Arabidopsis STAY-GREEN is a functional Mg-dechelatase that extracts Mg from free chlorophyll and from chlorophyll in complexes, thus acting in chlorophyll degradation and photosystem degradation.
Abstract
Pheophytin a is an essential component of oxygenic photosynthetic organisms because the primary charge separation between chlorophyll a and pheophytin a is the first step in the conversion of light energy. In addition, conversion of chlorophyll a to pheophytin a is the first step of chlorophyll degradation. Pheophytin is synthesized by extracting magnesium (Mg) from chlorophyll; the enzyme Mg-dechelatase catalyzes this reaction. In this study, we report that Mendel’s green cotyledon gene, STAY-GREEN (SGR), encodes Mg-dechelatase. The Arabidopsis thaliana genome has three SGR genes, SGR1, SGR2, and STAY-GREEN LIKE (SGRL). Recombinant SGR1/2 extracted Mg from chlorophyll a but had very low or no activity against chlorophyllide a; by contrast, SGRL had higher dechelating activity against chlorophyllide a compared with chlorophyll a. All SGRs could not extract Mg from chlorophyll b. Enzymatic experiments using the photosystem and light-harvesting complexes showed that SGR extracts Mg not only from free chlorophyll but also from chlorophyll in the chlorophyll-protein complexes. Furthermore, most of the chlorophyll and chlorophyll binding proteins disappeared when SGR was transiently expressed by a chemical induction system. Thus, SGR is not only involved in chlorophyll degradation but also contributes to photosystem degradation.
INTRODUCTION
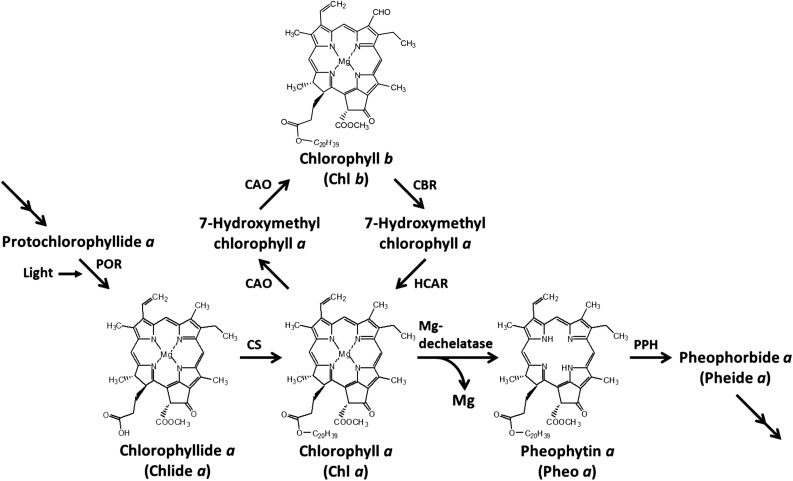
Chlorophyll and its derivatives play essential roles in photosynthesis, where chlorophyll harvests light energy and transfers it to the reaction center. Most chlorophyll molecules in photosynthesis are involved in this process. Green plants have two different chlorophyll species, chlorophyll a and b, which harvest light energy; the biosynthetic pathway for these chlorophylls has been studied extensively (Tanaka and Tanaka, 2007). Chlorophyll a is synthesized from 5-aminolevulinic acid through multiple steps. At the last step of chlorophyll synthesis, a portion of chlorophyll a is converted to chlorophyll b by chlorophyllide a oxygenase via 7-hydroxymethyl chlorophyll a. Chlorophyll b is reconverted to chlorophyll a by chlorophyll b reductase (CBR) and 7-hydroxymethyl chlorophyll a reductase (HCAR) (Meguro et al., 2011). Arabidopsis thaliana has two isozymes of CBR, NON-YELLOW COLORING1 (NYC1) and NYC1-LIKE (NOL) (Kusaba et al., 2007; Horie et al., 2009). This pathway, known as the chlorophyll cycle, interconverts chlorophyll a and chlorophyll b (Figure 1). All the enzymes responsible for chlorophyll synthesis and for the chlorophyll cycle have been identified, and the chlorophyll metabolic pathway has been determined.
Figure 1.
Chlorophyll Metabolic Pathway in Land Plants.
Mg-dechelatase was identified in this study. CAO, chlorophyllide a oxygenase; CBR, chlorophyll b reductase; CS, chlorophyll synthase; HCAR, 7-hydroxymethyl chlorophyll a reductase; PPH, pheophytin pheophorbide hydrolase; POR, NADPH:protochlorophyllide oxidoreductase.
Another important function of chlorophyll is to drive electron transfer, and pheophytin a plays a crucial role in this function. In the reaction center of PSII, the primary charge separation between P680 (chlorophyll a; PSII primary donor) and pheophytin a occurs; this is the first step in the conversion of light to chemical energy in photosynthesis (Holzwarth et al., 2006). Pheophytin a is synthesized by extracting magnesium (Mg) from chlorophyll a. The enzyme responsible for this reaction has been tentatively called Mg-dechelatase, although it is still not evident whether other enzymes catalyze Mg-dechelation or whether it occurs spontaneously under acidic conditions. Mg-dechelation is an important process in the formation of PSII because PSII assembly starts with the formation of the D1/D2 complex of which pheophytin a is an indispensable component (Nickelsen and Rengstl, 2013).
Mg-dechelatase also has a physiological function during senescence. A recent study showed that the first step of chlorophyll degradation is the conversion of chlorophyll a to pheophytin a (Christ and Hörtensteiner, 2013). Pheophytin a is then converted to pheophorbide a by pheophytin pheophorbide hydrolase (pheophytinase; PPH); pheophorbide a is then oxidatively ring-opened to the red chlorophyll catabolite by pheophorbide a oxygenase (PaO). This is followed by the reduction to fluorescent chlorophyll catabolite by red chlorophyll catabolite reductase (RCCR) (Rodoni et al., 1997; Schelbert et al., 2009). Interestingly, chlorophyll b cannot directly enter into this degradation pathway but must be converted to chlorophyll a before degradation; this is due to the substrate specificity of the latter degradation enzymes (Hörtensteiner, 2006). The degradation of chlorophyll is a key part of nitrogen recycling and is important in avoiding cellular damage. If chlorophyll degradation is not properly regulated, severe photodamage occurs and cell death is induced (Pruzinská et al., 2003; Hirashima et al., 2009; Hörtensteiner and Kräutler, 2011). Among chlorophyll degradation enzymes, Mg-dechelatase is especially important for regulation because it catalyzes the step in which chlorophyll is committed to degradation.
As Mg-dechelatase has indispensable functions in the formation of PSII and the degradation of chlorophyll, many attempts have been made to identify it; however, all these efforts failed. This has been partly due to the difficulty of detecting dechelation activity in vitro using chlorophyll(ide) as a substrate (Hörtensteiner and Kräutler, 2011). Instead of chlorophyll, an artificial substrate chlorophyllin (a semisynthetic derivative of chlorophyll) has been widely used to measure Mg-dechelatase activity. However, this might lead to a failure in identifying Mg-dechelatase because the real substrate of Mg-dechelatase is chlorophyll a.
Most of the mutants of chlorophyll degradation enzymes, such as PPH (Schelbert et al., 2009), PaO (Pruzinská et al., 2003), and CBR (Kusaba et al., 2007; Horie et al., 2009), exhibit a stay-green phenotype. It is therefore reasonable to assume that the mutation of Mg-dechelatase would also cause a strong stay-green phenotype because it catalyzes the first step of the chlorophyll degradation pathway. Mendel studied the mechanisms of inheritance using seven pea (Pisum sativum) mutants, including a green cotyledon mutant. Recently, Mendel’s green cotyledon gene was shown to encode the STAY-GREEN (SGR) protein. The SGR mutation induces a stay-green phenotype not only in Mendel’s green cotyledon (Armstead et al., 2007; Sato et al., 2007), but also in many other plants (Park et al., 2007; Ren et al., 2007). Many studies have been performed to elucidate the function of SGR and a hypothesis for SGR function was proposed based on protein-protein interaction experiments. Sakuraba et al. (2012) found that SGR physically interacted with the light-harvesting complex of PSII (LHCII) and also with six chlorophyll degradation enzymes including HCAR, NOL, NYC1, PaO, PPH, and RCCR; they proposed a complex of SGR with LHCII and chlorophyll degradation enzymes that allows the metabolic channeling of chlorophyll degradation intermediates. However, the question remained whether SGR can simultaneously bind six proteins and whether SGR has other functions.
We speculated that the SGR gene could encode Mg-dechelatase because all the sgr mutants showed strong stay-green phenotypes. To examine this possibility, we performed in vitro and in vivo experiments. When SGR was transiently expressed in Arabidopsis, chlorophyll was degraded and this was accompanied by the accumulation of a small amount of pheophytin a. Recombinant SGR proteins prepared using a wheat germ protein expression system converted chlorophyll a to pheophytin a, but SGR had no activity against chlorophyll b. When we incubated SGR with chlorophyll-protein complexes isolated with a sucrose density gradient, chlorophyll a was efficiently converted to pheophytin a. Based on these experiments, we concluded that Mendel’s green cotyledon gene (SGR) encodes Mg-dechelatase. We discuss the enzymatic properties of SGR in relation to the degradation of photosystems.
RESULTS
Mg-Dechelating Activity of Recombinant SGR
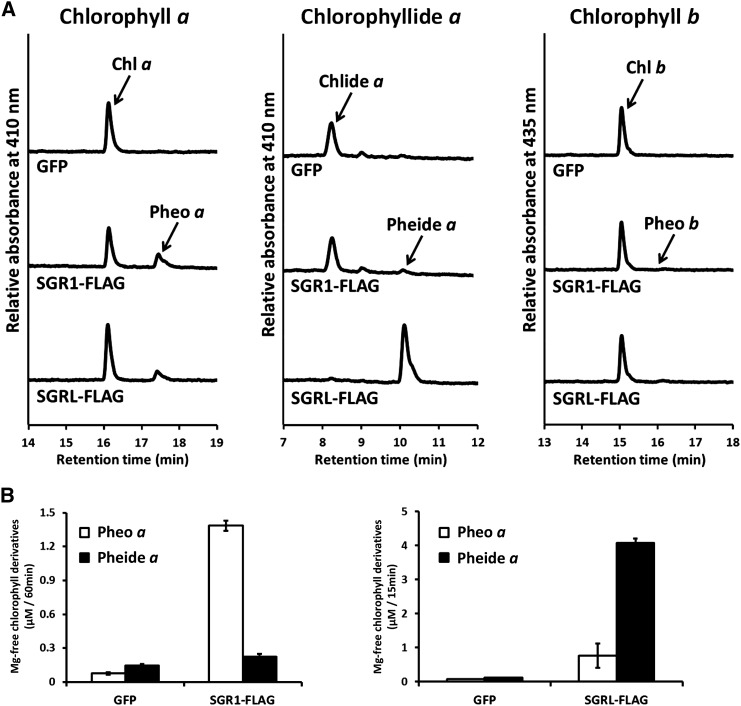
The Arabidopsis genome contains three SGR genes, SGR1 (AT4G22920), SGR2 (AT4G11910), and STAY-GREEN LIKE (SGRL; AT1G44000) (Sakuraba et al., 2014). First, we used recombinant mature SGR proteins expressed in Escherichia coli for enzymatic experiments, but we did not observe any Mg-dechelating activity. Next, we examined the Mg-dechelating activity of mature SGR proteins prepared by a wheat germ protein expression system (Supplemental Figure 1). Recombinant SGR1 had high dechelating activity against chlorophyll a but very low activity against chlorophyllide a (Figure 2A; Supplemental Data Set 1). Substrates and products were identified by their absorption spectra (Supplemental Figure 2) and by their HPLC retention time (Shimoda et al., 2012).The substrate specificity of SGR2 was almost the same as that of SGR1, which is consistent with the high amino acid sequence similarity between SGR1 and SGR2 (Supplemental Figure 3). In contrast, SGRL had much higher activity against chlorophyllide a than against chlorophyll a (Figure 2B). None of the three SGRs (SGR1, SGR2, and SGRL) extracted Mg from chlorophyll b. These results suggest that SGR has Mg-dechelating activity and that substrate specificity is different between SGR1/2 and SGRL.
Figure 2.
Mg-Dechelating Activity and Substrate Specificity of Recombinant SGR1 and SGRL.
(A) Pigment analysis after incubation of chlorophyll derivatives with SGR. Chlorophyll a and chlorophyllide a were incubated with recombinant GFP, SGR1 with a FLAG-tag (SGR1-FLAG) for 60 min, or with recombinant SGRL with a FLAG-tag (SGRL-FLAG) for 15 min. Recombinant proteins were prepared with a wheat germ protein expression system and diluted 3-fold with the reaction buffer without purification. GFP was used as a negative control because it has a similar molecular weight as SGR. Chlorophyll b was incubated with recombinant GFP, SGR1-FLAG, and SGRL-FLAG for 60 min. The concentration of substrates was 6 µM. After incubation, pigments were analyzed using HPLC. Pigments were detected at 410 nm for chlorophyll a derivatives or 435 nm for chlorophyll b derivatives.
(B) An increase in chlorophyll derivatives by SGR activity. The levels of pheophytin a and pheophorbide a were determined after incubation of recombinant GFP and SGR1 with a FLAG-tag (SGR1-FLAG and SGRL-FLAG) with 6 µM of chlorophyll a and chlorophyllide a (n = 3 ±sd). The incubation times of SGR1-FLAG and SGRL-FLAG were 60 and 15 min, respectively. Recombinant proteins were prepared with a wheat germ protein expression system and diluted with the reaction buffer without purification. GFP was used as a negative control because it has similar molecular weight as SGR.
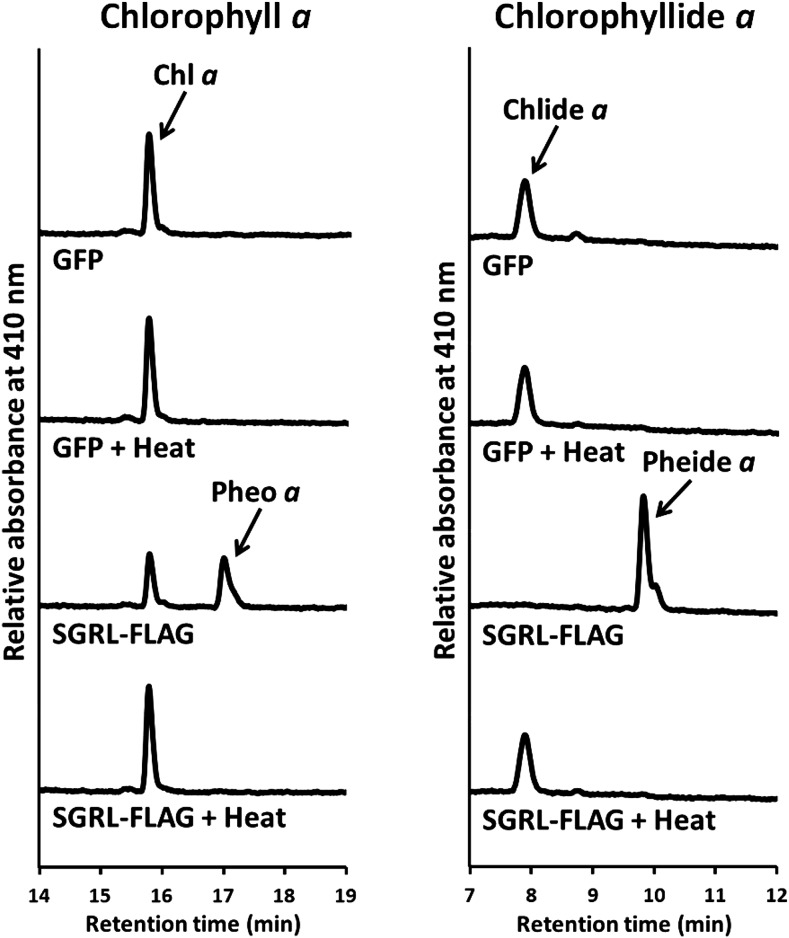
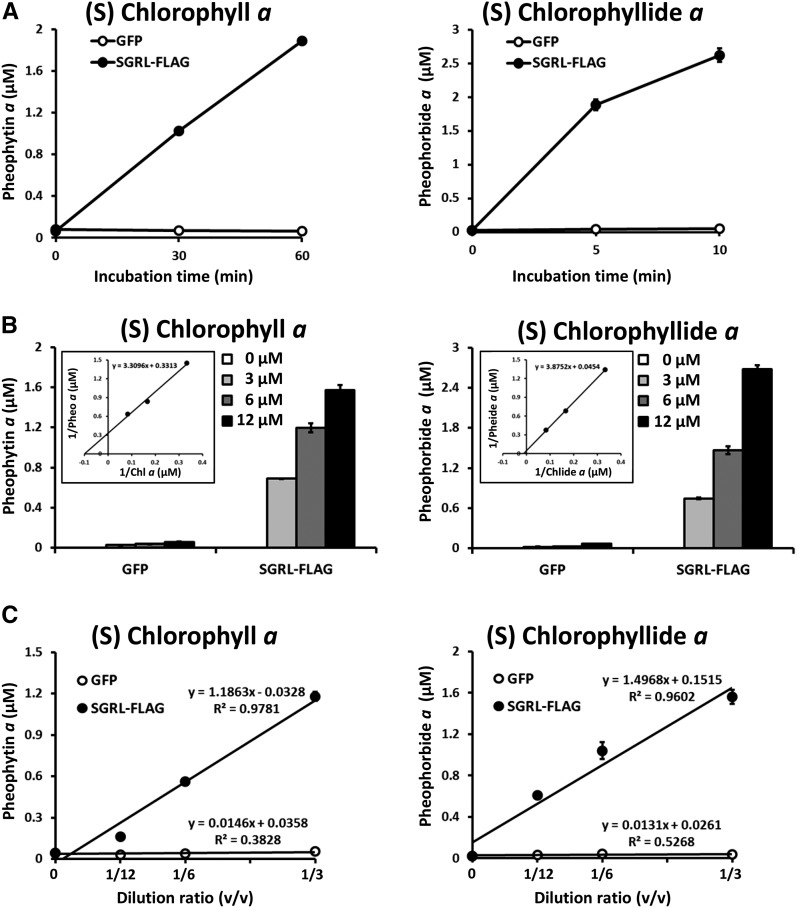
To confirm that in vitro Mg dechelation is an enzymatic reaction catalyzed by SGR, the following experiments were performed using recombinant SGRL protein because it has the highest activity among three SGRs. Mg-dechelating activity was completely lost by heating at 95°C (Figure 3). Purified SGRL, showing a single or a major band on SDS-PAGE, had Mg-dechelating activity (Supplemental Figure 4), suggesting that SGR has Mg-dechelating activity without any other factors. A time-course study showed that the amount of the products (pheophytin a or pheophorbide a) increased depending on the incubation time and the product never increased without SGRL proteins in the reaction mixture (Figure 4A). Increasing concentrations of chlorophyll a and chlorophyllide a substrates were accompanied by enhanced conversion to their respective products (Figure 4B). The nonlinearity observed using chlorophyll a as a substrate differs from the almost linear increase in product formation obtained with chlorophyllide a (Figure 4B, left panel); this could arise from a number of factors and a more detailed analysis is required. The amount of the product depended on the concentration of SGRL (Figure 4C). All these results strongly indicate that release of Mg from chlorophylls occurs enzymatically by SGR.
Figure 3.
Mg-Dechelating Activity of Heat-Denatured SGRL.
Recombinant GFP and SGRL-FLAG were denatured by heat treatment for 5 min at 95°C. Chlorophyll a and chlorophyllide a were incubated with nondenatured or denatured recombinant GFP and SGRL-FLAG for 60 min at 25°C. Recombinant proteins were prepared by a wheat germ protein expression system and diluted 3-fold with the reaction buffer without purification. GFP was used as a negative control because it has similar molecular weight as SGR. The concentration of substrates was 6 µM. After incubation, pigments were analyzed using HPLC. Pigments were detected at 410 nm.
Figure 4.
Biochemical Analysis of SGRL.
(A) Time-dependent formation of Mg-free chlorophyll derivatives by SGRL-FLAG. Chlorophyll a or chlorophyllide a were incubated with recombinant GFP (open circles) and SGRL-FLAG (closed circles) for up to 60 min or 10 min at 25°C, respectively. Recombinant proteins were prepared by a wheat germ protein expression system and diluted 3-fold with the reaction buffer without purification. GFP was used as a negative control because it has similar molecular weight as SGR. The concentration of substrates was 6 µM. After incubation, the level of pheophytin a and pheophorbide a was determined using HPLC (n = 3 ±sd).
(B) Kinetic analysis of Mg-dechelating of SGRL-FLAG. Various concentrations of chlorophyll a or chlorophyllide a were incubated with recombinant GFP and SGRL-FLAG for 30 or 5 min at 25°C, respectively. Recombinant proteins were prepared by a wheat germ protein expression system and diluted 3-fold with the reaction buffer without purification. GFP was used as a negative control because it has similar molecular weight as SGR. After incubation, the level of pheophytin a and pheophorbide a were determined using HPLC (n = 3 ±sd). The inset shows Lineweaver-Burk plot of kinetic data of Mg-dechelating of SGRL-FLAG.
(C) SGRL-FLAG concentration-dependent formation of Mg-free chlorophyll derivatives. Chlorophyll a or chlorophyllide a were incubated with various concentrations of recombinant GFP (open circles) and SGRL-FLAG (closed circles) for 30 or 5 min at 25°C, respectively. Translation solutions containing expressed GFP and SGRL-FLAG were diluted 3, 6, or 12 times in 50 μL of reaction buffer. GFP was used as a negative control because it has similar molecular weight as SGR. The concentration of substrates was 6 µM. After incubation, the levels of pheophytin a and pheophorbide a were determined using HPLC (n = 3 ±sd).
Mg-Dechelating Activity of SGR in Cells
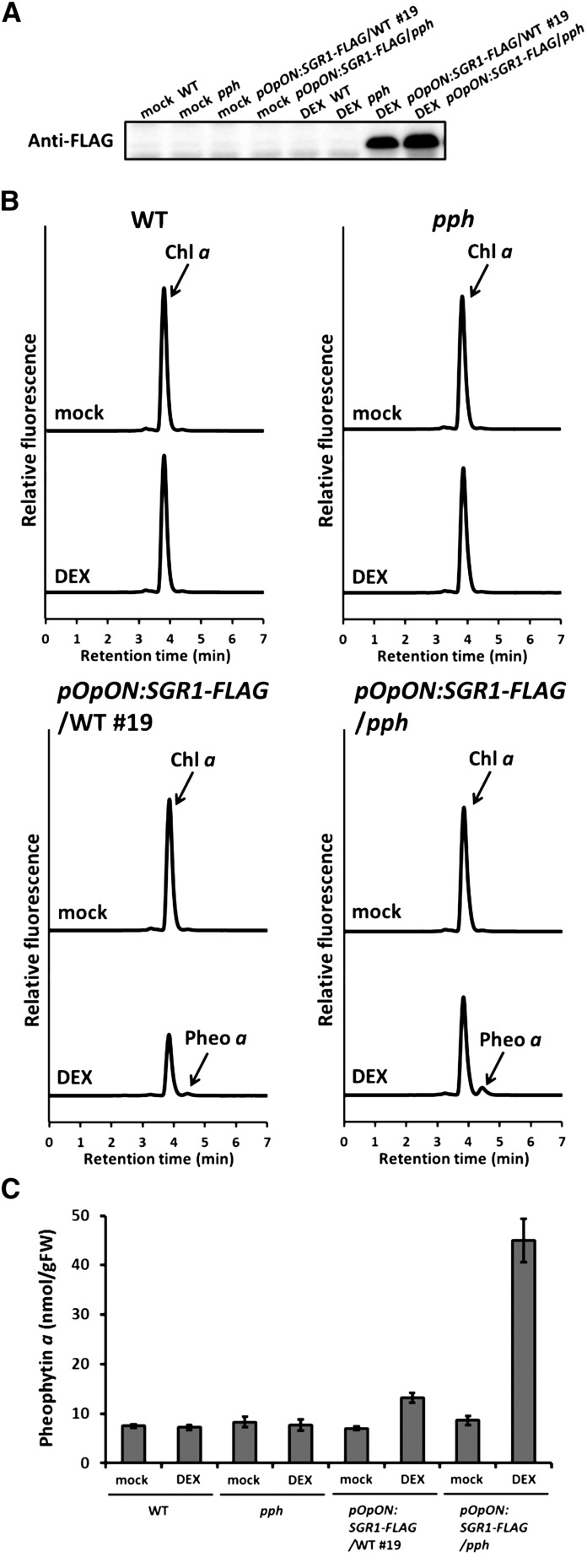
Recombinant SGR showed Mg-dechelating activity in vitro; however, it is not evident whether SGR functions as a Mg-dechelatase in cells. To answer this question, we transiently expressed SGR1 with a chemical induction system containing dexamethasone (DEX), and we examined the accumulation of pheophytin a, a product of Mg-dechelatase (Figure 5A). SGR1 expression increased the level of pheophytin a. Although the increase in pheophytin a suggested the occurrence of Mg-dechelation by SGR1, the absolute level of pheophytin a was very low (Figure 5B). One possible reason for this is that synthesized pheophytin a is immediately degraded by the next enzyme, PPH. In order to examine this possibility, SGR1 was transiently induced in pph background and the pigments were analyzed (Figure 5B). Pheophytin a accumulated more in the pph background than in the wild type by DEX treatment (Figure 5C), indicating that SGR could function as a Mg-dechelatase in cells.
Figure 5.
SGR1 Functions as a Mg-Dechelatase in Cells.
(A) SGR1 accumulation in the transformants. Inducible SGR1 with a FLAG-tag (SGR1-FLAG) was introduced into wild-type (pOpON:SGR1-FLAG/WT #19) and pph (pOpON:SGR1-FLAG/pph) plants. SGR1- FLAG was induced by DEX application in the transformants. After DEX or mock treatment for 24 h, proteins were extracted from the plants and SGR1 was detected by immunoblotting analysis using an anti-FLAG antibody.
(B) Pigment analysis after SGR1 induction. After DEX or mock treatment for 24 h, pigments were extracted from the plants and analyzed using HPLC. Fluorescence intensity was monitored (410 nm excitation; 680 nm fluorescence).
(C) Pheophytin a contents in the transformants. After DEX or mock treatment for 24 h, pigments were extracted from the plants and the amount of pheophytin a was determined (n = 4 ±sd).
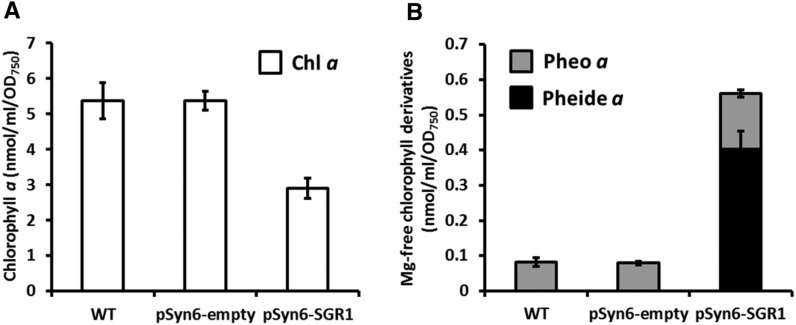
For further confirmation of SGR function, we introduced mature SGR1 into the cyanobacterium Synechococcus elongatus PCC7942 (hereafter Synechococcus) (Supplemental Figure 5A). The Synechococcus genome has no SGR, or any homologous gene, indicating that Synechococcus has no SGR system for Mg-dechelation. If SGR requires other protein components, it would not be expected to function as a Mg-dechelatase in Synechococcus cells. Our immunoblot analysis showed that SGR1 was successfully expressed in Synechococcus (Supplemental Figure 5B). Chlorophyll content was low (Figure 6A) and pheophytin a and pheophorbide a accumulated in large amounts (Figure 6B) in Synechococcus expressing SGR1, indicating that SGR1 functions as Mg-dechelatase in Synechococcus cells. Interestingly, the level of pheophorbide a was comparable to that of pheophytin a, which was quite different from the results obtained with the Arabidopsis leaves in which pheophorbide a was not detected (Figure 5B). Pheophorbide a might be synthesized from pheophytin a by an unknown PPH-like enzyme in Synechococcus cells. Based on these experiments, we finally concluded that SGR encodes a Mg-dechelatase and that no other protein is required for the dechelating activity of SGR.
Figure 6.
SGR1 Functions in Synechococcus.
(A) Chlorophyll a contents of Synechococcus. Chlorophyll a content of Synechococcus harboring the pSyn6 vector (pSyn6-empty) or SGR1 cloned into the pSyn6 vector (pSyn6-SGR1) was determined (n = 3 ±sd). Pigment content is shown based on OD750.
(B) Derivatives of chlorophyll a in Synechococcus. Pheophytin a and pheophorbide a contents of the wild type and transformed Synechococcus were determined (n = 3 ±sd).
Expression of SGR in Arabidopsis
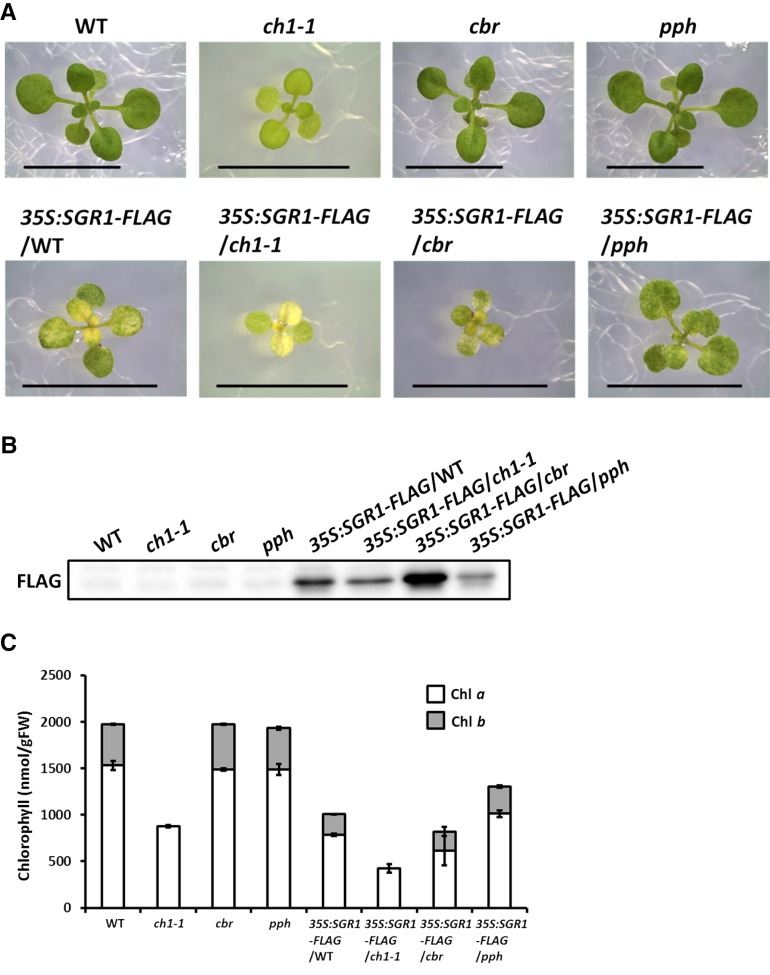
To elucidate the impact of SGR on chlorophyll metabolism and the relationship between SGR and other chlorophyll metabolic enzymes, we constitutively overexpressed the cDNA of the SGR1 gene in wild-type Arabidopsis plants and mutants, such as ch1-1 (mutant of chlorophyllide a oxygenase) and the cbr and pph mutants. These transgenic plants exhibited low chlorophyll content and retarded growth (Figure 7). We assumed that the plants would not grow when SGR1 was expressed in large amounts and that only the mutants with low expression levels of SGR would survive. Low chlorophyll content was also observed when SGR2 or SGRL was constitutively overexpressed (Supplemental Figures 6 and 7).
Figure 7.
SGR1 Overexpression in Arabidopsis.
(A) Visual phenotype of the transformants. SGR1 with a FLAG-tag (SGR1-FLAG) was overexpressed in wild-type plants and in the ch1-1, cbr, and pph mutants. Bars = 1 cm.
(B) SGR1 accumulation in the transformants. Proteins were extracted from the plants and SGR1 was detected by immunoblotting analysis using an anti-FLAG antibody.
(C) Chlorophyll content of the transformants. Chlorophyll was extracted from the plants and the amount of chlorophyll a and b was determined (n = 3 ±sd).
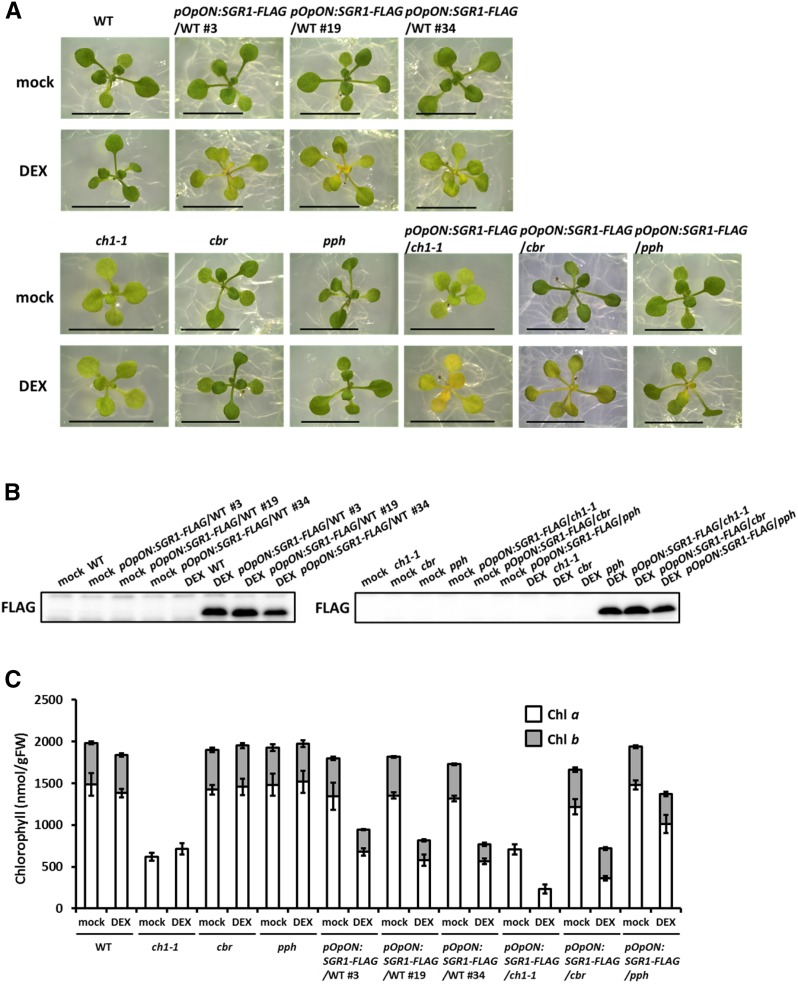
Based on these severe phenotypes, we concluded that constitutive overexpression is not appropriate for the study of SGR function. Instead, we transiently expressed SGR1 in fully greened leaves using a DEX induction system (Figure 8). Three independent transgenic lines transiently overexpressing SGR1 in a wild-type background (line numbers 3, 19, and 34) are shown in Figure 8A. After 24 h of DEX treatment, approximately half of the chlorophyll was degraded in the wild-type background (Figure 8C). Chlorophyll degradation was also observed in the pph mutant background; however, 70% of chlorophyll still remained after 24 h of DEX treatment. Interestingly, the level of chlorophyll b was not significantly changed by DEX treatment in a cbr mutant background, although chlorophyll a was extensively degraded. This is consistent with experiments demonstrating that SGR did not extract Mg from chlorophyll b (Figure 2A). Reduction of chlorophyll content was also observed when SGR2 or SGRL was transiently induced by DEX induction system (Supplemental Figures 6 and 7).
Figure 8.
SGR1 Induction in Arabidopsis.
(A) Visual phenotype of the transformants. SGR1 with a FLAG-tag (SGR1-FLAG) was induced by DEX application for 24 h in wild-type plants and in the ch1-1, cbr, and pph mutants grown for 2 weeks. Three independent transformants in a wild-type background are shown. Bars = 1 cm.
(B) SGR1 accumulation in the transformants. Proteins were extracted from the plants and SGR1 was detected by immunoblotting analysis using an anti-FLAG antibody.
(C) Chlorophyll contents of the transformants. Chlorophyll was extracted from the plants and the amount of chlorophyll a and b was determined (n = 4 ±sd).
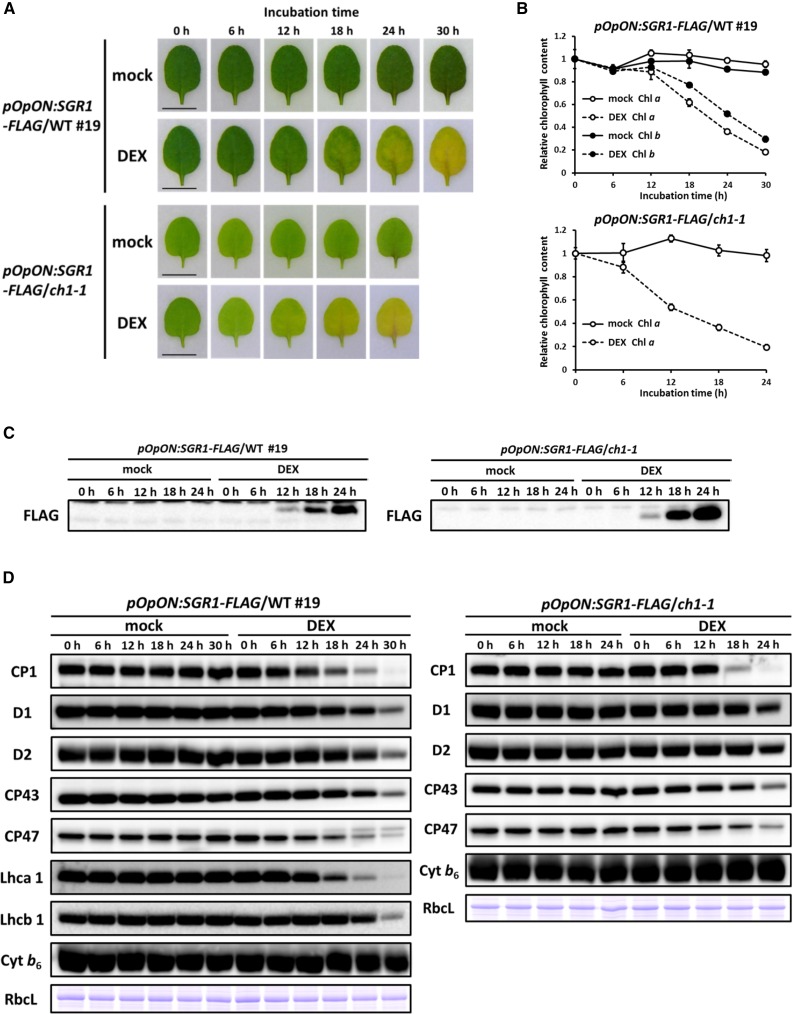
Next, we used excised leaves from either a wild-type or ch1-1 background to examine the effect of SGR1 expression on chloroplast proteins (Figure 9). After DEX treatment, chlorophyll levels decreased to 20% of the initial level in both wild-type and ch1-1 backgrounds (Figure 9B). The rate of chlorophyll degradation was slightly faster in the ch1-1 background than in the wild-type background. Upon DEX treatment, a reduction in chlorophyll content was accompanied by a decrease in chlorophyll binding proteins of both photosystems and LHC (Figure 9D). Degradation of PSI (CP1) and Lhca1 was slightly faster than that of PSII (CP43, CP47, D1, and D2) and Lhcb1. This was confirmed by a low-temperature fluorescence spectrum (Supplemental Figure 8) in which PSI fluorescence (∼735 nm) decreased rapidly, compared with PSII fluorescence (688 and 695 nm). By contrast, the levels of the cytochrome b6f complex, a thylakoid membrane protein, and ribulose-1,5-bisphosphate carboxylase/oxygenase, a soluble protein, were not significantly affected by DEX treatment. These results indicate that SGR regulates the first step of photosystem degradation by dechelating Mg from chlorophyll molecules. An experiment examining electrolyte leakage confirmed that degradation of chlorophyll and chlorophyll binding proteins was not caused by cell death in these plants (Supplemental Figure 9).
Figure 9.
Degradation of Chlorophyll and Chlorophyll Binding Protein by the Induction of SGR1.
(A) Color changes of leaves. Inducible SGR1 with a FLAG-tag was introduced into wild-type (pOpON:SGR1-FLAG/WT #19) or ch1-1 (pOpON:SGR1-FLAG/ch1-1) plants. DEX- or mock-treated excised leaves were observed for up to 30 h. Bars = 0.5 cm.
(B) Chlorophyll contents of leaves. Chlorophyll contents of pOpON:SGR1-FLAG/WT #19 and pOpON:SGR1-FLAG/ch1-1 were determined before and after DEX or mock treatment for up to 30 h (n = 4 ±sd). Comparisons were made to a 0 h control.
(C) SGR1 accumulation in leaves. Proteins were extracted from leaves and SGR1 was detected using immunoblotting analysis with an anti-FLAG antibody.
(D) Chloroplast protein content in leaves. Proteins were extracted from the pOpON:SGR1-FLAG/WT #19 and pOpON:SGR1-FLAG/ch1-1 excised leaves before and after DEX or mock treatment for up to 30 h. The large subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase (RbcL) was detected using Coomassie blue staining.
Chlorophyll a in the Pigment-Protein Complex Is a Substrate of SGR
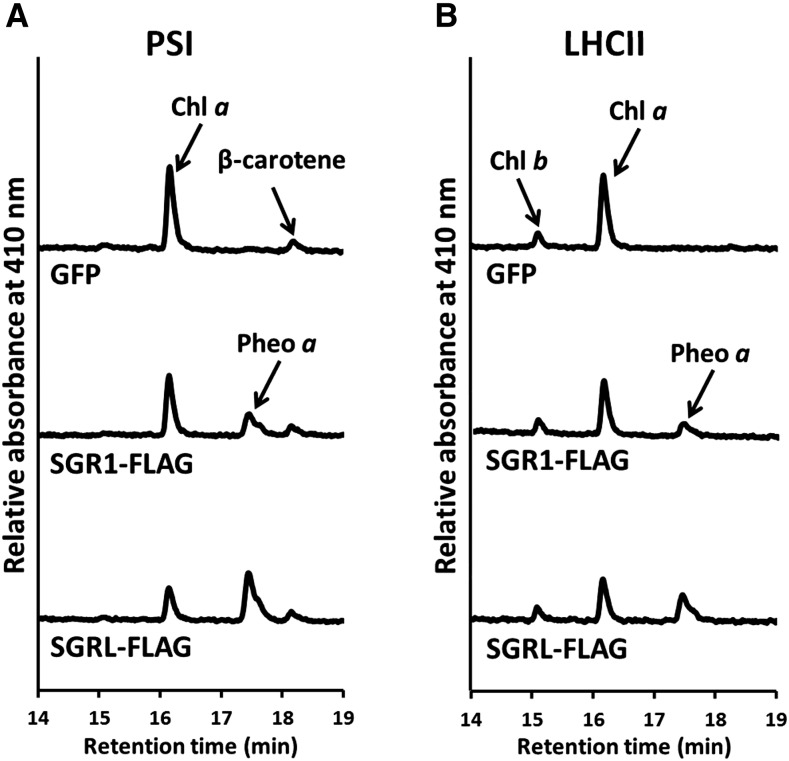
Most of the chlorophyll was degraded within 24 h of SGR1 expression. This suggests that SGR is able to release Mg not only from free chlorophyll but also from chlorophyll existing in photosystems because all of the chlorophyll binds to proteins in the chloroplast. To examine this possibility, we incubated recombinant SGR with PSI or LHCII purified by sucrose density gradient centrifugation. Pheophytin a accumulated after incubation with both substrates (Figure 10). The level of chlorophyll b was unchanged following incubation with SGR1 or SGRL, indicating that chlorophyll b is not a substrate of these proteins. SGR1 and SGRL had high catalytic activity against chlorophyll a in both PSI and LHCII. These observations suggest that SGR directly attacked the pigment-protein complexes and converted chlorophyll a to pheophytin a.
Figure 10.
Magnesium Extraction from Chlorophyll in the Chlorophyll-Protein Complex by SGR.
(A) Pigment analysis after incubation of PSI with SGR. PSI was isolated from Arabidopsis and incubated with recombinant GFP, SGR1 with a FLAG-tag (SGR1-FLAG), and SGRL with a FLAG-tag (SGRL-FLAG) for 60 min. Recombinant proteins were prepared by a wheat germ protein expression system and diluted with the same volume of reaction buffer without purification. GFP was used as a negative control because it has similar molecular weight as SGR. After incubation, pigments were analyzed using HPLC. Pigments were detected at 410 nm.
(B) Pigment analysis after incubation of LHCII with SGR. LHCII was isolated from Arabidopsis and incubated with recombinant GFP, SGR1-FLAG, and SGRL-FLAG for 60 min. Recombinant proteins were prepared with a wheat germ protein expression system and diluted with same volume of the reaction buffer without purification. GFP was used as a negative control because it has a similar molecular weight as SGR. After incubation, pigments were analyzed using HPLC. Pigments were detected at 410 nm.
DISCUSSION
SGR Encodes Mg-Dechelatase
The main enzymes of the chlorophyll degradation pathway have been identified previously, with the exception of Mg-dechelatase (Hörtensteiner and Kräutler, 2011). It has long been debated whether Mg-dechelation is brought about by an enzyme (Costa et al., 2002) or small substance (Suzuki et al., 2005) or whether it takes place spontaneously in a low pH environment (Christ and Hörtensteiner, 2013), as Mg-dechelatase has not been identified despite great efforts. In this study, we demonstrated that SGR encodes Mg-dechelatase.
There are no reports that discuss the metal dechelation mechanism. However, ferrochelatase catalyzes the reverse reaction of metal dechelation. According to the study of ferrochelatase, metal chelation consists of metal binding to the ferrochelatase, deprotonation from two -NH, and insertion of Fe into protoporphyrin IX (Wang et al., 2009). Glutamate, tyrosine, and histidine residues play a central role in these processes. Interestingly, these amino acid residues are conserved in SGRs. It is possible to speculate that Mg is dechelated by the reverse reaction of ferrochelatase, i.e., two protonations followed by Mg-dechelation. Amino acid substitution experiments will uncover the dechelation mechanism of SGR.
The sgr mutants have been extensively studied since Mendel and exhibit a strong stay-green phenotype without exception (Sato et al., 2007). This stay-green phenotype is consistent with our conclusion that SGR encodes Mg-dechelatase because it catalyzes the committed step of chlorophyll degradation. Sakuraba et al. (2012) proposed that SGR binds six chlorophyll degradation enzymes and forms a large complex (SGR-chlorophyll catabolic enzymes-LHCII complex) that enables efficient metabolic trafficking. The ch1-1 mutant lacks LHCII because chlorophyll b is not synthesized. However, SGR efficiently catalyzes the dechelating reaction in the ch1-1 mutant as in the wild-type background. These observations suggest that LHCII is not required for SGR function. In addition, if SGR binds many proteins (i.e., six chlorophyll degradation enzymes), it might be difficult for SGR to have access to the substrate of the chlorophyll-protein complexes. Another question is whether it is possible for SGR to simultaneously bind six proteins from a structural viewpoint. The hypothesis of the SGR-chlorophyll catabolic enzymes-LHCII complex should be reexamined. However, complexes consisting of two proteins (SGR-PPH, SGR-HCAR, and SGR-LHCII) should be considered because LHCII is a substrate of SGR and because SGR must accept chlorophyll a from HCAR and transfer pheophytin a to PPH.
Substrate Specificity and Physiological Functions of SGRs
Initially, we examined the dechelating activity of recombinant SGR expressed in E. coli; however, we did not observe any activity that was consistent with previous reports (Hörtensteiner, 2009). Then, we used the recombinant SGR prepared with a wheat germ protein expression system instead of E. coli; high dechelating activity was observed, indicating that the activity of SGR largely depends on protein-producing systems. Enzymatic experiments with recombinant SGR showed interesting substrate specificity among different SGRs; SGR1/2 extracted Mg from chlorophyll a but showed very low or no activity against chlorophyllide a. Considering the expression of SGR1/2 predominantly during senescence (Sakuraba et al., 2014) and its substrate specificity, SGR1/2 might be involved in chlorophyll degradation during senescence. This hypothesis is consistent with the strong stay-green phenotype of the sgr1/sgr2 double mutant (Wu et al., 2016). In contrast, the SGRL protein is expressed during greening (Sakuraba et al., 2014). Interestingly, SGRL showed higher activity against chlorophyllide a than against chlorophyll a. The conversion of chlorophyllide a to pheophorbide a by SGRL might not be an experimental artifact but might have a physiological function. Chidgey et al. (2014) proposed that chlorophyllide a is a component of the machinery involved in the formation of photosystems. Lin et al. (2014) reported that chlorophyllide a, which is derived from chlorophyll a, is reused for chlorophyll synthesis. The level of chlorophyllide a might partly be regulated by SGRL. Conversion of chlorophyllide a to pheophorbide a by SGRL suggests a new chlorophyll degradation pathway via chlorophyllide a (chlorophyllide pathway).
SGR1, SGR2, and SGRL could not extract Mg from chlorophyll b. This is consistent with the chemical experimental results that chlorophyll b is much more stable in acidic conditions compared with chlorophyll a (Saga and Tamiaki, 2012). The question remains as to whether SGR could not extract Mg from chlorophyll b due to the stabilization of Mg in chlorophyll b by the effect of 7-formyl group or whether SGR evolved to fit to chlorophyll a.
Regulation of Photosystem Dynamics by Chlorophyll Metabolic Enzymes
Two hypotheses exist for the degradation of photosystems. One is that some proteases are responsible for the first step of this process; chlorophyll degradation enzymes immediately degrade the resulting free chlorophylls. The other hypothesis is that chlorophyll degradation enzymes catalyze the first step of photosystem degradation and the resulting apoproteins are degraded by proteases. This SGR study supports the latter hypothesis. When we transiently induced SGR in fully greened leaves, chlorophyll levels decreased. This suggests that SGR extracts Mg from chlorophyll embedded in chlorophyll-protein complexes because all the chlorophyll molecules exist as chlorophyll-protein complexes. This idea was supported by in vitro experiments; chlorophyll a was converted to pheophytin a when isolated chlorophyll-protein complexes were incubated with SGR (Figure 10). Interestingly, chlorophyll binding proteins also disappeared along with a decrease in chlorophyll upon induction of SGR (Figure 9D), suggesting that chlorophyll-depleted apoproteins are immediately degraded in thylakoid membranes. If protein degradation occurred before chlorophyll degradation, a large amount of free chlorophyll would accumulate and the stay-green phenotype would not be observed in sgr mutants because free chlorophyll rapidly induces bleaching. These phenomena are similar to those of CBR (Horie et al., 2009); CBR converts chlorophyll b in LHCII to 7-hydroxymethyl chlorophyll a; this is the first step of chlorophyll b degradation. Chlorophyll b and LHCII are never degraded in the cbr mutant, although chlorophyll a is degraded as in wild-type plants. These in vitro and in vivo experiments with SGR and CBR strongly suggest that two enzymes, Mg-dechelatase (SGR) and CBR, primarily regulate the degradation of photosystems (PSI, PSII, LHCI, and LHCII) in green plants.
Another possible role of Mg-dechelatase is to supply pheophytin a for the formation of PSII. A supply of pheophytin a might also be required for the PSII repair cycle. Presently, we have no experimental evidence to support the involvement of SGR in these processes; even if the pheophytin a required for these processes is low, we cannot exclude the possibility that the pheophytin a required for the formation and repair cycle of PSII is generated spontaneously. However, the formation and repair cycle of PSII must be strictly regulated depending on the developmental stage and environmental conditions. It might be difficult to supply enough pheophytin a needed for these processes simply through spontaneous generation. Therefore, it is reasonable to assume that SGR or some other Mg-dechelatase participate in the formation and repair cycle of the PSII. Further study is required to understand the involvement of SGR (Mg-dechelatase) in these processes.
METHODS
Plant Materials and Growth Conditions
Arabidopsis thaliana wild-type (ecotype Columbia) and mutant ch1-1 (chlorophyllide a oxygenase) (Yamasato et al., 2005), cbr (nyc1 and nol) (Horie et al., 2009), and pph (Hu et al., 2015) plants were used in this study. We grew plants on soil or half-strength Murashige and Skoog (MS) medium containing 1% (w/v) sucrose, 0.8% (w/v) agar, and 0.05% MES buffer (pH 5.8) under 14-h-light/10-h-dark conditions (70 µmol photons m−2 s−1, white light, fluorescent bulbs) at 24°C.
We cultivated Synechococcus elongatus PCC7942 in BG-11 medium with shaking at 40 to 50 rpm under continuous light (20 to 30 µmol photons m−2 s−1) at 24°C; we used the logarithmically growing cells for pigment and immunoblot analysis.
Arabidopsis Transformation
We used a PCR assay (KOD-Plus-; Toyobo) to prepare Arabidopsis SGR1, SGR2, and SGRL cDNA with a C-terminal FLAG-tag, using the primers listed in Supplemental Table 1, and cloned it into the pGreenII vector (Hellens et al., 2000) under the control of the 35S promoter from CaMV using the SalI and NotI sites. To chemically induce expression of SGR in Arabidopsis, we expressed SGR under the control of the pOp6 promoter and the synthetic transcription factor, LhGR (Craft et al., 2005; Wielopolska et al., 2005). We subcloned SGR cDNA with a C-terminal FLAG-tag into a Gateway pENTR 4 Dual Selection Vector (Invitrogen) using an In-Fusion cloning system (Clontech Laboratories) and then introduced it into a binary vector, pOpON, using the Gateway recombination system. We constructed the pOpON vector from pOpOff2 by removing the antisense fragments with the KpnI and XbaI restriction enzymes. We introduced the glufosinate-resistant gene into the ClaI site. We transferred these constructs into the wild-type plants and into the ch1-1, cbr, and pph mutants.
Synechococcus Transformation
We transformed Synechococcus with a GeneArt Synechococcus Protein Expression Kit (Invitrogen). We used PCR (KOD- Plus-) to amplify the coding region of SGR1 lacking transit peptide using the primers listed in Supplemental Table 1 and cloned it into the pSyn6 vector (Invitrogen) using an In-Fusion system. We followed the manufacturer’s protocol to transform Synechococcus.
DEX Treatment
We grew plants on soil for 3.5 to 4.5 weeks under 14-h-light/10-h-dark conditions at 24°C. We placed excised third and fourth rosette leaves on wet filter paper containing 3 mM MES (pH 5.8). For DEX treatment, we prepared DEX as a 20 mM stock in dimethyl sulfoxide. We sprayed the plants with DEX (10 µM) supplemented with 0.015% Silwet L-77; plants were then incubated under continuous light (70 µmol photons m−2 s−1) for 24 to 30 h at 24°C. When whole plants were treated with DEX, they were grown on MS medium for 2 weeks under 14-h-light/10-h-dark conditions and sprayed with 10 µM DEX supplemented with 0.015% Silwet L-77 and then incubated under continuous light (70 µmol photons m−2 s−1) for 24 h at 24°C. The mock treatment consisted of a Silwet L-77 solution containing 0.05% dimethyl sulfoxide.
Mg-Dechelatase Assay
We obtained chlorophyllide a from chlorophyll a by hydrolysis with recombinant chlorophyllase (Tsuchiya et al., 1999; Shimoda et al., 2012); we isolated the LHCII trimer and PSI particles and purified them with sucrose gradient centrifugation (Shimoda et al., 2012). We synthesized recombinant SGR and GFP with an in vitro transcription/translation system (TNT SP6 High-Yield Wheat Germ Protein Expression System; Promega). We removed transit peptides and introduced a FLAG-tag at the C terminus of the SGR1, SGR2, and SGRL proteins (SGR1-FLAG, SGR2-FLAG, and SGRL-FLAG). We amplified the DNA fragments using the primers listed in Supplemental Table 1 and cloned them into the pF3A WG (BYDV) Flexi vector (Promega). We purified plasmid DNA with the PureYield Plasmid Miniprep System (Promega). After expression of the recombinant proteins according to the manufacturer’s protocol, we added one part mixture to three parts buffer in a 50-μL reaction buffer to a final concentration of 50 mM Tris-HCl (pH 7.5), 100 mM NaCl, and 0.05% polysorbate 20. We dissolved pigments in 80% acetone, and we added 0.8 μL of the acetone solution (375 µM substrate stock) to the reaction buffer. We used chlorophyll a, chlorophyllide a, and chlorophyll b (300 pmol) for the analyses (6 µM final concentration for standard assay). When we used PSI particles or LHCII as the substrate for the Mg-dechelatase assay, we diluted these translation solutions twice in 50 μL of reaction buffer to a final concentration of 50 mM Tris-HCl (pH 7.5), 100 mM NaCl, and 0.05% polysorbate 20; we added 1 μL of solution containing PSI particles or LHCII to the reaction buffer. For every reaction, we used 200 pmol of chlorophyll a in PSI particle or LHCII. We incubated the mixtures at 25°C in the dark for 60 min. In the case of SGRL incubated with chlorophyll a and chlorophyllide a, the incubation time was 15 min. We added 9 volumes of acetone after the reaction. After centrifugation at 21,600g for 15 min at 4°C, we analyzed the pigments with HPLC. All reported chlorophyll quantities are the mean values of three independent samples.
Pigment Analysis
We ground the leaves in pure acetone stored at −30°C using a Shake Master homogenizer (Biomedical Science) cooled in liquid nitrogen (Hu et al., 2013). We harvested Synechococcus cells by centrifugation at 4°C. We disrupted the cells in pure methanol stored at 4°C, with a Shake Master homogenizer cooled in liquid nitrogen. We separated the pigments on a Symmetry C8 column (150 × 4.6 mm; Waters). We analyzed the pigments extracted from the plants using the solvent (methanol/acetonitrile/acetone = 1:2:1 [v/v]) at the flow rate of 1.0 mL/min. We analyzed the pigments extracted from the reaction mixture according to Zapata et al. (2000). We monitored the elution profiles with a diode array detector (SPD-M10AVP; Shimadzu) and a fluorescence detector monitoring 680-nm fluorescence with 410-nm excitation (RF-20A; Shimadzu).
SDS-PAGE and Immunoblot Analysis
To extract all the proteins from the leaf tissue, we ground the leaf tissue in liquid nitrogen and homogenized it in 20 volumes (v/w) of protein extraction buffer containing 50 mM Tris-HCl (pH 8.0), 12% (w/v) sucrose, 2% (w/v) lithium lauryl sulfate, and 1.5% (w/v) DTT. We denatured the samples at 90°C for 2 min; then, we mixed the samples with an equal volume of urea lysis buffer containing 10 mM Tris-HCl (pH 8.0), 10% (w/v) sucrose, 2% (w/v) SDS, 1 mM ethylenediaminetetraacetic acid, 4 mM DTT, a small amount of bromophenol blue, and 10 M urea; finally, we centrifuged the samples at 21,600g for 5 min at 25°C. We harvested the Synechococcus cells by centrifugation at 4°C. We resuspended the pellets in the aforementioned protein extraction buffer. We disrupted the resuspended cells (300 µL) by vigorous shaking with glass beads (200 mg, 0.1 mm in diameter; M&S Instruments) using a Shake Master homogenizer for 5 min at 4°C. We denatured the samples at 90°C for 2 min and then centrifuged them at 21,600g for 5 min at 25°C. We determined protein concentrations using a Bradford Ultra Kit (Expedeon) with BSA (Sigma-Aldrich) as the protein standard. We subjected proteins to SDS-PAGE with a polyacrylamide gel (14%) containing 4 M urea. After electrophoresis, we transferred the proteins to polyvinylidene difluoride membranes. We normalized samples by their fresh weight for Arabidopsis and the volume of reaction mixture for recombinant proteins. We analyzed 5 μg of Synechococcus protein. We stained proteins with a Quick-CBB kit (Wako Chemicals). We purchased antibodies against the D1 (Arabidopsis D1 protein, C-terminal, AS05084, Lot 1207), D2 (Arabidopsis D2 protein, AS06146100), CP47 (Arabidopsis CP47, AS04038), Lhca1 (Arabidopsis Lhca1 protein, AS01005, Lot 0512), Lhcb1 (Arabidopsis Lhcb1 protein, AS01004, Lot 1501), and cytochrome b6f (Arabidopsis Cytb6 protein N-terminal, AS03034, Lot 0612) complex proteins from Agrisera. We purchased monoclonal antibodies against FLAG-tag from Sigma-Aldrich (F1804, Lot SLBK1346V). We purchased monoclonal antibodies against GFP from Roche (11814460001, Lot 12600500). We prepared the anti-CP1 (PsaA/PsaB) and anti-CP43 antibodies as previously described (Tanaka et al., 1991). We raised anti-SGR1 antibodies against peptides corresponding to residues GPLWEAVSPDGHKTETLPE of the Arabidopsis SGR1 protein.
RNA Isolation and Quantitative Real-Time PCR
We extracted total RNA from leaf tissues using the RNeasy Mini Kit (Qiagen) according to the manufacturer’s instructions. We synthesized the cDNA using the PrimeScriptRT reagent kit with gDNA eraser (TaKaRa). We performed quantitative real-time PCR using gene-specific primers as listed in Supplemental Table 1, the iQ SYBR Green Supermix (Bio-Rad), and a MyiQ2 Two-Color Real-Time PCR Detection System (Bio-Rad). We obtained the data using the iQ5 Optical System software (Bio-Rad).
Purification of Recombinant SGRL-FLAG
We synthesized recombinant SGRL with a FLAG-tag (SGRL-FLAG) using wheat germ expression system. We diluted translation solutions containing expressed SGRL-FLAG five times in 0.5 mL of buffer containing 50 mM Tris-HCl (pH 7.5) and 100 mM NaCl and were then incubated with FLAG antibody-linked magnetic beads (Wako Chemicals) using a rotator for 15 min at 20°C. We washed the beads four times with buffer containing 50 mM Tris-HCl (pH 7.5), 100 mM NaCl, and 0.05% polysorbate 20. We eluted SGRL-FLAG by incubation with buffer containing 50 mM Tris-HCl (pH 7.5), 100 mM NaCl, 0.05% polysorbate 20, and 1 mg/mL DYKDDDDK peptide for 15 min at 20°C. We detected purified SGRL-FLAG using gel electrophoresis followed by silver staining.
Spectroscopy of Low-Temperature Chlorophyll Fluorescence
We used a fluorescence spectrometer to measure the fluorescence spectra of leaves emitted at 77K (F-2500; Hitachi). The excitation wavelength was 440 nm. We normalized fluorescence intensities to an emission intensity of 690 nm.
Electrolyte Leakage
We measured electrolyte leakage in excised leaves before or after DEX treatment as previously described (Shimoda et al., 2012). We performed more than five replicates for each assay.
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under the following accession numbers: SGR1 (AT4G22920), SGR2 (AT4G11910), and SGRL (AT1G44000).
Supplemental Data
Supplemental Figure 1. Expression of recombinant proteins.
Supplemental Figure 2. In-line absorption spectra of pheophytin a and pheophorbide a.
Supplemental Figure 3. Mg-dechelating activity and substrate specificity of recombinant SGR2.
Supplemental Figure 4. Mg-dechelating activity of purified SGRL.
Supplemental Figure 5. SGR1 expression in Synechococcus.
Supplemental Figure 6. SGR2 overexpression and induction in Arabidopsis.
Supplemental Figure 7. SGRL overexpression and induction in Arabidopsis.
Supplemental Figure 8. Low-temperature fluorescence spectroscopy of SGR1-induced leaves.
Supplemental Figure 9. Electrolyte leakage of SGR1-induced leaves.
Supplemental Table 1. Primers used in this study.
Supplemental Data Set 1. Raw data of HPLC analysis of pigments.
Supplementary Material
Acknowledgments
We thank A. Takabayashi, Y. Akiyama, and K. Matsuda for their useful comments on this study. The Ministry of Education, Culture, Sports, Science, and Technology, Japan, supported this work with a Grant-in-Aid for Scientific Research 15H04381 to A.T. We thank CSIRO, Max-Planck-Gesellschaft zur Forderung der Wissenschaften c.V. (MPG), and Ian Moore of the University of Oxford for providing the pOpOff vector.
AUTHOR CONTRIBUTIONS
Y.S., H.I., and A.T. designed the research. Y.S. performed the research. Y.S., H.I., and A.T. analyzed the data. A.T. wrote the article.
Glossary
- DEX
dexamethasone
References
- Armstead I., et al. (2007). Cross-species identification of Mendel’s I locus. Science 315: 73. [DOI] [PubMed] [Google Scholar]
- Chidgey J.W., Linhartová M., Komenda J., Jackson P.J., Dickman M.J., Canniffe D.P., Koník P., Pilný J., Hunter C.N., Sobotka R. (2014). A cyanobacterial chlorophyll synthase-HliD complex associates with the Ycf39 protein and the YidC/Alb3 insertase. Plant Cell 26: 1267–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christ B., Hörtensteiner S. (2013). Mechanism and significance of chlorophyll breakdown. J. Plant Growth Regul. 33: 4–20. [Google Scholar]
- Costa M.L., Civello P.M., Chaves A.R., Martínez G.A. (2002). Characterization of Mg-dechelatase activity obtained from Fragaria × ananassa fruit. Plant Physiol. Biochem. 40: 111–118. [Google Scholar]
- Craft J., Samalova M., Baroux C., Townley H., Martinez A., Jepson I., Tsiantis M., Moore I. (2005). New pOp/LhG4 vectors for stringent glucocorticoid-dependent transgene expression in Arabidopsis. Plant J. 41: 899–918. [DOI] [PubMed] [Google Scholar]
- Hellens R.P., Edwards E.A., Leyland N.R., Bean S., Mullineaux P.M. (2000). pGreen: a versatile and flexible binary Ti vector for Agrobacterium-mediated plant transformation. Plant Mol. Biol. 42: 819–832. [DOI] [PubMed] [Google Scholar]
- Hirashima M., Tanaka R., Tanaka A. (2009). Light-independent cell death induced by accumulation of pheophorbide a in Arabidopsis thaliana. Plant Cell Physiol. 50: 719–729. [DOI] [PubMed] [Google Scholar]
- Holzwarth A.R., Müller M.G., Reus M., Nowaczyk M., Sander J., Rögner M. (2006). Kinetics and mechanism of electron transfer in intact photosystem II and in the isolated reaction center: pheophytin is the primary electron acceptor. Proc. Natl. Acad. Sci. USA 103: 6895–6900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie Y., Ito H., Kusaba M., Tanaka R., Tanaka A. (2009). Participation of chlorophyll b reductase in the initial step of the degradation of light-harvesting chlorophyll a/b-protein complexes in Arabidopsis. J. Biol. Chem. 284: 17449–17456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hörtensteiner S. (2006). Chlorophyll degradation during senescence. Annu. Rev. Plant Biol. 57: 55–77. [DOI] [PubMed] [Google Scholar]
- Hörtensteiner S. (2009). Stay-green regulates chlorophyll and chlorophyll-binding protein degradation during senescence. Trends Plant Sci. 14: 155–162. [DOI] [PubMed] [Google Scholar]
- Hörtensteiner S., Kräutler B. (2011). Chlorophyll breakdown in higher plants. Biochim. Biophys. Acta 1807: 977–988. [DOI] [PubMed] [Google Scholar]
- Hu X., Tanaka A., Tanaka R. (2013). Simple extraction methods that prevent the artifactual conversion of chlorophyll to chlorophyllide during pigment isolation from leaf samples. Plant Methods 9: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X., Makita S., Schelbert S., Sano S., Ochiai M., Tsuchiya T., Hasegawa S.F., Hörtensteiner S., Tanaka A., Tanaka R. (2015). Reexamination of chlorophyllase function implies its involvement in defense against chewing herbivores. Plant Physiol. 167: 660–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusaba M., Ito H., Morita R., Iida S., Sato Y., Fujimoto M., Kawasaki S., Tanaka R., Hirochika H., Nishimura M., Tanaka A. (2007). Rice NON-YELLOW COLORING1 is involved in light-harvesting complex II and grana degradation during leaf senescence. Plant Cell 19: 1362–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y.-P., Lee T.Y., Tanaka A., Charng Y.Y. (2014). Analysis of an Arabidopsis heat-sensitive mutant reveals that chlorophyll synthase is involved in reutilization of chlorophyllide during chlorophyll turnover. Plant J. 80: 14–26. [DOI] [PubMed] [Google Scholar]
- Meguro M., Ito H., Takabayashi A., Tanaka R., Tanaka A. (2011). Identification of the 7-hydroxymethyl chlorophyll a reductase of the chlorophyll cycle in Arabidopsis. Plant Cell 23: 3442–3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickelsen J., Rengstl B. (2013). Photosystem II assembly: from cyanobacteria to plants. Annu. Rev. Plant Biol. 64: 609–635. [DOI] [PubMed] [Google Scholar]
- Park S.Y., et al. (2007). The senescence-induced staygreen protein regulates chlorophyll degradation. Plant Cell 19: 1649–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruzinská A., Tanner G., Anders I., Roca M., Hörtensteiner S. (2003). Chlorophyll breakdown: pheophorbide a oxygenase is a Rieske-type iron-sulfur protein, encoded by the accelerated cell death 1 gene. Proc. Natl. Acad. Sci. USA 100: 15259–15264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren G., An K., Liao Y., Zhou X., Cao Y., Zhao H., Ge X., Kuai B. (2007). Identification of a novel chloroplast protein AtNYE1 regulating chlorophyll degradation during leaf senescence in Arabidopsis. Plant Physiol. 144: 1429–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodoni S., Muhlecker W., Anderl M., Krautler B., Moser D., Thomas H., Matile P., Hortensteiner S. (1997). Chlorophyll breakdown in senescent chloroplasts (cleavage of pheophorbide a in two enzymic steps). Plant Physiol. 115: 669–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saga Y., Tamiaki H. (2012). Demetalation of chlorophyll pigments. Chem. Biodivers. 9: 1659–1683. [DOI] [PubMed] [Google Scholar]
- Sakuraba Y., Park S.-Y., Kim Y.-S., Wang S.-H., Yoo S.-C., Hörtensteiner S., Paek N.-C. (2014). Arabidopsis STAY-GREEN2 is a negative regulator of chlorophyll degradation during leaf senescence. Mol. Plant 7: 1288–1302. [DOI] [PubMed] [Google Scholar]
- Sakuraba Y., Schelbert S., Park S.-Y., Han S.-H., Lee B.-D., Andrès C.B., Kessler F., Hörtensteiner S., Paek N.-C. (2012). STAY-GREEN and chlorophyll catabolic enzymes interact at light-harvesting complex II for chlorophyll detoxification during leaf senescence in Arabidopsis. Plant Cell 24: 507–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y., Morita R., Nishimura M., Yamaguchi H., Kusaba M. (2007). Mendel’s green cotyledon gene encodes a positive regulator of the chlorophyll-degrading pathway. Proc. Natl. Acad. Sci. USA 104: 14169–14174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schelbert S., Aubry S., Burla B., Agne B., Kessler F., Krupinska K., Hörtensteiner S. (2009). Pheophytin pheophorbide hydrolase (pheophytinase) is involved in chlorophyll breakdown during leaf senescence in Arabidopsis. Plant Cell 21: 767–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoda Y., Ito H., Tanaka A. (2012). Conversion of chlorophyll b to chlorophyll a precedes magnesium dechelation for protection against necrosis in Arabidopsis. Plant J. 72: 501–511. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Kunieda T., Murai F., Morioka S., Shioi Y. (2005). Mg-dechelation activity in radish cotyledons with artificial and native substrates, Mg-chlorophyllin a and chlorophyllide a. Plant Physiol. Biochem. 43: 459–464. [DOI] [PubMed] [Google Scholar]
- Tanaka A., Yamamoto Y., Tsuji H. (1991). Formation of chlorophyll-protein complexes during greening 2. Redistribution of chlorophyll among apoproteins. Plant Cell Physiol. 32: 195–204. [Google Scholar]
- Tanaka R., Tanaka A. (2007). Tetrapyrrole biosynthesis in higher plants. Annu. Rev. Plant Biol. 58: 321–346. [DOI] [PubMed] [Google Scholar]
- Tsuchiya T., Ohta H., Okawa K., Iwamatsu A., Shimada H., Masuda T., Takamiya K. (1999). Cloning of chlorophyllase, the key enzyme in chlorophyll degradation: finding of a lipase motif and the induction by methyl jasmonate. Proc. Natl. Acad. Sci. USA 96: 15362–15367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Shen Y., Ryde U. (2009). QM/MM study of the insertion of metal ion into protoporphyrin IX by ferrochelatase. J. Inorg. Biochem. 103: 1680–1686. [DOI] [PubMed] [Google Scholar]
- Wielopolska A., Townley H., Moore I., Waterhouse P., Helliwell C. (2005). A high-throughput inducible RNAi vector for plants. Plant Biotechnol. J. 3: 583–590. [DOI] [PubMed] [Google Scholar]
- Wu S., et al. (2016). NON-YELLOWING2 (NYE2), a close paralog of NYE1, plays a positive role in chlorophyll degradation in Arabidopsis. Mol. Plant 9: 624–627. [DOI] [PubMed] [Google Scholar]
- Yamasato A., Nagata N., Tanaka R., Tanaka A. (2005). The N-terminal domain of chlorophyllide a oxygenase confers protein instability in response to chlorophyll B accumulation in Arabidopsis. Plant Cell 17: 1585–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapata M., Rodríguez F., Garrido J.L. (2000). Separation of chlorophylls and carotenoids from marine phytoplankton: a new HPLC method using a reversed phase C8 column and pyridine-containing mobile phases. Mar. Ecol. Prog. Ser. 195: 29–45. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.