Mitochondrial mutations confer resistance towards cellulose inhibitors, indicating communication between mitochondria and the cell wall to cope with stresses that affect cell wall integrity.
Abstract
Because the plant cell wall provides the first line of defense against biotic and abiotic assaults, its functional integrity needs to be maintained under stress conditions. Through a phenotype-based compound screening approach, we identified a novel cellulose synthase inhibitor, designated C17. C17 administration depletes cellulose synthase complexes from the plasma membrane in Arabidopsis thaliana, resulting in anisotropic cell elongation and a weak cell wall. Surprisingly, in addition to mutations in CELLULOSE SYNTHASE1 (CESA1) and CESA3, a forward genetic screen identified two independent defective genes encoding pentatricopeptide repeat (PPR)-like proteins (CELL WALL MAINTAINER1 [CWM1] and CWM2) as conferring tolerance to C17. Functional analysis revealed that mutations in these PPR proteins resulted in defective cytochrome c maturation and activation of mitochondrial retrograde signaling, as evidenced by the induction of an alternative oxidase. These mitochondrial perturbations increased tolerance to cell wall damage induced by cellulose deficiency. Likewise, administration of antimycin A, an inhibitor of mitochondrial complex III, resulted in tolerance toward C17. The C17 tolerance of cwm2 was partially lost upon depletion of the mitochondrial retrograde regulator ANAC017, demonstrating that ANAC017 links mitochondrial dysfunction with the cell wall. In view of mitochondria being a major target of a variety of stresses, our data indicate that plant cells might modulate mitochondrial activity to maintain a functional cell wall when subjected to stresses.
INTRODUCTION
Cellulose is a main component of the plant cell wall, consisting of a linear chain of several hundred to many thousands of β-1,4-linked d-glucose units (McFarlane et al., 2014). Its synthesis is achieved through a plasma membrane-localized protein complex referred to as the cellulose synthase complex (CSC) that has a hexameric rosette-like structure (Endler and Persson, 2011). Each of the six lobes is thought to contain three distinct cellulose synthase catalytic subunits (CESAs) corresponding to CESA1, CESA3, and CESA6 (or CESA6-like proteins CESA2, CESA5, and CESA9) in the primary cell wall (Desprez et al., 2007; Persson et al., 2007; Vandavasi et al., 2016).
The activity of the CSC is modulated by abiotic and biotic stresses. For example, when exposed to osmotic stress, plants downregulate their cellulose production through the depletion of CSCs from the plasma membrane (Gutierrez et al., 2009; Lei et al., 2015). A similar depletion can be triggered by small molecules produced by pathogens, such as thaxtomin A, a potent inhibitor of cellulose biosynthesis produced by the plant pathogen Streptomyces scabies responsible for the scab disease (Crowell et al., 2009). Recently, a previously unidentified CSC inhibitor, acetobixan, was isolated from small molecule secretions derived from a library of switchgrass endophytes (Xia et al., 2014).
Because the cell wall is a key feature of plant cells, dedicated systems have evolved to monitor its integrity and to trigger changes in its composition and structure through tightly controlled enzymatic modifications and shifts in cellular metabolism (Hamann, 2015). Cell wall damage induces a wide range of responses, including ectopic lignin deposition, activation of jasmonate and ethylene signaling pathways, and upregulation of stress response genes (Caño-Delgado et al., 2000; Ellis and Turner, 2001; Ellis et al., 2002; Caño-Delgado et al., 2003). Several proteins have been identified to control these responses (Wolf et al., 2012). Among these, THESEUS1 (THE1) is one of the best-studied components, belonging to the family of the Catharanthus roseus receptor-like kinases. THE1 was originally identified in a screen for the suppression of the elongation defect in the cellulose-deficient mutant cesa6/procuste1. Depletion of THE1 partially restores growth inhibition and attenuates ectopic lignification of cellulose-deficient mutants, but fails to rescue the cellulose deficiency (Hématy et al., 2007).
In addition to the cell wall, mitochondria have been considered to be the target of a variety of stresses (Bartoli et al., 2004; Giraud et al., 2008). Generally, stresses can trigger the accumulation of reactive oxygen species (ROS), which in turn result in oxidative damage to mitochondria (Fujita et al., 2006; Gechev et al., 2006). Apart from being a target of ROS, mitochondria are a major source of cellular ROS (Rhoads et al., 2006). Mitochondria possess a large number of cellular enzymatic systems that catalyze the oxidation of various substrates and generate the reducing equivalents to reduce the pyridine and flavin nucleotides NAD and FAD. Reduced NAD and FAD in turn are oxidized by coenzyme Q in reactions catalyzed by several enzyme complexes located in the inner membrane of mitochondria. Ultimately, the flux of electrons from substrates through various redox carriers and centers is terminated in a four-electron reduction of molecular oxygen to water, catalyzed by the cytochrome c oxidase (Starkov, 2008). Stress-induced inhibition of the various mitochondrial electron transport protein complexes might result in an increase in the nonenzymatic single-electron reduction of oxygen, converting it into superoxide, a progenitor ROS (Starkov, 2008; Millar et al., 2011; Huang et al., 2016). Although many mitochondrial mutants exhibit increased ROS levels (Zsigmond et al., 2008; Liu et al., 2010; He et al., 2012; Yang et al., 2014; Zhu et al., 2014), a recent study reported that inhibiting mitochondrial activity might play a protective role to prevent the production of mitochondrial ROS and diminish the ROS-induced damage (Wu et al., 2015). Moreover, perturbing mitochondrial functions activates signaling cascades from this organelle to the nucleus, resulting in the modulation of gene expression (Rhoads and Subbaiah, 2007; Ng et al., 2014; Huang et al., 2016). In plants, several signaling components have been identified for this process, which include the cyclin-dependent kinase E1 (CDKE1), as well as the transcription factors ABSCISIC ACID INSENSITIVE4, WRKY40, NO APICAL MERISTEM/ARABIDOPSIS TRANSCRIPTION FACTOR/CUP-SHAPED COTYLEDON013 (ANAC013), and ANAC017 (Giraud et al., 2009; De Clercq et al., 2013; Ng et al., 2013b; Ng et al., 2013a; Van Aken et al., 2013). The depletion of either ANAC013 or ANAC017 affects mitochondrial retrograde signaling, resulting in plants being hypersensitive to abiotic stresses (De Clercq et al., 2013; Ng et al., 2013a), which highlights the importance of mitochondria in stress adaptation. Comparative analysis shows that among related NACs, ANAC017 is almost solely responsible for transcript induction of mitochondrial retrograde marker genes after chemical inhibition of organelle function (Van Aken et al., 2016).
Despite the crucial role of the plant mitochondria and the cell wall in stress response, it is unclear whether these two compartments are functionally linked. Here, we identify a CSC inhibitor named C17 that reduces cellulose production through perturbation of CSC activity. Surprisingly, C17-induced growth defects can be suppressed by perturbation of mitochondrial activity and by activation of mitochondrial retrograde signaling, indicating that modulation of mitochondrial activity might be required for the maintenance of functional cell walls.
RESULTS
C17 Interferes with Cytokinesis during Mitotic Division
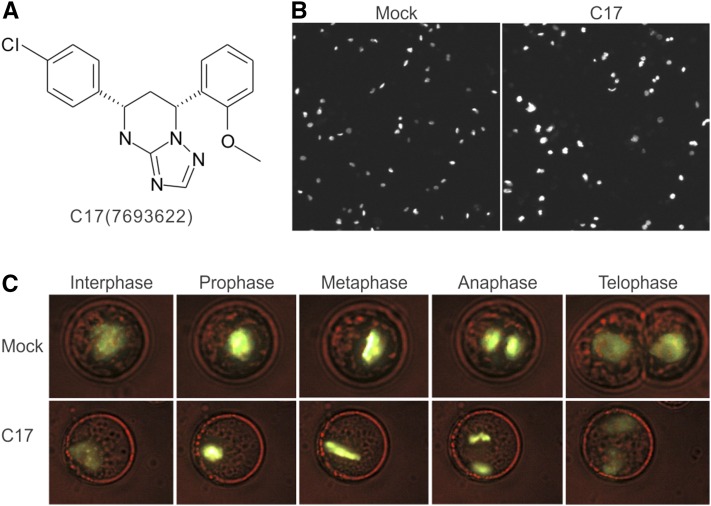
Plants are vulnerable to environmental stress conditions that interfere with a series of physiological processes, ultimately resulting in a cell cycle exit and growth inhibition. Compounds that interfere with cell division can be identified by assessment of intracellular DNA accumulation. To screen for novel cell division-interfering compounds, a high-throughput chemical screen was performed using an Arabidopsis thaliana cell suspension line producing a translational fusion between the Arabidopsis Histone 2B and Yellow Fluorescent Protein genes (H2B-YFP). Because H2B-YFP associates with chromatin and is fluorescently marked, DNA accumulation can be indirectly measured as enhanced YFP fluorescence. The H2B-YFP cells were treated with a chemical library of 12,000 organic molecules (DIVERSet; ChemBridge). Three compounds were found to induce polyploidy within 72 h of treatment, of which the synthetic molecule C17 (5-(4-chlorophenyl)-7-(2-methoxyphenyl)-1,5,6,7-tetrahydro-[1,2,4]triazolo[1,5-a] pyrimidine; ChemBridge, catalogue no. 7693622) was the most effective (Figure 1A). C17-treated cells showed larger nuclei and higher YFP fluorescence (Figure 1B). Through time-lapse imaging, C17 was found to inhibit cytokinesis without affecting mitosis, thus leading to endomitosis (Figure 1C; Supplemental Movies 1 and 2).
Figure 1.
C17 Interferes with Cytokinesis.
(A) C17 chemical structure (ChemDiv, catalog no. 7693622).
(B) H2B-YFP-labeled nuclei of Arabidopsis suspension cells in absence (mock) or presence (C17) of 50 µM C17 for 72 h.
(C) The five main phases (interphase, prophase, metaphase, anaphase, and telophase) of mitosis of suspension cells cultivated under control conditions (mock, upper panel) or in the presence of 50 µM C17 (C17, bottom panel).
Mutations in CESA1 and CESA3 Confer C17 Tolerance
In wild-type plants (Col-0), C17 administration resulted in a dose-dependent inhibition of cotyledon expansion and root growth, accompanied by the radial swelling of the root tip, with an IC50 < 0.1 µM (Figures 2A and 2B). To gain insight into the growth inhibitory activity of C17, an EMS-based genetic screen was performed to identify mutants that display tolerance to an extreme growth-inhibitory dose of C17 (2 µM), resulting in a total of 22 C17-tolerant mutants. All 22 mutants, except for 9R and 18A1 having a slight growth penalty, were phenotypically indistinguishable from wild-type plants in the absence of C17 (Figure 2C). The C17 inhibitory activity was attenuated in these C17-tolerant mutants that, in the presence of the compound, had longer roots and bigger cotyledons compared with wild-type seedlings (Figure 2D).
Figure 2.
C17-Tolerant Mutants.
(A) Five-day-old wild-type (Col-0) seedlings grown with (0.1, 0.2, 0.5, 1, 2, or 5 µM) or without (mock) C17.
(B) Quantification of the root length of seedlings shown in (A). Data represent mean ± sd (n > 10). Statistically significant differences compared with wild-type plants in absence of C17 are indicated; *P value < 0.01 (two-tailed Student’s t test).
(C) and (D) Roots of 5-d-old wild-type (Col-0) and 22 C17-tolerant mutants grown in the absence (C) or presence (D) of 2 µM C17. Bars = 5 mm.
Based on C17 sensitivity, the segregation ratio of F2 progenies indicated that 15 mutants displayed semidominant phenotypes (1:2:1 ratio, sensitive:intermediate tolerant:tolerant), whereas seven mutants exhibited a recessive phenotype (3:1 ratio, sensitive:tolerant) (Table 1), thus indicating that C17 tolerance resulted from single-gene mutations. All mutants were crossed with the Ler-0 ecotype to generate mapping populations. Linkage analysis with 24 simple sequence length polymorphism (SSLP) markers divided the 22 mutants into two groups labeled with the name of the closest corresponding markers, CH4-14494 and CH5-512, respectively (Supplemental Figure 1). Fine mapping and genome sequencing of the 7L and 2C mutant alleles identified the causal nucleotide mutation in the CESA1 and CESA3 loci, respectively (Figures 3A and 3B). The CESA1 and CESA3 loci of the remaining C17-tolerant mutants were sequenced, revealing that all identified C17-tolerant mutants carried a single-nucleotide missense change at either CESA1 or CESA3, all resulting in an amino acid change (Table 1). Collectively, this mutant series consisted of 10 mutant alleles of CESA1 and two of CESA3 (Table 1). Protein sequence analysis showed that most mutated amino acids clustered to the transmembrane regions of the CESA proteins (Figure 3C). Furthermore, amino acid alignment of CESA1/CESA3 homologs from seven species revealed that 10 of these 12 mutated amino acids are invariant (Supplemental Figure 2).
Table 1. CESA1 and CESA3 Mutant Alleles Conferring C17 Tolerance.
| C17-Tolerant Mutants | Genetic Property | Gene Mutated | Mutation Position |
|---|---|---|---|
| 1B | Semidominant | CESA1 | A1023T |
| 3D | Semidominant | CESA1 | V297M |
| 3E, 4H, 7L, 17Y, 19B1 | Semidominant | CESA1 | A1018V |
| 3G, 9Q | Recessive | CESA1 | L872F |
| 3F | Semidominant | CESA1 | S892N |
| 9R | Recessive | CESA1 | G1013R |
| 14V | Semidominant | CESA1 | K945R |
| 14U, 17Z, 20D1 | Recessive | CESA1 | G1013E |
| 18A1 | Recessive | CESA1 | S307L |
| 20C1 | Semidominant | CESA1 | P1010L |
| 2C, 5R, 6K, 15W | Semidominant | CESA3 | S983F |
| 8P | Semidominant | CESA3 | S1037F |
Figure 3.
Mapping of Mutations Rendering C17 Tolerance.
(A) and (B) Genetic mapping and gene structure of CESA1 (A) and CESA3 (B). The cesa17l and cesa32c loci were mapped to CESA1 (AT4G32410) and CESA3 (AT5G05170), respectively. The gene structure is shown below: Exons are represented as filled rectangles, and introns are shown as lines. The nucleotide replacement in the mutant allele is indicated. Bar = 100 kb.
(C) Schematic diagram of the domains and mutation locations in CESA1 and CESA3. CESA1 and CESA3 proteins are located in the plasma membrane (PM) with eight predicted transmembrane domains (black boxes). The C17 tolerance mutations are indicated by colored marks. The corresponding amino acid changes are listed.
C17 Inhibits Cellulose Biosynthesis and Acts via Clearance of CSCs from the Plasma Membrane
C17 application inhibits hypocotyl elongation (Figures 4A and 4B) and causes a decrease in cellulose content, as measured by the amount of glucose produced through hydrolysis of cellulose (Figure 4C). Consistent with this result, mutations conferring C17 tolerance reverted the inhibition of hypocotyl elongation (Figures 4A and 4B). The effect of C17 on CESA activity was addressed through live-cell imaging using CESA3-GFP reporter plants. Compared with untreated hypocotyl epidermal cells, administration of C17 resulted in a marked reduction of the CESA3-GFP signal associated with the plasma membrane (Figure 4D). These results demonstrate that C17’s effect on cellulose is caused by a removal of the CESA complexes from the plasma membrane. Although cortical microtubules guide the movement of the CESA complexes in the plasma membrane (Crowell et al., 2009; Gutierrez et al., 2009), C17 application did not affect microtubule organization, demonstrating that the observed CESA3-GFP depletion was not due to microtubule depolymerization (Supplemental Figure 3).
Figure 4.
C17 Inhibits Cellulose Biosynthesis and Depletes CSCs from the Plasma Membrane.
(A) Hypocotyl elongation of 5-d-old dark-grown wild type (Col-0, left panel) and a C17-tolerant mutant (cesa1A1018V, right panel) in the absence (mock) or presence of C17 (0.05, 0.1, 0.2, and 0.5 µM). Bars = 0.25 mm.
(B) Quantification of the hypocotyl length of plants shown in (A). Data represent mean ± sd (n > 10). Statistically significant differences compared with wild-type plants are indicated; *P value < 0.01 (two-sided Student’s t test).
(C) Glucose content of the hypocotyl of 5-d-old dark-grown wild type in the presence of C17 (0, 0.1, and 0.2 µM). Data represent mean ± sd (n = 4). Statistically significant differences compared with wild-type plants are indicated; *P value < 0.01 (two-tailed Student’s t test).
(D) GFP-CESA3 localization at the plasma membrane in the absence (mock) or presence of 50 µM C17 for 2 h. Single optical sections and time averages of 61 frames (5-min duration in 5-s intervals) of plasma membrane-localized GFP-CESA3. Bars = 10 µm.
C17-Induced Depletion of Membrane CESAs Results in a Weaker Cell Wall
Similar to its effect on the hypocotyl, C17 treatment resulted in the depletion of the CESA complex from the plasma membrane of wild-type root cells with a dramatic drop after 10 to 15 min of C17 application (Supplemental Figure 4). Because the cellulose synthesized by the CESA1/CESA3 complex is a primary cell wall component, it was expected that C17-treated plants would display a weaker cell wall, which can be visualized by the uptake of propidium iodide (PI) following the application of a gentle pressure on the root (see Methods). Indeed, cell wall weakening was observed in the root elongation zone within 2 h after applying C17 and increased over time (Figure 5A, left panel) along with growth inhibition (Figure 5C). Likewise, a weaker cell wall could be visualized in the root elongation zone of je5 plants, which are mutant in CESA3 (Figure 5B), indicating that the phenotypes observed upon C17 treatment reflect cellulose deficiency. No PI-positive cells and growth inhibition were observed in C17-tolerant mutants (Figures 5A, right panel, and 5C).
Figure 5.
C17 Results in a Brittle Cell Wall.
(A) Representative confocal microscopy images of plants stained with PI. Four-day-old wild-type (Col-0, left panel) and C17-tolerant mutant (cesa1A1018V, right panel) seedlings were treated with 200 nM C17 for 0, 1, 2, or 3 h and roots were collected and stained with PI. The broken cells with brittle cell walls were visualized by the uptake of PI. Bars = 50 µm.
(B) Representative confocal microscopy images of 4-d-old wild-type and je5 mutant seedlings (with a weak allele of CESA3) stained with PI. Bar = 50 µm.
(C) Root growth of 4-d-old wild-type (Col-0) and C17-tolerant mutant (cesa1A108V) seedlings in the presence of 200 nM C17. Data represent mean ± sd (n > 5). Statistically significant differences compared with wild-type plants are indicated; *P value < 0.01 (two-tailed Student’s t test).
Mutations in PPR-Like Genes Counteract the Growth Inhibition Induced by Cellulose Deficiency
To isolate putative components responding to CESA deficiency, a second mutagenesis screen was performed at a low dose of C17 (200 nM) using T-DNA insertion lines. At this moderate concentration of C17 root growth was inhibited instantly upon transfer to C17-containing medium (Figure 5C). Two insertion lines (SALK_017325C and SALK_020569C, hereafter referred to as cell wall maintainer1-1 [cwm1-1] and cwm2-1, respectively) exhibited significant suppression of the growth inhibition in the presence of C17 (Figures 6A and 6B).
Figure 6.
Suppression of C17 Sensitivity by the Mutations in CWM1 and CWM2.
(A) Root growth of the wild type (Col-0) and cwm1 and cwm2 mutants. Three-day-old seedlings grown on half-strength MS medium were transferred for 2 d to control medium (mock) or medium containing 200 nM C17. Arrowheads indicate the root tip position at the moment of transfer. Bars = 5 mm.
(B) Quantification of root elongation of plants after transfer. Data represent mean ± sd (n > 10). Statistically significant differences compared with wild-type plants are indicated; *P value < 0.01 (two-tailed Student’s t test).
The cwm1-1 line has a T-DNA insertion in the AT1G17630 locus, cwm2-1 in AT5G44570. Suppression of C17 sensitivity by AT1G17630 deficiency was confirmed with two independent T-DNA insertion lines (SALK_124160, cwm1-2; SALK_078133, cwm1-3) of this locus. The T-DNA inserts in these three lines are positioned in the region of AT1G17630 encoding a predicted pentatricopeptide repeat (PPR)-like protein (Supplemental Figure 5A).
Although the annotated cwm2-1 mutant (SALK_020569C) is a knockout of AT5G44570, an independent T-DNA insertion line (SAIL_699_C11) could not suppress the C17-induced growth inhibition (Supplemental Figure 5B). Furthermore, C17 tolerance in cwm2-1 was unlinked to the T-DNA insert in the AT5G44570 locus, confirming AT5G44570 is not the CWM2 gene. To isolate the CWM2 gene, we crossed cwm2-1 with the Ler ecotype and performed the positional cloning of the causal mutation. The CWM2 gene was mapped into a 171.8-kb region, between SSLP markers CH1_11687 and CH1_11859 (Supplemental Figure 5C). PCR analysis of T-DNA flanking sequences identified a second T-DNA insert positioned in the coding region of the AT1G32415 locus that also encodes a PPR-like superfamily protein. In addition to cwm2-1, the SALK_027874 line (cwm2-2) that harbors an independent T-DNA insert resulting in the depletion of the full-length mRNA transcribed from the AT1G32415 locus (Supplemental Figure 5C) also exhibits C17 tolerance (Figures 6A and 6B), thus demonstrating that AT1G32415 encodes the CWM2 protein. In addition, both cwm1 and cwm2 mutants displayed an enhanced tolerance to two other cellulose synthase inhibitors, isoxaben and indaziflam (Desprez et al., 2002; Brabham et al., 2014) (Supplemental Figure 6).
Both cwm1 and cwm2 Mutations Affect Mitochondrial Complexes
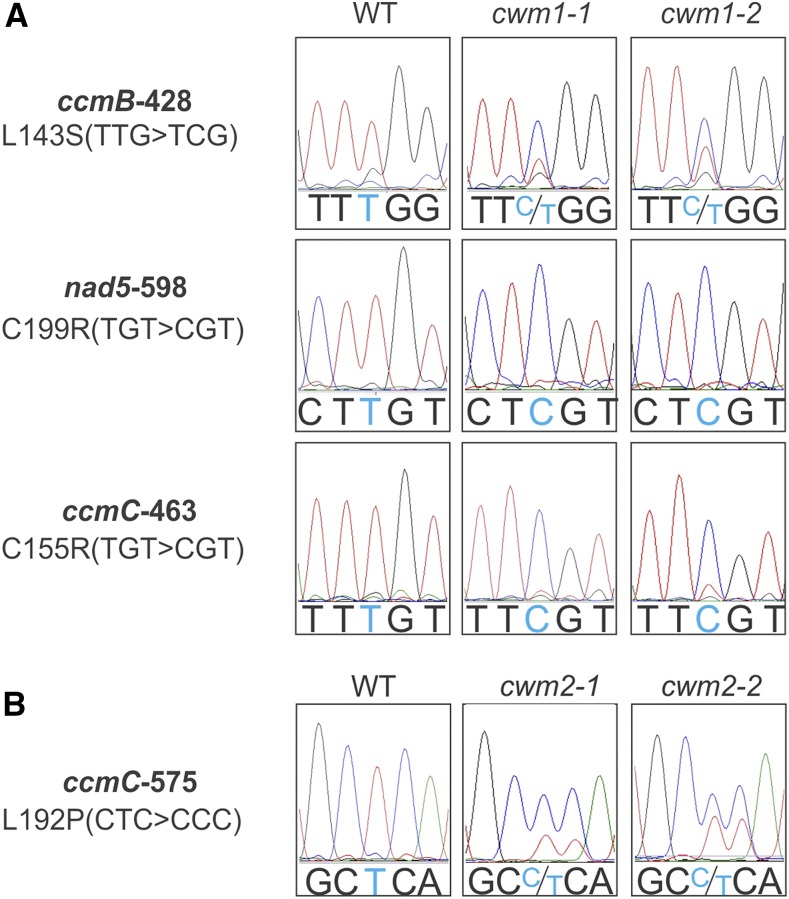
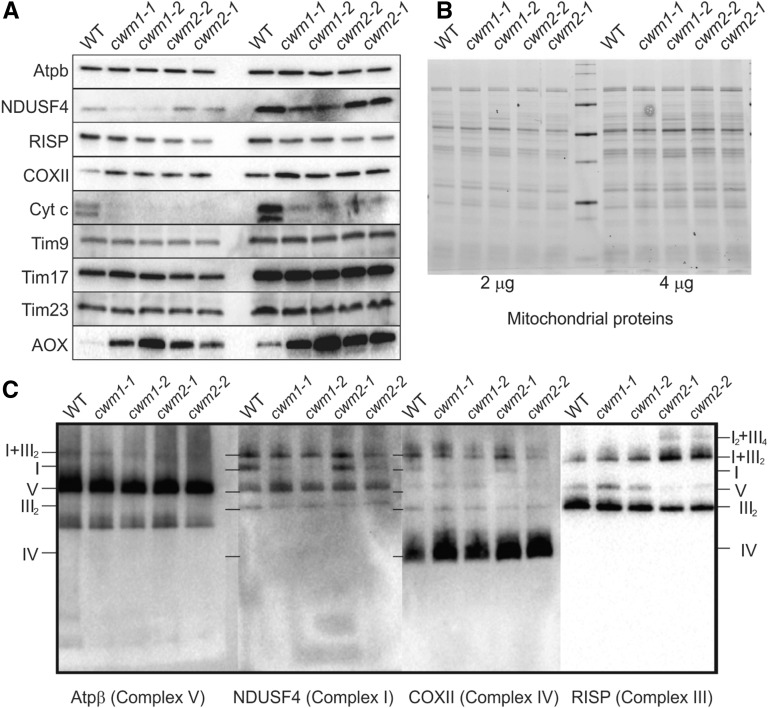
Protein domain analysis indicated that both CWM1 and CWM2 proteins contain an N-terminal mitochondrial targeting peptide, strongly suggesting that CWM1 and CWM2 are localized in the mitochondria. Since PPR proteins have been found to affect the maturation, stability, or expression of transcripts encoded in the mitochondrial genome (Barkan and Small, 2014), the mitochondrial transcriptome of the cwm1 and cwm2 mutants was sequenced. Among the transcripts from cwm1, editing defects were found in the cytochrome c maturation protein B (ccmB), ccmC, and NADH dehydrogenase subunit5 (nad5) sequences (Figure 7A), while an editing defect in the ccmC transcript was identified in cwm2 (Figure 7B). nad5 encodes a subunit of NADH dehydrogenase in mitochondrial complex I, whereas both ccmB and ccmC are required for the maturation of cytochrome c (Kranz et al., 2009; Millar et al., 2011). Consistent with this, a dramatic decrease in cytochrome c content was observed in all cwm mutants, whereas the level of the ubiquinone oxidoreductase Fe-S protein4 (NDUFS4, subunit of complex I) was affected only in the cwm1 mutants (Figures 8A and 8B). Three proteins were additionally found to display altered abundances in all cwm mutants, with cytochrome oxidase subunit II (COXII, subunit of complex IV) and alternative oxidase (AOX) being detected at higher levels, and the Rieske iron-sulfur protein (RISP, subunit of complex III) being reduced (Figures 8A and 8B). No significant change in protein abundance was observed for the beta subunit of ATP synthase (ATPβ, subunit of complex V) and three tested mitochondrial import inner membrane translocase subunits (Tim9, Tim17, and Tim23) (Figures 8A and 8B).
Figure 7.
Both CWM1 and CWM2 Are Involved in Mitochondrial RNA Editing Events.
(A) Multiple mitochondrial RNA editing defects in cwm1 mutants. Sequencing chromatograms of editing sites (ccmB-428, nad5-598, and ccmC-463) from the wild type (left panel), cwm1-1 (middle panel), and cwm1-2 (right panel) are displayed.
(B) ccmC-575 editing defect in cwm2 mutants. Sequencing chromatograms of the ccmC-575 editing site of the wild type (left panel), cwm2-1 (middle panel), and cwm2-2 (right panel) are displayed. The editing sites are marked with light blue color; amino acid changes caused by editing defects are listed on the left.
Figure 8.
Deficiency of CWM1 and CWM2 Perturbs Mitochondrial Function.
(A) Mitochondrial protein content was quantified using antibodies. Antibodies used detect ATP synthase (Atpb, subunit of complex V), ubiquinone oxidoreductase Fe-S protein4 (NDUFS4, subunit of complex I), Rieske iron-sulfur protein (RISP, subunit of complex III), cytochrome oxidase subunit II (COXII, subunit of complex IV), cytochrome c (Cyt c), AOX, and mitochondrial import inner membrane translocase subunits (Tim9, Tim17, and Tim23).
(B) Image of total mitochondrial proteins of the wild type, cwm1 mutants (cwm1-1 and cwm1-2), and cwm2 mutants (cwm2-1 and cwm2-2). Two or four micrograms of mitochondrial proteins were separated with SDS-PAGE and stained with Coomassie blue.
(C) Mitochondrial complexes in the wild type, cwm1 mutants (cwm1-1 and cwm1-2), and cwm2 mutants (cwm2-1 and cwm2-2). Mitochondrial proteins were separated with blue native polyacrylamide gel electrophoresis. Atpb antibody was used to visualize complex V, RISP for complex III, COXII for complex IV, and NDUFS4 for complex I. The identities of protein complexes are indicated on the left or right of the blots: I, complex I; IV, complex IV; V, complex V; III2, dimeric complex III; I+III2, supercomplex composed of complex I and dimeric complex III; I2+III4, a dimer of supercomplex I+III2.
To determine the abundance of assembled respiratory complexes, total membrane proteins were resolved by blue native polyacrylamide gel electrophoresis, and specific complexes were detected by probing immunoblots with antibodies against NDUFS4 (complex I), RISP (complex III), COXII (complex IV), and ATPβ (complex V) (Figure 8C). The results showed that assembled complex V accumulated to normal levels in all cwm mutants, but assembled complex IV accumulated to higher levels and assembled dimeric complex III to lower levels (Figure 8C). A clear accumulation of supercomplex I-III2 composed of complex I and a dimer of complex III and supercomplex I2III4, a dimer of I-III2 was observed in cwm2 mutants (Figure 8C). Consistent with the misedited NAD5 transcript in the cwm1 transcriptome, complex I was less abundant in these mutants (Figure 8C). Complex staining further confirmed the changes in assembled complex I and complex IV (Supplemental Figure 7).
As both cwm1 and cwm2 mutations resulted in changes in the abundance of assembled respiratory complexes, we measured the respiration rates of the mutants. Compared with wild-type seedlings, no significant difference was detected in all four cwm mutants under normal growth conditions. However, although all seedlings showed decreased respiration in the presence of potassium cyanide (KCN), which inhibits the cytochrome pathway, the cwm mutants displayed higher respiration than the wild type, indicating increased AOX capacity in all the cwm mutants (Supplemental Figure 8).
To explore whether mitochondrial defects lay at the origin of a signaling cascade that conferred C17-tolerance, we applied antimycin A (AA), a well-characterized inhibitor of mitochondrial complex III, and rotenone (RO), a complex I inhibitor, to wild-type plants. Similar to the cwm1 and cwm2 mutants, AA suppressed the C17-induced growth inhibition (Figures 9A and 9B) and reversed the brittle cell wall phenotype (Figure 9C). By contrast, RO did not confer C17 tolerance (Figures 9A and 9B). Consistent with these results, several mutants (ndufs4, bir6-2, and otp439) defective in complex I exhibited the same C17 sensitivity as wild-type plants (Supplemental Figure 9) (de Longevialle et al., 2007; Meyer et al., 2009; Koprivova et al., 2010). In conclusion, the C17 resistance phenotype of the cwm1 and cwm2 mutants can be attributed to mutations affecting mitochondrial complex III.
Figure 9.
Inhibition of Mitochondrial Complex III Phenocopies the C17-Tolerance Phenotype of cwm1 and cwm2 Mutants.
(A) Root elongation of wild-type control-treated (DMSO), 1 µM AA-treated, and 50 µM RO-treated plants. Three-day-old seedlings grown on half-strength MS medium were transferred to medium without (left panel) or with (right panel) 200 nM C17 for 2 d. Arrowheads indicate the root tip position at the moment of transfer. Bars = 5 mm.
(B) Quantification of the root elongation of plants after transfer. Data represent mean ± sd (n > 10). Statistically significant differences compared with wild-type plants in the absence of mitochondrial inhibitors are indicated; *P value < 0.01 (two-tailed Student’s t test).
(C) Representative confocal microscopy images of 4-d-old wild type (Col-0) control-treated with 0.1% DMSO (mock) or with 1 µM AA, 200 nM C17 (C17), or a combination of 1 µM AA with 200 nM C17 (C17+AA). Two-hour treated roots were stained with PI. The brittle cell wall was visualized by the uptake of PI. Bar = 100 µm.
(D) to (F) Representative spinning confocal microscopy images of 4-d-old GFP-CESA3 plants treated with 0.1% DMSO (D), 200 nM C17 (E), or a combination of 1 µM AA with 200 nM C17 (F). The images were taken at 20 min after treatment. Bars = 5 µm.
(G) Quantification of fluorescence in (D) to (F). The relative intensity is calculated by the fluorescence per unit area in the root elongation zone of each sample divided by that of the mock-treated plants. Data represent mean ± sd (n = 5). Statistically significant differences compared with wild-type plants in mock are indicated; *P value < 0.01 (two-tailed Student’s t test).
Although C17 inhibits cellulose production by depletion of CSCs from the plasma membrane, AA administration did not reverse this phenotype (Figures 9D to 9G; Supplemental Figure 10), indicating that the inhibition of mitochondrial activity did not restore CSC activity itself. Conversely, C17 had no significant effect on respiration rate, even at a concentration as high as 8 µM, ruling out the possibility that C17 directly acts on the cytochrome pathway of respiration (Supplemental Figure 11).
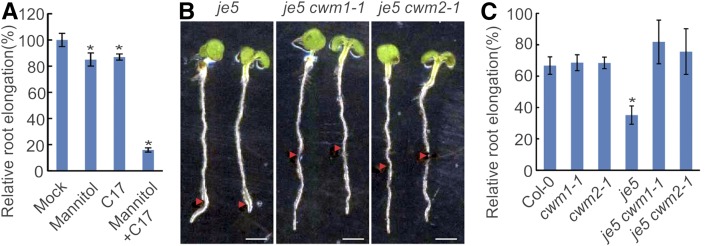
Both cwm1 and cwm2 Mutations Enhance the Tolerance of je5 against Osmotic Stress
The cell wall is anticipated to help plants to cope with environmental stimuli, such as osmotic stresses (Zhu et al., 2010; Tenhaken, 2015). Correspondingly, C17-treated plants and the je5 mutant show a severe root growth inhibition in response to mannitol (Figures 10A and 10B). Strikingly, whereas no significant difference in tolerance was observed between wild-type plants and cwm single mutants, both cwm1 and cwm2 mutations rescued the osmotic stress phenotype of the je5 mutant (Figures 10B and 10C), indicating that both cwm mutations confer a functional cell wall to the je5 mutant for growth in presence of mannitol.
Figure 10.
Both cwm1 and cwm2 Mutations Enhance the Tolerance of je5 against Osmotic Stress.
(A) Relative 2-d elongation of the wild type (Col-0) after transfer to the medium without (Mock) or with 200 mM mannitol, 100 nM C17 (C17), or the combination of 200 mM mannitol and 100 nM C17 (Mannitol+C17). Data represent mean ± sd (n > 10). Statistically significant differences compared with mock are indicated; *P < 0.01 (two-tailed Student’s t test).
(B) Root growth of je5, je5 cwm1, and je5 cwm2 mutants in presence of 250 mM mannitol. Three-day-old seedlings grown on half-strength MS medium were transferred to medium with 250 mM mannitol for 2 d. Arrowheads indicate root tip position at the moment of transfer. Bar = 2.5 mm.
(C) Relative 2-d elongation of the wild type (Col-0), cwm1-1, cwm2-1, je5, je5 cwm1-1, and je5 cwm2-1 after transfer to the medium supplemented with 250 mM mannitol. Data represent mean ± sd (n > 10). Statistically significant differences compared with wild-type plants are indicated; *P < 0.01 (two-tailed Student’s t test).
The C17 Tolerance Triggered by Dysfunctional Mitochondria Partially Depends on ANAC017
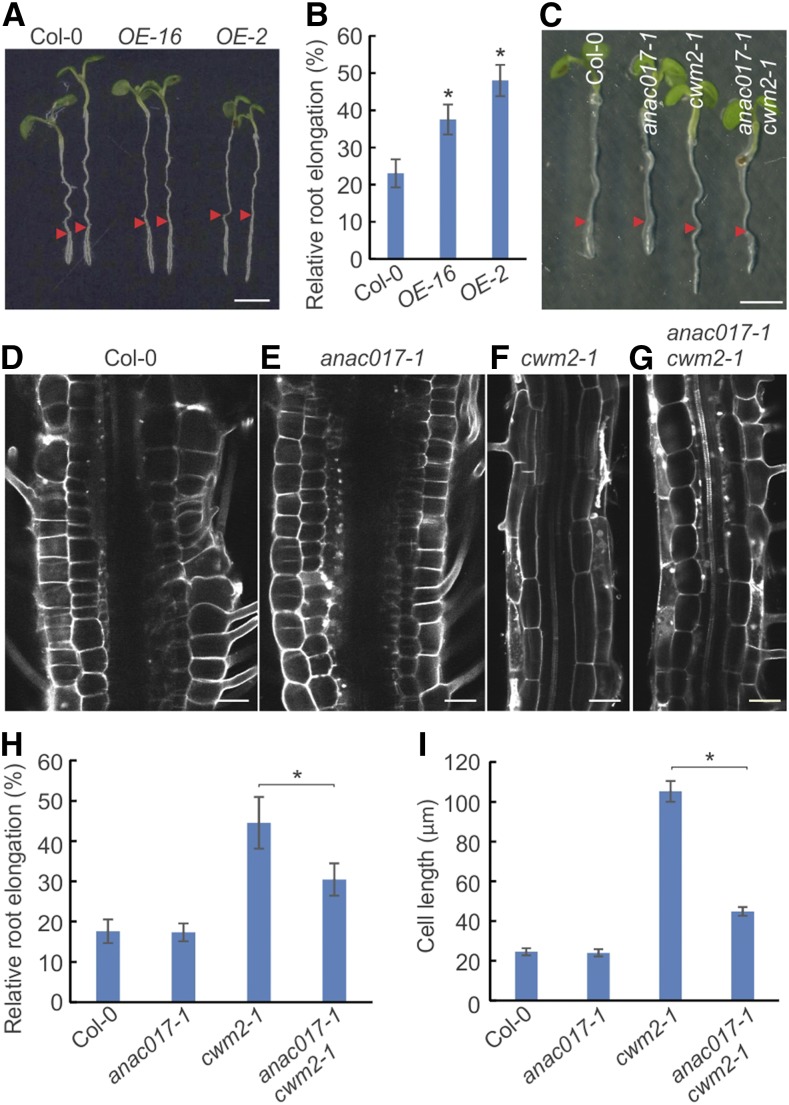
Recently, the ANAC017 transcription factor was characterized as a key regulator in the organelle signaling that links dysfunctional mitochondria and primary stress responses (Ng et al., 2013a). To test whether ANAC017-dependent signaling might contribute to the tolerance toward C17, ANAC017 overexpression plants were generated and tested for C17 sensitivity. Two independent ANAC017OE lines exhibited higher C17 tolerance than wild-type plants (Figures 11A and 11B). No difference in tolerance was observed between the wild type and ANAC017 knockout mutants (Figures 11C to 11I). Importantly, the loss of ANAC017 activity partially compromised the cwm2-1-triggered C17 tolerance, as revealed by the shorter roots and more isotropic-shaped cells of anac017-1 cwm2-1 double mutants compared with cwm2-1 single mutants. These data illustrate that ANAC017 is a signaling component linking mitochondria to the cell wall.
Figure 11.
ANAC017 Is a Component Linking Mitochondria and Cell Wall.
(A) Root growth of wild type (Col-0) and ANAC017-overexpressing lines (ANAC017OE-2 and ANAC017OE-16) in the presence of 200 nM C17. Three-day-old seedlings grown on half-strength MS medium were transferred to medium with 200 nM C17 for 2 d. Arrowheads indicate the root tip position at the moment of transfer. Bar = 2.5 mm.
(B) Relative root elongation of wild type (Col-0) and ANAC017-overexpressing lines (ANAC017OE-2 and ANAC017OE-16). Three-day-old seedlings grown on half-strength MS medium were transferred to medium without (mock) or with 200 nM C17 for 2 d. Data represent mean ± sd (n > 10).
(C) Root growth of the wild type (Col-0), ANAC017 knockout mutant (anac017-1), cwm2-1 single mutant, and cwm2-1 anac017-1 double mutant in the presence of 200 nM C17. Three-day-old seedlings grown on half-strength MS medium were transferred to medium with 200 nM C17 for 2 d. Arrowheads indicate the root tip position at the moment of transfer.
(D) to (G) Representative images of root mature zone of the wild type (Col-0; [D]), ANAC017 knockout mutant (anac017-1; [E]), cwm2-1 single mutant (F), and cwm2-1 anac017-1 double mutant (G) in the presence of 200 nM C17. Bar = 50µm.
(H) Relative root elongation of wild type (Col-0), ANAC017 knockout mutant (anac017-1), cwm2-1 single mutant, and cwm2-1 anac017-1 double mutant in the presence of 200 nM C17. Three-day-old seedlings grown on half-strength MS medium were transferred to medium without (mock) or with 200 nM C17 for 2 d. Data represent mean ± sd (n > 10).
(I) The length of mature cortical cells of the wild type (Col-0), ANAC017 knockout mutant (anac017-1), cwm2-1 single mutant, and cwm2-1 anac017-1 double mutant in the presence of 200 nM C17. Data represent mean ± sd (n > 20). Statistically significant differences are indicated; *P value < 0.01 (two-tailed Student’s t test).
DISCUSSION
Here, we report a CSC inhibitor (C17) severely affecting plant growth and development at the nanomolar level. C17-triggered growth inhibition can be overcome by a mutation in CESA1 or CESA3 without any growth penalty. Although structurally different from known CSC-inhibiting compounds, including isoxaben, thaxtomin, and quinoxyphen, C17 also triggers a rapid loss of CSCs from the plasma membrane and the inhibition of cellulose accumulation (Figure 4). The resulting cell wall is weakened, as shown by its rupture upon application of slight pressure on the surface of the organ (Figure 5). Mutations in CESA1 or CESA3 rescue this cell wall weakening, suggesting a causal link between C17, reduced CESA activity, and the weakening of the cell wall. A role as inhibitor of primary cell wall biosynthesis likely explains the ploidy-inducing effect of C17 in cell cultures, which arises because of impaired cytokinesis. During cytokinesis, the cell rapidly lays down a new cross wall, which requires cellulose. Cellulose and CESA1, CESA3, and CESA6 accumulate in the developing cell plate that forms during cytokinesis (Miart et al., 2014). Correspondingly, the cesa1rsw1-20 mutation results in incomplete cell plates (Beeckman et al., 2002).
The mechanism underlying the plasma membrane depletion of CSCs triggered by C17 remains unclear. This depletion is not an indirect consequence of cortical microtubule depolymerization. All 12 C17-tolerant EMS-induced mutant alleles encode an amino acid change in either CESA1 or CESA3, always located in or near the transmembrane spanning region (Figure 3C). Although the mutated amino acids are distributed over different transmembrane domains, it might be that all mutated amino acids cluster together. The amino acid changes in C17-tolerant mutants might either directly or indirectly alter the interaction between C17 and CSCs.
In addition to mutant cesa1 and cesa3 alleles, two mutant ppr-like genes (cwm1 and cwm2) were found to confer C17 tolerance (Figure 6). In recent years, a number of mitochondrial PPR-like proteins have been characterized and found to participate in virtually all posttranscriptional processes such as RNA editing, RNA splicing, and transcript processing (Schmitz-Linneweber and Small, 2008). Some are involved in RNA editing of single sites (Takenaka, 2010; Takenaka et al., 2010), and others control multiple sites (Zehrmann et al., 2009; Bentolila et al., 2010; Sung et al., 2010; Verbitskiy et al., 2010). Both CWM1 and CWM2 play a role in mitochondrial RNA editing: We found CWM1 to control three specific editing sites (ccmB, ccmC, and nad5), whereas CWM2 controls a single site (ccmC) (Figure 7). The cwm1 and cwm2 mutants have several common mitochondrial characteristics: reduced cytochrome c content, mutated ccmC, and altered assembly of complex III and increased abundance of complex IV (Figure 8), demonstrating that CWM1 or CWM2 deficiency causes dysfunctional mitochondria. This is further supported by an increased abundance of AOX, which is a hallmark for diverse mitochondrial dysfunctions (Vanlerberghe, 2013). The most likely explanation for the defective complex III might be the altered cytochrome c maturation machinery, which is needed to attach the heme to both the cytochrome c1 in complex III, as well to the soluble cytochrome c (Mavridou et al., 2013). The increased abundance of complex IV is interesting because the same changes do not occur in a partial loss-of-function cytochrome c mutant (Welchen et al., 2012). These mutants display a reduction in cytochrome c levels (Welchen et al., 2012). In our case, both CWM1 and CWM2 encode editing proteins required for the maturation of cytochrome c, rather than the components in mitochondrial complexes. This may indicate that the signals resulting from a lack of a structural gene differ from those preventing assembly of a functional complex.
C17 tolerance of both cwm1 and cwm2 mutants may be attributed to the decreased activity of mitochondrial complex III. In support of this hypothesis, administration of the mitochondrial complex III inhibitor AA partially reverses the C17 sensitivity phenotype (Figure 9A). AA blocks the Qi site and loss of the heme on cytochrome c1 would block the final electron transfer to cytochrome c. Both would leave the Qo site active, which is linked to ROS signaling (Bleier and Dröse, 2013). There is likely some specific ROS production at the blocked complex III, resulting in retrograde signaling that leads to C17 tolerance. Contrastingly, the complex I mutants (ndufs4, bir6-2, and otp439) and the chemical inhibitor RO could not confer C17 tolerance to plants, suggesting that a defective mitochondrial complex I in cwm1 mutants is not linked to C17 tolerance. These observations exclude the possibility that the alleviated deleterious effects in cwm mutants are caused by reduced growth as a result of reduced respiration with dysfunctional mitochondria. Although we cannot rule out the possibility that C17 acts on mitochondria, it seems unlikely because a high dosage of C17 did not cause inhibition of the cytochrome pathway of respiration (Supplemental Figure 11). Additionally, the increased tolerance of cwm1 and cwm2 toward two other cellulose synthase inhibitors emphasizes that cwm1 and cwm2 mutations counteract the growth inhibition induced by cellulose deficiency rather than other effects triggered by C17.
Perturbing mitochondria alters nuclear gene expression via organelle signaling regulation (Rhoads and Subbaiah, 2007; Ng et al., 2014). Organelle signaling is active in the cwm1 and cwm2 mutants, as marked by AOX accumulation, and might mediate a crosstalk between the mitochondria and the cell wall. This hypothesis is supported by the observation that ANAC017 overexpression confers C17 tolerance, whereas ANAC017 depletion results in a partial loss of the cwm2-1-triggered C17 tolerance phenotype (Figure 11). ANAC017 is the core cellular component in mitochondrial organelle signaling, particularly for retrograde signaling, accounting for more than 85% of H2O2-mediated primary stress responses in plants (Ng et al., 2013a). Because ANAC017 is latent in the endoplasmic reticulum (ER) and activated by mitochondrial dysfunction (Ng et al., 2013a), C17 tolerance upon inhibition of mitochondrial activity might find its origin in the ER; all the cell wall-synthesizing enzymes are transported via the ER. ANAC017 deficiency only partially abolished the C17 tolerance triggered by cwm2-1 mutation. It is possible that ANAC017 functions redundantly with another ER-bound NAC transcription factor, such as ANAC013 (De Clercq et al., 2013) or ANAC017-independent organelle signaling cascades. Indeed, at least two other stress-responsive systems in the ER are known, including the activation of bZIP transcription factors by the S1P and S2P proteases and the IRE1 and IRE2 splicing systems (Deng et al., 2013; Howell, 2013; Srivastava et al., 2013).
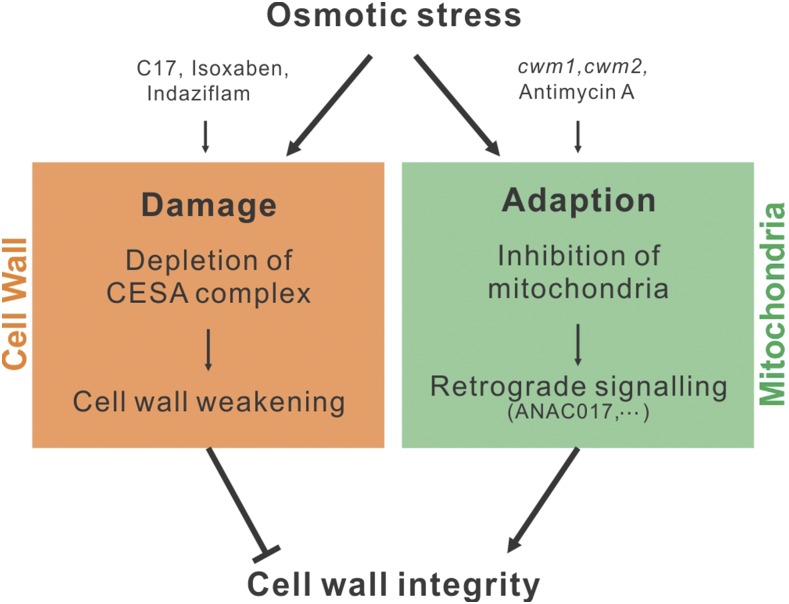
Our data suggest that the inhibition of mitochondria contributes to the maintenance of a functional cell wall under osmotic stress conditions. In agreement, CWM1 or CWM2 loss of function in cellulose-deficient plants increased the tolerance to osmotic stresses (Figure 10), indicating that the cell wall in these plants copes better with stress. Enhanced osmotic tolerance was observed only in a cellulose-deficient background, indicating that the connection between mitochondrial function and cell wall is masked in wild-type plants. Alternatively, regarding the fact that osmotic stress naturally results in decreased cellulose production by depletion of membrane-associated CESAs (Crowell et al., 2009), these changes might be dependent on the cell wall status. Taken together, our results suggest that perturbing mitochondrial activity results in attenuation of the growth inhibition that is caused by CESA depletion from the plasma membrane, likely through a change in cell wall composition. Moreover, in view of the fact that mitochondrial activity is inhibited under osmotic stresses (Skirycz et al., 2010; Vanderauwera et al., 2012), our data point to a possible mechanism underlying adaptation to osmotic stress, in which the modulation of mitochondrial activity and subsequent retrograde signaling controls the maintenance of cell wall integrity (Figure 12).
Figure 12.
Model Depicting the Relationship between the Cell Wall and Mitochondria under Osmotic Stress Conditions.
Osmotic stress exerts at least two different effects on the plants: damage and adaption. Damage occurs through the depletion of CESA complexes from the plasma membrane, resulting in decreased cellulose production that in turn leads to cell wall weakening and loss of cell wall integrity. Adaptation occurs in response to inhibition of mitochondrial activity, which triggers retrograde signaling that eventually results in cell wall fortification. CESA inhibitors (including C17, isoxaben, and indaziflam) mimic the process of cell wall damage, whereas inhibition of mitochondrial activity can be simulated by mutations in mitochondrial editing genes (such as CWM1 and CWM2) or application of chemical inhibitors (such as AA). The presence of both CESA and mitochondrial inhibitors likely mimics osmotic stress conditions, in which a decrease in cellulose content is matched by retrograde-induced cell wall modifications. Cell wall, orange; mitochondria, green.
METHODS
Plant Materials and Growth Conditions
Arabidopsis thaliana plants were grown under long-day conditions (16 h of light/8 h of darkness) at 22°C on half-strength Murashige and Skoog (MS) germination medium (Murashige and Skoog, 1962). The cwm1-1 (SALK_017325C), cwm1-2 (SALK_124160), cwm1-3 (SALK_078133), cwm2-1 (SALK_020569C), cwm2-2 (SALK_027874), and SAIL_699_C11 were acquired from the ABRC. The otp439, bir6-2, ndufs4, je5, and anac017-1 mutants, the GFP-CESA3 line in the je5 background, and the GFP-CESA3 mCherry-MBD double fluorescence line have been described previously (de Longevialle et al., 2007; Desprez et al., 2007; Meyer et al., 2009; Koprivova et al., 2010; Ng et al., 2013a; Miart et al., 2014).
Chemical Treatments
AA and RO were purchased from Sigma-Aldrich and applied from 50 mM stock solutions in DMSO to the final concentration described in the text. Mock treatments of 0.1% (v/v) DMSO were used as a control. For the treatments of chemical compounds, plants were grown on the control medium for three days and then transferred to medium without or with the indicated drugs for 2 d. The 2-d elongation of seedling roots after treatment was measured.
Generation of Transgenic Arabidopsis Plants
Overexpressing plants were generated by cloning the open reading frame of ANAC017 into pK7WG2D (Karimi et al., 2002). Cloning primers are listed in Supplemental Table 1. Constructs were transformed into Arabidopsis Col-0 by Agrobacterium tumefaciens-mediated floral dipping (Clough and Bent, 1998).
Chemical Screen
The PSB-L_H2B-YFP cell line was obtained through cocultivation of the Arabidopsis PSB-L cell suspension culture (Landsberg erecta) (May and Leaver, 1993) with an Agrobacterium strain carrying a binary T-DNA vector containing a transgene encoding a translational fusion between Arabidopsis Histone 2B (AT5G22880) and Yellow Fluorescent Protein (H2B-YFP) (Boisnard-Lorig et al., 2001). Protoplasts were isolated from 100 mL 3-d-old Arabidopsis PSB-L_H2B-YFP cell suspension culture expressing the H2B-YFP transgene. Cells were harvested through sedimentation in 2 × 50 mL tubes (Corning) and removing the supernatants. After cell wall digestion with 100 mL cellulase (15 mg mL−1; Yakult Onozuka R10) in 0.4 M mannitol, 5 mM MES, pH 5.7, for 3 to 4 h at 25°C, 50 rpm in a large (142/20 mm) vented Petri dish (Greiner), isolated cells were transferred to 2 × 50 mL tubes (Corning) and washed with and resuspended in 25 mL MSMO containing 0.35 M mannitol, 0.5 mg L−1 NAA, and 0.05 mg L−1 kinetin using centrifugation at 145g. Protoplasts were then filtered through a 40-µm mesh and incubated overnight in the dark at room temperature for partial cell wall regeneration in MSMO containing 0.35 M mannitol, 0.5 mg L−1 NAA, and 0.05 mg L−1 kinetin. Dead cells were removed by sucrose gradient (0.5 M) decantation at 100g during 10 min. Subsequently, the protoplasts were washed in 2 × 40 mL and resuspended in 2 × 2.5-5 mL MSMO containing 0.35 M mannitol, 0.5 mg L−1 NAA, and 0.05 mg L−1 kinetin in 2 × 50 mL tubes. Cell density was estimated with a cell counter (Countess Automated Cell Counter; Invitrogen), and subsequently diluted to obtain a standard density of 105 cells mL−1. Diluted cells were then seeded in a Nunc 96-well CC2 Coverglass Bottom Plate (Thermo Scientific) at 100 μL per well using a Biomek 2000 robot (Beckman Coulter). Subsequently, compounds of the DIVERSet library (12,000 compounds) were added with a Te-MO robot (Tecan) to a final concentration of 50 µM (1% [v/v] DMSO). Plates were shaken for 3 min to homogenize the medium. Images of the plate were acquired using the Scan^R screening station from Olympus. This platform includes an epifluorescence microscope IX81 with a MT20 illumination system, an automated motorized stage and a CCD camera. YFP fluorescence was recorded using the fluorescein isothiocyanate parameters (YFP FITC, DAPI/FITC/TxRED filter cube, fluorescence illumination, FITC 492/18 excitation filter), the UPLSAPO 10× objective and an exposure time of 15 ms. Time-lapse movies were recorded with a resolution of 30 min between the pictures for up to 120 h. Because cells need between 60 and 90 min to divide in optimal conditions, two to three images were obtained capturing the mitotic figures of each cell. This time resolution allowed the acquisition of four pictures for each of the 96 wells, covering ∼10% of the well surfaces.
Screen of C17-Tolerant Mutants and Map-Based Cloning
To obtain mutants tolerant to C17, seeds from an EMS-treated seed collection with Col-0 background were plated on half-strength MS medium containing 2 μM C17. After growing for 7 d under long-day conditions (16 h of light/8 h of darkness) at 22°C, plants with a long root were identified as C17- tolerant mutants. From 300,000 independent EMS-mutagenized seeds (divided over 20 pools), 22 mutants were isolated (from 12 independent pools). To define the mutations underlying C17 tolerance, all C17- tolerant mutants were backcrossed with wild-type (Col-0) plants to obtain F1 progenies. F2 progenies from self-pollinated F1 plants were grown in the presence of 2 μM C17 for 7 d, allowing calculation of the segregation ratio of C17 tolerance within the F2 progenies. All mutants were used to generate mapping populations through crossing with another ecotype (Ler-0). SSLP markers (Supplemental Table 1) were used to map the position of the mutated genes in the Arabidopsis genome. Subsequently, the mutant genes were identified through candidate gene sequencing.
For the cwm mutant screen, seeds of a set of confirmed T-DNA lines (CS27941) were germinated on half-strength MS medium. Three-day-old seedlings were transferred to medium with a low dose of C17 (200 nM), a concentration at which root growth was inhibited instantly upon transfer to C17-containing medium. Two insertion lines (SALK_017325C and SALK_020569C) were obtained with significant suppression of the growth inhibition in presence of C17. Because the T-DNA insert within the AT5G44570 locus in SALK_020569C was not linked with the C17-tolerant phenotype, SALK_020569C was crossed with the Ler-0 ecotype to generate a mapping population. SSLP markers (Supplemental Table 1) were used to map the position of the mutated genes in the Arabidopsis genome. Subsequently, using PCR, a second T-DNA insert was found positioned at the coding region of AT1G32415.
Cellulose Measurement
The analysis of glucose content was performed on an AIR (alcohol-insoluble residue) prepared as follows. One hundred milligrams (fresh weight) of ground 4-d-old dark-grown seedlings were washed twice in 4 volumes of absolute ethanol for 15 min, then rinsed twice in 4 volumes of acetone at room temperature for 10 min and left to dry under a fume hood overnight at room temperature. AIR was then submitted to hydrolysis in 2.5 M trifluoroacetic acid for 1.5 h at 100°C. To determine the cellulose content, the residual pellet obtained after the trifluoroacetic acid hydrolysis was rinsed twice with 10 volumes of water and hydrolyzed with H2SO4 as described previously (Updegraff, 1969). The released glucose was diluted 500 times and then quantified using a high-performance anion exchange chromatography with pulsed amperometric detection as described previously (Harholt et al., 2006). Quantification was done with biological quadruplicates and the average values and standard errors are indicated in the graphs.
Detection of Cell Wall Weakening
For the detection of cell wall weakening, 3-d-old seedlings grown on half-strength MS medium were transferred to liquid medium without or with 200 nM C17. The root tips were stained with 10 mg mL−1 PI for 3 min. The stained root tips were put on the Nunc Lab-Tek Chambered Coverglass (catalog no. 155361) without pressure or on a microscope slide with a cover slip that gently exerted pressure. Using confocal laser scanning microscopy (LSM710; Zeiss), the brittle cells could be visualized by the uptake of PI.
Spinning-Disk Microscopy and Image Analysis
For live-cell imaging, hypocotyls of 3-d-old etiolated je5 seedlings harboring the GFP-CESA3 marker were treated without or with 50 µM C17 for 3 h were analyzed on an Axiovert 200M microscope (Zeiss) equipped with a Yokogawa CSU22 spinning disk, Zeiss 100/1.4 numerical aperture oil objective, and Andor EMCCD iXon DU 895 camera (Plateforme d'Imagerie Dynamique, Institut Pasteur, Paris, France). A 488-nm diode-pumped solid-state laser was used for excitation, and emission was collected using band-pass 488/25 for GFP. For root tip observations, 3-d-old light-grown seedlings were treated without or with the indicated drugs and the change of GFP were observed with a time-series scanning from 0 to 20 min.
Isolation of Mitochondria from Hydroponic Cultures
Mitochondria were isolated from 14-d-old hydroponically grown Arabidopsis plants as previously described (Millar et al., 2001) with slight modifications. Plant material was homogenized in grinding buffer (0.3 M sucrose, 25 mM tetrasodium pyrophosphate, 1% [w/v] PVP-40, 2 mM EDTA, 10 mM KH2PO4, 1% [w/v] BSA, and 20 mM ascorbic acid, pH 7.5) using mortar and pestle for 2 to 5 min twice. The homogenate was filtered through four layers of Miracloth and centrifuged at 2500g for 5 min; the resulting supernatant was then centrifuged at 14,000g for 20 min. The resulting pellet was resuspended in sucrose wash medium (0.3 M sucrose, 0.1% [w/v] BSA, and 10 mM TES, pH 7.5) and carefully layered over 35 mL PVP-40 gradient (30% Percoll, 0 to 4% PVP). The gradient was centrifuged at 40,000g for 40 min. The mitochondrial band was collected and washed three times in sucrose wash buffer without BSA by 20,000g for 20 min.
Oxygen Consumption of Whole Seedlings and Isolated Mitochondria
Oxygen consumption of 6-d-old Arabidopsis seedlings was measured using a computer-controlled Clark-type O2 electrode (Hansatech Instruments). All reactions were performed at 25°C using 2 mL of whole tissue reaction medium (10 mM HEPES, 10 mM MES, and 2 mM CaCl2, pH 7.2) and 40 to 60 mg plant material. To investigate AOX-dependent respiration rate, 1 mM KCN was added. To inhibit AOX activity, 2 mM SHAM was added.
To measure oxygen uptake of isolated mitochondria, 1 mL reaction medium (0.3 M sucrose, 10 mM TES, 10 mM NaCl, 4 mM MgSO4, and 0.1% [w/v] BSA, pH 7.2) together with 50 µg mitochondria protein was used. Then, 5 mM succinate and 1 mM NADH were added. To investigate the effect of C17 on isolated mitochondria, concentrations ranging from 1 to 8 µM were added to mitochondria using succinate and NADH as substrates and compared with the effect of 100 µM KCN as a cytochrome pathway inhibitor.
RNA Editing Analysis
For analysis of RNA editing, total RNA was isolated from the root tips (3 to 5 mm) of 5-d-old seedlings using an RNeasy plant mini kit (Qiagen) and treated with DNase I (Invitrogen). DNA-free RNA (2 μg) was reverse transcribed and sequences including the editing sites were amplified by PCR. Primers to amplify the mitochondrial transcripts are described previously (Bentolila et al., 2013). The RT-PCR products were sequenced immediately.
Isolation of Mitochondria and Immunoblots
For analysis of mitochondrial protein content through immunoblotting, mitochondria were isolated from 2-week-old hydroponically grown Arabidopsis seedlings, according to the method described previously (Murcha and Whelan, 2015). Mitochondrial proteins were separated by SDS-PAGE (Bio-Rad) or blue-native gels as described previously (Eubel et al., 2005), followed by transfer to Hybond-C extra nitrocellulose (Bio-Rad). Immunodetections were performed as described previously (Wang et al., 2012). To ensure linearity of detection, two dilutions of mitochondria were loaded. Antibodies used were raised against Ndufs4 (Meyer et al., 2009), AOX (Elthon et al., 1989), Tim9 (Wang et al., 2012), Tim17 (Wang et al., 2012), RISP (Duncan et al., 2011), and Tim23 (Wang et al., 2012). The antibodies against to Atpb (catalog number AS05 085), Cyt c (AS08 343A), and COXII (AS04 0543A) were obtained from Agrisera.
For mitochondrial complex staining, mitochondrial proteins (20 μg) were solubilized with digitonin (5.0 g/g protein final) in digitonin extraction buffer (30 mM HEPES. 150 mM K-Acetate, and 10% [v/v] glycerol, pH 7.4) and incubated on ice for 20 min. The samples were centrifuged for 10 min at 15,000g, and Serva Blue G (0.2% [v/v] final) was added to the supernatant. The samples were loaded onto NativePAGE Novex 4% to 16% Bis-Tris gels (Life Technologies). Gels were washed twice for 10 min with distilled water and incubated in complex I staining medium (0.1 M Tris, pH 7.4, 0.14 mM NADH, and 1 mg mL−1 Nitro tetrazolium blue) and in peroxidase staining medium (10 mM phosphate buffer, pH 6.0, 20 mM guaiacol, and 0.03% H2O2). After 2 to 3 h of staining, gels were transferred to Coomassie-colloidal fixing solution (40% methanol and 10% acetic acid) to stop the reactions.
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under the following accession numbers: CWM1 (AT1G17630), CWM2 (AT1G32415), CESA1 (AT4G32410), CESA3 (AT5G05170), ANAC017 (AT1G34190), and SLO2 (AT2G13600).
Supplemental Data
Supplemental Figure 1. Rough map position on the Arabidopsis genome of the mutated genes rendering C17 tolerance.
Supplemental Figure 2. Sequence alignment of CESA1 and CESA3 of several plant species.
Supplemental Figure 3. C17 does not trigger microtubule polymerization.
Supplemental Figure 4. C17 results in the depletion of CSCs from the root plasma membrane.
Supplemental Figure 5. Isolation of cwm1 and cwm2 mutants.
Supplemental Figure 6. Both cwm1-1 and cwm2-1 mutations counteract the growth inhibition induced by cellulose deficiency.
Supplemental Figure 7. Staining of respiratory protein complexes from the wild type, cwm1, and cwm2.
Supplemental Figure 8. Respiration rates of cwm1 and cwm2 mutants.
Supplemental Figure 9. C17 sensitivity of the mutants with defective mitochondrial complex I.
Supplemental Figure 10. Inhibition of mitochondrial activity did not restore CSC activity.
Supplemental Figure 11. C17 does not directly inhibit isolated mitochondrial respiration.
Supplemental Table 1. Primer sequences used for mapping and cloning.
Supplemental Movie 1. Time-lapse imaging of Arabidopsis H2B-YFP suspension cells in absence of C17.
Supplemental Movie 2. Time-lapse imaging of Arabidopsis H2B-YFP suspension cells in the presence of 50 µM C17.
Supplementary Material
Acknowledgments
We thank Annick Bleys for help preparing manuscript. This work was supported by grants from the Integrated Project AGRONOMICS, in the Sixth Framework Programme of the European Commission (LSHG-CT-2006-037704), Research Foundation-Flanders (G.0236.16N), and the Interuniversity Attraction Poles Programme (IUAP P7/29 “MARS”), initiated by the Belgian Science Policy Office. T.C. and I.D.C. are Postdoctoral Fellows of the Research Foundation-Flanders. I.D.C. is also supported by FWO travel grant 12N2415N. F.V.B. is supported by grants from the Interuniversity Attraction Poles Programme (IUAP P7/29 ‘MARS’) initiated by the Belgian Science Policy Office and Ghent University (Multidisciplinary Research Partnership ‘Biotechnology for a Sustainable Economy’, Grant 01MRB510W). A.H.M., K.B., Y.W., I.S., and J.W. were funded by the ARC Centre of Excellence Plant Energy Biology (CE140100008).
AUTHOR CONTRIBUTIONS
Z.H., J.W., H.H., and L.D.V. conceived and designed the research. Z.H., R.V., T.C., Y.W., I.D.C., O.L., K.B., G.M., and S.V. performed the experiments. Z.H., I.S., A.H.M., S.V., F.V.B., J.W., H.H., and L.D.V. analyzed the data. P.H. provided the platform for high-throughput chemical screen. Z.H. and L.D.V. wrote the article. All authors read, revised, and approved the article.
Glossary
- CSC
cellulose synthase complex
- ROS
reactive oxygen species
- SSLP
simple sequence length polymorphism
- PI
propidium iodide
- AOX
alternative oxidase
- AA
antimycin A
- RO
rotenone
- ER
endoplasmic reticulum
- MS
Murashige and Skoog
References
- Barkan A., Small I. (2014). Pentatricopeptide repeat proteins in plants. Annu. Rev. Plant Biol. 65: 415–442. [DOI] [PubMed] [Google Scholar]
- Bartoli C.G., Gómez F., Martínez D.E., Guiamet J.J. (2004). Mitochondria are the main target for oxidative damage in leaves of wheat (Triticum aestivum L.). J. Exp. Bot. 55: 1663–1669. [DOI] [PubMed] [Google Scholar]
- Beeckman T., Przemeck G.K.H., Stamatiou G., Lau R., Terryn N., De Rycke R., Inzé D., Berleth T. (2002). Genetic complexity of cellulose synthase a gene function in Arabidopsis embryogenesis. Plant Physiol. 130: 1883–1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentolila S., Knight W., Hanson M. (2010). Natural variation in Arabidopsis leads to the identification of REME1, a pentatricopeptide repeat-DYW protein controlling the editing of mitochondrial transcripts. Plant Physiol. 154: 1966–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentolila S., Oh J., Hanson M.R., Bukowski R. (2013). Comprehensive high-resolution analysis of the role of an Arabidopsis gene family in RNA editing. PLoS Genet. 9: e1003584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleier, L., and Dröse, S. (2013). Superoxide generation by complex III: from mechanistic rationales to functional consequences. Biochim. Biophys. Acta 1827: 1320–1331. [DOI] [PubMed] [Google Scholar]
- Boisnard-Lorig C., Colon-Carmona A., Bauch M., Hodge S., Doerner P., Bancharel E., Dumas C., Haseloff J., Berger F. (2001). Dynamic analyses of the expression of the HISTONE:YFP fusion protein in Arabidopsis show that syncytial endosperm is divided in mitotic domains. Plant Cell 13: 495–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brabham C., Lei L., Gu Y., Stork J., Barrett M., DeBolt S. (2014). Indaziflam herbicidal action: a potent cellulose biosynthesis inhibitor. Plant Physiol. 166: 1177–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caño-Delgado A., Penfield S., Smith C., Catley M., Bevan M. (2003). Reduced cellulose synthesis invokes lignification and defense responses in Arabidopsis thaliana. Plant J. 34: 351–362. [DOI] [PubMed] [Google Scholar]
- Caño-Delgado A.I., Metzlaff K., Bevan M.W. (2000). The eli1 mutation reveals a link between cell expansion and secondary cell wall formation in Arabidopsis thaliana. Development 127: 3395–3405. [DOI] [PubMed] [Google Scholar]
- Clough S.J., Bent A.F. (1998). Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16: 735–743. [DOI] [PubMed] [Google Scholar]
- Crowell E.F., Bischoff V., Desprez T., Rolland A., Stierhof Y.-D., Schumacher K., Gonneau M., Höfte H., Vernhettes S. (2009). Pausing of Golgi bodies on microtubules regulates secretion of cellulose synthase complexes in Arabidopsis. Plant Cell 21: 1141–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clercq I., et al. (2013). The membrane-bound NAC transcription factor ANAC013 functions in mitochondrial retrograde regulation of the oxidative stress response in Arabidopsis. Plant Cell 25: 3472–3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Longevialle A.F., Meyer E.H., Andrés C., Taylor N.L., Lurin C., Millar A.H., Small I.D. (2007). The pentatricopeptide repeat gene OTP43 is required for trans-splicing of the mitochondrial nad1 Intron 1 in Arabidopsis thaliana. Plant Cell 19: 3256–3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y., Srivastava R., Howell S.H. (2013). Endoplasmic reticulum (ER) stress response and its physiological roles in plants. Int. J. Mol. Sci. 14: 8188–8212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desprez T., Vernhettes S., Fagard M., Refrégier G., Desnos T., Aletti E., Py N., Pelletier S., Höfte H. (2002). Resistance against herbicide isoxaben and cellulose deficiency caused by distinct mutations in same cellulose synthase isoform CESA6. Plant Physiol. 128: 482–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desprez T., Juraniec M., Crowell E.F., Jouy H., Pochylova Z., Parcy F., Höfte H., Gonneau M., Vernhettes S. (2007). Organization of cellulose synthase complexes involved in primary cell wall synthesis in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 104: 15572–15577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan O., Taylor N.L., Carrie C., Eubel H., Kubiszewski-Jakubiak S., Zhang B., Narsai R., Millar A.H., Whelan J. (2011). Multiple lines of evidence localize signaling, morphology, and lipid biosynthesis machinery to the mitochondrial outer membrane of Arabidopsis. Plant Physiol. 157: 1093–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis C., Turner J.G. (2001). The Arabidopsis mutant cev1 has constitutively active jasmonate and ethylene signal pathways and enhanced resistance to pathogens. Plant Cell 13: 1025–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis C., Karafyllidis I., Wasternack C., Turner J.G. (2002). The Arabidopsis mutant cev1 links cell wall signaling to jasmonate and ethylene responses. Plant Cell 14: 1557–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elthon T.E., Nickels R.L., McIntosh L. (1989). Monoclonal antibodies to the alternative oxidase of higher plant mitochondria. Plant Physiol. 89: 1311–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endler A., Persson S. (2011). Cellulose synthases and synthesis in Arabidopsis. Mol. Plant 4: 199–211. [DOI] [PubMed] [Google Scholar]
- Eubel H., Braun H.-P., Millar A.H. (2005). Blue-native PAGE in plants: a tool in analysis of protein-protein interactions. Plant Methods 1: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita M., Fujita Y., Noutoshi Y., Takahashi F., Narusaka Y., Yamaguchi-Shinozaki K., Shinozaki K. (2006). Crosstalk between abiotic and biotic stress responses: a current view from the points of convergence in the stress signaling networks. Curr. Opin. Plant Biol. 9: 436–442. [DOI] [PubMed] [Google Scholar]
- Gechev T.S., Van Breusegem F., Stone J.M., Denev I., Laloi C. (2006). Reactive oxygen species as signals that modulate plant stress responses and programmed cell death. BioEssays 28: 1091–1101. [DOI] [PubMed] [Google Scholar]
- Giraud E., Van Aken O., Ho L.H.M., Whelan J. (2009). The transcription factor ABI4 is a regulator of mitochondrial retrograde expression of ALTERNATIVE OXIDASE1a. Plant Physiol. 150: 1286–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraud E., Ho L.H.M., Clifton R., Carroll A., Estavillo G., Tan Y.-F., Howell K.A., Ivanova A., Pogson B.J., Millar A.H., Whelan J. (2008). The absence of ALTERNATIVE OXIDASE1a in Arabidopsis results in acute sensitivity to combined light and drought stress. Plant Physiol. 147: 595–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez R., Lindeboom J.J., Paredez A.R., Emons A.M.C., Ehrhardt D.W. (2009). Arabidopsis cortical microtubules position cellulose synthase delivery to the plasma membrane and interact with cellulose synthase trafficking compartments. Nat. Cell Biol. 11: 797–806. [DOI] [PubMed] [Google Scholar]
- Hamann T. (2015). The plant cell wall integrity maintenance mechanism-concepts for organization and mode of action. Plant Cell Physiol. 56: 215–223. [DOI] [PubMed] [Google Scholar]
- Harholt J., Jensen J.K., Sørensen S.O., Orfila C., Pauly M., Scheller H.V. (2006). ARABINAN DEFICIENT 1 is a putative arabinosyltransferase involved in biosynthesis of pectic arabinan in Arabidopsis. Plant Physiol. 140: 49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J., Duan Y., Hua D., Fan G., Wang L., Liu Y., Chen Z., Han L., Qu L.J., Gong Z. (2012). DEXH box RNA helicase-mediated mitochondrial reactive oxygen species production in Arabidopsis mediates crosstalk between abscisic acid and auxin signaling. Plant Cell 24: 1815–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hématy K., Sado P.-E., Van Tuinen A., Rochange S., Desnos T., Balzergue S., Pelletier S., Renou J.-P., Höfte H. (2007). A receptor-like kinase mediates the response of Arabidopsis cells to the inhibition of cellulose synthesis. Curr. Biol. 17: 922–931. [DOI] [PubMed] [Google Scholar]
- Howell S.H. (2013). Endoplasmic reticulum stress responses in plants. Annu. Rev. Plant Biol. 64: 477–499. [DOI] [PubMed] [Google Scholar]
- Huang S., Van Aken O., Schwarzlander M., Belt K., Millar A.H. (2016). Roles of mitochondrial reactive oxygen species in cellular signalling and stress response in plants. Plant Physiol. 171: 1551–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi M., Inzé D., Depicker A. (2002). GATEWAY vectors for Agrobacterium-mediated plant transformation. Trends Plant Sci. 7: 193–195. [DOI] [PubMed] [Google Scholar]
- Koprivova A., des Francs-Small C.C., Calder G., Mugford S.T., Tanz S., Lee B.R., Zechmann B., Small I., Kopriva S. (2010). Identification of a pentatricopeptide repeat protein implicated in splicing of intron 1 of mitochondrial nad7 transcripts. J. Biol. Chem. 285: 32192–32199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranz R.G., Richard-Fogal C., Taylor J.-S., Frawley E.R. (2009). Cytochrome c biogenesis: mechanisms for covalent modifications and trafficking of heme and for heme-iron redox control. Microbiol. Mol. Biol. Rev. 73: 510–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei L., Singh A., Bashline L., Li S., Yingling Y.G., Gu Y. (2015). CELLULOSE SYNTHASE INTERACTIVE1 is required for fast recycling of cellulose synthase complexes to the plasma membrane in Arabidopsis. Plant Cell 27: 2926–2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., He J., Chen Z., Ren X., Hong X., Gong Z. (2010). ABA overly-sensitive 5 (ABO5), encoding a pentatricopeptide repeat protein required for cis-splicing of mitochondrial nad2 intron 3, is involved in the abscisic acid response in Arabidopsis. Plant J. 63: 749–765. [DOI] [PubMed] [Google Scholar]
- Mavridou D.A.I., Ferguson S.J., Stevens J.M. (2013). Cytochrome c assembly. IUBMB Life 65: 209–216. [DOI] [PubMed] [Google Scholar]
- May M.J., Leaver C.J. (1993). Oxidative stimulation of glutathione synthesis in Arabidopsis thaliana suspension cultures. Plant Physiol. 103: 621–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlane H.E., Döring A., Persson S. (2014). The cell biology of cellulose synthesis. Annu. Rev. Plant Biol. 65: 69–94. [DOI] [PubMed] [Google Scholar]
- Meyer E.H., Tomaz T., Carroll A.J., Estavillo G., Delannoy E., Tanz S.K., Small I.D., Pogson B.J., Millar A.H. (2009). Remodeled respiration in ndufs4 with low phosphorylation efficiency suppresses Arabidopsis germination and growth and alters control of metabolism at night. Plant Physiol. 151: 603–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miart F., Desprez T., Biot E., Morin H., Belcram K., Höfte H., Gonneau M., Vernhettes S. (2014). Spatio-temporal analysis of cellulose synthesis during cell plate formation in Arabidopsis. Plant J. 77: 71–84. [DOI] [PubMed] [Google Scholar]
- Millar A.H., Sweetlove L.J., Giegé P., Leaver C.J. (2001). Analysis of the Arabidopsis mitochondrial proteome. Plant Physiol. 127: 1711–1727. [PMC free article] [PubMed] [Google Scholar]
- Millar A.H., Whelan J., Soole K.L., Day D.A. (2011). Organization and regulation of mitochondrial respiration in plants. Annu. Rev. Plant Biol. 62: 79–104. [DOI] [PubMed] [Google Scholar]
- Murashige T., Skoog F. (1962). A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 15: 473–497. [Google Scholar]
- Murcha M.W., Whelan J. (2015). Isolation of intact mitochondria from the model plant species Arabidopsis thaliana and Oryza sativa. Methods Mol. Biol. 1305: 1–12. [DOI] [PubMed] [Google Scholar]
- Ng S., De Clercq I., Van Aken O., Law S.R., Ivanova A., Willems P., Giraud E., Van Breusegem F., Whelan J. (2014). Anterograde and retrograde regulation of nuclear genes encoding mitochondrial proteins during growth, development, and stress. Mol. Plant 7: 1075–1093. [DOI] [PubMed] [Google Scholar]
- Ng S., et al. (2013a). A membrane-bound NAC transcription factor, ANAC017, mediates mitochondrial retrograde signaling in Arabidopsis. Plant Cell 25: 3450–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng S., et al. (2013b). Cyclin-dependent kinase E1 (CDKE1) provides a cellular switch in plants between growth and stress responses. J. Biol. Chem. 288: 3449–3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson S., Paredez A., Carroll A., Palsdottir H., Doblin M., Poindexter P., Khitrov N., Auer M., Somerville C.R. (2007). Genetic evidence for three unique components in primary cell-wall cellulose synthase complexes in Arabidopsis. Proc. Natl. Acad. Sci. USA 104: 15566–15571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoads D.M., Subbaiah C.C. (2007). Mitochondrial retrograde regulation in plants. Mitochondrion 7: 177–194. [DOI] [PubMed] [Google Scholar]
- Rhoads D.M., Umbach A.L., Subbaiah C.C., Siedow J.N. (2006). Mitochondrial reactive oxygen species. Contribution to oxidative stress and interorganellar signaling. Plant Physiol. 141: 357–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz-Linneweber C., Small I. (2008). Pentatricopeptide repeat proteins: a socket set for organelle gene expression. Trends Plant Sci. 13: 663–670. [DOI] [PubMed] [Google Scholar]
- Skirycz A., De Bodt S., Obata T., De Clercq I., Claeys H., De Rycke R., Andriankaja M., Van Aken O., Van Breusegem F., Fernie A.R., Inzé D. (2010). Developmental stage specificity and the role of mitochondrial metabolism in the response of Arabidopsis leaves to prolonged mild osmotic stress. Plant Physiol. 152: 226–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava R., Deng Y., Shah S., Rao A.G., Howell S.H. (2013). BINDING PROTEIN is a master regulator of the endoplasmic reticulum stress sensor/transducer bZIP28 in Arabidopsis. Plant Cell 25: 1416–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkov A.A. (2008). The role of mitochondria in reactive oxygen species metabolism and signaling. Ann. N. Y. Acad. Sci. 1147: 37–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung T.-Y., Tseng C.-C., Hsieh M.-H. (2010). The SLO1 PPR protein is required for RNA editing at multiple sites with similar upstream sequences in Arabidopsis mitochondria. Plant J. 63: 499–511. [DOI] [PubMed] [Google Scholar]
- Takenaka M. (2010). MEF9, an E-subclass pentatricopeptide repeat protein, is required for an RNA editing event in the nad7 transcript in mitochondria of Arabidopsis. Plant Physiol. 152: 939–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takenaka M., Verbitskiy D., Zehrmann A., Brennicke A. (2010). Reverse genetic screening identifies five E-class PPR proteins involved in RNA editing in mitochondria of Arabidopsis thaliana. J. Biol. Chem. 285: 27122–27129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenhaken R. (2015). Cell wall remodeling under abiotic stress. Front. Plant Sci. 5: 771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Updegraff D.M. (1969). Semimicro determination of cellulose in biological materials. Anal. Biochem. 32: 420–424. [DOI] [PubMed] [Google Scholar]
- Van Aken O., Zhang B., Law S., Narsai R., Whelan J. (2013). AtWRKY40 and AtWRKY63 modulate the expression of stress-responsive nuclear genes encoding mitochondrial and chloroplast proteins. Plant Physiol. 162: 254–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Aken, O., De Clercq, I., Ivanova, A., Law, S.R., Van Breusegem, F., Millar, A.H., and Whelan, J. (2016). Mitochondrial and chloroplast stress responses are modulated in distinct touch and chemical inhibition phases in Arabidopsis. Plant Physiol. 171: 2150–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandavasi V.G., et al. (2016). A structural study of CESA1 catalytic domain of Arabidopsis cellulose synthesis complex: evidence for CESA trimers. Plant Physiol. 170: 123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderauwera S., Vandenbroucke K., Inzé A., van de Cotte B., Mühlenbock P., De Rycke R., Naouar N., Van Gaever T., Van Montagu M.C., Van Breusegem F. (2012). AtWRKY15 perturbation abolishes the mitochondrial stress response that steers osmotic stress tolerance in Arabidopsis. Proc. Natl. Acad. Sci. USA 109: 20113–20118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanlerberghe G.C. (2013). Alternative oxidase: a mitochondrial respiratory pathway to maintain metabolic and signaling homeostasis during abiotic and biotic stress in plants. Int. J. Mol. Sci. 14: 6805–6847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbitskiy D., Zehrmann A., van der Merwe J.A., Brennicke A., Takenaka M. (2010). The PPR protein encoded by the LOVASTATIN INSENSITIVE 1 gene is involved in RNA editing at three sites in mitochondria of Arabidopsis thaliana. Plant J. 61: 446–455. [DOI] [PubMed] [Google Scholar]
- Wang Y., Carrie C., Giraud E., Elhafez D., Narsai R., Duncan O., Whelan J., Murcha M.W. (2012). Dual location of the mitochondrial preprotein transporters B14.7 and Tim23-2 in complex I and the TIM17:23 complex in Arabidopsis links mitochondrial activity and biogenesis. Plant Cell 24: 2675–2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welchen, E., Hildebrandt, T.M., Lewejohann, D., Gonzalez, D.H., and Braun, H.-P. (2012). Lack of cytochrome c in Arabidopsis decreases stability of Complex IV and modifies redox metabolism without affecting Complexes I and III. Biochim. Biophys. Acta 1817: 990–1001. [DOI] [PubMed] [Google Scholar]
- Wolf S., Hématy K., Höfte H. (2012). Growth control and cell wall signaling in plants. Annu. Rev. Plant Biol. 63: 381–407. [DOI] [PubMed] [Google Scholar]
- Wu J., et al. (2015). Deficient plastidic fatty acid synthesis triggers cell death by modulating mitochondrial reactive oxygen species. Cell Res. 25: 621–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y., Lei L., Brabham C., Stork J., Strickland J., Ladak A., Gu Y., Wallace I., DeBolt S. (2014). Acetobixan, an inhibitor of cellulose synthesis identified by microbial bioprospecting. PLoS One 9: e95245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Zhang J., He J., Qin Y., Hua D., Duan Y., Chen Z., Gong Z. (2014). ABA-mediated ROS in mitochondria regulate root meristem activity by controlling PLETHORA expression in Arabidopsis. PLoS Genet. 10: e1004791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehrmann A., Verbitskiy D., van der Merwe J.A., Brennicke A., Takenaka M. (2009). A DYW domain-containing pentatricopeptide repeat protein is required for RNA editing at multiple sites in mitochondria of Arabidopsis thaliana. Plant Cell 21: 558–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J., Lee B.H., Dellinger M., Cui X., Zhang C., Wu S., Nothnagel E.A., Zhu J.K. (2010). A cellulose synthase-like protein is required for osmotic stress tolerance in Arabidopsis. Plant J. 63: 128–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q., Dugardeyn J., Zhang C., Mühlenbock P., Eastmond P.J., Valcke R., De Coninck B., Oden S., Karampelias M., Cammue B.P., Prinsen E., Van Der Straeten D. (2014). The Arabidopsis thaliana RNA editing factor SLO2, which affects the mitochondrial electron transport chain, participates in multiple stress and hormone responses. Mol. Plant 7: 290–310. [DOI] [PubMed] [Google Scholar]
- Zsigmond L., Rigó G., Szarka A., Székely G., Otvös K., Darula Z., Medzihradszky K.F., Koncz C., Koncz Z., Szabados L. (2008). Arabidopsis PPR40 connects abiotic stress responses to mitochondrial electron transport. Plant Physiol. 146: 1721–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.