Abstract
The pyridine ring is a potent pharmacophore in alkaloid natural products. Nonetheless, its biosynthetic pathways are poorly understood. Rubrolones A and B are tropolone alkaloid natural products possessing a unique tetra-substituted pyridine moiety. Here, we report the gene cluster and propose a biosynthetic pathway for rubrolones, identifying a key intermediate that accumulates upon inactivation of sugar biosynthetic genes. Critically, this intermediate was converted to the aglycones of rubrolones by non-enzymatic condensation and cyclization with either ammonia or anthranilic acid to generate the respective pyridine rings. We propose that this non-enzymatic reaction occurs via hydrolysis of the key intermediate, which possesses a 1,5-dione moiety as an amine acceptor capable of cyclization. This study suggests that 1,5-dione moieties may represent a general strategy for pyridine ring biosynthesis, and more broadly highlights the utility of non-enzymatic diversification for exploring and expanding natural product chemical space.
The biosynthesis of pyridine rings is still poorly understood. Here the authors propose a biosynthetic pathway for pyridine-containing rubrolones, which is characterized by a non-enzymatic condensation and cyclization of the pyridine moiety.
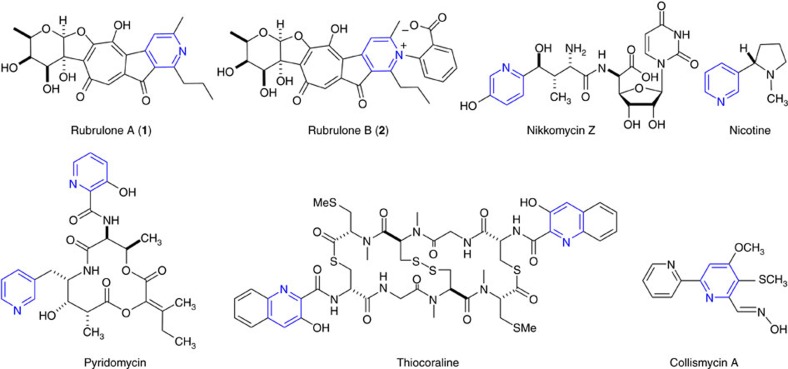
Rubrolone A (1) was first reported in 1978 as a red pigment produced by Streptomyces enchinoruber1, and subsequent structure elucidation revealed a nearly coplanar and highly conjugated aglycone consisting of a tropolone ring, a five-membered cyclopentanone, and a pyridine ring2 (Fig. 1). A unique carbon–carbon bond links C-2 of the deoxysugar to the tropolone ring of the aglycone. This highly oxygenated hybrid structure with distinct ring assembly has inspired chemists and microbiologists, but only two total synthetic routes to the rubrolone aglycone have been devised3,4 and the biosynthetic pathway has not been elucidated.
Figure 1. Chemical structures of rubrolones A (1) and B (2) and selected pyridine alkaloids.
Pyridine rings are highlighted in blue.
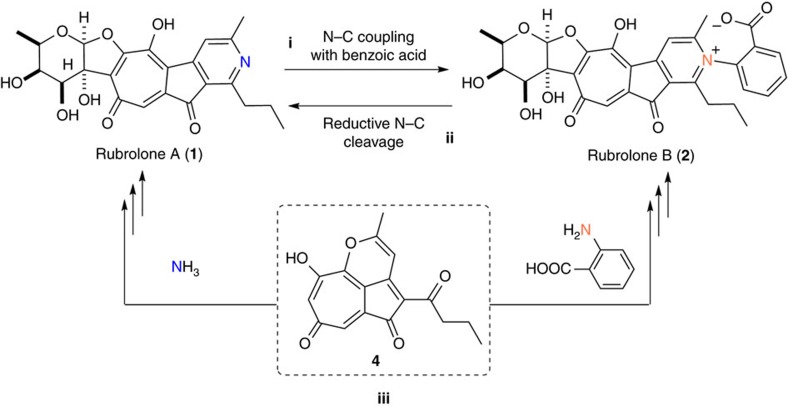
Although rubrolone A has shown no significant biological activity in cytotoxicity and antimicrobial assays1, we recently isolated rubrolone B (2) (Fig. 1) as a new potentially cardioprotective rubrolone from the endophytic strain Streptomyces sp. KIB-H033 (S. sp. KIB-H033)5. Importantly, feeding experiments with [13C]-acetate demonstrated aglycone assembly via type II PKS chemistry followed by complex oxidative rearrangements to afford the tropolone, which differs from the pinacol rearrangement-based ring expansion during fungal stipitatic acid tropolone biosynthesis6. Moreover, the additional benzoic acid moiety of rubrolone B (2) compared with rubrolone A (1) provides an example of a unique cationic inner salt moiety, the presence of which raises questions about biosynthetic pathway logic and timing; for example, is rubrolone A the biosynthetic precursor to rubrolone B, or vice versa?
The rubrolones are members of the pyridine alkaloid family of natural products (Fig. 1) found widely in nature and possessing antimalarial, anticancer and antibacterial activities7,8,9,10,11. The biologically significant pyridyl moiety is assembled via diverse biosynthetic pathways that primarily originate from amino acid precursors. For example, the pyridine rings of virginiamycin S (ref. 12) and nikkomycin13 derive from L-lysine, while aspartate generates the pyridyl moiety of nicotine14 and pyridomycin15 and L-tryptophan is the precursor for the pyridyl moiety of thiocoraline16 (Fig. 1). Only in rare cases are pyridine alkaloids derived from other sources; for example, one of the collismycin pyridine rings is derived from L-cysteine and acetate (Fig. 1)17. However, because most of these insights have been achieved from feeding isotope-labelled precursors, the detailed mechanisms of alkaloid pyridine ring formation are poorly understood.
To understand the mechanism of rubrolone biosynthesis in S. sp. KIB-H033, and pyridyl construction in particular, we herein report the biosynthetic gene cluster together with heterologous expression experiments that enable us to propose a biosynthetic pathway. Critically, inactivation of rubrolone sugar biosynthetic genes led to the accumulation of a key intermediate that was shown to react non-enzymatically with either ammonia or anthranilic acid to afford the respective aglycones of rubrolones A (1) and B (2). This study provides important new insight into the non-enzymatic and non-amino acid origins of the pyridyl moiety in alkaloid natural products.
Results
Identification of the rubrolone biosynthetic gene cluster
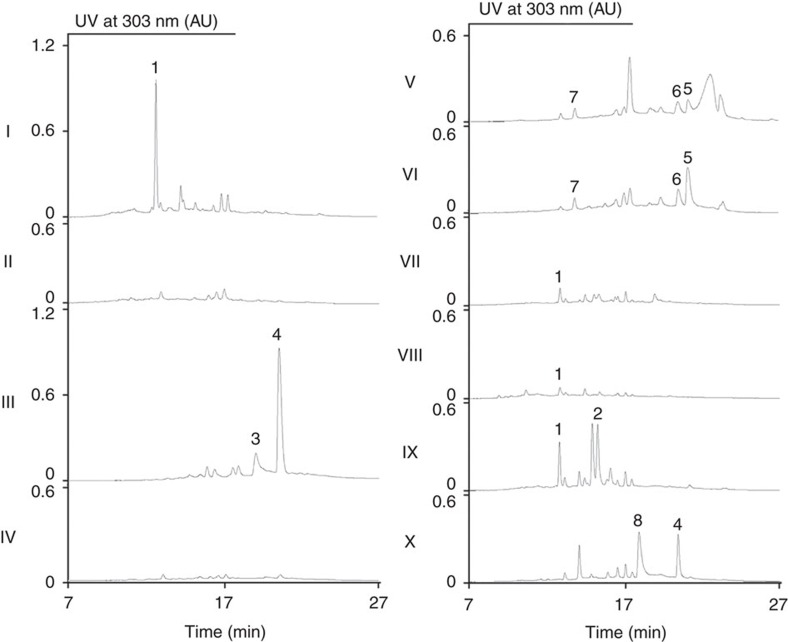
Our strategy for identifying the rubrolone (rub) biosynthetic gene cluster was based on our hypotheses that (i) the aglycone of rubrolones A and B is of polyketide synthase origin5, and (ii) a sugar 4,6-dehydratase installs the C-6 methyl group of the deoxysugar. The dehydratase-encoding gene has been used as a probe to successfully identify the gene cluster of the enediyne antibiotic C-1027 (ref. 18). To search for these two gene loci, the genomic deoxyribonucleic acid (DNA) of S. sp. KIB-H033 was subjected to Illumina sequencing. Using KSα and sugar 4,6-dehydratase genes as sequence queries to scan the assembled scaffolds, we identified a 20-kb region of the genome possessing both genes. To validate the involvement of this DNA region in rubrolone biosynthesis, a genomic library of S. sp. KIB-H033 was constructed based on cosmid pJTU2554 (ref. 19), from which the cosmid p9B10 containing this DNA region was identified through library screening. Integration of p9B10 into the chromosome of Streptomyces albus J1074 (ref. 20) by conjugation generated strain S. albus 9B10, from which heterologous expression of the putative rubrolone gene cluster could be monitored by a colour change on an agar plate; the recombinant strain produced a ruby red pigment, whereas control strain containing the relevant empty vector pJTU2554 did not (Supplementary Fig. 1). To confirm the identity of this red metabolite, liquid culture extracts of recombinant strain S. albus 9B10 and appropriate control were analysed by high-performance liquid chromatography (HPLC). The putative rub-containing clone produced a peak with retention time identical to that of rubrolone A (Fig. 2, trace I), and this peak was absent from the S. albus control strain containing the empty vector (Fig. 2, trace II). The presence of rubrolone A (1) was further confirmed by high-resolution electrospray ionization mass spectrometry (HRESIMS) and 1H NMR spectroscopy. Overall, these results demonstrated that cosmid 9B10 possesses the requisite gene cluster encoding the biosynthesis of rubrolone A.
Figure 2. HPLC profiles of the fermentation extracts of engineered heterologous expression strains.
I: S. albus 9B10; II: S. albus pJTU2554; III: S. albus 9B10-ΔS1; IV: S. albus 9B10-ΔE9; V: S. albus 9B10-ΔB; VI: S. albus 9B10-ΔC; VII: S. albus 9B10-ΔE9 feeding with 3; VIII: S. albus 9B10-ΔE9 feeding with 4; IX: S. albus 9B10 feeding with anthranilic acid; and X: S. albus 9B10-ΔS1 feeding with anthranilic acid.
Determining the rub gene cluster boundaries
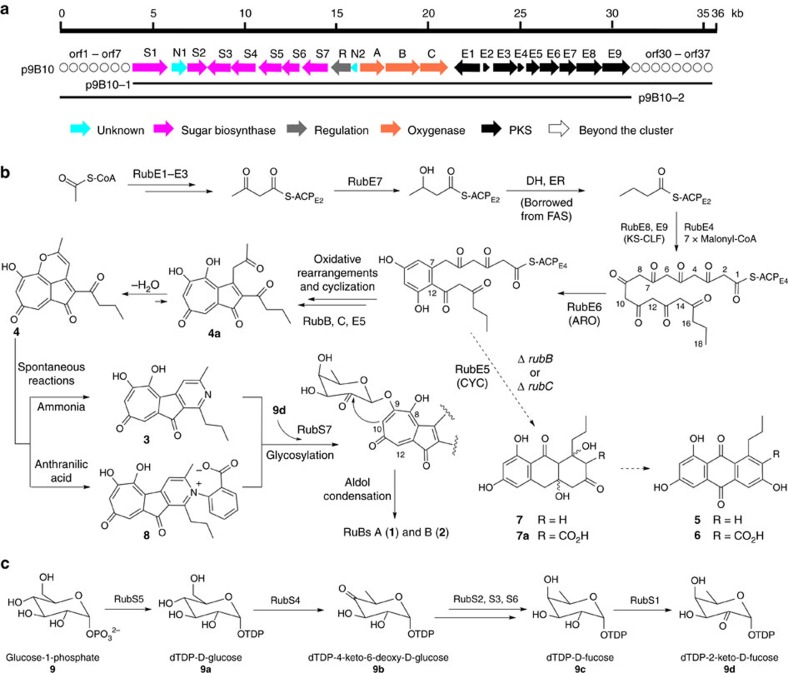
The sequence of the 37 open reading frames identified in cosmid 9B10 (Fig. 3a) has been deposited in Genbank with accession number KX218108. To determine the rub gene cluster boundaries, we first constructed cosmid p9B10-1 (from genes rubS1 to orf37, Fig. 3a) using λ red-mediated polymerase chain reaction (PCR) targeting mutagenesis21, and this strain still produced rubrolone A (Supplementary Fig. 2, trace I). In contrast, inactivation of the oxidoreductase-encoding rubS1 gene abolished production of 1 in the new recombinant strain S. albus 9B10-ΔS1, but resulted in the accumulation of two new compounds 3 and 4 (Fig. 2, trace III). Therefore, the left boundary of this gene cluster was determined to be rubS1. To locate the right boundary of the gene cluster, cosmid p9B10-2 (genes from orf1 to rubE9, Fig. 3a) was constructed and was then conjugated into S. albus generating the engineered strain S. albus 9B10-2, which did not affect the production of 1 (Supplementary Fig. 2, trace II). The gene at the right boundary of cosmid 9B10-2 is the putative ketosynthase (KS) chain length factor (CLF) encoding gene rubE9, in-frame deletion of which in strain S. albus 9B10-ΔE9 abolished production of 1 (Fig. 2, trace IV), confirming type II PKS involvement in rubrolone biosynthesis. Having established rubS1 and rubE9 as the boundaries of the ∼22.5 kb rub gene cluster (Fig. 3a), we then analysed all 22 ORFs using a basic local alignment search tool to assign putative functions (Supplementary Table 1). In summary, the rub biosynthetic genes encode: (i) PKS and associated enzymes (RubE1–E9), (ii) sugar biosynthesis enzymes (RubS1–S7), (iii) oxygenases (RubA–C) and the regulatory protein RubR and (iv) two unknown proteins (RubN1 and RubN2).
Figure 3. Biosynthetic gene cluster and proposed biosynthetic pathway of rubrolones.
(a) Organization of the rub biosynthetic gene cluster, with functional assignment of genes including PKS (black), oxygenases (orange), deoxysugar synthases (pink), regulation (green), unknown (cyan) and genes outside the cluster (white). (b) Proposed biosynthetic pathway for PKS and post-PKS modifications, with dashed arrows indicating the pathway generating the shunt metabolites R1128A (5), 6 and 7. (c) Proposed biosynthetic pathway for the deoxysugar dTDP-2-keto-D-fucose.
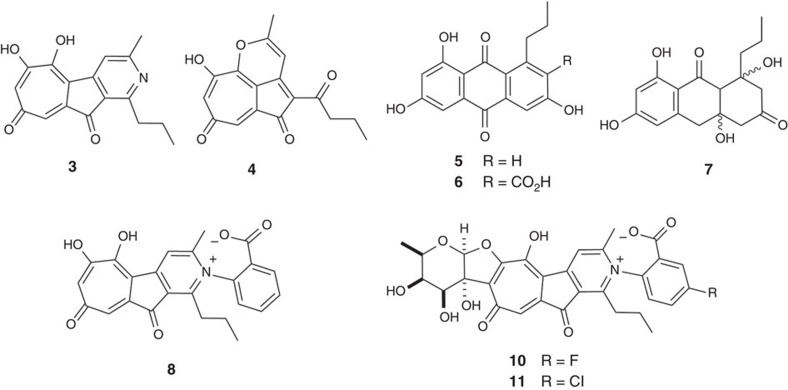
A scale-up fermentation of S. albus 9B10-ΔS1 was carried out to provide sufficient amounts of compounds 3 and 4 for structural characterization. The HRESIMS peak at m/z 298.1077 [M+H]+ for C17H16NO4 (calcd. 298.1074), together with the nuclear magnetic resonance (NMR) data comparison with literature values confirmed that 3 was the rubrolone aglycone3,4. For compound 4, the observed exact mass at m/z 297.0780 [M−H]− for C17H13O5 (calcd. 297.0769) indicated that the nitrogen atom in 3 was replaced by oxygen in 4. Full NMR analysis including 1H and 13C NMR as well as two-dimensional (2D) NMR experiments (Supplementary Table 2; Supplementary Fig. 3), 1H−1H COSY, HSQC, and HMBC, identified the structure of 4 as shown in Fig. 4.
Figure 4. Chemical structures of rubrolone analogues and related metabolites produced by mutants.
Compounds 3 and 4 were isolated from mutant S. albus 9B10-ΔS1; 5–7 were isolated from mutant S. albus 9B10-ΔB; 8 was obtained from S. albus 9B10-ΔS1 feeding with anthranilic acid; and compounds 9 and 10 were obtained from S. albus 9B10 feeding with 2-amino-5-fluorobenzoic acid and 2-amino-5-chlorobenzoic acid, respectively.
Enzymes in the biosynthesis of the rubrolone scaffold
Our previous 13C-acetate feeding experiments demonstrated that the aglycone originates from type II PKS chemistry followed by complex oxidative rearrangements5. Bioinformatic analysis yields the expected minimal type II PKS encoded by rubE8 (KSα), rubE9 (CLF/KSβ) and rubE4 (acyl carrier protein, ACP), which share high sequence similarity with the type II PKS involved in R1128 biosynthesis (ZhuB, 79% identity; ZhuA, 71% identity; and ZhuN, 68% identity)22. Additionally, rubE1, rubE2 and rubE3 encode β-ketoacyl/ACP synthase III (KSIII), ACP and an acyltransferase, respectively, which share high sequence similarity with a set of starter unit butyryl-ACP generating enzymes (ZhuH, 61% identity; ZhuG, 53% identity; and ZhuC, 56% identity) in R1128 biosynthesis23,24. Two putative polyketide cyclases, RubE5 and RubE6, are also found within the rub gene cluster and share high sequence similarity with the cyclase/dehydratase protein ZhuJ (69% identity) and cyclase ZhuI (79% identity), respectively25. Cyclase RubE6 may catalyse the C7/C12 cyclization and aromatization of the linear polyketide intermediate as does the ZhuI ARO/CYC in R1128 biosynthesis (Fig. 3b), with subsequent cyclization catalysed by the ZhuJ cyclase homologue RubE5. Significantly, the R1128 and rubrolone biosynthetic gene clusters share the same suite of PKS genes (Supplementary Fig. 4, comparison of the two gene clusters), which is consistent with common structural features of a propyl side chain and the same PKS chain length in both R1128 and rubrolones (Fig. 3b). Interestingly, the putative reductase RubE7 is not found in the R1128 biosynthetic gene cluster, and may function as the missing ketoreductase (KR) responsible for generating the butyryl starter unit (Fig. 3b). Inactivation of rubE7 slightly reduced the production of 1 to ∼60% of production in S. albus 9B10 (Supplementary Fig. 2, trace III), consistent with previous assumption upon partial complementation by the KR from fatty acid biosynthesis22.
Overall, bioinformatic analysis of the rub gene cluster has identified PKS genes with high sequence similarity to genes responsible for the biosynthesis of R1128 (Supplementary Table 1)22. This supports our previous proposal of KSIII-catalysed formation of the butyryl-ACP starter unit for type II PKS-catalysed extension with seven malonyl-CoA additions to generate the highly reactive poly-β-ketone backbone (Fig. 3b).
Enzymes for oxidative formation of the tropolone scaffold
Although clearly of polyketide origin, the rubrolone carbon skeleton possesses a labelling pattern consistent with a series of complex oxidative rearrangements5. There are three putative oxygenases in the robulone gene cluster: RubA belongs to the bacterial luciferase-like monooxygenase superfamily26; RubB possesses 55% identity to ZhuM, a cytochrome P450 oxygenase catalysing anthraquinone formation in R1128 biosynthesis22; RubC is similar to naphthocyclinone hydroxylases like ActVA-orf5 (59% identity) that catalyse ring hydroxylation in the actinorhodin biosynthetic pathway27.
To determine if any of these three oxygenases may be involved in tropolone ring formation, we constructed the respective in-frame gene deletion mutants S. albus 9B10-ΔA, S. albus 9B10-ΔB and S. albus 9B10-ΔC for metabolite analysis by HPLC. Although inactivation of rubA did not affect rubrolone production (Supplementary Fig. 2, trace IV), both S. albus 9B10-ΔB and S. albus 9B10-ΔC shared a similar metabolite profile that included abolished production of 1 together with three new peaks 5–7 (Fig. 2, traces V and VI). Large-scale fermentation of S. albus 9B10-ΔB enabled the purification of sufficient quantities of 5–7 for structure elucidation by mass spectrometry (MS) and NMR. The known structure of R1128A (5) was determined by comparison to the 1H and 13C NMR data in the literature28. The structures of the new compounds 6 and 7 were elucidated by a combination of HRESIMS and one-dimensional (1D) and 2D NMR spectroscopic analyses (Supplementary Table 3; Supplementary Fig. 3).
Because deletion of either rubB or rubC completely abolished production but resulted in the accumulation of R1128A (5) and the related compounds 6 and 7 (Fig. 3b), both RubB and RubC are required for tropolone ring formation, and without both of these enzymes present RubE5 and/or RubE6 can catalyse cyclization and dehydration reactions analogous to those catalysed by their homologues ZhuJ and ZhuI to furnish R1128A. In addition, RubB, RubC and/or an oxidase in the host strain may catalyse oxidation and possibly also the dehydration reactions that transform 7 to R1128A (5) (Fig. 3b); a similar bifunctional oxygenase/dehydratase has been found in jadomycin biosynthesis29. The isolation of 5–7 from mutant strains is unexpected but consistent with the presence of homologues of all R1128 biosynthetic genes in the rub gene cluster (Supplementary Fig. 4). Taken together, the data suggest that RubB and RubC together may catalyse the complex oxidative rearrangements leading to tropolone ring formation, and that these oxygenases may work on the ACP-bound intermediate before the second cyclization to direct biosynthetic divergence between rubrolones and R1128 (Fig. 3b).
Sugar gene mutants accumulate 3 and 4 as precursors to 1
Glycosylation can vastly expand natural product chemical space due to the structural diversity of sugars and their varying modes of aglycone attachment30,31. Rubrolones possess a rare D-fucose deoxysugar with a unique 1,2-linkage to the seven-membered tropolone ring that implies a dTDP-2-keto-D-fucose precursor. Seven genes (rubS1–rubS7) in the rub gene cluster encode enzymes putatively assigned to the biosynthesis of the deoxysugar moiety and its attachment to the aglycone. We propose that the glucose-1-phosphate thymidylyltransferase (RubS5)32 and dTDP-glucose 4,6-dehydratase (RubS4)33 sequentially transform glucose-1-phosphate (9) to dTDP-4-keto-6-deoxy-D-glucose (9b) via dTDP-D-glucose (9a), followed by RubS3-catalysed ketoreduction to dTDP-D-fucose (9c) (Fig. 3c)34. RubS2 (ref. 35) and RubS6 (ref. 36) are putative NAD-dependent epimerases that may also catalyse this ketoreduction. RubS1 possesses an FAD binding motif and 52% identity to the AknOx oxidoreductase catalysing the final two steps of aclacinomycin biosynthesis, the oxidation of the terminal sugar rhodinose to L-aculose37. We therefore propose that RubS1 transforms dTDP-D-fucose to dTDP-2-keto-D-fucose (9d) (Fig. 3c) before installation on the rubrolone scaffold by the glycosyltransferase RubS7 (Fig. 3b). Finally, we propose an intramolecular aldol reaction between the 2-keto group of the deoxysugar and C-10 of the aglycone to yield the rubrolones (Fig. 3b).
To probe the biosynthetic functions of RubS1–S7, we inactivated the rubS2, rubS3 and rubS7 genes to afford mutant strains S. albus 9B10-ΔS2, S. albus 9B10-ΔS3 and S. albus 9B10-ΔS7, respectively. All three mutations completely abolished rubrolone production, but accumulated 3 and 4 and shared the same metabolite profile as that of S. albus 9B10-ΔS1 (Supplementary Fig. 2, traces V–VII). Both 3 and 4 possess the characteristic tropolone ring, and 3 is the rubrolone aglycone (Fig. 4).
To determine if these compounds are biosynthetic intermediates or shunt products, 3 and 4 were fed to a mutant with rubrolone biosynthesis blocked at an early stage. RubE8 (KSα) and RubE9 (CLF) form a heterodimer catalysing Claisen-like condensation of malonyl-ACP extender units. The rubE9-inactivated mutant S. albus 9B10-ΔE9 generated a clean background with no rubrolone-related metabolites detected by HPLC (Fig. 2, trace IV), and therefore was used as the host to carry out feeding experiments with 3 and 4. HPLC chromatograms revealed that both compounds were completely converted to 1 (Fig. 2, traces VII and VIII), suggesting that both 3 and 4 are biosynthetically competent intermediates en route to rubrolone A (1).
To investigate the timing and specificity of the putative glycosyltransferase RubS7, we sought to present this enzyme with large quantities of 4, which is the precursor of the pyridyl-containing aglycone. This was achieved by decreasing the amount of nitrogen source in the culture media, which dramatically decreased the production of 1 and accumulated large amounts of 4 in the heterologous expression strain S. albus 9B10 (Supplementary Fig. 2, trace VIII). This suggests that the downstream glycosyltransferase RubS7 is specific for the pyridyl-containing aglycone, and therefore pyridyl moiety formation occurs before glycosylation.
Our collective interrogation of the sugar biosynthetic genes suggests a biosynthetic pathway from glucose-1-phosphate to dTDP-2-keto-D-fucose, which is installed on the fully maturated pyridyl-containing rubrolone aglycone by the glycosyltransferase RubS7 (Fig. 3c). An unprecedented carbon–carbon bond between C-2 of the sugar and C-10 of the aglycone is then formed by an unknown mechanism. Significantly, the compounds 3 and 4 that accumulate upon sugar biosynthetic gene inactivation are biosynthetic intermediates en route to rubrolones.
Anthranilic acid is a biosynthetic precursor to product 2
Having established the intermediacy of 3 and 4 in the biosynthesis of rubolone A (1), we next sought to understand the biosynthetic incorporation of the benzoic acid moiety of rubrolone B (2). Although a 2-l fermentation of the wild type strain S. sp. KIB-H033 produces large quantities of 1 (60 mg l−1) and 2 (50 mg l−1) under even suboptimal conditions5, the heterologous expression strain S. albus 9B10 showed a similar titre to the WT strain for 1, but only a very small amount of 2 (∼0.2 mg) was isolated from 2-l fermentation broth. The varying titre ratios of rubrolones A (1) and B (2) in different strains may arise from biosynthetic pathways in which (i) one is the precursor of the other or (ii) they arise from divergent amination of a common intermediate 4 (Fig. 5). For example, if 1 is the precursor of 2, then the benzoic acid moiety would need to be attached to the nitrogen atom of 1 by oxidative N–C coupling to afford 2 (Fig. 5). Conversely, initial production of 2 would require subsequent N–C bond cleavage to liberate the benzoic acid moiety and yield 1 (Fig. 5). Alternatively, 3 and 4 may generate 1 and 2 via divergent elaboration of 4 with either ammonia or anthranilic acid as respective nitrogen sources (Fig. 5). Interestingly, the latter hypothesis is favored by the presence of large quantities of anthranilic acid only in the wild type strain, which produced large quantities of 2. In contrast, anthranilic acid was not detected in the heterologous expression strain S. albus 9B10, which yielded only trace amount of 2.
Figure 5. Different possible biosynthetic relationships between 1 and 2.
(i) 2 being generated by the oxidative N–C coupling of benzoic acid and 1, (ii) 1 generated by the reductive N–C cleavage of 2 and (iii) both 1 and 2 arising from divergent amination of 4.
To begin to differentiate among these three possible routes, we fed 15N-labelled anthranilic acid to the fermentation media of wild type strain S. sp. KIB-H033 at 24 h (the time point at which 1 and 2 could first be detected) and 48 h after inoculation, and then isolated and analysed 1 and 2 by HRMS, 15N NMR and 1H–15N long range correlation experiments to characterize 15N incorporation. Consistent with the divergent pathway hypothesis, adding 200 mg l−1 of 15N-labelled anthranilic acid to cultures yielded 15N enrichment in 2 but not 1 (Supplementary Fig. 5). Moreover, adding exogenous anthranilic acid to the heterologous expression strain S. albus 9B10 completely restored production of 2 (Fig. 2, trace IX). In summary, the incorporation of exogenous anthranilic acid supports its role as a biosynthetic intermediate en route to rubrolone B (2).
The pyridyl moiety arises from non-enzymatic amination of 4
The divergent biosynthetic pathways for 1 and 2 implied by anthranilic acid incorporation, together with the production of intermediate 4 in sugar biosynthetic gene knockout mutants, strongly suggested that 4 can react with free ammonia and anthranilic acid to generate the pyridine ring and pyridine inner salt moiety, respectively (Fig. 5). To further test this hypothesis, we fed 200 mg l−1 anthranilic acid to the 4-producing mutant S. albus 9B10-ΔS1. HPLC analyses of the fermentation extracts revealed production of a new compound 8 (Fig. 2, trace X), of which we isolated 7.9 mg from a 2-l culture. Evaluation of the HRESIMS at m/z 416.1140 [M−H]− for C24H18NO6 and 1D and 2D NMR spectra of 8 (Supplementary Table 3; Supplementary Fig. 3) in comparison to the NMR data of 2 and 3 (Supplementary Table 2) led to the structural assignment of 8 (Fig. 4) as the aglycone of rubrolone B (2).
The chemical structure and reactivity of the biosynthetic intermediate 4, the presence of excess anthranilic acid in the fermentation culture of the wild type strain S. sp. KIB-H033, and the absence of suitable gene candidates in the rub cluster together suggest that amination may occur non-enzymatically. This would enable production of designer RUB analogues with altered benzoate moieties if the RubS7 glycosyltransferase were sufficiently promiscuous to attach different aglycones to the NDP sugar. Feeding F, Cl, Br or OH substituted anthranilic acids to the culture medium of S. albus 9B10 led to the production of several novel rubrolones based on HRMS data (Supplementary Figs 6–11), two of which (Fig. 4) were isolated and characterized in detail by NMR (rubrolones C (10) and D (11), Supplementary Fig. 3; Supplementary Table 4).
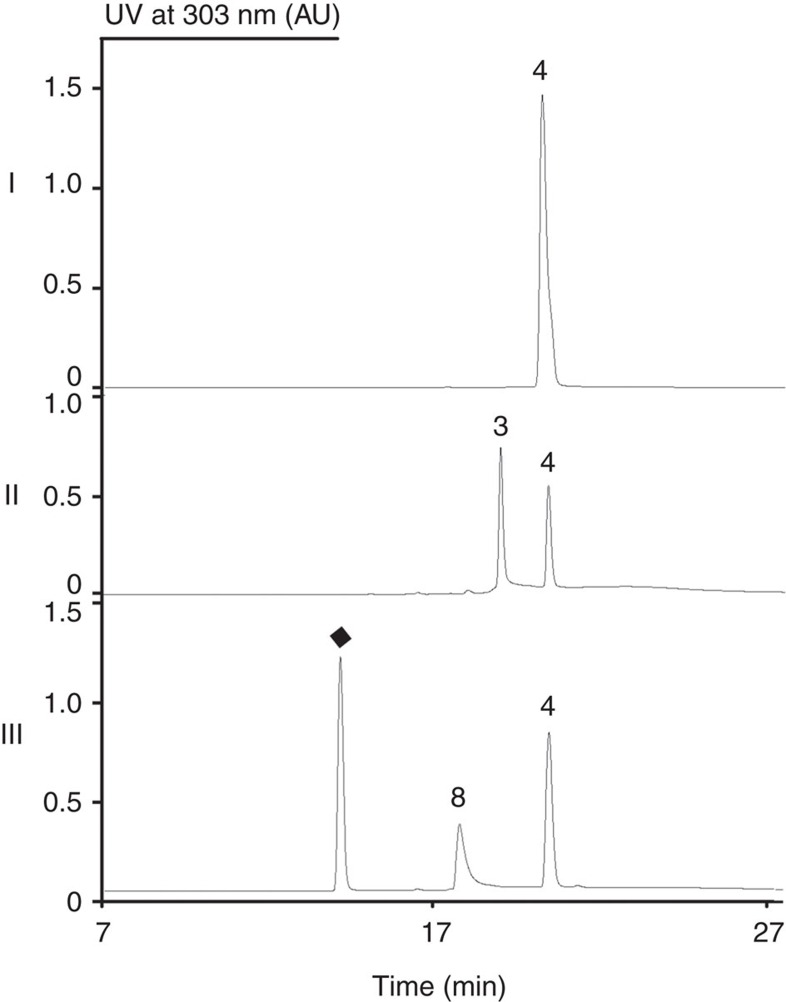
Encouraged by the in vivo results, we next sought to obtain evidence for the non-enzymatic reactions by performing in vitro reactions between the isolated biosynthetic intermediate 4 and either ammonia or anthranilic acid analogues in phosphate buffer at pH 8.0 to mimic the fermentation media. As expected, incubation of 4 with ammonium acetate yielded a new HPLC peak with a retention time corresponding to that of the intermediate 3 (Fig. 6, trace II), while a peak corresponding to 8 appeared when anthranilic acid was used in place of ammonium acetate (Fig. 6, trace III). Furthermore, incubation of 4 with F, Cl, Br or OH substituted anthranilic acids yielded new rubrolone aglycones based on HRMS data (Supplementary Figs 12–15). These results clearly demonstrate the non-enzymatic divergent elaboration of 4 with either ammonia or anthranilic acid analogues to afford diverse rubrolone aglycones.
Figure 6. HPLC analysis of in vitro chemical conversions |:
4 was incubated with buffer only; II: 4 was incubated with ammonium acetate; and III: 4 was incubated with anthranilic acid (black diamond).
Discussion
Rubrolones are tropolonoid natural products with an unusual carbon skeleton of polyketide origin containing a unique 2,3,4,6-tetra-substituted pyridine ring or pyridine inner salt moiety. In this work, we have identified a 22 ORF rubrolone biosynthetic gene cluster and performed a series of experiments that are consistent with the following features of rubrolone biosynthesis (Fig. 3): (i) a KSIII generates a butyryl-ACP starter unit, and a type II PKS catalyses seven rounds of chain extension; (ii) oxidative enzymes RubB and RubC together catalyse the complex oxidative rearrangements leading to tropolone ring formation; (iii) glucose-1-phosphate is transformed to dTDP-2-keto-D-fucose, which is then attached to the fully maturated pyridyl-containing rubrolone aglycone by glycosyltransferase RubS7 and (iv) 4 is a common intermediate in rubrolone biosynthesis, from which structural divergence to rubolones A (1) and B (2) occurs by non-enzymatic condensation with respective amine donors ammonia and anthranilic acid.
Several experimental results demonstrated that free ammonia and anthranilic acid can react with 4 to generate the pyridyl moieties of rubrolone A (1) and rubrolone B (2), respectively. First, only 2 was enriched with 15N after feeding 15N-labelled anthranilic acid to the wild type strain, demonstrating that direct conversion of 2 to 1 via N–C bond cleavage did not occur (Fig. 5). Second, exogenous anthranilic acid could completely restore production of 2 to the anthranilic acid-deficient heterologous expression strain S. albus 9B10 (Fig. 2, trace IX). Third, the rubrolone B aglycone 8 (Fig. 4; Supplementary Fig. 3) was directly observed when anthranilic acid was supplemented in the fermentation media of the sugar biosynthesis-deficient strain S. albus 9B10-ΔS1. Overall, this data clearly implicates a biosynthetic pathway that diverges from 4 in the presence of ammonia or anthranilic acid to respectively afford either rubrolone A (1) or rubrolone B (2).
Although our in vivo data support divergent amination of 4, the absence of suitable aminotransferase enzymes in the rub gene cluster suggested a non-enzymatic mechanism of pyridine formation. This hypothesis was proven by observing in vitro transformations of 4 to either 8 or 3 in the presence of anthranilic acid or ammonium acetate, respectively. Non-enzymatic reactions are increasingly being found in natural product biosynthetic pathways and have been exploited to expand chemical diversity of several classes of natural products. In this work, we demonstrated the facile non-enzymatic incorporation of several substituted anthranilic acids to explore rubrolone chemical space. Similar non-enzymatic condensations between carbonyl and amine functional groups are well known in natural products biosynthesis and have afforded discoipyrroles38, ammosamides39, elansolids40 and jadomycins41,42. Non-enzymatic Diels–Alder reactions have been widely observed en route to natural products such as paracaseolide A (ref. 43). Additional pericyclic reactions have been observed to generate impressive diversity of fungal polyketides by exploiting the intrinsic reactivity of an isochromene intermediate44. In summary, our non-enzymatic incorporation of various amine donors into the rubrolone scaffold highlights the potential for further non-enzymatic elaboration of rubrolones.
Most alkaloid pyridine ring biosyntheses involve proposed cyclization between carbonyl and amine functional groups, with one recent exception being an enzyme-catalysed formal [4+2] cycloaddition between two dehydroalanines and subsequent aromatization en route to the pyridyl moiety of thiocillins45. We propose a non-enzymatic rubrolone pyridine construction that does not involve an amino acid precursor, but instead features the capture of free amines from solution by the reactive 1,5-dione moiety of 4 (Supplementary Fig. 16A). Specifically, we propose that the large distance between the C-15 ketone and C-3 could require hydrolytic ring opening and isomerization to the 1,5-dicarbonyl intermediate 4a, which rapidly interconverts with 4 as shown by the disappearance of the H-2 and H-4 NMR signals of 4 in D2O (Supplementary Fig. 16B). The reactive 1,5-dicarbonyl intermediate 4a would readily capture free amines to generate the ketimine 4b. Subsequent dehydration and aromatization would afford the rubrolone pyridyl moiety 4c (Supplementary Fig. 16A). The condensation of 1,5-diones with amines is a general approach to pyridyl formation that has been widely exploited in chemical synthesis46,47,48, but suffers from required extremes of temperature and pH (refs 47, 48). Nature has solved this problem in rubrolone biosynthesis by generating a highly reactive 1,5-dione intermediate to non-enzymatically capture free amines en route to pyridyl assembly. We propose that such non-enzymatic capture of diverse free amine metabolites may represent a general biosynthetic strategy for furnishing pyridine rings in natural products such as cimicifugadine49, beatrines50 and cassiarins51 (Supplementary Fig. 17), and this study sets the stage for further discovery of an expanded set of diverse pyridine-containing natural products.
Methods
General materials and experimental procedures
All reagents, solvents and restriction enzymes were purchased from standard commercial sources and used directly. DNA isolation and manipulation in Escherichia coli (E. coli) and Streptomyces were performed according to standard protocols. PCR amplifications were carried out on Biometra professional thermocycler (070-851, An Analytik Jena Company, Germany) using either Taq DNA polymerase (TaKaRa) or Pfu DNA polymerase (Thermo scientific). Primer synthesis and DNA sequencing were performed at Beijing Zixi Bio Tech Co., Ltd.
Column chromatography (CC) was performed using silica gel 60 RP-18 (EMD Chemicals Inc., Germany), Sephadex LH-20 (GE Healthcare Bio-Sciences Corp., Piscataway, NJ, USA) and D101 macroporous absorbent resin (Tianjin Haiguang Chemical Co. Ltd.). Semipreparative HPLC was conducted on a HITACHI Chromaster system equipped with a DAD detector, a YMC-Triart C18 column (250 mm × 10 mm i.d., 5 μm), and a flow rate of 3.0 ml min−1 at a column temperature of 25 °C. HPLC analysis was carried out on HITACHI Chromaster system equipped with a DAD detector, a YMC-Triart C18 column (250 mm × 4.6 mm i.d., 5 μm, Japan). NMR spectra were recorded in dimethylsulphoxide (DMSO)-d6 or CD3OD using a Bruker AVANCE III-600 spectrometer (Bruker Corp., Switzerland), and tetramethylsilane was used as internal standard. HRESIMS data were obtained using an Agilent G6230 Q-TOF mass instrument (Agilent Corp., USA).
Strain culture conditions and plasmids
The bacterial strains and plasmids used in this study are summarized in Supplementary Table 5. The primer sequences are listed in Supplementary Table 6. E. coli strains or E. coli strains carrying plasmids were grown in Luria-Bertani medium with appropriate antibiotic selection. Streptomyces stains were routinely cultured in mannitol soya flour (MS) medium (Soybean flour 20 g l−1, mannitol 20 g l−1, agar 20 g l−1, pH 7.2) with appropriate antibiotic selection. E. coli–Streptomyces conjugations were performed on MS solid medium freshly supplemented with 20 mM MgCl2, and TSB liquid medium (tryptone 17 g l−1, phytone 3 g l−1, NaCl 5 g l−1, K2HPO4 2.5 g l−1, glucose 2.5 g l−1, pH 7.2) was used as seed medium, and the rubrolone production medium (dextrin 40 g l−1, lactose 40 g l−1, yeast extract 5 g l−1, MOPS sodium salt 20 g l−1, ammonium acetate 1 g l−1, pH 7.2) was used for fermentation. For selection of recombinant clones, antibiotics were supplemented as follows: kanamycin (50 μg ml−1), apramycin (50 μg ml−1), erythromycin (200 μg ml−1) and carbenicillin (100 μg ml−1).
Genome sequencing and genomic library construction
The genome sequencing of S. sp. KIB-H033 was performed with the Illumina Genome Analyzer (Illumina, San Diego, CA) by BGI (BGI-Shenzhen, China). High-molecular-mass genomic DNA isolated from S. sp KIB-H033 was used to construct small (200–500 bp) and large (2–3 kb) random sequencing libraries. The reads were first filtered and assembled into 85 contigs using SOAPdenovo (http://soap.genomics.org.cn/). We then used the paired-end information, step-by-step from the shortest (200 bp) to the longest (2,000 bp) insert size, to join the contigs into 17 scaffolds. Putative protein-coding sequences were predicted using the GLIMMER program, and the annotation was accomplished by BlastP analysis of sequences in the Nr, Nt, and SwissProt databases and by manual curation of the outputs of a variety of similarity searches. According to standard procedures, the genomic DNA was partially digested with MboI. Then the 30–45 kb DNA fragments were isolated and ligated to cosmid pJTU2554. MaxPlax Lambda packaging extracts were used to packaging. About 2,000 E. coli clones were picked, and stored in 20 96-well microplates at −80 °C.
Heterologous expression and mutant construction
The positive cosmids were introduced to the selected model Streptomyces host by E. coli–Streptomyces conjugation according to the standard protocol21. Cosmid 9B10 which contains the whole rub gene cluster was used for the construction of gene deletion mutant. Since the cosmid pJTU2554 has the apramycin resistance gene aac(3)IV and oriT, the target gene was replaced with erythromycin resistance cassette ermE which was amplified from plasmid pJTU6722 (provided by Prof Meifeng Tao (Shanghai Jiao Tong University, China)) using λ RED-mediated PCR targeting mutagenesis with corresponding primers (Supplementary Table 6). To isolate the markerless gene deletion mutant, the gene inactivation cosmids were introduced into E. coli DH5α/BT340 via electroporation; incubation overnight at 42 °C to induce expression of FLP recombinase resulted in the loss of ermE cassette and the generation of an 81-bp in-frame scar, affording final markerless gene inactivation vectors. All the gene inactivation vectors used in this study were constructed by the same strategy (Supplementary Figs 18–28).
The gene inactivation vectors were introduced by transformation into E. coli ET12567/pUZ8002 and the vectors were then transferred to S. albus J1074 by intergeneric conjugation according to the standard methods21. The spores of S. albus J1074 were produced in the MS medium. E. coli–Streptomyces conjugations were performed on MS solid medium freshly supplemented with 20 mM MgCl2. The mutants were selected with 50 μg ml−1 apramycin after 4 days cultivation and subsequently identified through PCR with corresponding primers (Supplementary Table 6). Finally, 11 markerless gene inactivation mutants were constructed in this study.
Chemical purification of compounds 1–8 and 10–11
For HPLC analysis of the fermentation extracts of engineered heterologous expression strains, the frozen spore stocks (100 μl) of strains were inoculated into 250 ml baffled-flask containing 50 ml of seed medium and shaken at 250 r.p.m. and 28 °C. After 30 h, an aliquot of seed culture (2.5 ml) was transferred into a 250 ml baffled-flask containing 50 ml of production medium and shaken at 250 r.p.m. and 28 °C for 4 days. The 50 ml fermentation culture broth was separated into mycelia and supernatant by centrifugation at 3,500g for 30 min. The resulting supernatant was subsequently mixed with D101 macroporous absorbent resin (5 ml) and stirred for 20 min. After removal of supernatant, the resin was washed with 20 ml H2O, and then 10 ml methanol. The 10 ml CH3OH was evaporated to dryness, and then the residues were dissolved in 1 ml CH3OH. The samples were applied to reverse-phase HPLC analysis eluted with a flow rate of 1 ml min−1 over a 28 min gradient as follows: T=0, 10% B; T=20, 100% B; T=24, 100% B; T=25, 10% B; T=28, 10% B (A, H2O; B, CH3OH), and the column temperature is 25 °C.
A 2 -l fermentation culture broth of S. albus 9B10 was separated into mycelia and supernatant by centrifugation at 3,500g for 30 min. The resulting supernatant was subsequently applied to a D101 macroporous absorbent resin column (0.5 l), the column was washed with H2O and then successively eluted with 100% methanol. The CH3OH fraction was evaporated to dryness, and the residue was dissolved in 50% CH3OH/H2O and subjected to Sephadex LH-20 CC eluting with 50% CH3OH/H2O to yield 18 fractions. All the fractions were analysed by HPLC and combined. Selected fractions contained the rubrolone analogues were further purified by semipreparative C18 HPLC to yield compounds 1 (26.8 mg) and 2 (∼0.2 mg).
A 2-l fermentation culture broth of S. albus 9B10-ΔS1 was separated into mycelia and supernatant by centrifugation at 3,500g for 30 min. The resulting supernatant was subsequently applied to a D101 macroporous absorbent resin column (0.5 l), the column was washed with H2O and then successively eluted with 100% methanol. The CH3OH fraction was evaporated to dryness, and the residue was dissolved in CH3OH and subjected to Sephadex LH-20 CC eluting with CH3OH to yield 21 fractions. The fractions contained the compounds 3 and 4 were combined by HPLC analysis. Finally, the compounds 3 (15.6 mg) and 4 (33.7 mg) were purified by semipreparative C18 HPLC.
Compound 3: Purple red powder; 1H NMR data was summarized in Supplementary Table 2 (Supplementary Fig. 29); HRESIMS m/z 298.1077 [M+H]+ (calculated for C17H15NO4, 298.1074), see Supplementary Fig. 30.
Compound 4: Purple red powder; 1H, 13C, COSY and HMBC NMR data was summarized in Supplementary Table 2 (Supplementary Figs 31–35); HRESIMS m/z 297.0780 [M−H]− (calculated for C17H14O5, 297.0769), see Supplementary Fig. 36.
A 5-l fermentation culture broth of S. albus 9B10-ΔB was separated into mycelia and supernatant by centrifugation at 3,500g for 30 min. The resulting supernatant was extracted with ethyl acetate (3 l) for three times, then evaporation of EtOAc generated the residue. This residue was dissolved in CH3OH and mixed with an appropriate amount of polyamide, then the sample was applied to a reverse-phase C18 column and subsequently eluted with gradient CH3OH–H2O (from 10:90 to 100:0) to afford 27 fractions, each of which was analysed by HPLC. The fractions containing the 5–7 were combined and evaporated, then subjected to Sephadex LH-20 CC eluting with CH3OH to yield 25 fractions, and combined by HPLC analysis. The compounds 5 (6.9 mg), 6 (11.3 mg), and 7 (4.2 mg) were finally purified by semipreparative C18 HPLC from selected fractions.
Compound 5: Yellow powder; 1H and 13C NMR data was summarized in Supplementary Table 2 (Supplementary Figs 37 and 38); HRESIMS m/z 297.0770 [M−H]− (calculated for C17H14O5, 297.0769), see Supplementary Fig. 39.
Compound 6: Yellow powder; 1H, 13C, COSY and HMBC NMR data was summarized in Supplementary Table 3 (Supplementary Figs 40–44); HRESIMS m/z 341.0669 [M−H]− (calculated for C18H14O7, 341.0667), see Supplementary Fig. 45.
Compound 7: White powder; 1H, 13C, COSY, and HMBC NMR data was summarized in Supplementary Table 3 (Supplementary Figs 46–50); HRESIMS m/z 319.1189 [M−H]− (calculated for C17H20O6, 319.1187), see Supplementary Fig. 51.
The S. albus 9B10-ΔS1 was grown in seed medium for 30 h, then about 2.5 ml seed culture (5% inoculums) was inoculated into 50 ml production medium at 30 °C. The anthranilic acid was added to the fermentation media at 24 h (10 mg in 100 μl DMSO) after inoculation, and then fermented for additional 3 days The extract was analysed by HPLC and found the production of compound 8. For isolation of compound 8, a 2-l fermentation culture broth of S. albus 9B10-ΔS1 feeding with anthranilic acid (200 mg l−1) was separated into mycelia and supernatant by centrifugation at 3,500g for 30 min. The resulting supernatant was subsequently applied to a D101 macroporous absorbent resin column (0.5 l), the column was washed with H2O and then successively eluted with 100% CH3OH. The CH3OH fraction was evaporated to dryness, and the residue was dissolved in CH3OH and subjected to Sephadex LH-20 CC eluting with CH3OH to yield fractions, and combined by HPLC analysis. The fractions containing 8 was finally subjected to semipreparative C18 HPLC to yield the pure compound 8 (7.9 mg).
Compound 8: Purple red powder; 1H, 13C, COSY, and HMBC NMR data was summarized in Supplementary Table 3 (Supplementary Figs 52–56); HRESIMS m/z 416.1140 [M−H]− (calculated for C24H19NO6, 416.1140), see Supplementary Fig. 57.
The S. albus 9B10 was grown in seed medium for 30 h, then about 2.5 ml seed culture (5% inoculums) was inoculated into 50 ml production medium at 30 °C. After 24 h incubation, 2-amino-5-fluoro-benzoic acid (10 mg), 2-amino-5-chloro-benzoic acid (10 mg), 2-amino-5-bromo-benzoic acid (10 mg), 2-amino-5-hydroxy-benzoic acid (10 mg), 2-amino-3-chloro-benzoic acid (10 mg) and 2-amino-4-chloro-benzoic acid (10 mg) dissolved in 100 μl DMSO were added into culture, respectively, and fermented for additional 3 days. The extracts were analysed by HPLC and the HPLC peaks corresponding to rubrolone analogues 10–15 were collected for HRESIMS analysis. For isolation of compounds 10 and 11, the 2-l fermentation culture broth of S. albus 9B10 feeding with 2-amino-5-fluoro-benzoic acid (200 mg l−1) and 2-amino-5-chloro-benzoic acid (200 mg l−1), respectively, were separated into mycelia and supernatant by centrifugation at 3,500g for 30 min. The resulting supernatant was subsequently applied to a D101 macroporous absorbent resin column (0.5 l), the column was washed with H2O and then successively eluted with 100% methanol. The CH3OH fraction was evaporated to dryness, and the residue was dissolved in CH3OH and subjected to Sephadex LH-20 CC eluting with CH3OH to yield fractions, and combined by HPLC analysis. The compounds 10 (9.2 mg) and 11 (4.7 mg) were finally purified by semipreparative C18 HPLC, respectively.
Compound 10: Purple red powder; 1H, 13C, 19F, COSY and HMBC NMR data was summarized in Supplementary Table 4 (Supplementary Figs 58–63); HRESIMS m/z 580.1615 [M+H]+ (calculated for C30H26NO10F, 580.1614), see Supplementary Fig. 6.
Compound 11: Purple red powder; 1H, 13C, COSY and HMBC NMR data was summarized in Supplementary Table 4 (Supplementary Figs 64–68); HRESIMS m/z 596.1319 [M+H]+ (calculated for C30H26NO10Cl, 596.1318), see Supplementary Fig. 7.
Feeding the S. albus 9B10-ΔE9 with compounds 3 and 4
The S. albus 9B10-ΔE9 mutant was grown in seed medium for 30 h, then about 2.5 ml seed culture (5% inoculums) was inoculated into 50 ml production medium at 30 °C. After 24 h incubation, compounds 3 (6 mg) and 4 (6 mg) in 100 μl DMSO were added into culture, respectively, and fermented for additional 3 days. The extracts were analysed by HPLC.
Feeding the S. albus 9B10 with anthranilic acid
The S. albus 9B10 was grown in seed medium for 30 h, then about 2.5 ml seed culture (5% inoculums) was inoculated into 50 ml production medium at 30 °C. After 24 h incubation, anthranilic acid (10 mg) in 100 μl DMSO was added into culture and fermented for additional 3 days. The extract was analysed by HPLC.
Synthesis of 15N-anthranilic acid and feeding experiment
The 15N-labelled anthranilic acid was synthesized from 15N urea and phthalic anhydride by the modified Hofmann reaction52. Before isotope-labelled precursor feeding, the production of rubrolones was monitored and found that rubrolones was produced at around 24 h after the start of fermentation. So, the 15N-labelled anthranilic acid at a final concentration of (200 mg l−1) was fed to the fermentation media of the wild type strain S. sp. KIB-H033 at 24 and 48 h after inoculation. From 1-l fermentation culture broth, rubrolones A (10.8 mg) and B (12.6 mg) were isolated following the purification procedure previously published5. Both compounds were analysed by HRESIMS. Finally, the same amounts (8.0 mg) of 1 and 2 in 0.45 ml DMSO-d6 solvent were subjected to 15N NMR measurements to determine the 15N incorporation (Supplementary Figs 5 and 69).
In vitro reactions
All reactions were performed in sodium phosphate buffer (pH 8.0, 45 mmol). A mixture of compound 4 (1.5 mg, 5.03 μmol) and ammonium acetate (1.95 mg, 25.14 μmol) or anthranilic acid (3.45 mg, 25.14 μmol) was suspended in 1.0 ml of phosphate buffer and the mixture was stirring at 30 °C for 4 days. After reaction, 10 μl mixture was subject to HPLC analysis eluted with a flow rate of 1 ml min−1 over a 28 min gradient as follows: T=0, 10% B; T=20, 100% B; T=24, 100% B; T=25, 10% B; T=28, 10% B (A, H2O; B, CH3OH), and the column temperature was 25 °C. The same condition was used for generation of new rubrolone aglycones 16–19 using the 5-F, -Cl, -Br or -OH substituted anthranilic acids as the amine donors. The HPLC peaks corresponding to 16–19 were collected for HRESIMS analysis.
Measurement of 1H NMR spectra of 4 in D2O
Compound 4 (5 mg) in NMR tube was mixed with 450 μl D2O. The 1H NMR spectrum of 4 was measured every 2 h until the disappearance of H-2 and H-4 NMR signals in 4, see Supplementary Figs 70–72.
Data availability
Sequence data that support the findings of this study has been deposited in GenBank with accession codes KX218108. The authors declare that all other relevant data supporting the findings of this study are available within the article and its Supplementary Information Files and from the corresponding author upon reasonable request.
Additional information
How to cite this article: Yan, Y. et al. Non-enzymatic pyridine ring formation in the biosynthesis of the rubrolone tropolone alkaloids. Nat. Commun. 7, 13083 doi: 10.1038/ncomms13083 (2016).
Supplementary Material
Supplementary Figures 1-72, Supplementary Tables 1-6, Supplementary References.
Acknowledgments
We thank Profs Yihua Chen (Institute of Microbiology, CAS), Tao Liu (Tianjin Institute of Industrial Biotechnology, CAS) and Shuangjun Lin (Shanghai Jiao Tong University) for informative discussions and careful reading of the manuscript. We thank Prof Meifeng Tao (Shanghai Jiao Tong University) for providing the plasmids pJTU2554 and pJTU6722. This work was supported financially by the National Natural Science Foundation of China to S.-X.H (Nos. 81522044 and 21372230), the Science Foundation of Yunnan to S.-X.H (Nos. 2013HA022 and 2016FA003) and to Y.Y. (No. 2015FB175), and the Thousand Young Talents Program of China.
Footnotes
Author contributions S.-X.H. designed the research, and Y.Y., J.Y., G.P.H. and S.-X.H. wrote the paper. Y.Y. constructed all the engineered strains and mutants, and carried out the in vitro chemical reactions and all the precursors feeding experiments as well as HPLC analysis. Z.Y. performed the fermentation and compounds isolation. M.Y. synthesized 15N-labelled substrate. M.Y., Y.-T.M., J.Y. and L.W. analysed the NMR data and performed the structures determination of isolated compounds. C.S. and J.L. did the bioinformatics analysis and mutants construction. G.P.H. made a contribution to proposals of the biosynthetic pathway and the in vitro reaction mechanism.
References
- Palleroni N. J. et al. Production of a novel red pigment, rubrolone, by Streptomyces echinoruber sp. nov. I. Taxonomy, fermentation and partial purification. J. Antibiot. 31, 1218–1225 (1978). [DOI] [PubMed] [Google Scholar]
- Schüep W., Blount J. F., Williams T. H. & Stempel A. Production of a novel red pigment, rubrolone, by Streptomyces echinoruber sp. nov. II. Chemistry and structure elucidation. J. Antibiot. 31, 1226–1232 (1978). [DOI] [PubMed] [Google Scholar]
- Kelly T. R., Echavairen A., Whiting A., Weibel F. R. & Miki Y. Synthesis of the chromophore of rubrolone. Tetrahedron Lett. 27, 6049–6050 (1986). [Google Scholar]
- Boger D. L., Ichikawa S. & Jiang H. Total synthesis of the rubrolone aglycon. J. Am. Chem. Soc. 122, 12169–12173 (2000). [Google Scholar]
- Yan Y. et al. Tropolone ring construction in the biosynthesis of rubrolone B, a cationic tropolone alkaloid from endophytic Streptomyces. Org. Lett. 18, 1254–1257 (2016). [DOI] [PubMed] [Google Scholar]
- Davison J. et al. Genetic, molecular, and biochemical basis of fungal tropolone biosynthesis. Proc. Natl Acad. Sci. USA 109, 7642–7647 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinder A. R. Pyrrole, pyrrolidine, piperidine, pyridine, and azepine alkaloids. Nat. Prod. Rep. 9, 17–23 (1992). [DOI] [PubMed] [Google Scholar]
- Plunkett A. O. Pyrrole, pyrrolidine, pyridine, piperidine, and azepine alkaloids. Nat. Prod. Rep. 11, 581–590 (1994). [DOI] [PubMed] [Google Scholar]
- O'Hagan D. Pyrrole, pyrrolidine pyridine, piperidine, azepine and tropane alkaloids. Nat. Prod. Rep. 14, 637–651 (1997). [DOI] [PubMed] [Google Scholar]
- O'Hagan D. Pyrrole, pyrrolidine, pyridine, piperidine and tropane alkaloids. Nat. Prod. Rep. 17, 435–446 (2000). [DOI] [PubMed] [Google Scholar]
- Dewick P. M. Medicinal Natural Products: A Biosynthetic Approach 3rd edn John Wiley & Sons Ltd. (2009). [Google Scholar]
- Namwat W., Kinoshita H. & Nihira T. Identification by heterologous expression and gene disruption of VisA as L-lysine 2-aminotransferase essential for virginiamycin S biosynthesis in Streptomyces virginiae. J. Antibiot. 184, 4811–4818 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruntner C. & Bormann C. The Streptomyces tendae Tü901 L-lysine 2-aminotransferase catalyzes the initial reaction in nikkomycin D biosynthesis. Eur. J. Biochem. 254, 347–355 (1998). [DOI] [PubMed] [Google Scholar]
- Shoji T. & Takashi H. Plant Metabolism and Biotechnology John Wiley & Sons Ltd. (2011). [Google Scholar]
- Huang T. et al. Identification and characterization of the pyridomycin biosynthetic gene cluster of Streptomyces pyridomyceticus NRRL B-2517. J. Biol. Chem. 286, 20648–20657 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheoran A., King A., Velasco A., Pero J. M. & Garneau-Tsodikova S. Characterization of TioF, a tryptophan 2,3-dioxygenase involved in 3-hydroxyquinaldic acid formation during thiocoraline biosynthesis. Mol. Biosyst. 4, 622–628 (2008). [DOI] [PubMed] [Google Scholar]
- Qu X. et al. Caerulomycins and collismycins share a common paradigm for 2,2'-bipyridine biosynthesis via an unusual hybrid polyketide-peptide assembly Logic. J. Am. Chem. Soc. 134, 9038–9041 (2012). [DOI] [PubMed] [Google Scholar]
- Liu W., Christenson S. D., Standage S. & Shen B. Biosynthesis of the enediyne antitumor antibiotic C-1027. Science 297, 1170–1173 (2002). [DOI] [PubMed] [Google Scholar]
- Li L. et al. The mildiomycin biosynthesis: initial steps for sequential generation of 5-hydroxymethylcytidine 5'-monophosphate and 5-hydroxymethylcytosine in Streptoverticillium rimofaciens ZJU5119. ChemBioChem 9, 1286–1294 (2008). [DOI] [PubMed] [Google Scholar]
- Chater K. F. & Wilde L. C. Streptomyces albus G mutants defective in the SalGI restriction-modification system. J. Gen. Microbiol. 116, 323–334 (1980). [DOI] [PubMed] [Google Scholar]
- Gust B., Challis G. L., Fowler K., Kieser T. & Chater K. F. PCR-targeted Streptomyces gene replacement identifies a protein domain needed for biosynthesis of the sesquiterpene soil odor geosmin. Proc. Natl Acad. Sci. USA 100, 1541–1546 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti T., Hu Z., Pohl N. L., Shah A. N. & Khosla C. Cloning, nucleotide sequence, and heterologous expression of the biosynthetic gene cluster for R1128, a non-steroidal estrogen receptor antagonist insights into an unusual priming mechanism. J. Biol. Chem. 275, 33443–33448 (2000). [DOI] [PubMed] [Google Scholar]
- Meadows E. S. & Khosla C. In vitro reconstitution and analysis of the chain initiating enzymes of the R1128 polyketide synthase. Biochemistry 40, 14855–14861 (2001). [DOI] [PubMed] [Google Scholar]
- Tang Y., Koppisch A. T. & Khosla C. The acyltransferase homologue from the initiation module of the R1128 polyketide synthase is an acyl-ACP thioesterase that edits acetyl primer units. Biochemistry 43, 9546–9555 (2004). [DOI] [PubMed] [Google Scholar]
- Ames B. D. et al. Structural and biochemical characterization of ZhuI aromatase/cyclase from the R1128 polyketide pathway. Biochemistry 50, 8392–8406 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aufhammer S. W. et al. Crystal structure of methylenetetrahydromethanopterin reductase (Mer) in complex with coenzyme F420: architecture of the F420/FMN binding site of enzymes within the nonprolyl cis-peptide containing bacterial luciferase family. Protein Sci. 14, 1840–1849 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi T. et al. Biosynthetic conclusions from the functional dissection of oxygenases for biosynthesis of actinorhodin and related Streptomyces antibiotics. Chem. Biol. 20, 510–520 (2013). [DOI] [PubMed] [Google Scholar]
- Hori Y. et al. R1128 substances, novel non-steroidal estrogen-receptor antagonists produced by a Streptomyces. II. Physico-chemical properties and structure determination. J. Antibiot. 46, 1063–1068 (1993). [DOI] [PubMed] [Google Scholar]
- Chen Y. H. et al. Functional analyses of oxygenases in jadomycin biosynthesis and identification of JadH as a bifunctional oxygenase/dehydrase. J. Biol. Chem. 280, 22508–22514 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibodeaux C. J., Melancon C. E. & Liu H. W. Unusual sugar biosynthesis and natural product glycodiversification. Nature 446, 1008–1016 (2007). [DOI] [PubMed] [Google Scholar]
- Elshahawi S. I., Shaaban K. A., Kharel M. K. & Thorson J. S. A comprehensive review of glycosylated bacterial natural products. Chem. Soc. Rev. 44, 7591–7697 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson D. F., Manning P. A. & Morona R. Characterization of the dTDP-rhamnose biosynthetic genes encoded in the rfb iocus of Shigella flexneri. Mol. Microbiol. 11, 281–292 (1994). [DOI] [PubMed] [Google Scholar]
- Thuy T. T. T., Lee H. C., Kim C. G., Heide L. & Sohng J. K. Functional characterizations of novWUS involved in novobiocin biosynthesis from Streptomyces spheroides. Arch. Biochem. Biophys. 436, 161–167 (2005). [DOI] [PubMed] [Google Scholar]
- Giraud M. F. et al. Overexpression, purification, crystallization and preliminary structural study of dTDP-6-deoxy-L-lyxo-4-hexulose reductase (RmlD), the fourth enzyme of the dTDP-L-rhamnose synthesis pathway, from Salmonella enterica serovar Typhimurium. Acta. Crystallogr. Sect. D: Biol. Crystallogr. 55, 2043–2046 (1999). [DOI] [PubMed] [Google Scholar]
- Dong C. J. et al. RmlC, a C3′ and C5′ carbohydrate epimerase, appears to operate via an intermediate with an unusual twist boat conformation. J. Mol. Biol. 365, 146–159 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blümel S., Knackmuss H. J. & Stolz A. Molecular cloning and characterization of the gene coding for the aerobic azoreductase from Xenophilus azovorans KF46F. Appl. Environ. Microbiol. 68, 3948–3955 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexeev I., Sultana A., Mäntsälä P., Niemi J. & Schneider G. Aclacinomycin oxidoreductase (AknOx) from the biosynthetic pathway of the antibiotic aclacinomycin is an unusual flavoenzyme with a dual active site. Proc. Natl Acad. Sci. USA 104, 6170–6175 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colosimo D. A. & MacMillan J. B. Detailed mechanistic study of the non-enzymatic formation of the discoipyrrole family of natural products. J. Am. Chem. Soc. 138, 2383–2388 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan E. et al. Precursor-directed generation of amidine containing ammosamide analogs: ammosamides E-P. Chem. Sci. 4, 482–488 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz H. et al. Precursor-directed syntheses and biological evaluation of new elansolid derivatives. ChemBioChem 13, 1813–1817 (2012). [DOI] [PubMed] [Google Scholar]
- Rix U. et al. The dynamic structure of jadomycin B and amino acid incorporation step of its biosynthesis. J. Am. Chem. Soc. 126, 4496–4497 (2004). [DOI] [PubMed] [Google Scholar]
- Robertson A. W. et al. Eight-membered ring-containing jadomycins: implications for non-enzymatic natural products biosynthesis. J. Am. Chem. Soc. 137, 3271–3275 (2015). [DOI] [PubMed] [Google Scholar]
- Wang T. & Hoye T. R. Diels-Alderase-free, bis-pericyclic, [4+2] dimerization in the biosynthesis of (±)-paracaseolide A. Nat. Chem. 7, 641–645 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai T. et al. Use of a biosynthetic intermediate to explore the chemical diversity of pseudo-natural fungal polyketides. Nat. Chem. 7, 737–743 (2015). [DOI] [PubMed] [Google Scholar]
- Wever W. J. et al. Chemoenzymatic synthesis of thiazolyl peptide natural products featuring an enzyme-catalyzed formal [4+2] cycloaddition. J. Am. Chem. Soc. 137, 3494–3497 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill M. D. Recent strategies for the synthesis of pyridine derivatives. Chem. Eur. J. 16, 12052–12062 (2010). [DOI] [PubMed] [Google Scholar]
- Henry G. D. De novo synthesis of substituted pyridines. Tetrahedron 60, 6043–6061 (2004). [Google Scholar]
- Kelly T. R. & Lebedev R. L. Synthesis of some unsymmetrical bridged terpyridines. J. Org. Chem. 67, 2197–2205 (2002). [DOI] [PubMed] [Google Scholar]
- Dan C. et al. Cimicifugadine from Cimicifuga foetida, a new class of triterpene alklaoids with novel reactivity. Org. Lett. 9, 1813–1816 (2007). [DOI] [PubMed] [Google Scholar]
- Baghdikian B. et al. Two new pyridine monoterpene alkaloids by chemical conversion of a commercial extract of Harpagophytum procumbens. J. Nat. Prod. 62, 211–213 (1999). [DOI] [PubMed] [Google Scholar]
- Oshimi S. et al. Cassiarins C-E, antiplasmodial alkaloids from the flowers of Cassia siamea. J. Nat. Prod. 72, 1899–1901 (2009). [DOI] [PubMed] [Google Scholar]
- Liu Z., Yuan Q. & Wang W. Biosynthesis of [1–15N] l-tryptophan from 15N labeled anthranilic acid by fermentation of Candida utilis mutant. Amino Acids 36, 71–73 (2009). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures 1-72, Supplementary Tables 1-6, Supplementary References.
Data Availability Statement
Sequence data that support the findings of this study has been deposited in GenBank with accession codes KX218108. The authors declare that all other relevant data supporting the findings of this study are available within the article and its Supplementary Information Files and from the corresponding author upon reasonable request.