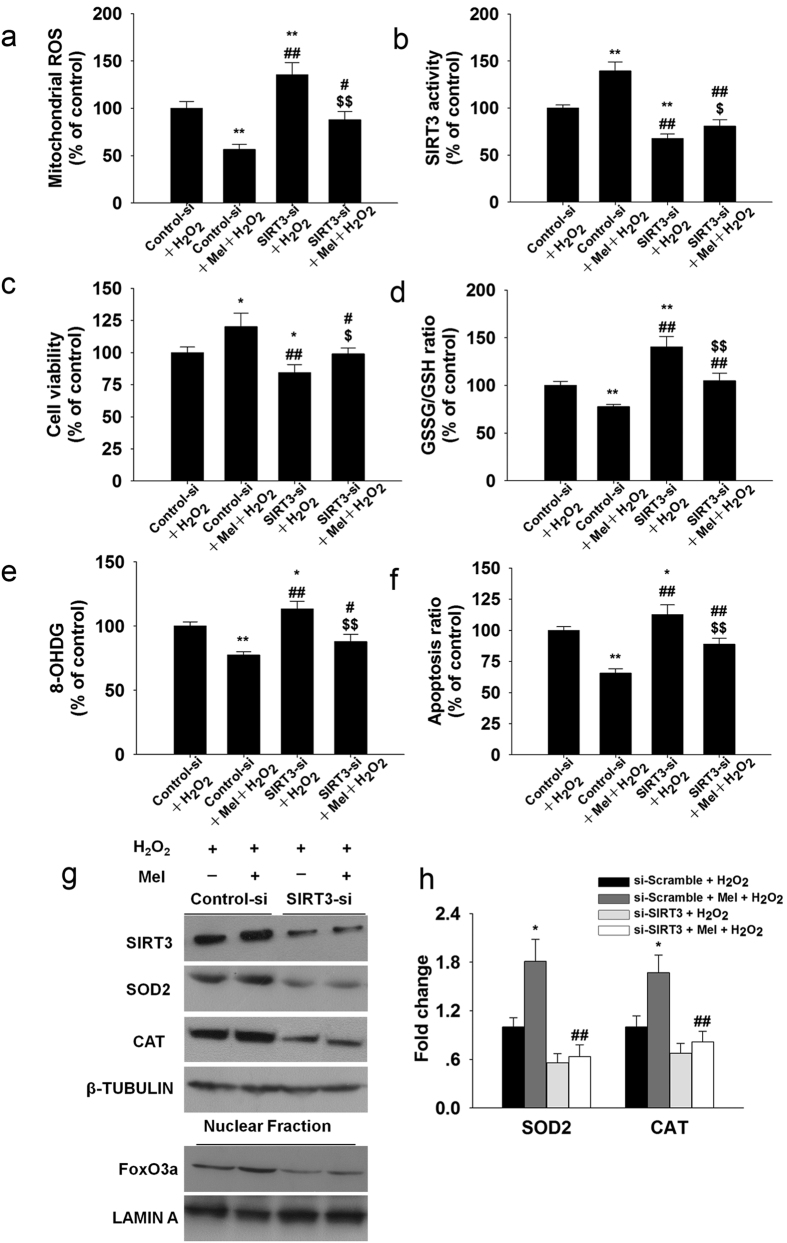
SIRT3 siRNA treatment significantly attenuated the protective effects of melatonin on H2O2-induced mitochondrial ROS generation (Fig. 8a) and cell viability (Fig. 8c). Furthermore, GSSG content (Fig. 8d), 8-OHdG (Fig. 8e) and apoptosis (Fig. 8f) were decreased in cells with melatonin, but these beneficial effects were abolished in the presence of SIRT3 siRNA. SIRT3 activity (Fig. 8b) and protein level (Fig. 8g) and FoxO3a nuclear localization (Fig. 8g) were significantly reduced in granulosa cells subjected to siRNA-mediated SIRT3 knockdown. Upon SIRT3 knockdown by siRNA, SOD2 and CAT levels were significantly downregulated in granulosa cells (Fig. 8g), and the binding affinity of FoxO3a to SOD2 and CAT promoters were significantly decreased (Fig. 8h).
Figure 8. SiRNA-mediated knockdown of SIRT3 abolishes the protective effects of melatonin in granulosa cells. SIRT3 was knocked down by SIRT3 siRNA transfection as described in the Materials and Methods section.
(a) Mitochondrial ROS, (b) SIRT3 activity, (c) Cell viability, (d) GSSG/GSH ratio, (e) 8-OHdG, (f) Apoptosis ratio. (g) The expressions of SIRT3, SOD2, CAT and the nuclear translocation of FoxO3a were examined using immunoblotting. (h) Granulosa cells were harvested for ChIP analysis and the FoxO3a binding affinity was measured by real-time PCR assay. The values are presented as the mean ± SD of the results from three independent experiments. *p < 0.05, **p < 0.01 versus the Control siRNA + H2O2 group; #p < 0.05, ##p < 0.01 versus the Control siRNA + H2O2 + melatonin group; $p < 0.05, $$p < 0.01 versus the SIRT3 siRNA + H2O2 group. For ChIP assay, *p < 0.05 versus the si-Scramble + H2O2 group; ##p < 0.01 versus the si-Scramble + Mel + H2O2 group.
Melatonin increased the SIRT3 and FoxO3a expression in vivo
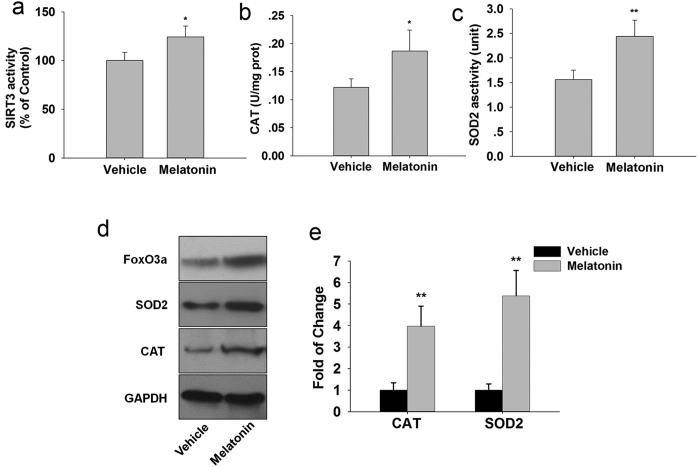
Mice receiving melatonin had significantly higher activities of SIRT3 (Fig. 9a), CAT (Fig. 9b) and SOD2 (Fig. 9c) in the ovaries than did the untreated aged mice. We further investigated the role of FoxO3a in melatonin-induced CAT and SOD2 expression (Fig. 9d) using chromatin immunoprecipitation. In ChIP assay, we found that melatonin promoted the binding of FoxO3a to the promoters of CAT and SOD2 (Fig. 9e).
Figure 9. Melatonin suppresses ovarian mitochondrial oxidative stress by enhancing SIRT3 activity in vivo.
(a) The effects of melatonin on SIRT3 activity. Mitochondrial antioxidants activities of CAT (b) and SOD2 (c) were measured. (d) Western blot were further confirmed the expression of FoxO3a, SOD2 and CAT. (e): The binding of FoxO3a to the promoters of SOD2 and CAT were performed by ChIP-qPCR in ovary. Data are presented as the mean ± SD (n = 7/group). *p < 0.05, **p < 0.01 versus the Vehicle group.
Discussion
In the present study we show that exogenous melatonin on the protects against the reduction during the aging of mice as indicated by litter size. Furthermore, melatonin improves the healthy follicle number, telomerase activity and telomere length, as well as the oocyte quality and quantity, indicating that it staves off the ovarian aging.
The well-established paradigm of reproduction in mammals holds that females are born with a fixed number of oocytes which continuously decline until few or none remain27. Our results are in agreement with studies, that aging ovaries exhibit reduced numbers, as well as reduced quantity and quality of oocytes. We show that melatonin may provide long-term beneficial effect by maintaining female reproductive capacity in mice. The beneficial effects of melatonin on ovarian aging might be contribute to the slowed loss of non-renewable female germ cells. But, we should note that melatonin rescue experiments showed that melatonin cannot prevent but can significantly delay declined fertility associated with reproductive age.
It has been suspected that the reproductive failure can be attributed to the functional and structural qualities of the oocytes28. Reports indicate that sub-normal mtDNA levels and ATP contents could be directly associated with poor oocyte quality and detrimental embryonic development29. It seems that the reduced number of oocytes retrieved with age may be of less importance than the decline in oocyte quality. Age-related fertility decline of the mice is not only due to a reduced oocyte pool, but also the result of a decrease in oocyte quality and embryonic development potential. Melatonin treatment both improves the quantity and quality of oocytes with age.
Various theories propose an essential role of mitochondria in cellular events associated with the aging process, via accumulation of mitochondrial ROS and oxidative damage to mitochondrial and cytoplasmic components30,31. There is evidence that mitochondrial respiratory complex activity and mitochondrial membrane potential decline during aging and that endogenous antioxidant system activities exhibit an age-dependent decrease, making redox imbalance play a more predominant role in the aged cells and tissues. Here we showed that melatonin was effective in ameliorating mitochondrial oxidative damage, apoptosis and preserving mitochondrial function in the aged ovarian. There is increasing evidence for beneficial effects of the antioxidant melatonin in preventing ROS-induced damage and pathology32,33. Reducing oxidative stress by supplementation of melatonin could potentially reduce mitochondrial ROS-induced damage, thus maintaining the number and quality of oocytes and follicles. Telomere shortening is considered as a biomarker of cellular senescence and is highly sensitive to oxidative stress34,35. Telomere dysfunction may contribute to reproductive aging-associated meiotic defects, miscarriage and infertility36. Thus, protection against oxidative stress by melatonin (Supplementary Fig. S2) could directly contribute to maintenance of telomeres in aging mouse ovaries.
As one of the main components of ovary, granulosa cell is a appropriate object for in vitro experiments. Ovarian ageing involves the accumulation of damage by oxidative stress associated with an increasingly compromised microcirculation around the leading follicle37. Previous study have established that advanced maternal age have altered expression of genes involved in mitochondrial metabolic pathways in granulosa and cumulus cells compared with young38,39. Communication between the oocyte and its surrounding cells is essential for the development of a viable oocyte, and impaired mitochondria (Supplementary Fig. S3) within the follicular cells ultimately affect oocyte development. Moreover, follicular atresia is the main process responsible for the loss of follicles and oocytes from the ovary, and it is the root cause of ovarian aging. More than 99% of follicles undergo atresia that has been associated with apoptosis of granulosa cells40. Therefore primary ovarian granular cells were further selected for in vitro experimentation.
SIRT3 is the primary mitochondrial deacetylase, and it regulates biological functions directly involved in mitochondrial function and limits the generation of mitochondrial ROS41. Residing predominantly in the mitochondria, SIRT3 is known to deacetylate and activate complex I of the respiratory chain in mitochondria, leading to an increase in levels of ATP and thereby protecting the cells from ROS-mediated oxidative damage42. Ectopic SIRT3 expression has antioxidant regulatory effects in primary cardiomyocytes, suggesting that SIRT3 may regulate antioxidant systems43. The activity of sirtuin 3 (SIRT3) was decreased in granulosa and cumulus cells from women of advanced maternal age44. SIRT3 attenuation or ablation is associated with accelerated development of several diseases of aging45.
SIRT3 has also been shown to interact with FoxO3a, the transcription factor implicated in ROS regulation and to activate FoxO3a-dependent gene expression46,47. Increased nuclear translocation of FoxO3a strengthens antioxidant defense systems, which leads to extended longevity, in several experimental organisms48,49. An age-mediated decrease in FoxO3a activity was observed in aged rats, which led to a decrease in the mitochondrial antioxidant enzymes50. In this study as FoxO3a was found to bind to the promoter regions of SOD2 and CAT genes. These results indicate that melatonin enhances the SIRT3 activity and activates FoxO3a nuclear translocation which leads to transactivation of the anti-oxidant genes, thereby resulting in inhibition of mitochondrial oxidative damage. Notably, though melatonin could scavenge the H2O2 directly, H2O2 radical scavenging ability of melatonin works mainly through the SIRT3 pathway (Supplementary Fig. S4). Overall, SIRT3 and its target FoxO3a appear to function against mitochondrial oxidative damage.
In mammals, overall reproductive lifespan is predetermined at birth by the number of oocytes in the ovary and the ovarian aging manifestations of menopause occur when the finite supply of oocytes is depleted. However, our results using mice as a model suggest that this depletion can be significantly delayed, with potential clinical benefits for women who wish to postpone childbearing. More extensive research will be needed, however, prior to conducting clinical trials to test this proposal.
Additional Information
How to cite this article: Song, C. et al. Melatonin improves age-induced fertility decline and attenuates ovarian mitochondrial oxidative stress in mice. Sci. Rep. 6, 35165; doi: 10.1038/srep35165 (2016).
Supplementary Material
Acknowledgments
This work was funded by the National High Technology Research and Development Program of China (863 Program) (No. 2011AA100303), the State Key Program (No. 31530075) of National Natural Science Foundation of China and China Postdoctoral Science Foundation Grant (No. 2016M590978).
Footnotes
Author Contributions C.S. Conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing W.P. Collection and assembly of data S.Y. Collection and assembly of data J.Z. Collection and assembly of data B.F. Collection and assembly of data J.Z. Collection and assembly of data T.M. Collection and assembly of data H.W. Conception and design, Collection and assembly of data, data analysis and interpretation Y.Z. Conception and design, data analysis and interpretation, financial support, final approval of manuscript
References
- Leridon H. Can assisted reproduction technology compensate for the natural decline in fertility with age? A model assessment. Hum Reprod 19, 1548–1553 (2004). [DOI] [PubMed] [Google Scholar]
- Mills M. et al. Why do people postpone parenthood? Reasons and social policy incentives. Human reproduction update 17, 848–860 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M. et al. Resveratrol protects against age-associated infertility in mice. Human reproduction 28, 707–717 (2013). [DOI] [PubMed] [Google Scholar]
- Tatone C. et al. Cellular and molecular aspects of ovarian follicle ageing. Human reproduction update 14, 131–142 (2008). [DOI] [PubMed] [Google Scholar]
- Meldrum D. R. et al. Aging and the environment affect gamete and embryo potential: can we intervene? Fertility and sterility 105, 548–559 (2016). [DOI] [PubMed] [Google Scholar]
- Liu J. et al. Delay in oocyte aging in mice by the antioxidant N-acetyl-L-cysteine (NAC). Human reproduction 27, 1411–1420 (2012). [DOI] [PubMed] [Google Scholar]
- Stangenberg S. et al. Oxidative stress, mitochondrial perturbations and fetal programming of renal disease induced by maternal smoking. The international journal of biochemistry & cell biology 64, 81–90 (2015). [DOI] [PubMed] [Google Scholar]
- Medkour Y. & Titorenko V. I. Mitochondria operate as signaling platforms in yeast aging. Aging 8, 212–213 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H. C. et al. Neural activity and CaMKII protect mitochondria from fragmentation in aging Caenorhabditis elegans neurons. Proceedings of the National Academy of Sciences of the United States of America 112, 8768–8773 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratic A. & Larsson N. G. The role of mitochondria in aging. The Journal of clinical investigation 123, 951–957 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter R. J., Tan D. X. & Galano A. Melatonin: exceeding expectations. Physiology 29, 325–333 (2014). [DOI] [PubMed] [Google Scholar]
- Martin-Cano F. E., Camello-Almaraz C., Acuna-Castroviejo D., Pozo M. J. & Camello P. J. Age-related changes in mitochondrial function of mouse colonic smooth muscle: beneficial effects of melatonin. Journal of pineal research 56, 163–174 (2014). [DOI] [PubMed] [Google Scholar]
- Manchester L. C. et al. Melatonin: an ancient molecule that makes oxygen metabolically tolerable. Journal of pineal research 59, 403–419 (2015). [DOI] [PubMed] [Google Scholar]
- Ramis M. R., Esteban S., Miralles A., Tan D. X. & Reiter R. J. Protective Effects of Melatonin and Mitochondria-targeted Antioxidants Against Oxidative Stress: A Review. Current medicinal chemistry 22, 2690–2711 (2015). [DOI] [PubMed] [Google Scholar]
- Tamura H. et al. Melatonin and the ovary: physiological and pathophysiological implications. Fertility and sterility 92, 328–343 (2009). [DOI] [PubMed] [Google Scholar]
- Carretero M. et al. Long-term melatonin administration protects brain mitochondria from aging. Journal of pineal research 47, 192–200 (2009). [DOI] [PubMed] [Google Scholar]
- Zhang J. Q. et al. 3-Nitropropionic acid induces ovarian oxidative stress and impairs follicle in mouse. PloS one 9, e86589 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q. et al. Maternal diabetes causes mitochondrial dysfunction and meiotic defects in murine oocytes. Molecular endocrinology 23, 1603–1612 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye R. et al. Ginsenoside Rd attenuates redox imbalance and improves stroke outcome after focal cerebral ischemia in aged mice. Neuropharmacology 61, 815–824 (2011). [DOI] [PubMed] [Google Scholar]
- Wu H. et al. Vitamin C enhances Nanog expression via activation of the JAK/STAT signaling pathway. Stem cells 32, 166–176 (2014). [DOI] [PubMed] [Google Scholar]
- Yang Y. et al. Melatonin prevents cell death and mitochondrial dysfunction via a SIRT1-dependent mechanism during ischemic-stroke in mice. Journal of pineal research 58, 61–70 (2015). [DOI] [PubMed] [Google Scholar]
- Wu J., Yuan M., Song Y., Sun F. & Han X. MC-LR Exposure Leads to Subfertility of Female Mice and Induces Oxidative Stress in Granulosa Cells. Toxins 7, 5212–5223 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J. L. et al. URI regulates tumorigenicity and chemotherapeutic resistance of multiple myeloma by modulating IL-6 transcription. Cell death & disease 5, e1126 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spikings E. C., Alderson J. & St John J. C. Regulated mitochondrial DNA replication during oocyte maturation is essential for successful porcine embryonic development. Biology of reproduction 76, 327–335 (2007). [DOI] [PubMed] [Google Scholar]
- Rodriguez-Alvarez L., Manriquez J., Velasquez A. & Castro F. O. Constitutive expression of the embryonic stem cell marker OCT4 in bovine somatic donor cells influences blastocysts rate and quality after nucleus transfer. In vitro cellular & developmental biology. Animal 49, 657–667 (2013). [DOI] [PubMed] [Google Scholar]
- Sun Q., Zhong W., Zhang W. & Zhou Z. Defect of mitochondrial respiratory chain is a mechanism of ROS overproduction in a rat model of alcoholic liver disease: role of zinc deficiency. American journal of physiology. Gastrointestinal and liver physiology 310, G205–G214 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flurkey K. & Harrison D. E. Reproductive ageing: of worms and women. Nature 468, 386–387 (2010). [DOI] [PubMed] [Google Scholar]
- Sfontouris I. A. et al. Artificial oocyte activation to improve reproductive outcomes in women with previous fertilization failure: a systematic review and meta-analysis of RCTs. Human reproduction 30, 1831–1841 (2015). [DOI] [PubMed] [Google Scholar]
- Zhang X., Wu X. Q., Lu S., Guo Y. L. & Ma X. Deficit of mitochondria-derived ATP during oxidative stress impairs mouse MII oocyte spindles. Cell research 16, 841–850 (2006). [DOI] [PubMed] [Google Scholar]
- Yang W. & Li X., Li, X., Li, X. & Yu S. Neuronal hemoglobin in mitochondria is reduced by forming a complex with alpha-synuclein in aging monkey brains. Oncotarget (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Freire M. et al. Reconsidering the Role of Mitochondria in Aging. The journals of gerontology. Series A, Biological sciences and medical sciences 70, 1334–1342 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu P. et al. Melatonin protects ADSCs from ROS and enhances their therapeutic potency in a rat model of myocardial infarction. Journal of cellular and molecular medicine 19, 2232–2243 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schallreuter K. U. et al. Blunted epidermal L-tryptophan metabolism in vitiligo affects immune response and ROS scavenging by Fenton chemistry, part 1: Epidermal H2O2/ONOO(-)-mediated stress abrogates tryptophan hydroxylase and dopa decarboxylase activities, leading to low serotonin and melatonin levels. FASEB journal: official publication of the Federation of American Societies for Experimental Biology 26, 2457–2470 (2012). [DOI] [PubMed] [Google Scholar]
- Huang J., Okuka M., McLean M., Keefe D. L. & Liu L. Telomere susceptibility to cigarette smoke-induced oxidative damage and chromosomal instability of mouse embryos in vitro. Free radical biology & medicine 48, 1663–1676 (2010). [DOI] [PubMed] [Google Scholar]
- Kalmbach K. H., Antunes D. M., Kohlrausch F. & Keefe D. L. Telomeres and Female Reproductive Aging. Seminars in reproductive medicine 33, 389–395 (2015). [DOI] [PubMed] [Google Scholar]
- Keefe D. L. & Liu L. Telomeres and reproductive aging. Reproduction, fertility, and development 21, 10–14 (2009). [DOI] [PubMed] [Google Scholar]
- Tatone C. et al. Age-dependent changes in the expression of superoxide dismutases and catalase are associated with ultrastructural modifications in human granulosa cells. Molecular human reproduction 12, 655–660 (2006). [DOI] [PubMed] [Google Scholar]
- McReynolds S. et al. Impact of maternal aging on the molecular signature of human cumulus cells. Fertility and sterility 98, 1574–1580 e1575 (2012). [DOI] [PubMed] [Google Scholar]
- Wilding M. et al. Mitochondrial aggregation patterns and activity in human oocytes and preimplantation embryos. Hum Reprod 16, 909–917 (2001). [DOI] [PubMed] [Google Scholar]
- Yu Y. S. et al. Apoptosis in granulosa cells during follicular atresia: relationship with steroids and insulin-like growth factors. Cell research 14, 341–346 (2004). [DOI] [PubMed] [Google Scholar]
- Pi H. et al. SIRT3-SOD2-mROS-dependent autophagy in cadmium-induced hepatotoxicity and salvage by melatonin. Autophagy 11, 1037–1051 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn B. H. et al. A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proceedings of the National Academy of Sciences of the United States of America 105, 14447–14452 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai V. B. et al. Exogenous NAD blocks cardiac hypertrophic response via activation of the SIRT3-LKB1-AMP-activated kinase pathway. The Journal of biological chemistry 285, 3133–3144 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacella-Ince L., Zander-Fox D. L. & Lan M. Mitochondrial SIRT3 and its target glutamate dehydrogenase are altered in follicular cells of women with reduced ovarian reserve or advanced maternal age. Human reproduction 29, 1490–1499 (2014). [DOI] [PubMed] [Google Scholar]
- Zhang C. Z. et al. Low SIRT3 expression correlates with poor differentiation and unfavorable prognosis in primary hepatocellular carcinoma. PloS one 7, e51703 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaresan N. R. et al. Sirt3 blocks the cardiac hypertrophic response by augmenting Foxo3a-dependent antioxidant defense mechanisms in mice. The Journal of clinical investigation 119, 2758–2771 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng A. H., Shieh S. S. & Wang D. L. SIRT3 deacetylates FOXO3 to protect mitochondria against oxidative damage. Free radical biology & medicine 63, 222–234 (2013). [DOI] [PubMed] [Google Scholar]
- Rangarajan P., Karthikeyan A., Lu J., Ling E. A. & Dheen S. T. Sirtuin 3 regulates Foxo3a-mediated antioxidant pathway in microglia. Neuroscience 311, 398–414 (2015). [DOI] [PubMed] [Google Scholar]
- Qi X. F. et al. FoxO3a suppresses the senescence of cardiac microvascular endothelial cells by regulating the ROS-mediated cell cycle. Journal of molecular and cellular cardiology 81, 114–126 (2015). [DOI] [PubMed] [Google Scholar]
- Luo L. et al. Chronic resistance training activates autophagy and reduces apoptosis of muscle cells by modulating IGF-1 and its receptors, Akt/mTOR and Akt/FOXO3a signaling in aged rats. Experimental gerontology 48, 427–436 (2013). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.